Antisolvent Crystallization of Papain
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
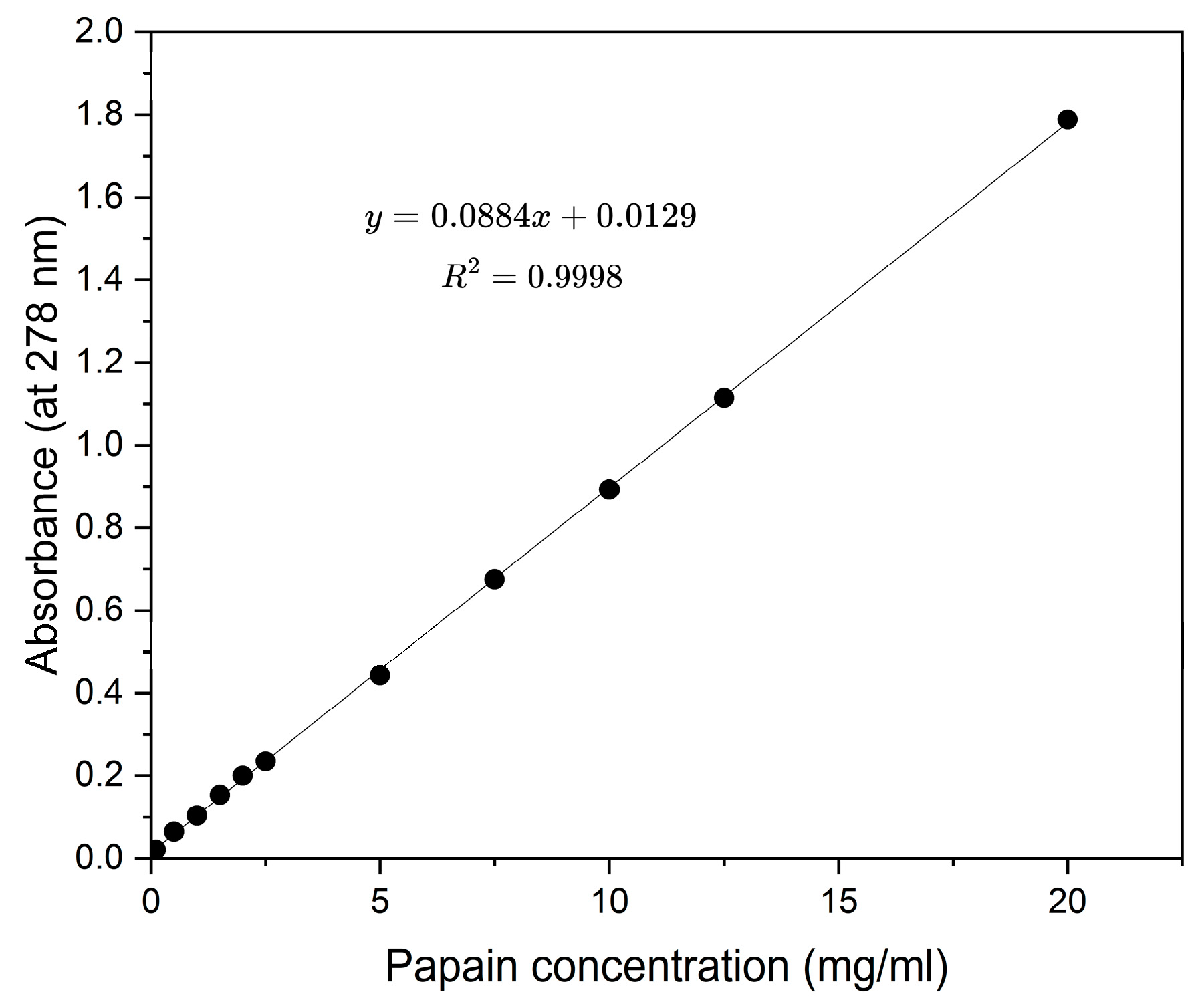
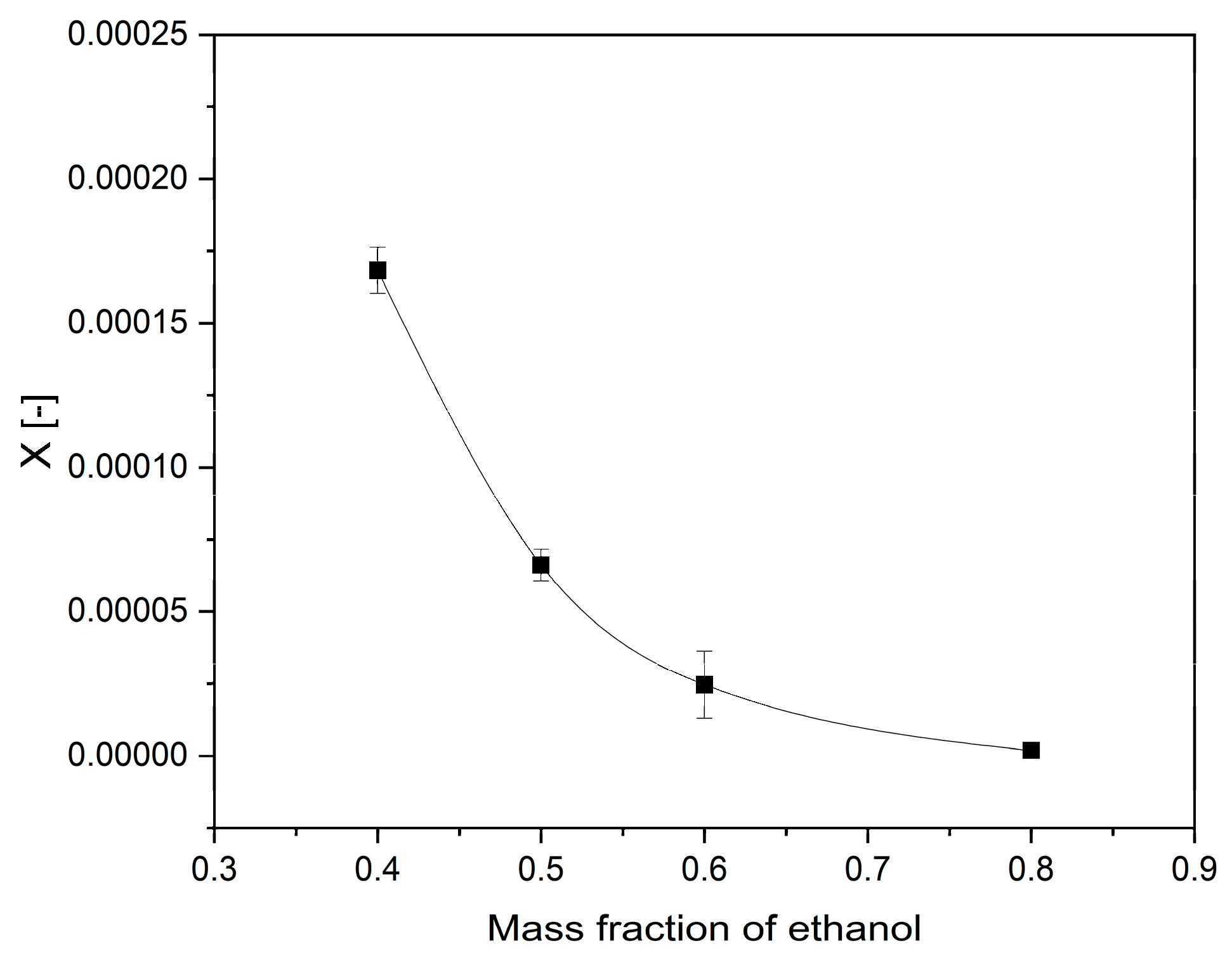
2.2. Solubility Measurement
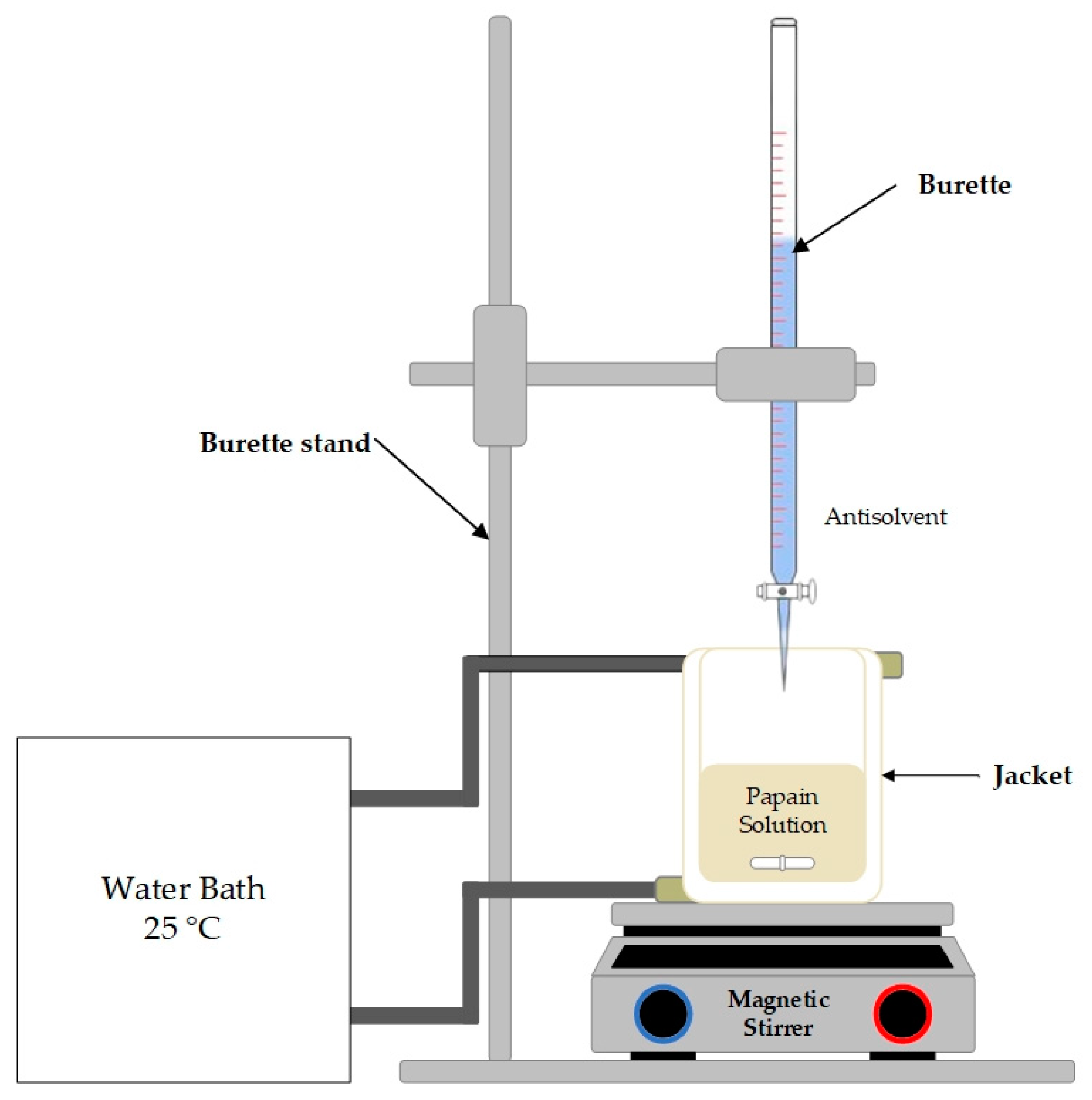
2.3. Crystallization Experiment
2.4. Crystallization Yield
2.5. Residual Activity
2.6. Particle Characterization
3. Results and Discussion
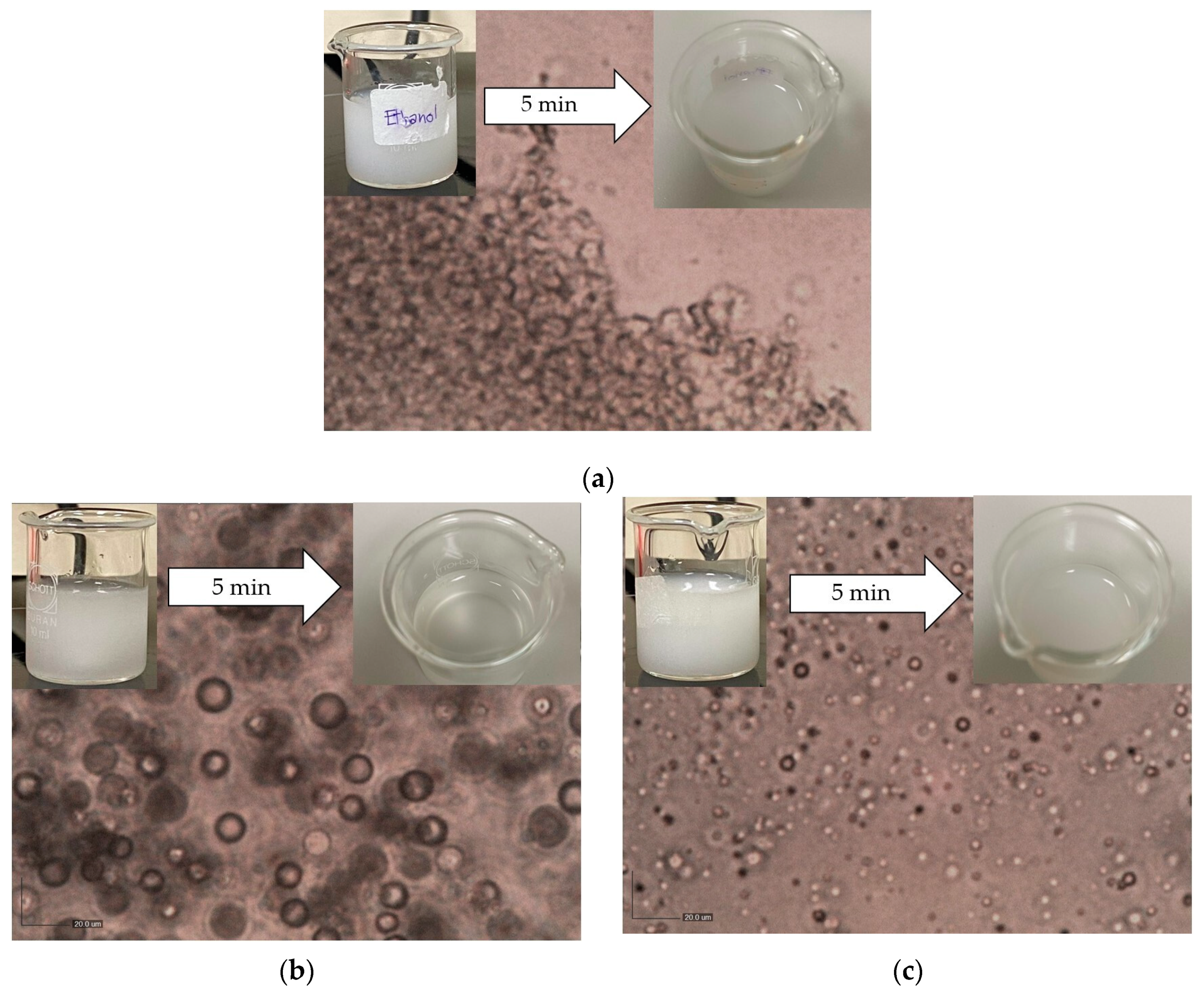
3.1. The Effect of Antisolvent Types
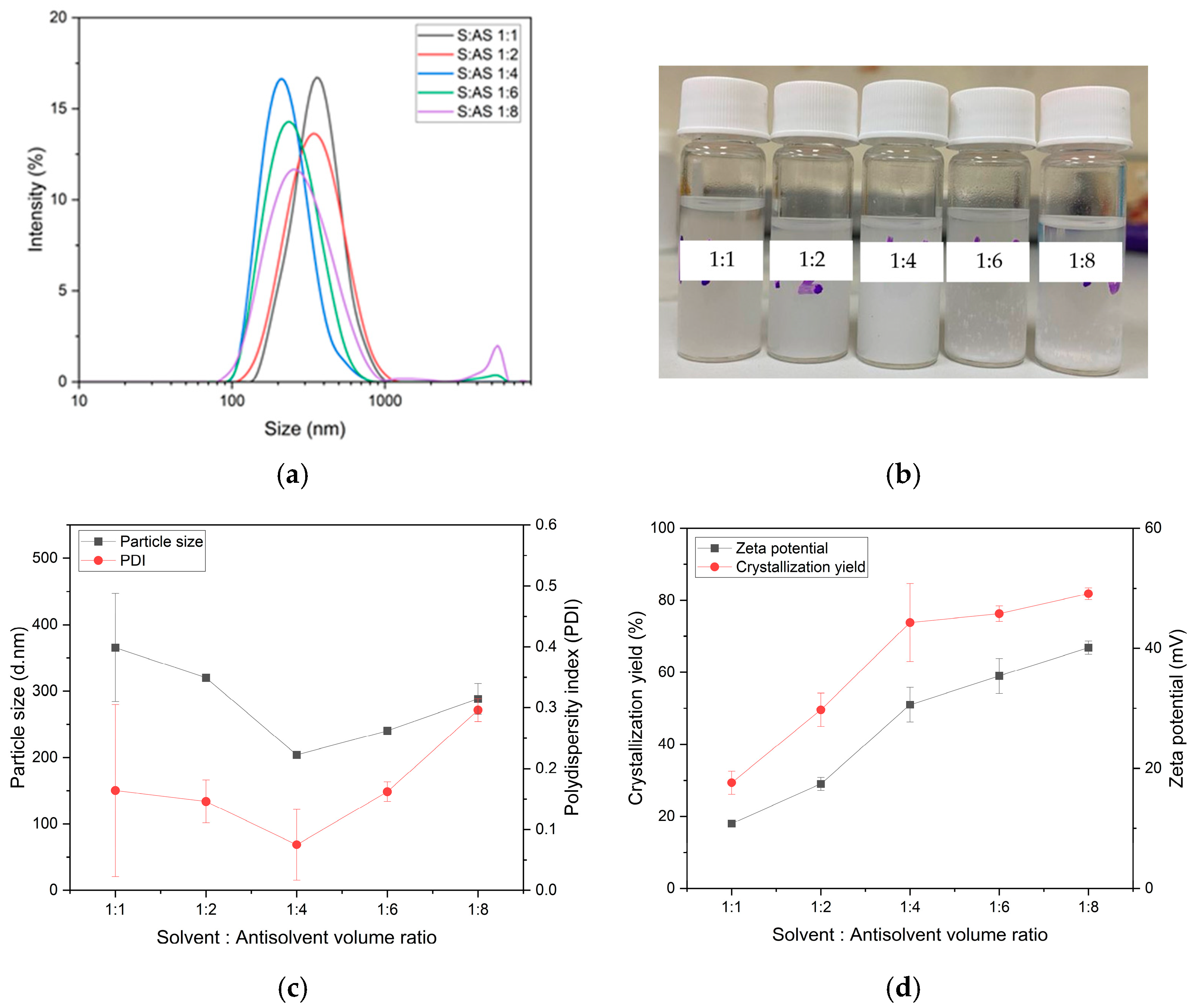
3.2. The Effect of Solvent-to-Antisolvent Volume Ratios
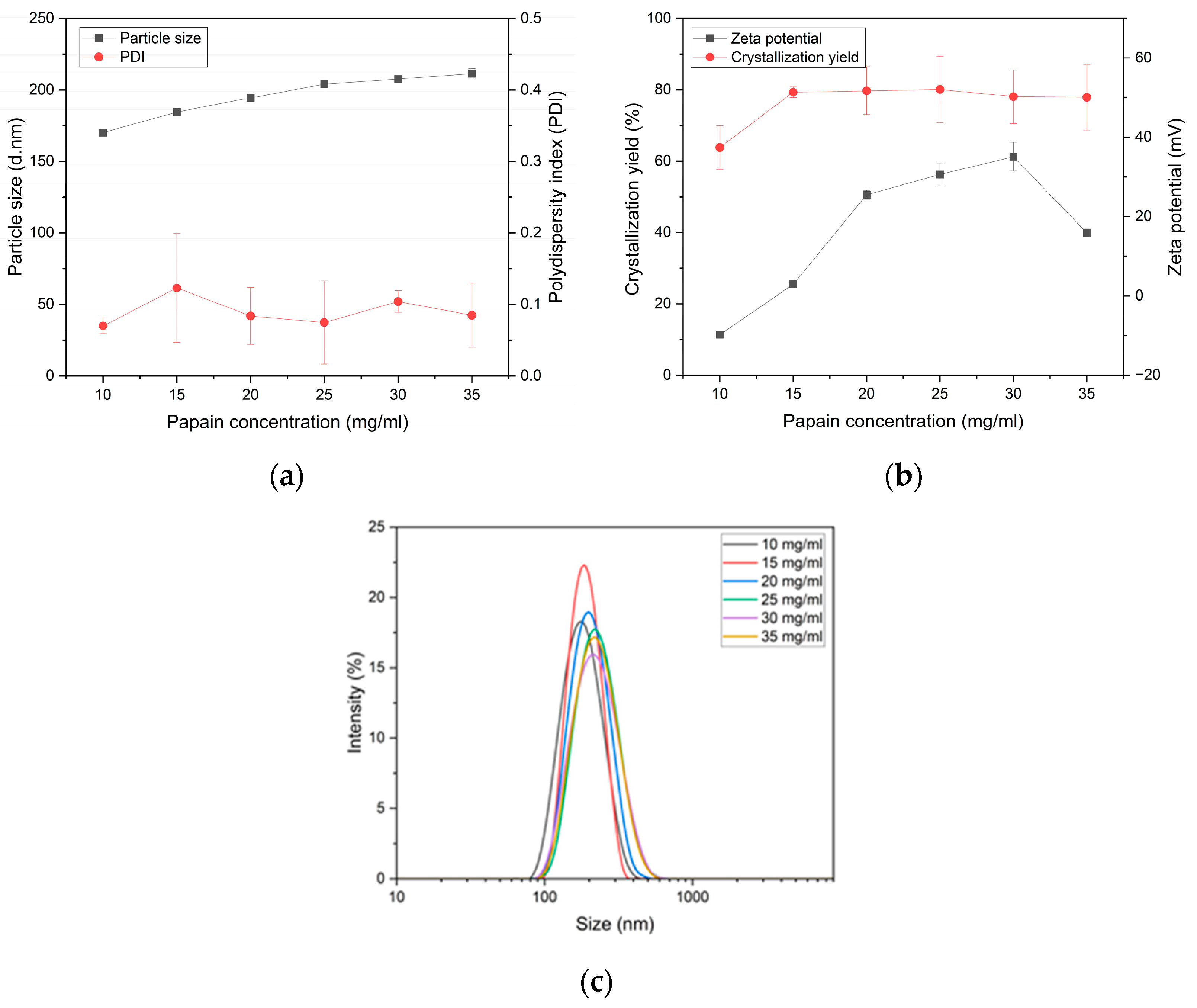
3.3. The Effect of Papain Concentration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Zalaki, E. Preparation and properties of papain precipitated from fresh latex of papaya fruits (Carica papaya). Alex. J. Food Sci. Technol. 2021, 18, 27–32. [Google Scholar] [CrossRef]
