Abstract
The continuous and irresponsible addition of environmental pollutants into aqueous reservoirs due to excessive industrialization is a significant contemporary challenge. Nanomaterial-based catalytic reduction provides an effective way to convert these materials into environmentally useful products. Responsive polymeric assemblies, complemented with nanomaterials, represent advanced nanocatalysts that are gaining interest within the scientific community. These assemblies exhibit reversible morphological transitions in response to variations induced by external factors such as temperature, pH, or electromagnetic irradiation treatment. The term hybrid microgels has been coined for assemblies that contain both nanomaterial and smart polymeric components. This review presents recent advancements in the field of hybrid microgels as nanocatalysts for conducting reduction reactions on pollutants present in aqueous media. Apart from placing detailed emphasis on the advancements documented for these assemblies, the fundamentals associated with hybrid microgels, as well as the typical catalytic reduction, are also emphasized to develop an understanding for new academicians looking to explore this field. The author hopes that this critical review of the most recent academic literature, including the years spanning 2020 to 2023, will serve as a tutorial for the identification of research gaps in this field, along with its prospective solutions.
1. Introduction
Responsive microgels are recently developed complex assemblies that are capable of exhibiting the peculiar property of swelling/shrinkage when developed in an appropriate solvent [1]. These colloidal materials exhibit varying morphological characteristics as a response to the variation in external incentives associated with the reaction medium, including pH, temperature, radiation exposure, ionic strength, etc. This peculiar property makes microgels an attractive material for the fabrication of exclusive assemblies capable of modulating their own morphology or hydrodynamics with respect to the applied conditions for the applications of catalysis, photocatalysis, the biomedical field, photonics, and environmental sciences [2]. Among the variety of polymeric assemblies, the synthetic polymer poly(N-isopropyl acrylamide) (PNIPAM) is the most renowned smart polymer utilized for the fabrication of smart microgels. The extensive usage of PNIPAM is ascribed to the fact that PNIPAM exhibits a swelling/shrinkage property at the specific temperature of 32 °C, which is quite close to human physiological temperature, making PNIPAM an appropriate material for biomedical applications [1]. This temperature, at which the assembly undergoes transition, i.e., from the swelled to deswelled state or deswelled to swelled state, is regarded as the volume phase transition temperature (VPTT), and, in terms of the application, VPTT is the most significant parameter for the modulation of the morphology of the assembly. VPTT is influenced by several factors, but the composition and nature of the polymer utilized are the two most crucial factors that need to be considered for the fabrication of microgels [3].
As indicated in the above paragraph, VPTT is directly affiliated with the composition of microgels. Therefore, several responsive characteristics or variations in VPTT values are imparted to microgels by changing the composition of the assembly. The generation of PNIPAM-based copolymerized assembly (COP), where two or more polymers are simultaneously copolymerized in a single medium, is currently the best-documented way to achieve a microgel assembly capable of showing sensitivity towards multiple reaction parameters. For instance, Haleem et al. [4] synthesized homogenous microgels containing 2-acrylamido-2-methylpropane sulfonic acid (AMPSA) as a comonomer alongside PNIPAM as a main polymer and indicated that the utilization of AMPSA imparted pH and temperature sensitivity to the whole assembly. Ali et al. [5] engineered a COP containing methacrylic acid (MAC) as a comonomer and established that the repulsions generated by the negatively carboxylic groups (COO−1) of MAC at the suitable pH were responsible for the swelling properties of the whole assembly. Begum et al. [6] engineered a COP assembly containing acrylamide (AA) as the comonomer via free-radical emulsion polymerization. All these examples establish that the utilization of comonomers in microgels alongside the main monomer is a crucial method for obtaining enhanced characteristics in microgel assembly.
Recently, highly efficient assemblies have been synthesized for surface-dependent applications, such as catalysis, adsorption, and photocatalysis, through the combination of polymer chemistry and nanotechnology. Owing to the extremely small size and exponential surface area of nanomaterials (NMs), NMs have emerged as a copacetic alternative to the conventional catalysts used in water purification or water treatment applications. However, these NMs rapidly undergo aggregation upon synthesis because of the high surface energy values associated with their surface plasmons. Therefore, the presence of a fabrication/stabilization/supporting medium is an essential requirement for the confinement of NMs. Crosslinked polymeric microgels provide a solution to the challenge of stabilizing nanoparticles (NMs) by harnessing the swelling and shrinking properties of these gels. This capability is employed for the in situ fabrication of NMs. Upon synthesis, microgels, because of the sieves formed within them through polymer crosslinking, serve as a medium for producing synthesized NMs. An assembly that contains a combination of NMs stabilized in microgels is termed a “hybrid microgel” (HMG) assembly. The term “hybrid” was coined based on the observation that this complex assembly exhibits characteristics associated with both nanomaterials (NMs) and polymeric gels within a single unit.
The incorporation of NMs into microgels has significantly enhanced the utility of these complex assemblies as catalysts for various reactions related to water pollution. The relentless and excessive release of pollutants into natural aqueous reservoirs stands as one of the foremost scientific concerns in today’s industrialized world. The industrial boom witnessed worldwide in the 21st century has markedly increased the influx of pollutants into these reservoirs. Utilizing the catalytic reduction reactions (CRRs) as a removal methodology is considered advantageous in comparison to other removal methodologies (including adsorption, photocatalysis, advanced oxidation processes, and membrane filtration, etc.) as it does not suffer from the various drawbacks associated with these conventional methods. The adsorption process requires additional recovery setups for the acquisition of adsorbed pollutants. Photocatalysis and advanced oxidation processes are time-consuming and ineffective, as complete degradation is not achieved in many cases. Moreover, the advanced oxidation process requires strong oxidizing agents, which are mostly toxic. The membrane filtration process is effective, but the breakage of the membrane during filtration is a typical problem that is observed mostly in these assemblies. The catalytic reduction reaction (CRR) utilizes the reducing agents and converts the potent group of aromatic pollutants into less potent synthetically useful products. The economical, rapid, and complete conversion of the substrate are the few advantages associated with this methodology, which make this methodology significant in comparison to other routes. HMGs are largely utilized to carry out the reduction reactions of the pollutants as catalysts.
This tutorial review summarizes recent advancements in HMGs for CRRs of pollutants. The first section provides a brief description of HMGs and introduces the reader to the significant terminologies used in this research domain. The second section focuses on the fundamentals of HMGs and details the basics and properties affiliated with each component of HMGs (polymeric material and NMs) to develop a deeper understanding of the functioning of HMGs. The third section focuses on the numerous fundamentals of CRRs and explains the variety of pollutants, progression detection, kinetics, and thermodynamics of the underlying reactions performed via the use of HMGs as catalysts. A literature survey associated with the utilization of HMGs for CRRs of pollutants is presented in the fourth section. For classification, two different reference points (i.e., the morphology of HMGs and the nature of the NMs used in HMGs) were used. The future perspective and conclusions are presented in the last section. This review is developed by utilizing the recent literature from the years 2020 to 2023 and will serve as a guide for readers considering the exploration of HMGs for numerous applications.
