Abstract
Vegetable oil methyl ester has promising properties for bio-based resin production due to its higher degree of unsaturation. The initial low methyl ester yield from corn oil compared to soybean and canola oils requires further investigation of the influence of neutralization at the end of the transesterification reaction. To evaluate the neutralization effect with HCl, corn, canola, and soybean oil were transesterified using NaOH at 60 °C with a 6:1 methanol–oil ratio. This research also investigated the effect of reaction times (0.5–1.5 h) with varying neutralization levels (0–100%) on the corn oil methyl ester yield. The yield of corn, canola, and soybean methyl ester was increased significantly by 16–25% through neutralization, indicating the positive impact of neutralization. The corn oil methyl ester yield ranged from 45 to 79% across different neutralization levels and reaction times. With 25% neutralization, the yield increased by 20%. On the other hand, the yield reduced by 18–24% over time when there was no neutralization. A statistical model was developed where the yield varied significantly with the acid amount, reaction time, and their interactions. The quality of the corn methyl ester was found to be within the limits of standard pure methyl ester. Overall, the effect of neutralization showed promise in increasing the yield of quality methyl ester from commercial corn oil.
Keywords:
corn oil; methyl ester; transesterification; yield recovery; neutralization; reaction time 1. Introduction
There has been growing interest in using sustainable and eco-friendly materials, chemicals, and energy. One of the latest advancements in this area is the synthesis of biodegradable and non-toxic resins derived from renewable resources. Vegetable oils are an excellent choice for this purpose due to their functionality and ease of biodegradation [1]. Bio-based resins, used to make biomaterials, are produced through the epoxidation of unsaturated double bonds in vegetable oils or their fatty acid methyl esters. Currently, the production of fatty acid methyl esters, also known as biodiesel, is obtaining significant attention as an environmentally friendly fuel. Additionally, the use of fatty acid methyl esters in epoxy resin production has numerous opportunities in coatings and applications of thermoset materials [2]. The transesterification of vegetable oils is the most effective process for transforming triglyceride molecules into fatty acid methyl esters. As a renewable resource, vegetable oils, especially corn oil (usually rich in oleic and linoleic acids), can be epoxidized using hydrogen peroxide in the presence of either acetic acid or formic acid. The same reaction principle can be applied to the methyl ester of corn oil to produce a bio-based resin. However, high methyl ester yields are crucial for the large-scale production of bioresin, ensuring that the process is cost-effective and competes favorably with petrochemical alternatives.
The transesterification process involves three catalyzed steps that use triacylglycerols in vegetable oil and three moles of methanol to produce methyl esters and glycerol. Initially, the triacylglycerols of vegetable oil react with methanol to form diglycerides and methyl esters. This reaction continues as methanol combines with diglycerides, generating monoglycerides and additional methyl esters. Finally, monoglycerides react with methanol to produce glycerol and more methyl esters. The transesterification reaction generally yields 10% by weight of glycerol and 90% by weight of methyl ester [3]. Several published reports have claimed that corn oil methyl esters yield in the range of 85–95% [4,5,6]. However, these yields often need more uniformity, as various researchers have defined methyl ester yield differently. Okwundu et al. [7] reported that methyl ester yield notation can be based on the mass, volume, fraction, and molecular weight of methyl esters. Some studies in the literature have also reported methyl ester yield as the purity and conversion rates of reactants. Often, methyl ester yield is calculated using 1H-NMR, capillary gas chromatograph, and high-performance liquid chromatograph peaks. These differences make comparing methyl ester yields and methods challenging, except when the yield is well defined. To define product yield accurately, one should refer to the basics of chemical reactions. The total mass yield or recovery yield depends on various factors, such as the degree of reactant conversion, side reactions, and inefficiency in purifying or recovering the product during washing. There may be a complete conversion of triacylglycerol, but recovery or mass yield may be low if all the methyl esters cannot be retrieved during the washing process. On the other hand, all the recovered methyl esters may not meet the desired quality requirements and contain impurities (glycerol/free fatty acids/residual soap or methanol) other than methyl esters, as impurities are miscible with methyl esters.
KOH and NaOH are commonly used catalysts in the transesterification of vegetable oils due to their low cost, high efficiency, and ease of availability [8]. Singh et al. [9] reviewed the effectiveness of different catalysts in transesterification reactions and concluded that homogeneous base catalysts perform better in low-temperature operations. In contrast, heterogeneous catalysts need extreme reaction conditions (high temperature and pressure) that do not favor industrial applications, although heterogeneous catalysts can be used repeatedly. However, homogenous base catalysts initiate a saponification reaction, making the separation of methyl esters from the glycerol phase difficult [10,11,12]. Sodium ions, which are formed by a reaction between sodium hydroxide and methanol (Equation (1)), initiate soap formation. Soaps (RCOONa) are formed when the free fatty acids produced during transesterification react with NaOH (Equation (2)) or by the saponification of glycerides with NaOH (Equation (3)) [13].
Vávra et al. [13] stated that the transesterification reaction is reversible and has to be stopped before methanol is removed. During the methanol removal process from the entire reaction mixture in the presence of the base catalyst, reverse transesterification to raw materials occurred [14], leading to the low recovery yield of methyl ester. The degree of the reversible reaction depends on the equilibrium between reactants and the products, and also the reaction conditions. Hence, neutralization of the base catalyst with acid helps to curb soap formation or reduces the amount in the solution (Equation (4)) and neutralizes catalytically active methoxide ions (Equation (5)) to stop the transesterification reaction [15,16].