- Budama-Kilinc, Y.; Cakir-Koc, R.; Kecel-Gunduz, S.; Zorlu, T.; Kokcu, Y.; Bicak, B.; Karavelioglu, Z.; Ozel, A.E. Papain loaded poly(epsilon-caprolactone) nanoparticles: In-silico and in-vitro studies. J. Fluoresc. 2018, 28, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Shouket, H.; Ameen, I.; Tursunov, O.; Kholikova, K.; Pirimov, O.; Kurbonov, N.; Ibragimov, I.; Mukimov, B. Study on industrial applications of papain: A succinct review. IOP Conf. Ser. Earth Environ. Sci. 2020, 614, 012171. [Google Scholar] [CrossRef]
- Moreira Filho, R.N.F.; Vasconcelos, N.F.; Andrade, F.K.; Rosa, M.F.; Vieira, R.S. Papain immobilized on alginate membrane for wound dressing application. Colloids Surf. B Biointerfaces 2020, 194, 111222. [Google Scholar] [CrossRef] [PubMed]
- Amri, E.; Mamboya, F. Papain, a plant enzyme of biological importance: A review. Am. J. Biochem. Biotechnol. 2012, 8, 99–104. [Google Scholar]
- dos Anjos, M.M.; da Silva, A.A.; de Pascoli, I.C.; Mikcha, J.M.G.; Machinski, M.; Peralta, R.M.; de Abreu Filho, B.A. Antibacterial activity of papain and bromelain on Alicyclobacillus spp. Int. J. Food Microbiol. 