2. Fundamentals of HMGs for CRRs
CRRs utilize mild reducing agents for the reduction of pollutants in the presence of HMGs. This reaction was found to be thermodynamically favorable, but the kinetics do not favor this reaction in the absence of catalysts. Consequently, HMGs are utilized to overcome this kinetic barrier. The fundamentals associated with HMGs in terms of synthesis, characterization, and properties are discussed in this section.
2.1. Synthesis of Hybrid Microgels
2.1.1. Synthesis of Microgels in HMGs
The typical synthesis of PNIPAM microgels involves the utilization of free-radical emulsion polymerization (FEPol). The FEPol route is utilized because PNIPAM is a soluble monomer, and only those routes are used that deal with the soluble polymers. The identified components associated with FEPol have been found to be free-radical initiator, monomer, crosslinker, and surfactant (optional) compounds. In a conventional route, the monomer (N-isopropyl acrylamide; NIPAM) is first homogenized using constant magnetic stirring followed by heating to a temperature of 70 °C. The temperature of 70 °C is responsible for the lysis of ammonium persulfate (initiator), which generates the free radicals in the medium. To avoid any side reaction of these initiators with atmospheric oxygen molecules, this reaction is carried out under nitrogen-purging conditions (for inertness). The radicals interact with the monomers as well as crosslinkers to generate polymeric chains. The onset of polymerization is marked by the observation of turbid gel dispersion in the medium. With the further progression of the polymerization reaction, the extent of turbidity increases, as PNIPAM is an insoluble polymer. The acquired crosslinked microgels are then purified via dialysis to acquire pure PNIPAM gels. The use of a free-radical initiator in this route makes this type of polymerization the most frequent type of polymerization used for the fabrication of PNIPAM gels. This technique is effective, easy, and time-efficient. The presence of a surfactant provides effective control over the manipulation of the size of microgels. However, a surfactant-free route might also be used for this purpose. The reports documented by Haleem et al. [4], Din et al. [7], Zahid et al. [8], and Farooqi et al. [9] use this peculiar methodology for the fabrication of PNIPAM-based HMGs using FEPol methodology.
To enhance the scope of the applicability potential of the microgels, certain functional moieties are introduced into pure microgels as comonomers. For instance, Moon and Kim [10] utilized AA as a functional moiety alongside the PNIPAM polymer and identified hydrogen bonding between the acrylamide group of AA and water molecules to be responsible for swelling purposes. With the increment in temperature, water (present as a dispersed phase in microgels) content is decreased, and hydrogen bonding interactions are reduced, leading to stronger polymer–polymer hydrophobic interactions in the medium. Consequently, the microgels change from their linear phase to globule phase and are termed as being in a “deswelled” state of microgels. Rahimkhoei et al. [11] identified the comonomer of itaconic acid (IA) as the main moiety responsible for the swelling of the microgels. The authors further supplemented the assembly with polyhedral oligomeric silsesquioxane (POSS) to improve the compactness of the assembly, and fabricated silver (Ag) nanoparticles (NPs) for the development of HMGs. Agnihotri and Dan [12] developed gold (Au) NP-based HMGs and identified the MAC moiety as the main functional moiety responsible for the phase transitions of gels. In all the abovementioned cases, FEPol was utilized to develop these COP assemblies. The only difference in the synthesis is the addition of a comonomer alongside the monomer in the reaction medium. Still, it should be noted that the concentration of the added comonomer is kept greatly less in comparison to the concentration of PNIPAM in the medium. Owing to this fact, the main polymeric chains formed in microgels are of PNIPAM, and the added comonomer acts as a hanging or pendant group on the main chains. These pendant groups, in turn, can develop an interaction with the water phase (in hydrogels) and impart morphological changes into the whole assembly. The synthesis of pure PNIPAM microgels and COP microgels via FEPol is presented in Figure 1.
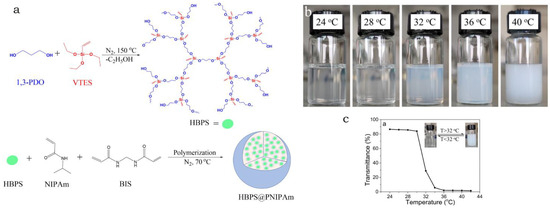
Figure 1.
(a) Development of hyperbranched polysiloxane (HBPS) core via reaction of 1,3-propane diol (1,3-PDO) with vinyltriethoxysilane (VTES), followed by the synthesis of core@shell HBPS@PNIPAM microgels by FEPol methodology (using NIPAM as the main monomer and BIS as the crosslinker); (b) Digital photographs of the microgels at various temperatures; and (c) VPTT studies performed for the HBPS@PNIPAM microgels indicating the critical temperature for phase transition to be 32 °C. Figure adapted with permission from Ref. [13]—copyright 2023 Taylor & Francis.
2.1.2. Synthesis of NMs in HMGs
Metal NPs, including Ag, Au, Pt, Pd, etc., are synthesized by the reduction reaction of metal ions in the presence of reductant NaBH4. The typical synthetic route of metal NPs via the utilization of a reductant can be divided into the following steps:
- Upon the dissolution of the precursor salt () in an aqueous medium, the salt is dissociated into ions (Equation (1)).
- These metal ions are then reduced (the species is capable of giving electrons to the interacting molecule) into zero-valent metal atoms () (Equation (2)).
- Almost 30 metal atoms combine to give NMs.
- In the absence of any stabilization medium, the NMs undergo aggregation due to higher surface energy values.
- The presence of the stabilization medium/surfactant confines the fabricated materials in the nano range (Equation (3)).
Most of the NMs stabilized in HMGs follow the reduction methodology presented in Figure 2. Apart from metal NMs, metal oxide NMs can also be utilized as CRRs of pollutants. In these cases, oxidants such as sodium hydroxide (NaOH) are utilized to carry out the oxidation (addition of an oxygen atom) of metal ions to achieve metal oxide NMs. The presence of a stabilization medium is crucial for keeping the fabricated metal oxide in the nano range as well. It should be mentioned that, in the case of metal oxide NMs, the process of photocatalysis is preferred rather than the reduction process. This preference is attributed to the fact that these metal oxides exhibit copacetic light-absorption capabilities, appropriate band-gap values, and charge-transduction/transport characteristics necessary for performing photocatalysis. However, a few academic reports documenting the usage of metal oxide NMs as catalysts for performing the CRRs of the pollutants exist. Din et al. [7] engineered CuO NPs fabricated inside COP microgels and utilized the engineered assembly for the CRR of methylene blue (MB). The authors identified the reaction to operate via pseudo-first-order (PFO) kinetics and the significant parameters of the apparent rate constant () and reaction time were documented for this reaction. Naz et al. [14] documented the engineering of manganese (Mn)-doped tin oxide NPs and employed it to perform the CRR of the pollutants.