In some previous studies, a problem associated with phase separation between glycerol and methyl ester was reported [17,18], but the occurrence of the neutralization step by adding acid after the transesterification reaction can minimize this separation problem [19,20]. However, the neutralization step is typically used in transesterification kinetics studies, where the conversion and purity of methyl esters are prioritized over recovery yield. There is a lack of scientific literature regarding the recovered yields of methyl esters via the neutralization of transesterified corn oil compared to other vegetable oils. The only study found that has attempted to address this separation issue is the one conducted by El Boulifi et al. [4], that reduced the pH of the transesterified product by washing it with distilled water during phase separation. However, their yield calculation (~90%) was based on the purity of methyl ester, which did not accurately reflect the actual methyl ester recovery yield of their process.
Achieving high methyl ester yields in industrial processes requires precise control over transesterification parameters. Some process parameters include the alcohol-to-oil ratio, catalyst amount, temperature, reaction duration, degree of agitation, and nature of feedstock. These parameters have been studied extensively for the methyl ester production of vegetable oils. Veljković et al. [21] reviewed these conditions, and they concluded that higher corn oil triacylglycerol conversion (>90%) was achieved using a 6:1 methanol-to-oil molar ratio with NaOH at a catalyst concentration of 1.25% at 60 °C. The same study reported that heterogeneous catalysts, such as alkali and alkaline earth metal oxides and hydroxides, are commonly used for corn oil transesterification. However, they generally result in lower ester yields and require longer reaction times than homogeneous catalysts [21]. The right oil-to-alcohol ratio is crucial to avoid complicated methanol recovery processes, and appropriate catalyst levels are necessary for complete oil conversion without complicating the purification of the final product. According to the literature, alkaline catalysts require approximately a 6:1 molar ratio of methanol-to-oil for methyl ester production, sufficient to enhance fatty acid–glycerol chains [22]. Increasing the reaction temperature can improve the methyl ester yield by increasing the reaction rate, creating a better mixing of oil and alcohol, and enhancing the separation rate of glycerol from methyl ester by reducing the oil viscosity. However, a temperature increase beyond the permissible range may cause a considerable reduction in methyl ester yield. It was reported in the literature that raising the reaction temperature above 65 °C insignificantly influences oil conversion [22]. When the reaction temperature reached 70 °C, the conversion dropped because the methanol in the mixture evaporated at a temperature beyond the methanol boiling point (64.7 °C), resulting in an undesirable oil-to-methanol ratio that can inhibit the reaction. Moreover, various reports have mentioned different reaction times for corn oil transesterification, ranging from 0.5 to 1.5 h [21]. Reaction time is critical for complete conversion, while excessively long periods can lead to potential side reactions. Besides time-dependent reactions, it is necessary to investigate the methyl ester yield at different reaction times to see the effect of side reactions on recovery yield. Nevertheless, most previous studies on different process conditions of corn oil methyl ester production emphasized optimizing combustion properties rather than maximizing the yield recovery of methyl esters.
In this context, the present research work intends to contribute towards increasing methyl ester recovery yield via acid neutralization. To assess the influence of neutralization on methyl ester yield from corn oil, it is crucial to compare it with the methyl ester yield of other commonly used vegetable oils in the US, such as soybean and canola. The expectation is that the methyl ester yield will be comparable since the refining steps are also similar for these oils. Then, the recovered yield of corn oil methyl ester needs to be optimized at different neutralization levels and reaction times. The optimization step is vital in minimizing process time and can be achieved by developing a robust model that can best predict recovery yield at different process conditions. Finally, the overall methyl ester quality needs to be compared with the required standards needed for epoxy resin production.
2. Materials and Methods
2.1. Materials
Commercial edible grade (brand name: Our Family) corn oil, soybean oil, and canola oil were bought from the local supermarket. Methanol of 99.8% purity was supplied from VWR (Radnor, PA, USA). Sodium hydroxide (NaOH) pellets (reagent grade, ≥98%, anhydrous) were purchased from Sigma-Aldrich. Magnesium sulfate (MgSO4), anhydrous ≥99%, powder was purchased from J.T. Baker (Center Valley, PA, USA). A working solution of 6 M HCl was prepared from ACS grade HCl solution (supplied from EMD Millipore, Darmstadt, Germany). All the chemicals were used without any further purification.
2.2. Methyl Ester Production
The methyl esters were produced via a transesterification process in which NaOH was used as a catalyst. A total of 100 g of different oil samples (corn, canola, and soybean) was heated to 50 °C in a 250 mL Erlenmeyer flask. The heating helped to decrease the viscosity of the oil and thus facilitated the activity of methanol. Sodium methoxide solution was prepared separately by dissolving 1.25 g of NaOH in 22 mL of methanol using a magnetic hotplate stirrer. The methanol amount for the sodium methoxide solution was based on the molar ratio of the methanol and corn oil at 6:1. Prepared sodium methoxide solution was poured into the reaction flask when the corn oil reached 50 °C. Heating continued and was maintained at 60 ± 2 °C, below the boiling point of methanol (64.7 °C). The temperature of the reactant was monitored using Mini data logger Model GL220 (Graphtec Instruments, Irvine, CA, USA). Stirring on the hot plate was performed using a magnetic stirrer at 600 rpm. The reaction was timed as soon as the methanol/catalyst mixture was added. The total reaction time was 1 h for the neutralization experiment when different vegetable oils (corn, canola, and soybean) were used for methyl ester production. In the corn oil methyl ester yield optimization study, the reaction time varied from 0.5 to 1.5 h (Table 1).

Table 1.
Experimental design matrix with corn oil and comparison of experimental and predicted yields of corn oil methyl ester at different reaction times and acid amounts (% neutralization).