2016, 216, 121–126. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.R.; Oliveira, M.B.; Motta, E.S.; de Almeida, G.S.; Varanda, L.L.; de Padula, M.; Leitao, A.C.; Caldeira-de-Araujo, A. Genotoxic and cytotoxic safety evaluation of papain (Carica papaya L.) using in vitro assays. J. Biomed. Biotech. 2010, 2010, 197898. [Google Scholar]
- Muss, C.; Mosgoeller, W.; Endler, T. Papaya preparation (Caricol®) in digestive disorders. Neuro Endocrinol. Lett. 2013, 34, 38–46. [Google Scholar]
- Patel, A.; Cholkar, K.; Mitra, A.K. Recent developments in protein and peptide parenteral delivery approaches. Ther. Deliv. 2014, 5, 337–365. [Google Scholar] [CrossRef]
- Arisanti, C.I.S.; Rachmawati, H.; Pamudji, J.S.; Sumirtapura, Y.C. Chitosan reinforced alginate microcapsules retained the release of papain in simulated gastric fluid. J. Pharm. Sci. Appl. 2012, 1, 47–61. [Google Scholar]
- Chandran, S.P.; Nachinmuthu, K.P.; Natarajan, S.B.; Inamdar, M.G.; Shahimi, M.S.B.M. Papain loaded solid lipid nanoparticles for colorectal cancer therapy. Curr. Cancer Ther. Rev. 2018, 14, 75–87. [Google Scholar] [CrossRef]
- Basu, S.K.; Govardhan, C.P.; Jung, C.W.; Margolin, A.L. Protein crystals for the delivery of biopharmaceuticals. Expert Opin. Biol. Ther. 2004, 4, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.D.; Mallet, F.P.; Ricard, F.; Heng, J.Y.Y. Pharmaceutical nanocrystals. Curr. Opin. Chem. Eng. 2012, 1, 102–107. [Google Scholar] [CrossRef]
- Hartje, L.F.; Snow, C.D. Protein crystal based materials for nanoscale applications in medicine and biotechnology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1547. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, Y.; Sun, J. Heterogeneous nucleation in protein crystallization. Biomimetics 2023, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Myerson, A.S. Handbook of Industrial Crystallization; Butterworth-Heinemann: Oxford, UK, 2002. [Google Scholar]