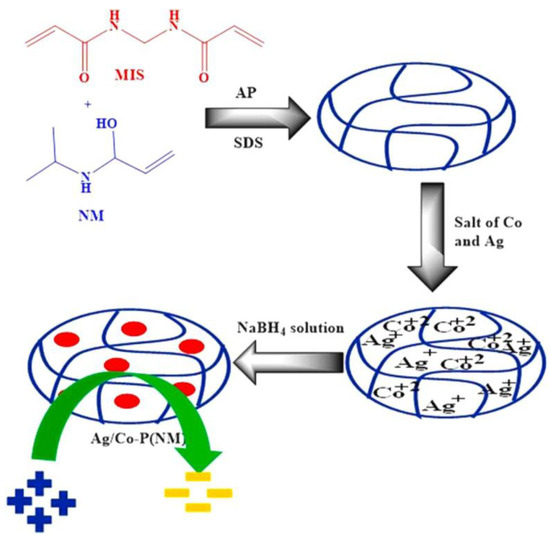
Figure 2.
Synthesis of Ag and Co NMs inside microgels via in situ reduction methodology. Figure adapted with the permission from Ref. [15]—copyright 2022 Elsevier.
2.1.3. Synthesis of Microgels in HMGs
The literature survey of the recent studies reveals that two different approaches can be implemented for the fabrication of HMGs.
In Situ Generation of NMs within Microgels
The utilization of microgels as a microreactor for the in situ fabrication of NMs is the most common way to achieve the fabrication of HMGs. The frequent usage of this methodology is employed owing to its easy nature. First, the synthesized microgels are homogenized and swelled by utilizing the factors of pH or dilution (addition of water content), followed by the addition of the reductant and the salt in these homogenized assemblies. The fabrication of the metal/metal oxide NMs inside the sieves of the microgels is largely achieved by carrying out the in situ reduction/oxidation of precursor salts. The electrostatic interactions between the pendant groups (of microgels) and the metal ions facilitate the spontaneous diffusion of metal ions into microgels. Given the small sizes of the meshes in the microgels, only a small fraction of ions are diffused into the mesh of the gels. Upon addition of the reductant (mostly NaBH4)/oxidant (mostly NaOH), these ions are either reduced or oxidized into metal or metal oxide NMs, respectively. The presence of the surfactant, as well as the small mesh size of the microgels, confines the synthesized materials to the nanorange. Consequently, this methodology serves the dual purpose of not only acting as a microreactor for the NMs synthesis but also facilitating the stabilization of the synthesized NMs via the polymeric mesh of the gels. Du et al. [16] fabricated platinum (Pt), Au, and palladium (Pd) NPs within the sieves of pure PNIPAM microgels via in situ reduction processes and implemented the synthesized nanocatalyst for the reduction of 4-nitroaniline (4NA), nitrobenzene (NB), and 4-nitrophenol (4NP). Hussain et al. [17] fabricated Ag NPs via a similar process in the core@shell microgels and also utilized the assembly for the CRR of 4NP. The typical in situ reduction methodology associated with the homogenous HMGs is presented in Figure 2.
Another thing to note is the morphology of the HMGs. If the HMGs are homogenous, i.e., the crosslinking density of the microgels as well as the NMs fabricated inside is the same, then the morphology of the HMGs is termed homogenous. This methodology of in situ reduction of salts for the generation of NMs is preferred over other methods for the synthesis of HMGs. Egemole et al. [18] synthesized Au NPs inside PNIPAM microgels via the in situ reduction methodology and utilized it as a catalyst for performing numerous reactions. Interestingly, the authors indicated that the amount of the crosslinker added during the emulsion polymerization of PNIPAM was found to be a significant contributing factor modulating catalytic efficacy. The decreased crosslinker values generated loosely bound PNIPAM microgels, and consequently, Au NPs of extremely small size were fabricated inside these meshes. These NPs exhibited enhanced turnover frequency (TOF) values, as the pollutants introduced into the medium of HMGs containing lower crosslinking density faced decreased diffusional barrier, and NPs were assessable to the incoming pollutant particles for removal. Jang et al. [19] investigated another factor associated with the in situ reduction-based synthesis of HMGs and utilized a variety of mild reducing agents for the synthesis of Au NPs in PNIPAM microgels. The authors modulated the size and morphology of Au NPs by systematic variation in the dose ratio of Au ions to reducing agents. No significant effect on the catalytic efficacy of synthesized HMGs was documented, but the morphology of Au NPs was strongly influenced by the reducing agent used for carrying out the reduction of the Au ions. An interesting study was documented by Issasi et al. [20], where the process of electrical discharge was utilized to synthesize PNIPAM-Ag NP HMGs in a single step. The initiator, monomer, and precursor salt were added to the reaction pot, and the polymerization reaction, as well as the reduction reaction, was carried out using the extreme heat generated by the electrical discharge, leading to the formation of HMGs. This process is novel, and the authors claim to be the first to document this methodology.
In some cases, core@shell microgels were first synthesized using the combination of emulsion polymerization and seeded-based emulsion polymerization. Emulsion polymerization was used to develop the PS core while the PNIPAM-acrylic acid (AAc) shell was incorporated over it using the PS core as a seed, leading to seed-based emulsion polymerization. These PS@PNIPAM-AAc microgels were supplemented with Ag NPs using the in situ reduction methodology. The authors utilized a pH of 8.4 as the main factor for the swelling of the synthesized microgels and established that in the swelled state, the effective fabrication of Ag NPs was successfully achieved. Synthesized HMGs were utilized for performing the CRR of 4NP. The authors further investigated the impact of VPTT on the CRR. The spontaneous nature of the reaction was found to increase with the increment in temperature before and after the transition temperatures. Sabadasch et al. [21] also synthesized core@shell COP microgels via FEPol and introduced Pd NPs inside the sieves of the microgels for the synthesis of HMGs. The HMGs were further used as a nanocatalytic material for the CRR of 4NP. Besold et al. [22] engineered PS@PNIPAM core@shell microgels using the combination of emulsion polymerization (PS core) and seed-mediated emulsion polymerization (for PNIPAM shells) and then synthesized PS@PNIPAM/Ag NPs HMGs using the in situ reduction methodology for the synthesis of Ag NPs. The authors carried out the CRR of the 4NP using the prepared HMGs and performed complete kinetic studies to acquire specific insights to improve the catalytic efficacy of the underlying reaction. The typical in situ reduction methodology associated with heterogeneous core@shell HMGs is presented in Figure 3.