After completion of the reaction time mentioned, a fixed amount of acid (5.2 mL) was added in the neutralization experiment with corn, canola, and soybean oil. However, different acid amounts (0–5.2 mL) were used for the optimization study with corn oil (Table 1) to produce methyl ester. Neutralization was performed by using 6 M HCl, and the amounts were calculated based on the stoichiometry between HCl and NaOH used. The neutralized reaction mixture was transferred into a 500 mL separatory funnel and left overnight for cooling and separation of the phases of glycerol and methyl ester. Dark glycerol phase was discarded followed by phase separation, and the methyl esters phase was washed and purified with distilled water. Firstly, the excess methanol from the methyl esters phase was removed by evaporation under vacuum. This was achieved by heating the separated methyl ester under vacuum at 65 °C for 30 min. The methyl esters were further purified by gentle washing with distilled water to remove residual catalyst, glycerol, and soaps (aqueous phase). The mixture was allowed to settle for 20 min, followed by each washing for complete removal of the aqueous phase. After three successive washes with distilled water (25 mL in each wash), the aqueous phase became clear. Afterwards, the resulting solution was dried overnight using anhydrous MgSO4 (5% w/w). Finally, the produced methyl ester was filtered under vacuum to remove the hydrated MgSO4.
2.3. Neutralization Experiment Using Three Different Oils
To investigate the effect of neutralization on recovery yield (simply known as yield), methyl ester was produced from corn, canola, and soybean oil. Two different reaction conditions, each with three replicates, were used in this experiment. The first reaction condition was to produce methyl ester without adding any acid, and the other was to add 5.2 mL of 6 M HCl to stop the reaction before separation. The reaction ran for 60 min, and the other parameters were the same as described in the previous section. The yield of the produced methyl ester was determined using Equation (6).
The percent yield in Equation (6) is the recovered yield, indicating how much methyl ester can be isolated from the reaction mixture, and the theoretical weight of the methyl ester is the actual reaction yield, which means how much oil is converted to methyl ester. The theoretical weight of the methyl ester was calculated by multiplying the weight of oil used with a conversion factor based on the average molecular weight of the corn oil triglyceride, the fatty acid methyl esters of triglyceride, and the stoichiometry of the transesterification reaction. The fatty acid profile of corn oil (Table 2) was used to calculate the average molecular weight of the triglyceride in corn oil (871 g/mol) and its fatty acid methyl ester (291 g/mol). For soybean oil, the average molecular weight of the triglyceride and its fatty acid methyl ester was 872 g/mol and 292 g/mol, respectively. For canola oil, it was 894 g/mol and 299 g/mol, respectively.

Table 2.
Fatty acid weight percentage of corn, canola, and soybean oils, including distribution of saturated and unsaturated fatty acids, average chain length, and saponification value.
2.4. Experimental Design to Optimize Product Yield of Corn Oil Methyl Ester
A full factorial design with two factors was conducted to optimize the methyl ester production from corn oil. The two selected factors were reaction time (A) and the acid amounts used to stop the reaction (B). Factor A had three levels (0.5, 1, and 1.5 h) and factor B had five levels (0, 1.3, 2.6, 3.9, and 5.2 mL). The experimental matrix is tabulated in Table 1. A total of 15 treatment combinations were obtained, and each treatment was conducted in triplicate. The response variable was the yield of methyl ester. Following the completed experiments, the response variable (methyl ester yield) was fitted into a second-order polynomial model in order to correlate the response variable to the independent variable. The general form of the model was as follows in Equation (7):
where Y is the recovered yield of methyl ester, Xi and Xij are the factors/independent variables, b0 is the constant regression coefficient, and bi, bii, and bij (i, j = 1, 2) are linear, quadratic, and two-way regression coefficients, respectively.
2.5. Statistical Analysis
Statistical analysis was performed using Minitab 21 (State College, PA, USA; Version 21.2) at a 95% confidence interval. One-way analysis of variance (ANOVA) was also computed to evaluate the statistical significance and validity of the model. The influence of the process factors on methyl ester yield was analyzed using the main effect and interaction effect. Student’s t-test was also conducted to test the significant difference in means when the p-value < 0.05 based on the ANOVA.
2.6. Analytical Methods
The Official American Oil Chemists’ Society [23] methods were used to determine the fatty acid composition (Ce 1e-62) and saponification value (Cd 3a-94) of the oils. From the fatty acid composition, the total percent of saturated (e.g., palmitic, stearic, and arachidic acid), monounsaturated (e.g., oleic acid), and polyunsaturated fatty acids (e.g., linoleic and linolenic acid) was calculated by adding the different percentages of their respective fatty acids. The polyunsaturated/saturated (P/S) index was obtained by dividing the total polyunsaturated fatty acids (%) and the total saturated fatty acids (%). To calculate the average chain length, the product of each fatty acid’s carbon atoms and its percentage in the oil were added and then divided by 100. The quality of the produced methyl ester was analyzed using the methods according to the American Society for Testing Materials (ASTMs) standards [24] in terms of viscosity (ASTMs D445), acid value (ASTMs D664), cloud point (ASTMs D2500), and pour point (ASTMs D97). The water content of the methyl ester was determined using Karl Fischer titration. Total glycerin was calculated using the SafTestTM total glycerin kit [25]. This glycerin kit was brought from MP Biomedical; Solon, OH, USA. A total glycerin reagent, a lipase enzyme, was used to convert bound glycerin into the free glycerin of the methyl ester sample. Then, a SafTest™ analyzer, which is a spectrophotometer at 570 nm, was used to quantify the total free glycerin content. The obtained characteristics of the methyl ester were then compared with the standards, such as the American Society for Testing and Materials (ASTMs D6751), and the previously published literature. 1H NMR spectroscopy was performed using a Bruker Ascend 400 MHz magnet with an Avance III HD console, and CDCl3 was used as a solvent. The 1H-NMR spectra were plotted, peaks were identified, and integration values were determined using OriginPro software (OriginLab Corporation, Northampton, MA, USA; Version: 2023b).