- Teng, H.; Chen, L.; Lee, W.Y. Anti-solvent crystallization of l-alanine and effects of process parameters and ultrasound. Food Sci. Technol. Res. 2017, 23, 495–502. [Google Scholar] [CrossRef][Green Version]
- Lonare, A.A.; Patel, S.R. Antisolvent crystallization of poorly water soluble drugs. Int. J. Chem. Eng. Appl. 2013, 4, 337–341. [Google Scholar] [CrossRef]
- Park, M.W.; Yeo, S.D. Antisolvent crystallization of roxithromycin and the effect of ultrasound. Sep. Sci. Technol. 2010, 45, 1402–1410. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Production of nanoparticles by anti-solvent precipitation for use in food systems. Trends Food Sci. Technol. 2013, 34, 109–1230. [Google Scholar] [CrossRef]
- de Boer, F.Y.; Imhof, A.; Velikov, K.P. Encapsulation of colorants by natural polymers for food applications. Color. Technol. 2019, 135, 183–194. [Google Scholar] [CrossRef]
- Nelemans, L.C.; Buzgo, M.; Simaite, A. Optimization of protein precipitation for high-loading drug delivery systems for immunotherapeutics. In Proceedings of the 1st International Electronic Conference on Pharmaceutics, Online, 10–15 December 2020; MDPI: Basel, Switzerland, 2020. [Google Scholar]
- Andrade-Mahecha, M.M.; Morales-Rodriguez, O.; Martinez-Correa, H.A. Study of the extraction process of papain from the latex of the fruit of papaya (Carica papaya L.) cv. Maradol. Acta Agron. 2011, 60, 218–224. [Google Scholar]
- Morales-Cruz, M.; Flores-Fernandez, G.M.; Morales-Cruz, M.; Orellano, E.A.; Rodriguez-Martinez, J.A.; Ruiz, M.; Griebenow, K. Two-step nanoprecipitation for the production of protein-loaded PLGA nanospheres. Results Pharma. Sci. 2012, 2, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Kongsamai, P.; Phoumixay, C.; Wantha, L. Experiments and correlations of the solubility of γ-DL-methionine in binary solvent mixtures. Chem. Eng. Technol. 2020, 43, 1079–1086. [Google Scholar] [CrossRef]