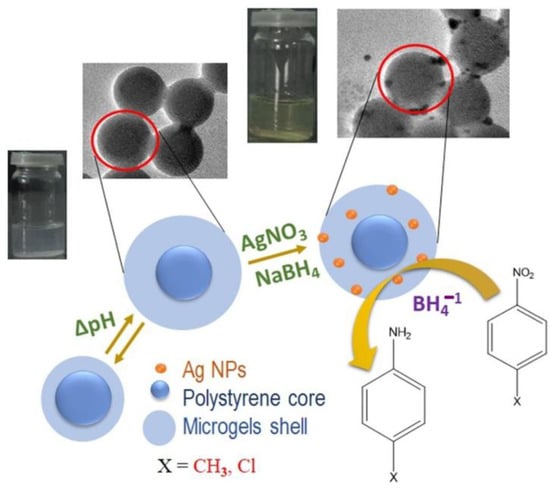
Figure 3.
Synthesis of Ag NMs inside core@shell microgels for HMGs via in situ reduction methodology. Figure adapted with the permission from Ref. [23]—copyright 2020 Elsevier.
Microgels Grown on Pre-Synthesized NMs Core
Another route adopted for the synthesis of HMGs is to develop smart microgels on pre-synthesized NMs. This route provides a rigid and compact assembly, as the central component of HMGs is the solid NMs rather than the soft microgel assembly. Consequently, these synthesized HMGs are more stable in comparison to the homogenous assembly. The hard core of the HMG assembly can be synthesized by a process of the reduction of precursor salt, co-precipitation reaction, or any other route. The soft, responsive polymeric shell was then developed as a shell using any polymerization technique. However, the range of polymerization techniques used for the development of the shell is quite wide in the case of this type of synthetic route. For instance, the polymerization techniques of reversible-addition chain-fragmentation transfer (RAFT) [24] and selective bond formation techniques [25] have been used alongside FEPol for the development of HMGs. Li et al. [26] synthesized Au NPs using citrate reduction methodology and anchored two successive shells of PNIPAM layers (via FEPol) over these NPs by carrying out a polymerization reaction containing the dispersion of Au NPs in the reaction pot.
Bera et al. [24] developed iron oxide (Fe3O4) NPs via a co-precipitation reaction where two soluble salts (including ferric chloride and ferric sulfate) were simultaneously used in the presence of NaOH to precipitate in the form of Fe3O4 NPs. This methodology is crude but effective at synthesizing NPs that are stable at lower temperatures (80 to 150 °C). The synthesized NPs were further modified by the amine (NH2) group using the process of grafting ((3-Aminopropyl) triethoxysilane; APTES modification). These simple NH2-modified Fe3O4 NPs were then further capped with two block co-polymeric chains synthesized by RAFT polymerization. Usually, the components involved in this type of polymerization include the monomer, solvent, RAFT agent, and radical source. The polymerization reaction is initiated by utilizing free radicals that produce living propagating polymeric chains of the monomers. In the next pre-equilibrium step, the RAFT agent interacts with the propagating polymeric radical to form an adduct (known as a RAFT adduct intermediate) that can either simultaneously go towards the parent side or can undergo decomposition to generate a leaving group alongside the polymeric chain. The leaving radical starts interacting with new monomers and generates new active polymeric chains via the re-initiation process. The most significant part of RAFT polymerization is the establishment of the RAFT equilibrium between the established polymeric chain and the newly formed active polymeric chains. The polymerization opportunities are shared among both chains, leading to the development of a polymeric assembly with a very narrow polydispersity index (PDI). After the formation of sufficient polymeric chains, bi-radical termination is carried out to form dead polymers. The synthesized assembly was utilized as a nanocatalyst for the CRR of nitroarenes.
The development of HMGs presented by Bera et al. [24] is presented in Figure 4. As indicated in Figure 4a, the poly(tert-butyl acrylate) macro-chain transferring agent [p(t-BuA macro-CTA)] was synthesized using S-1-dodecyl-S′-(α,α′-dimethyl-α″-acetic acid) trithiocarbonate (DDMAT) (initiator/chain transferring agent) in the presence of azobis(isobutyronitrile) (AIBN; initiator) and 1,4-dioxane (solvent). Synthesized CTA was further utilized for the formation of two different block co-polymers of poly[(N-isopropylacrylamide)-b-(acrylic acid)] (P1a) and poly[(N-isopropylacrylamide-r-N-vinylpyrrolidone)-b-(acrylic acid)] (P2a) using a similar RAFT methodology to that indicated in Figure 4b. The functionalized magnetic nanoparticles (MNPs) were first synthesized via co-precipitation methodology while using ferric chloride and ferric sulfate as precursor salts in the presence of ammonium hydroxide as the oxidizing agent, as indicated in Figure 4c. The grafting of the polymers over NH2-MNPs was achieved by the development of the covalent amide bond between the NH2 of MNPs and the carboxylic group (COOH/COO−1) using N-ethyl-N′-(3-(dimethylamino)propyl)carbodiimide (EDC) and N-hydroxysuccinimide (NHS) coupling. This EDC/NHS coupling is a typical example of the selective bond formation technique using covalent bonds between the MNPs and PNIPAM-based block polymeric gels for the development of HMGs, as presented in Figure 4c.
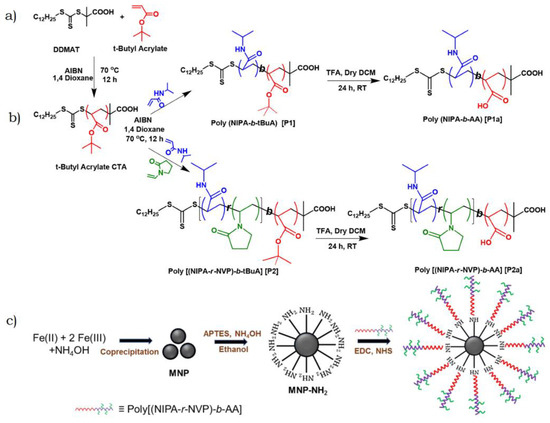
Figure 4.
Schematic representation of (a) the synthesis of p(t-BuA macro-CTA) by RAFT polymerization; (b) the development of block polymers P1a and P2a using the RAFT polymerization; and (c) the synthesis of MNPs by co-precipitation followed by grafting of the block polymers using EDC/NHS coupling. Figure adapted with permission from Ref. [24]—copyright 2022 American Chemical Society.