3. Results and Discussion
3.1. Effect of Neutralization on Recovered Yield of Methyl Ester from Three Different Oils
This research started by producing corn oil methyl ester on a lab scale using a homogeneous base catalyst (NaOH pellets). However, the recovered yield of the corn methyl ester was very low (<50%) because it was difficult to separate the methyl ester from the glycerol phase (Figure 1). There was no distinct separation phase for the corn methyl ester compared to that of soybean oil methyl ester, as shown in Figure 1. The corn methyl ester had significant soap formation and was mixed in the aqueous phase. It was essential to investigate the best way to address the poor separation of corn methyl ester.

Figure 1.
Difference between separation of glycerol from methyl ester of soybean oil (left) and corn oil (right).
Poor separation of corn oil methyl ester can be explained by looking at the fatty acid distribution of the different oils tabulated in Table 2. Corn oil is composed of nearly 55% linoleic acid (C18:2), 28% oleic acid (C18:1), and 13% saturated fatty acids (predominately palmitic acid). Canola oil exhibits the highest monounsaturated fatty acids (63.51%) content, primarily due to oleic acid, while soybean oil has the lowest monounsaturated fatty acids (21.20%) content. The P/S index is notably higher in soybean oil (4.11), suggesting the presence of more polyunsaturated fats than other oils. The average chain length and saponification number of soybean oil are similar to corn oil. Nevertheless, it is already evident from Figure 1 that soybean oil had less soap formation during the reaction than corn oil, resulting in a better methyl ester yield. On the other hand, canola oil had the lowest saponification value (Table 2), causing less soap formation with the same catalyst concentration during the transesterification reaction compared to corn and soybean oils.
The saponification number is inversely proportional to the average molecular weight or the chain length of the fatty acids [26], which is also evident in Table 2. Saponification is a side reaction with transesterification, producing soap in the system. The soap increases the methyl ester solubility in glycerol and emulsifies the ester and glycerol, which causes difficulties in separating the esters, thus reducing the recovered methyl ester yield. Saponification occurs when the free hydroxide of a catalyst breaks the ester bonds between fatty acids and glycerol in a triglyceride, resulting in more free fatty acids and glycerol. The ester bond is more prone to break down with short-chain fatty acids. The sodium used for a catalyst is then bound with the fatty acid and unusable, thus complicating the separation and recovery of esters.
The distribution of fatty acids and other components of different oils presented in Table 2 is consistent with the previously published literature [27,28]. Other past studies have highlighted some critical aspects regarding the inter-dependence between fatty acid composition and the physical and chemical properties of methyl ester [29,30,31,32]. However, none of these studies explained the methyl ester yield with a changing fatty acid composition.
The effect of neutralizing the reaction of the different corn, canola, and soybean methyl esters was studied to improve the recovered yield. The results illustrated in Figure 2 revealed that neutralization has a positive effect on the yield of methyl ester. The recovered yield increased with the use of acid to stop the reaction. The methyl ester yield was 52–55% for corn and canola oil samples that were not neutralized. This yield significantly increased to 75% when the reaction was neutralized using acid. However, it was also visible that the soybean methyl ester yield in response with no neutralization was around 72%, which was not significantly different from the corn and canola methyl ester yield neutralized with acid. Neutralizing the reaction of soybean methyl ester significantly increased the yield to 88%. The different yields do indicate the effective phase separation between the glycerol and methyl ester rather than the completeness of the transesterification reaction.
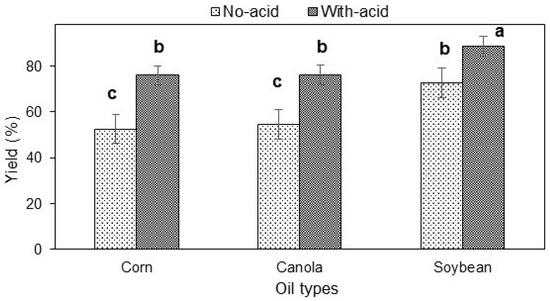
Figure 2.
Effect of neutralization on methyl ester yield of different oils (response is methyl ester yield and different letters indicate significant differences at α = 0.05).
This finding is interesting because it suggests that the recovered yield is dependent on how well the organic phase separated from the aqueous phase and also the formation of soap that usually dissolves in the aqueous phase. The higher recovered yield seen with soybean oil is likely due to less soap formation. Moreover, the added HCl is more strongly attracted to the metal ion on the sodium soap than the fatty acid chain. So, the metal ion (Na+) combines with the Cl− from the HCl to produce NaCl, and the hydrogen freed from the HCl converts the fatty acid chain to free fatty acid. In this way, the soap formation was reduced, and an increasing recovered yield was observed for all types of oil when acid was added after the transesterification reaction.
Most previous studies on the transesterification of these three mentioned oils were mainly focused on the property of methyl ester from different oils and their combustion performance [33,34]. The findings of this present study are quite similar to Karademir and Karademir [35], who measured the efficiency of biodiesel production from soybean, corn, and canola oil. Their result also showed a better ester conversion rate in soybean compared to corn or canola. But their yield measurement was based on the total ester rate and the percent of linoleic acid conversion through a gas chromatography instrument rather than the recovered yield. Moreover, the previous studies mentioned did not use any neutralization step during their experiment. The neutralization effect on the recovered yield on the basis of stoichiometry differentiates the present study from previous studies.
3.2. Modeling the Factors Influencing the Recovery Yield of Corn Oil Methyl Ester
With the finding of the importance of neutralization towards increasing the recovered yield during transesterification, it was important to identify the amount of acid needed and its interaction with the total reaction time. The experimental runs corresponding to the factorial design along with the values of the yield for each run are presented in Table 1, where the predicted value is based on the statistical model. The model to predict the yield of methyl ester was first developed by considering both the linear and quadratic terms in Equation (7). A statistical analysis was carried out on these experimental values, and the main effects and interaction effects of the factors were determined. Initially, both time (A) and square of time (A2) in the model were statistically insignificant at p-value < 0.05. Therefore, a reduced model was developed, as seen in Equation (8), and the ANOVA results of the developed model are shown in Table 3. However, time (A) was found to be significant during the process of removing the square term of time (A2), indicating the absence of a non-linear relationship with the variables.