- Qureshi, A.; Vyas, J.; Upadhyay, U. Determination of solubility by gravimetric method: A brief review. Nat. J. Pharm. 2022, 2, 1–3. [Google Scholar]
- Arnon, R. Papain. In Methods in Enzymology; Perman, G.E., Lorand, L., Eds.; Elsevier: New York, NY, USA, 1970; Volume 19, pp. 226–244. [Google Scholar]
- Moreno-Cortez, I.E.; Romero-Garcia, J.; Gonzalez-Gonzalez, V.; Garcia-Gutierrez, D.I.; Garza-Navarro, M.A.; Cruz-Silva, R. Encapsulation and immobilization of papain in electrospun nanofibrous membranes of PVA cross-linked with glutaraldehyde vapor. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 52, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Mahajan, A.; Ramana, E. Patents on magnetoelectric multiferroics and their processing by electrophoretic deposition. Recent Pat. Mater. Sci. 2014, 7, 109–130. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Babaei, Z. Protein nanoparticle: A unique system as drug delivery vehicles. Afr. J. Biotechnol. 2008, 7, 604. [Google Scholar]

| Antisolvent | Crystallization Yield (%) | Residual Activity (%) |
|---|---|---|
| Acetonitrile | 98.67 ± 1.15 | N/A ** |
| Acetone | 89.33 ± 2.31 | 25.16 ± 5.47 |
| Ethanol | 80.10 ± 9.33 | 82.47 ± 7.00 |
| Concentration (mg/mL) | Residual Activity (%) |
|---|---|
| 25 | 89.68 ± 0.57 |
| 30 | 100 ± 6.55 |
| 35 | 90.87 ± 2.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonkerd, S.; Wantha, L. Antisolvent Crystallization of Papain. ChemEngineering 2024, 8, 4. https://doi.org/10.3390/chemengineering8010004
Boonkerd S, Wantha L. Antisolvent Crystallization of Papain. ChemEngineering. 2024; 8(1):4. https://doi.org/10.3390/chemengineering8010004
Chicago/Turabian StyleBoonkerd, Sasitorn, and Lek Wantha. 2024. "Antisolvent Crystallization of Papain" ChemEngineering 8, no. 1: 4. https://doi.org/10.3390/chemengineering8010004
APA StyleBoonkerd, S., & Wantha, L. (2024). Antisolvent Crystallization of Papain. ChemEngineering, 8(1), 4. https://doi.org/10.3390/chemengineering8010004