Yin et al. [25] also engineered Ag NPs coated with the hierarchal hollow silica spheres (HHS) with the shell of the PNIPAM shell using the selective development of hydrogen bonding between silica and PNIPAM moieties. The selective development of hydrogen bonding as a means of immobilization of microgels over the hierarchical assembly of SiO2@Ag NPs-SiO2@PNIPAM is presented in Figure 5. The functionalized Ag NPs were coated with a PNIPAM layer via a copolymerization reaction between allyltriethoxysilane (ATES) and the NIPAM monomer, obtaining an Ag-HHSPN nanocatalyst, as indicated in Figure 5a. The preparation of isomorphological as well as ordered Ag-HHSPN structures was documented via scanning electron microscopy (SEM), as indicated in Figure 5b, which was further validated by the transmission electron microscopy (TEM) results. The TEM analysis, as presented in Figure 5c, affirms the PNIPAM shell encapsulation of Ag nanostructures, as observed by the appearance of two rings in the TEM micrographs. High-resolution (HR)-TEM analysis was further utilized to identify the mean size of Ag NPs (0.204 nm), as indicated by Figure 5d. The compositional analysis of the catalyst performed via energy-dispersive X-ray spectrophotometry (EDX) indicated that the sample was extremely pure, as presented in Figure 5e. The scanning-TEM (STEM; Figure 5f) analysis further highlighted the distribution of the elements within the whole assembly with silicon (Si; Figure 5g) and oxygen (O; Figure 5h) rings to be present as the double-layer rings, indicating the proper coating of Ag with SiO2. The nitrogen (N; Figure 5i) ring (present in PNIPAM) also covered the whole assembly, and Ag (Figure 5j) and carbon (C; Figure 5j) rings indicated that Ag NPs were fully encapsulated in the case of the Ag-HHSPN nanocatalyst. The synthesized assembly was utilized as a nanocatalyst for the CRR of nitroarenes.
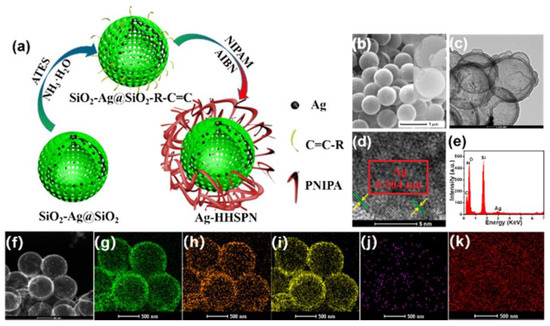
Figure 5.
(a) Synthesis of Ag NMs coated with HHS followed by PNIPAM coat; (b) SEM; (c) TEM; (d) HRTEM; (e) EDX; (f) STEM; (g–k) Image mapping concerning the Si (g), Ni (h), O (i), Ag (j), and C (k). Figure adapted with the permission from Ref. [25]—copyright 2020 American Chemical Society.
2.2. Characterization Techniques for HMGs
Several different characterization techniques for investigating numerous functionalities of synthesized HMGs have been documented in the academic literature. The summary and purpose of the implemented technique are summarized in Table 1.

Table 1.
Characterization techniques used for investigating the peculiar properties of HMGs.
2.3. Properties of HMGs
HMGs are composite materials containing two different components, namely microgels and NMs, available in a single system. Consequently, the properties associated with both these components will also be present in HMGs.
2.3.1. Temperature Responsiveness of HMGs
The N-isopropylacrylamide (NIPAM) constituent of PNIPAM-based HMGs is accountable for the acquisition of the thermosensitivity of HMGs owing to the balancing of polymer–polymer and polymer–solvent interactions. Numerous studies documenting the temperature sensitivity of PNIPAM have been carried out. At lower temperature values, the acrylamide group (with functionalities of C=O and –NH) can interact strongly with water molecules (present as the dispersed phase in gels) via hydrogen bonding. Consequently, the polymer–solvent interactions are regarded as the dominant force under these lower-temperature conditions. Increasing the temperature enhances the kinetic energy of the medium, which disrupts the hydrogen bonds present in the HMGs, and polymer–polymer interaction becomes dominant. The disruption of the interactions leads to the transition from coil form (observed at lower temperatures) to globular form (observed at higher temperatures), and the temperature of this transition is termed the lowest critical solution temperature (LCST). The VPTT and LCST temperature was found to be 32 °C. The general trend observed in the case of PNIPAM-based HMGs is presented in Figure 6, where increasing the temperature reduces the size or hydrodynamic radius of fabricated HMGs. As presented in Figure 6, a sharp change in the hydrodynamic radius was documented at the VPTT value.
The VPTT value is a function of the parameters of the comonomer dose, solvent nature, and the dose of NMs fabricated inside the sieves. Pure PNIPAM microgels have a VPTT value of 33.7 °C, which can be modulated using a variety of factors. Han et al. [32] documented that the VPTT of PNIPAM microgels shifted to 32 °C by the introduction of the hydrophobic azobenzene group into PNIPAM as a comonomer. The addition of the Au NPs further reduced the VPTT to 29.5 °C. Darini and Ghorbanloo [33] functionalized PNIPAM with the comonomer of 2-acrylamido-2-methyl-propane sulfonic acid (AMPSA) and observed that the VPTT of engineered PNIPAM-AMPSA MGs was found to be greater than the general VPTT of the PNIPAM monomer. The authors identified that the hydrophilic moieties result in an increment in the VPTTs (i.e., 42–45 °C in the case of AMPSA), while the hydrophobic moieties result in a decrease in the VPTT values, as previously explained by Han et al. [32]. This discussion affirms that the selection of the proper comonomer is essential for the acquisition of the desired thermo-responsiveness for catalytic application.
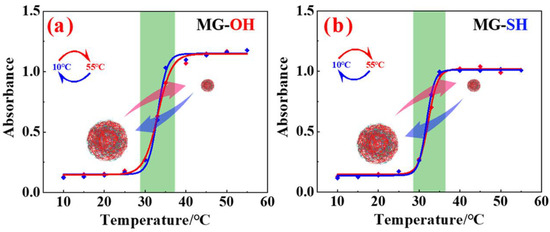
Figure 6.
Temperature responsiveness of (a) hydroxyl-functionalized PNIPAM microgels (MG-OH) and (b) thiol-functionalized PNIPAM microgels (MG-SH). The red curves represent the heating process carried out from 10 °C to 55 °C while the blue curve represents the cooling process carried out from 55 °C to 10 °C. The compact and swelled states of the microgels are represented by the smaller and larger balls. At higher temperatures, the MG-OH and MG-SH exhibited collapsed or shrunken morphology, while at lower temperatures, both assemblies exhibited a swollen state with higher hydrodynamic values. The green highlighted region represents the range of the temperature responsible for the morphological transitions of the microgels. Figure adapted with the permission from Ref. [34]—copyright 2023 American Chemical Society.