Table 3.
ANOVA results for the reduced quadratic model.
According to the ANOVA results (Table 3), the reaction time (A), the acid amounts used to stop the reaction (B), square term of acid amount (B2), and interaction term (A × B) significantly affected the yield at the 95% confidence level. The p-values (<0.0001) indicate that all of the factors were important in modeling the transesterification reaction. The ANOVA table shows that almost 82% of the source of variation (SOV) in the process was due to the variation in the levels of the selected factors. Acid amount (B) contributed more than 50% to the variation in the model. Both the squared term of acid amount (B2) and interaction term (A × B) had similar contributions to predict the yield of methyl ester (12.8% and 10.7%, respectively). Time (A) contributed only 6% in the variation in the developed model. The source of variation for each factor is represented in the Pareto chart in Figure 3 to present the magnitude and the importance of the effects. On the Pareto chart, bars that cross the reference line are statistically significant.
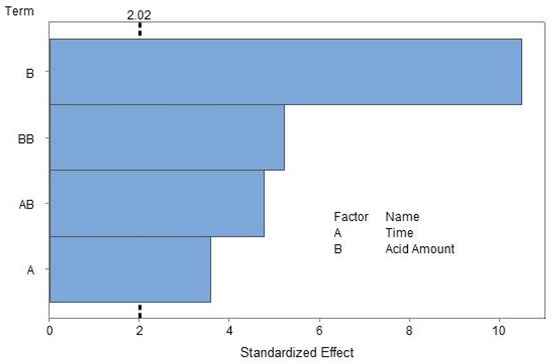
Figure 3.
Pareto chart of the standardized effects of reaction time and acid amount (response is methyl ester yield, α = 0.05).
However, the lack of fit was slightly significant at p-value = 0.042 in Table 3, which was very close to being insignificant at p-value > 0.05. The significant error term was probably due to the elimination of the square term of time (A2) from the initial model, and higher relative deviation (greater than 5%) occurred in experimental run 1 to 6 (Table 1). The developed model indicates that for the reactions with no acid addition, the separation between methyl ester and glycerol becomes more difficult mostly when the reaction time increases.
The significance of each coefficient in Equation (8) was evaluated by the p-value shown in Table 4. The smaller the magnitude of the p-value, the more significant is the corresponding coefficient. From Table 4, it can be seen that all the terms in the model were found to be statistically significant. The acid amount (B) term had the most significant effect, followed by the square of acid amount (B2) and interaction (A × B) term. The reaction time (A) had the least impact, and a similar trend can also be seen at the 5% significance level with the Pareto chart in Figure 3.

Table 4.
Regression coefficients of the reduced quadratic model.
Reaction time (A), amount of acid (B), and time–acid amount interaction effects (A × B) were fitted by multiple regression analysis to develop a linear model. This linear model was developed to improve the quadratic models. The adequacy of all the statistical models (linear, quadratic, reduced quadratic) was compared by model summary statistics (Table 5). The linear model maximizes the value of R2 and R2adj and minimizes the standard deviation compared to the other quadratic models. The high R2 value refers to the acceptable goodness of fit of the linear model. The R2 value 0.893 of the linear model revealed that 89.3% of the variation in the response was due to the difference observed in the factors. The linear model to predict methyl ester yield (response function) for the significant main effects and interactions is in Equation (9).
Yield (%) = 70.66 − 2.91 A + 9.84 B + 5.48 AB

Table 5.
Summary statistics of the developed models.
3.3. Main Effect and Interaction Effect on Corn Oil Methyl Ester Yield
Figure 4 and Figure 5 illustrate the main effects and interaction effects of the treatment combinations on the yield of corn oil methyl ester. The model results indicate that both the main effects, such as reaction time and acid amounts, were statistically significant. Moreover, the interaction between the reaction time and amount of acid added was also significant. In the main effects plot (Figure 4), reaction time at 0.5 h and 1 h was associated with the highest mean yield (>70%). The methyl ester yield at 1.5 h was significantly lower than the yield at 0.5 h and 1 h. The decreasing trend of the yields with increasing time is also evident in Table 4. The negative coefficient of factor time (A) in Table 4 also indicates a reduction in methyl ester yield with the increasing reaction time. This is due to the saponification reaction, where more soap formed over time and reduced the methyl ester yield.
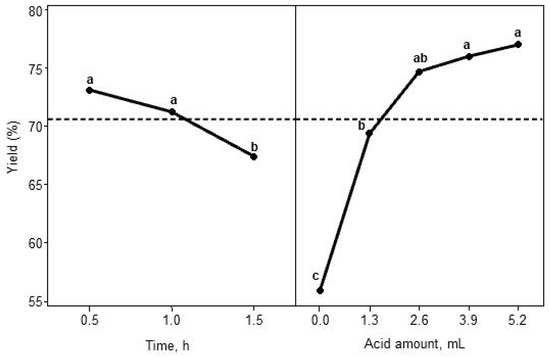
Figure 4.
Main effects plot for methyl ester yield (different letters indicate significant differences (at α = 0.05) between time and acid amount main effects).
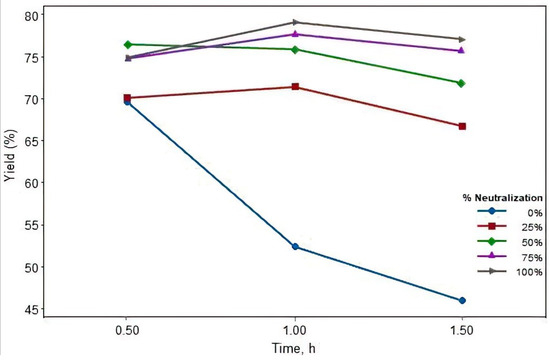
Figure 5.