2.3.2. pH Responsiveness of HMGs
The functional moieties introduced into comonomers are also found to be responsible for the pH responsiveness of HMGs. The functional moieties present in the polymeric material become either protonated or deprotonated/charged materials, which ultimately results in the swelling/shrinkage of HMGs. For instance, the functional moieties of AAc or MAC exhibit pH responsiveness by utilizing the protonated (COOH) or deprotonated (COO−1) carboxylic groups of these groups. At lower pH values, the medium becomes acidic (with a high concentration of hydrogen ion [H+]), which results in the protonation of the acid groups (COOH). Consequently, HMGs remain deswelled under these extreme conditions. Under basic conditions, the medium becomes enriched with hydroxyl groups (OH−1). These OH−1 ions extract protons from the COOH group, resulting in the formation of COO−1 groups. These charged pendent groups repel each other, and the size of the mesh increases, resulting in the swelling of the HMGs. This pH responsiveness is an essential tool for the modulation of the catalytic efficacy of the HMGs, where the pH of the medium acts as a switch that activates the catalyst. The pH responsiveness of the HMGs is presented in Figure 7.
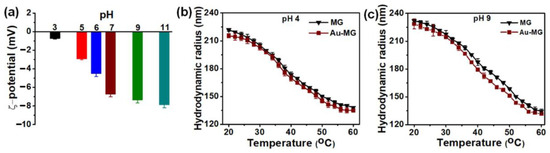
Figure 7.
(a) Zeta potential of HMGs (Au-PNIPAM HMGs) in terms of pH; Hydrodynamic radius values concerning the variation in temperature at the pH values of 4.00 (b) and 9.00 (c). Figure adapted with the permission from Ref. [12]—copyright 2022 American Chemical Society.
The zeta potential values of Au-based HMGs at different pH values are shown in Figure 7a. At lower pH values or acidic conditions, the H+ ions are in excess, keeping the carboxylic groups (from MAC) in their protonated form (COOH), and the negative values of zeta potential were recorded. With an increment in pH, the zeta potential became even more negative, which indicated that, owing to the presence of the OH−1 ions (basic medium), the protons were extracted from the COOH groups, leaving behind the assembly with the ever-increasing negatively charged (COO−1) groups. The presence of the COO−1 groups further facilitated the morphological transitions, as observed in the case of the Au-PNIPAM HMGs studied at pH 4.00 (Figure 7b) and 9.00 (Figure 7c) over a wide range of temperatures. At 20 °C, high hydrodynamic radius values were recorded for HMGs at the pH value of 9.00 in comparison to 4.00, indicating that the COO−1 groups available at the basic condition caused repulsions leading to the assembly comparatively existing in the swollen state (i.e., microgels with larger sieve sizes). At pH 4.00, the COOH group does not have any repulsions, leading to the lower value of the hydrodynamic radius of the assembly under acidic conditions. Moreover, the assemblies at pH 4.00 and pH 9.00 both underwent morphological changes from swollen to deswelled with the increase in temperature, as indicated by the lowering of the assembly radius values with the temperature increase for Figure 7b,c, respectively. This lowering was attributed to the rapid removal of water from hydrogel assembly via the evaporation process with the increase in temperature.
2.3.3. Optical Responsiveness of HMGs
The optical responsiveness of HMGs is imparted into these assemblies, owing to the presence of NMs or NPs. NMs are well-known plasmonic materials, which is their characteristic feature. The electron or surface plasmons of NMs start oscillating in response to the oscillating electric field of the electromagnetic radiations. When the frequency of these oscillations matches, a resonance occurs, which appears as the surface plasmonic resonance (SPR) band in the ultraviolet–visible spectroscopy (UV–VIS) spectrum. This feature is essential in the case of the photocatalytic degradation process of the pollutants [2]. Since photocatalysis is not included in the scope of this review, it will not be discussed in detail here. However, it should be mentioned here that the optical responsiveness of NMs indirectly facilitates the conformational variation of HMGs. The radiation interactions of NMs (as the SPR process) allow NMs to cool down using a vibration-based radiation-less relaxation process. The SPR band is not only a significant factor used for the validation of the successful development of NMs in HMGs, but it also provides essential information regarding the shape and yield of NMs fabricated in the HMGs. The presence of the single band (as presented in Figure 8) affirms the formation of iso-morphological spherical NMs [35]. In the case of Au nanorods (NRs), two SPR bands are observed, which indicates that two types of resonance (longitudinal and transversal) happen in the case of rod-shaped morphology [36]. This resonance process heats the assembly and leads to morphological changes in HMGs indirectly by causing temperature responsiveness. The heating process enhances the process of evaporation, resulting in a lower water content in the hydrogels. This allows polymer–polymer interaction to dominate rather than polymer–solvent interaction, and causes the shrinkage of the overall assembly. The optical responsiveness of HMGs is presented in Figure 8.
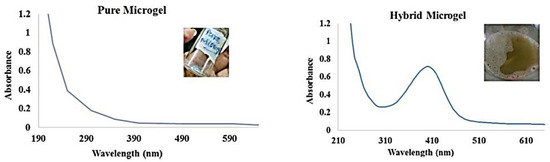
Figure 8.
UV–VIS spectrum of pure MGs and CuO NM-based HMGs. Figure adapted with the permission from Ref. [7]—copyright 2022 Elsevier.
3. Fundamentals of CRRs in HMG
The fundamental peculiarities associated with benchmark CRRs are discussed below.
3.1. Main Process and Progress Detection
CRRs, particularly CRRs associated with nitroaromatic compounds and dyes, are regarded as the benchmark reaction for investigating the efficacy of any newly engineered assembly. The reason behind this convention is ascribed to numerous phenomena, most prominently that this reaction being a part of wastewater treatment plants. The other reasons include the fact that dyes or pollutants utilized during these studies are colored, and the reduction of the pollutants can be visually observed as well. Consequently, this makes the study of these reactions quite easy. Moreover, most of these pollutants exhibit a maximum absorption wavelength () in the UV–VIS region, indicating that the progression of this reaction can also be studied using the simple instrument of UV–VIS. The typical CRRs investigated using the UV–VIS are presented in Figure 9. As per Beer–Lambert law, the absorbance documented in the UV–VIS is directly proportional to the amount of the UV–VIS active pollutant in the medium. Consequently, the absorbance of the pollutant observed at the maximum absorption wavelength of the pollutant is a direct indication of the pollutant dose available in the system. This law not only allows us to study the progress of the CRR but also provides an easy way to calculate the reduction rate using the kinetics equation. The absorbance values associated with the particular pollutant are observed to be highest at the start of the reaction, as there is no reduction/degradation at that time. Upon the addition of the catalyst and reductant into the medium, pollutant concentration starts decreasing in the medium as it starts reducing. Consequently, the absorption intensity of the pollutant also starts decreasing over time, as per Beer–Lambert law. The main process involved in the CRRs of pollutants performed while using HMGs as a catalyst is summarized in the following steps:
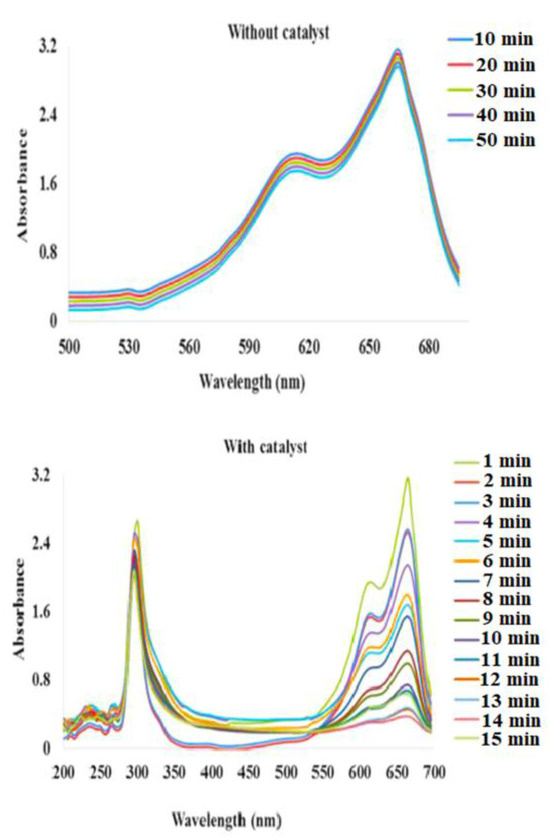
Figure 9.
UV–VIS spectrum of the methylene blue dye without the catalyst exhibited a negligible decrease in absorbance. With the addition of the catalyst, the characteristic peaks of MB (664 nm and 615 nm) exhibited a decrease in absorbance with time. Figure adapted with the permission from Ref. [7]—copyright 2022 Elsevier.
- HMGs are first placed in the swelled state to overcome any possible diffusional barrier that can be observed by the pollutant in reaching the NPs present in the HMGs.
- The pollutant then becomes physically adsorbed on the surface of the NPs present in the HMGs.
- The reducing agent either becomes absorbed on the surface of NPs (by the Langmuir–Hinshelwood (LH) mechanism), or remains present in the solution (by the Eley–Rideal (ER) mechanism) during this process.
- The reductant provides the electron to the pollutant by utilizing the electron transferring/transducing surface of the NPs present in the HMGs, and the pollutant is reduced.
- The formed reduced product is then desorbed from the surface of the NPs and is diffused out of the HMGs into the solution.
3.2. Kinetics and Thermodynamics
In terms of the kinetic studies, CRRs are regarded as kinetically unfavorable reactions, indicating that the presence of the catalytic surface responsible for the shifting of the electron from the reductant to the pollutant is essential for the occurrence of the reaction. Most conventional CRRs are documented as following pseudo-first-order (PFO) kinetics. The typical reaction associated with pollutant reduction can be summarized as follows:
The pollutant exhibits the UV–VIS absorption band within the range of 200–800 nm, and the absorbance measured at the start of the reaction is labeled as . As the absorbance is directly proportional to the concentration of the pollutant, the value will be the highest, as the reduction reaction is not carried out at the start. Upon the addition of a reducing agent and HMGs, the reduction reaction of the pollutant will start, and the concentration of the pollutant in the medium will be decreased. Consequently, the absorption intensity with the increase in the reaction time will be reduced as well. This absorption intensity documented at any reaction time (t) is represented as . The rate reaction equation will be written as follows:
where represents the apparent rate constant of the reaction, which is the main kinetic parameter investigated for any newly synthesized HMG-based nanocatalyst. This parameter serves as the quantitative parameter for comparing the efficacy of the synthesized catalyst. The greater the value of , the better the synthesized catalyst in comparison to the catalysts with lower values of . The rate constant also serves as the bridge to investigate several thermodynamic parameters. For instance, the activation energy (; the minimum amount of energy essential for the reactant molecules to convert into the product) can be investigated using the Arrhenius equation (Equation (6)).
where exhibits the Arrhenius factor (associated with the fraction of excited molecules converting into the product), is the gas constant (8.314 J K−1 mol−1), and exhibits the temperature value of the underlying reaction. and can be graphically calculated using the linear form of the Arrhenius equation (Equation (7)).
Here, represents the slope of the graph, and represents the intercept of the plot developed between (x-axis) and (y-axis). Both slope and intercept can be utilized to investigate and , respectively. Moreover, if the rate constants ( and ) are measured at two different temperature values ( and ), then the can be effectively measured by performing just two experiments (Equation (8) in comparison to a series of experiments presented in Equation (7).
Here, also represents the slope of the graph and can be calculated by multiplying the numerical value of the slope with . Other factors, including Gibbs free energy of activation (), enthalpy of activation (), and entropy of activation () can also be studied using the Eyring equation, as given by Equation (9).
Here, represents Plank’s constant (6.626 × 10−34 Js) and represents Boltzmann’s constant (1.381 × 10−23 J K−1). These parameters explain the spontaneous or non-spontaneous nature of the underlying chemical reaction. Generally, kinetic analysis is performed in most academic studies documenting the usage of HMGs for CRRs, while thermodynamic studies are rarely performed.
3.3. Mechanism
Two different types of mechanisms, including the LH and ER mechanisms, were documented for HMGs acting as nanocatalysts for reduction reactions. In the case of the LH mechanism, the following steps can be identified for the overall process: (a) the addition of pollutant, reductant, and HMGs in the medium; (b) the diffusion of the reductant and pollutant into the sieves of the microgels; (c) the adsorption of the reductant and pollutant into the NP surface of HMGs; (d) the provision of electrons from the reductant to the NP surface and then transduction of the electrons to the pollutants; (e) the reduction of the pollutant; (f) the desorption of the product and reductant from the NPs in HMGs; and (g) the diffusion of the product and reductant from the sieves of the microgels. Most of the academic studies document the LH type of mechanism for HMGs. It should also be noted that competition occurs between the reductant and the pollutant to be attached to the surface of the NPs present in the HMGs. Therefore, the optimization of the reaction becomes even more essential, and the reductant concentration must be managed such that the active sites associated with the NPs should remain available for the pollutants to become attached. If the pollutant does not reach the surface of NPs, it will not receive the electron from the reductants, and the pollutant will not be reduced.