Interaction plot for methyl ester yield.
The main effect plot also shows that the highest yield (~78%) was achieved by stopping the transesterification reaction with 5.2 mL of 6 M HCl (100% neutralization). However, the highest yield was not statistically different for 2.6 mL (50% neutralization) and 3.9 mL (75% neutralization) of acid addition (Figure 4). The lowest yield of methyl ester was found when no acid was added. This was again due to the continuous saponification reaction, which makes methyl ester separation from glycerol difficult. The added acid neutralizes the base catalyst and converts some soaps to free fatty acid, which helps to increase the methyl ester yield. Only 1.3 mL (25% neutralization) of acid addition helped to raise the yield significantly from 55.9% without acid to 69.4%. The positive coefficient of factor acid amount (B) in Table 4 indicates an increasing trend of methyl ester yield with the increasing acid amount. The negative coefficient of the square of acid amount (B2) indicates a concave curvature relationship with yield (Table 4; Figure 4).
In the interaction plot (Figure 5), the highest yield (79.1%) of methyl ester was achieved with the treatment condition of 1 h reaction time and 5.2 mL acid amounts used to stop the reaction. The significant differences in the means are not illustrated here in Figure 5, but they are presented in Table 1 and Figure 4. From Figure 5, it is evident that the methyl ester yield of corn oil depends both on reaction time and the amount of acid added to stop the reaction. Figure 5 exhibits a trend of increasing yield with the increasing time and acid amount. The only exception happened when no acid was added to stop the reaction. When no acid was used, the yield of methyl ester reduced significantly, from 69.6% at 0.5 h to 52.3% and 45.9% at 1.0 h and 1.5 h, respectively. From a practical observation, the separation between glycerol and methyl ester became very difficult when no acid was added. Moreover, an interaction effect only occurred between 0.5 and 1 h. As the reaction time extended beyond 1 h, the interaction was not seen, and the methyl ester yield was also slightly reduced in all treatment conditions.
3.4. Quality of Corn Oil Methyl Ester
The characteristics of the produced methyl ester from the experimental runs were tabulated and compared with the standard methyl ester values and other previous studies, as shown in Table 6. The selected characteristics were water content, viscosity, total glycerin content, acid value, cloud point, and pour point. For bioresin production using methyl esters, the water content of the methyl ester is considered to be the most important parameter. This is because a high water content slows down the catalysts by not only participating in the formation of emulsions but also causing hydrolysis or hydrolytic oxidation during the esterification reaction [36]. The water content of the produced corn oil methyl ester was well below the limit of ASTMs D6751 (Table 6). Some previous studies have reported a very high water content of the methyl ester, which might be an indication of using a different drying agent (diatomaceous earth) than that used in the present study [37]. Both diatomaceous earth and MgSO4 have been reported to be good drying agents for oil, but vacuum drying may have helped lower the moisture content. The amount of drying agent can also impact the water content in the final product. The kinematic viscosity and total glycerin content were also found to be within the limits of ASTMs standards and comparable to previous studies as well (Table 6). The low total glycerin content indicated that the conversion was good and very little impurity was present.

Table 6.
Characterization of corn oil methyl ester.
The successful conversion of methyl ester from corn oil can also be seen through the 1H-NMR spectra (Figure 6). The 1H-NMR spectra can be used to show complete transesterification and other impurities that may be present in the methyl ester. The singlet peak at 3.6 ppm corresponds to the methoxy/methyl ester peak, indicating that methanol reacted with fatty acids, and the methyl ester is now free from the glycerol core. A slightly higher peak at 2.34 ppm in the methyl ester compared to corn oil also indicates the presence of α-CH2 protons. The multiplet peaks in parent corn oil around 4.1 and 4.3 ppm correspond with the hydrogen in the glycerol backbone. Their disappearance in the methyl ester spectrum indicates that all the glycerol was successfully removed (Figure 6). A similar peak at 1.3 ppm (doublet) and 2.1 ppm (triplet) in both spectra represents the methylene protons of the carbon chain and α-carbonyl methylene protons, respectively [39]. The 1H-NMR spectra of corn oil methyl ester can be helpful for subsequent bioresin synthesis processes and used for quality control.
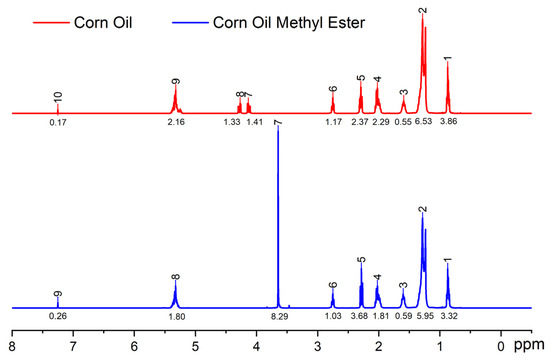
Figure 6.
1H-NMR spectra of corn oil and corn methyl ester showing successful conversion of methyl ester.
The acid value found in this study was very similar to Moser and Vaughn [38]. The low acid value reported in previous studies may be due to the difference in the acid value in the corn oil used. Cloud point and pour point were the main indicators of the cold flow properties of methyl esters. However, ASTMs D6751 requires that cloud point and pour point be reported. The observed cloud point and pour point values for the present study were −2 °C and −4.5 °C, respectively. Other studies have reported lower cloud point and pour point values for their methyl esters, indicating good cold flow properties (Table 6). In our study, this characteristic is paid less attention as it was mainly related with fuel properties. Overall, it was evident that despite having a low yield in some of the experimental runs, the quality was still within acceptable limits.
4. Conclusions and Future Directions
Neutralization during transesterification increased the methyl ester yield from different vegetable oils (corn, canola, and soybean). The production of methyl ester from commercial corn oil via transesterification was optimized using a full factorial design. The results indicated that the acid amount used to stop the reaction (both linear and quadratic terms), the reaction time (only linear term), and their two-way interaction were statistically significant at a 0.05 significance level. The developed quadratic model adequately described the transesterification process. After 1.5 h of reaction time and complete neutralization of the NaOH catalyst included in the experiment, a maximum corn oil methyl ester yield of 78.9% was predicted. The acid amount factor and its quadratic terms had the most influence on the transesterification reaction. Compared to the treatment with the minimum amount of neutralization (25%), the yield was found to be much lower with no neutralization. The characteristics of the corn oil methyl ester generated in this research complemented those of existing standards and prior reports. The present findings of this study will pave the way to synthesize quality epoxy resin from corn oil. Future studies will look at how the properties of corn oil methyl ester influence the production of the epoxidized sucrose ester of fatty acid.
Author Contributions
Conceptualization, M.S.H., D.C.W. and E.M.; methodology, M.S.H. and M.O.; validation, M.S.H. and E.M.; formal analysis, M.S.H., M.O. and N.C.S.; investigation, M.S.H., M.O., D.C.W. and E.M.; resources, M.S.H., N.C.S. and E.M.; writing—original draft preparation, M.S.H. and M.O.; writing—review and editing, N.C.S., D.C.W. and E.M.; visualization, M.S.H.; supervision, D.C.W. and E.M.; project administration, D.C.W. and E.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the North Dakota Corn Utilization Council (FAR0032322) for funding this research study. Additional support comes from North Dakota Agricultural Experiment Station and USDA-NIFA Hatch multistate ND01491.
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors are very grateful to Brent Hulke from USDA, Sunflower and Plant Biology Research in Fargo, ND, for providing the fatty acid profile of different oils used in the experiment. The authors also would like to acknowledge Andrew Taylor of the North Dakota State University Center for Writers for his writing consultation to prepare the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Alam, M.; Akram, D.; Sharmin, E.; Zafar, F.; Ahmad, S. Vegetable oil based eco-friendly coating materials: A review article. Arabian J. Chem. 2014, 7, 469–479. [Google Scholar] [CrossRef]
- Karis, D.; Cain, R.; Young, K.; Shand, A.; Holm, T.; Springer, E. Non-fuel uses for fatty acid methyl esters. Biofuels Biopro. Bioref. 2022, 16, 1893–1908. [Google Scholar] [CrossRef]
- Peters, M.A.; Alves, C.T.; Wang, J.; Onwudili, J.A. Subcritical Water Hydrolysis of Fresh and Waste Cooking Oils to Fatty Acids Followed by Esterification to Fatty Acid Methyl Esters: Detailed Characterization of Feedstocks and Products. ACS Omega 2022, 7, 46870–46883. [Google Scholar] [CrossRef] [PubMed]
- El Boulifi, N.; Bouaid, A.; Martinez, M.; Aracil, J. Process optimization for biodiesel production from corn oil and its oxidative stability. Int. J. Chem. Eng. 2010, 2010, 518070. [Google Scholar] [CrossRef]
- Velázquez, J.M. Conversion of Corn Oil to Alkyl Esters. Master’s Thesis, Iowa State University, City of Ames, IA, USA, 2007. [Google Scholar]
- Khan, N.; Dessouky, H. Biodiesel production from corn oil by transesterification process. Nucleus 2009, 46, 241–252. [Google Scholar]
- Okwundu, O.S.; El-Shazly, A.H.; Elkady, M. Comparative effect of reaction time on biodiesel production from low free fatty acid beef tallow: A definition of product yield. SN Appl. Sci. 2019, 1, 140. [Google Scholar] [CrossRef]
- Leung, D.Y.; Wu, X.; Leung, M. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Bangar, S.P.; Purewal, S.S.; Trif, M.; Maqsood, S.; Kumar, M.; Manjunatha, V.; Rusu, A.V. Functionality and applicability of starch-based films: An eco-friendly approach. Foods 2021, 10, 2181. [Google Scholar] [CrossRef]
- Vicente, G.; Martınez, M.; Aracil, J. Integrated biodiesel production: A comparison of different homogeneous catalysts systems. Bioresour. Technol. 2004, 92, 297–305. [Google Scholar] [CrossRef]
- Anuar, M.R.; Abdullah, A.Z. Challenges in biodiesel industry with regards to feedstock, environmental, social and sustainability issues: A critical review. Renew. Sustain. Energy Rev. 2016, 58, 208–223. [Google Scholar] [CrossRef]
- Elsayed, M.; Eraky, M.; Osman, A.I.; Wang, J.; Farghali, M.; Rashwan, A.K.; Yacoub, I.H.; Hanelt, D.; Abomohra, A. Sustainable valorization of waste glycerol into bioethanol and biodiesel through biocircular approaches: A review. Environ. Chem. Lett. 2023, 1–26. [Google Scholar] [CrossRef]
- Vávra, A.; Hájek, M.; Skopal, F. The removal of free fatty acids from methyl ester. Renew. Energy 2017, 103, 695–700. [Google Scholar] [CrossRef]
- Hájek, M.; Skopal, F.; Černoch, M. Effect of phase separation temperature on ester yields from ethanolysis of rapeseed oil in the presence of NaOH and KOH as catalysts. Bioresour. Technol. 2012, 110, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Vávra, A.; Hájek, M.; Kocián, D. The influence of vegetable oils composition on separation of transesterification products, especially quality of glycerol. Renew. Energy 2021, 176, 262–268. [Google Scholar] [CrossRef]
- Georgogianni, K.; Katsoulidis, A.; Pomonis, P.; Manos, G.; Kontominas, M. Transesterification of rapeseed oil for the production of biodiesel using homogeneous and heterogeneous catalysis. Fuel Process. Technol. 2009, 90, 1016–1022. [Google Scholar] [CrossRef]
- Savaliya, M.L.; Dhorajiya, B.D.; Dholakiya, B.Z. Current trends in separation and purification of fatty acid methyl ester. Separ. Purif. Rev. 2015, 44, 28–40. [Google Scholar] [CrossRef]
- Akgün, N.; İşcan, E. Effects of process variables for biodiesel production by transesterification. Eur. J. Lipid Sci. Technol. 2007, 109, 486–492. [Google Scholar] [CrossRef]
- Hájek, M.; Skopal, F.; Vávra, A.; Kocík, J. Transesterification of rapeseed oil by butanol and separation of butyl ester. J. Clean. Prod. 2017, 155, 28–33. [Google Scholar] [CrossRef]
- Vávra, A.; Hájek, M.; Skopal, F. Acceleration and simplification of separation by addition of inorganic acid in biodiesel production. J. Clean. Prod. 2018, 192, 390–395. [Google Scholar] [CrossRef]
- Veljković, V.B.; Biberdžić, M.O.; Banković-Ilić, I.B.; Djalović, I.G.; Tasić, M.B.; Nježić, Z.B.; Stamenković, O.S. Biodiesel production from corn oil: A review. Renew. Sustain. Energy Rev. 2018, 91, 531–548. [Google Scholar] [CrossRef]
- Trirahayu, D.A.; Abidin, A.Z.; Putra, R.P.; Hidayat, A.S.; Safitri, E.; Perdana, M.I. Process Simulation and Design Considerations for Biodiesel Production from Rubber Seed Oil. Fuels 2022, 3, 563–579. [Google Scholar] [CrossRef]
- Firestone, D. (Ed.) Official Methods and Recommended Practices, 7th ed.; American Oil Chemists’ Society: Champaign, IL, USA, 2017. [Google Scholar]
- ASTM. Standards for Petroleum Products, Lubricants, and Fossil Fuels; ASTM International: West Conshohocken, PA, USA, 2023. [Google Scholar]
- Tuntiwiwattanapun, N.; Monono, E.; Wiesenborn, D.; Tongcumpou, C. In-situ transesterification process for biodiesel production using spent coffee grounds from the instant coffee industry. Ind. Crop. Prod. 2017, 102, 23–31. [Google Scholar] [CrossRef]
- Neupane, D. Biofuels from Renewable Sources, a Potential Option for Biodiesel Production. Bioengineering 2022, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Willams, K.J.; Goldberg, A.C. Plant-Based Oils. In Prevention and Treatment of Cardiovascular Disease: Nutritional and Dietary Approaches; Wilkinson, M.J., Garshick, M.S., Taub, P.R., Eds.; Humana: Cham, Switzerland, 2021; pp. 115–127. [Google Scholar] [CrossRef]
- Folayan, A.J.; Anawe, P.A.L.; Aladejare, A.E.; Ayeni, A.O. Experimental investigation of the effect of fatty acids configuration, chain length, branching and degree of unsaturation on biodiesel fuel properties obtained from lauric oils, high-oleic and high-linoleic vegetable oil biomass. Energy Rep. 2019, 5, 793–806. [Google Scholar] [CrossRef]
- Giakoumis, E.G. A statistical investigation of biodiesel physical and chemical properties, and their correlation with the degree of unsaturation. Renew. Energy 2013, 50, 858–878. [Google Scholar] [CrossRef]
- Anwar, M. Biodiesel feedstocks selection strategies based on economic, technical, and sustainable aspects. Fuel 2021, 283, 119204. [Google Scholar] [CrossRef]
- Ramírez-Verduzco, L.F.; Rodríguez-Rodríguez, J.E.; del Rayo Jaramillo-Jacob, A. Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 2012, 91, 102–111. [Google Scholar] [CrossRef]
- Jahirul, M.; Rasul, M.; Brown, R.; Senadeera, W.; Hosen, M.; Haque, R.; Saha, S.; Mahlia, T. Investigation of correlation between chemical composition and properties of biodiesel using principal component analysis (PCA) and artificial neural network (ANN). Renew. Energy 2021, 168, 632–646. [Google Scholar] [CrossRef]
- Efe, Ş.; Ceviz, M.A.; Temur, H. Comparative engine characteristics of biodiesels from hazelnut, corn, soybean, canola and sunflower oils on DI diesel engine. Renew. Energy 2018, 119, 142–151. [Google Scholar] [CrossRef]
- Ramos, M.J.; Fernández, C.M.; Casas, A.; Rodríguez, L.; Pérez, Á. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 2009, 100, 261–268. [Google Scholar] [CrossRef]
- Karademir, C.; Karademir, E. Efficiency of Biodiesel Production from Soybean, Corn, and Canola Oil. In Proceedings of the International Mesopotamia Agriculture Congress, Diyarbakir, Turkey, 22–25 September 2015. [Google Scholar] [CrossRef]
- Knothe, G. Analyzing biodiesel: Standards and other methods. JAOCS J. Am. Oil Chem. Soc. 2006, 83, 823–833. [Google Scholar] [CrossRef]
- Mata, T.M.; Sousa, I.R.; Vieira, S.S.; Caetano, N.S. Biodiesel production from corn oil via enzymatic catalysis with ethanol. Energy Fuels 2012, 26, 3034–3041. [Google Scholar] [CrossRef]
- Moser, B.R.; Vaughn, S.F. Biodiesel from corn distillers dried grains with solubles: Preparation, evaluation, and properties. Bioenrg. Res. 2012, 5, 439–449. [Google Scholar] [CrossRef]
- Saini, P.; Gupta, C.; Shankar, R. Characterization of corn oil biodiesel and its application in diesel engine. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 9498–9512. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).