Apart from the LH mechanism, another mechanism, namely the ER mechanism, is also documented for CRRs using HMGs as the nanocatalysts. The steps involved in the ER mechanism are as follows: (a) the addition of pollutant, reductant, and HMGs in the medium; (b) the diffusion of the reductant and pollutant into the sieves of the microgels; (c) the adsorption of the pollutant on the NPs surface of the HMGs; (d) the provision of electrons from the reductant (present in the solution) to the NPs surface and then transduction of the electrons to the pollutants; (e) the reduction of the pollutant; (f) the desorption of the product from the NPs in HMGs; and (g) the diffusion of the product and reductant out of the sieves of the microgels. The main advantage of this mechanism is that there is no competition between the reductant and pollutant in this case.
4. Literature Survey/Recent Advancements
4.1. Advancements in Terms of Morphology
Modifications introduced into the morphology of synthesized HMGs are the major advancements documented in this research domain, as they directly provide ways to modulate the catalytic efficacy of the underlying reaction. The morphology of HMGs has been developed based on the desired application. Typically, PNIPAM-based HMGs are categorized into two broader types, namely homogenous and heterogeneous, based on morphology.
4.1.1. Homogenous HMGs
Homogenous HMGs are one of the pre-existing morphologies associated with HMGs, where the crosslinking density of PNIPAM microgels remains consistent throughout the assembly, and NMs are uniformly distributed in the sieves of the microgels. To prepare such assemblies, microgels were prepared first, and then NMs were fabricated inside the sieves of the microgels by the in situ reduction of salt. Ahmad et al. [37] first engineered copolymerized PNIPAM microgels (containing PNIPAM and chitosan) and then introduced Ag NPs inside the meshes of gels by chemical reduction. The synthesized HMGs were used as a catalyst for the CRRs of 4NP, 2NP, 4NA, and 2NA. The authors performed the individual as well as simultaneous reduction of the pollutants to better stimulate the conditions of industrial effluents. Hou et al. [38] also developed PNIPAM-based HMGs containing Au NPs as a homogenous catalyst for catalyzing the homo-coupling reactions.
Agnihotri and Dan [12] synthesized the homogenous Ag NPs/PNIPAM-MAC HMGs for carrying out the catalytic reduction of MB. The zeta potential of these studies revealed that the assembly remained negative at all pH values, and an increase in the negative values was documented by increasing the pH value from 3 to 11 values. The observance of negative charge is expected at basic conditions because deprotonation of the COOH groups to generate COO−1 groups of the MGs occurs, leading it to become swelled. However, the observance of the negative charge was not expected under acidic conditions because the protonation of COOH groups is expected. The authors attributed the observance of the negative charge under acidic conditions to the utilization of potassium persulfate as an initiator. Consequently, the polymeric chains were terminated with negative sulfate ions, leading to the presence of a negative charge at all conditions. The presence of this negative charge makes these HMGs excellent catalytic assemblies for cationic pollutants. The authors performed the CRR of MB and carried out kinetics and thermodynamic analyses for the underlying reaction. Higher reduction efficacies were documented at conditions of high pH (alkaline condition, pH 11), low temperature (lower than VPTT value, maximum 20 °C, and under low ionic strength. The authors also tested anionic dye for reduction, but better catalytic efficacy was documented for cationic dye rather than anionic dye, indicating that the presence of charge in the HMGs is not only essential for morphological changes but also makes HMGs selective catalysts.
The major disadvantages associated with these homogenous HMGs must also be discussed. First, homogenous HMGs are mechanically less stable than other morphologies and cannot be used as an effective catalyst at higher temperatures. For instance, Jabeen et al. [29] engineered polydispersed cobalt (Co) NPs within the sieves of the PNIPAM microgels and employed these HMGs as a catalyst for CRRs of 4NP, 2NP, 4NA, and 2NA. At high temperatures, the catalytic efficacy of the catalyst was greatly reduced owing to the contraction of the HMGs due to loss of water, indicating that these homogenous HMGs were not as effective at higher temperatures for catalysis. This limits the usage of the homogenous HMGs at high temperatures. Raza et al. [39], Jang et al. [19], Mohan et al. [40], and Hussain et al. [41] documented the abovementioned results in terms of the rate of kinetics. A literature survey associated with homogenous HMGs for CRRs of pollutants is presented in Table 2. Furthermore, the generalized synthesis scheme for the fabrication of homogenous HMGs is presented in Figure 10.

Table 2.
Literature survey of the PNIPAM-based homogenous HMGs for CRRs.
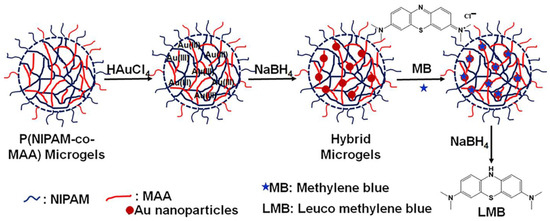
Figure 10.
Synthesis of the homogenous HMGs for CRRs. Figure adapted with permission from Ref. [12]—copyright 2022 American Chemical Society.
4.1.2. Heterogeneous HMGs
In the case of the heterogeneous HMGs, the crosslinking density does not remain consistent throughout the assembly but can be divided into different regions of core or corona/shells. The NMs can be introduced into any of the regions, and the presence of the NMs at the specific region helps in the modulation of the catalytic efficacy of HMGs.
Rigid Core@Polymeric Shell/NM-Based HMGs
Rigid core@polymeric shell-based HMGs address one of the basic problems associated with homogenous HMGs, which is leakage issues of NPs from HMGs. In these types of assemblies, NPs or NMs are developed separately first, and then the smart shell is developed around it [46]. As the shell completely caps the NMs, leakage issues are avoided effectively. Moreover, the mechanical characteristics of HMGs are extensively improved owing to the presence of the rigid core, which makes this overall assembly quite stable [47]. An interesting study providing an effective way to modulate the catalytic efficacy of engineered HMGs using the core@shell morphology was proposed by Zbonikowski et al. [48]. The authors developed an extremely thin polymeric shell (around 5 nm) of PNIPAM around FexOy@SiO2 NPs and investigated the impact of the conformational changes observed in the PNIPAM shell at the air–water interface concerning the changing temperature. This approach can be implemented for the modulation of the catalytic efficacy in that at higher temperatures (40 °C or above) or in air, the polymeric shell exists as a highly crosslinked structure and acts as a capping agent surrounding the NPs. Consequently, the catalyst will be switched off under these conditions. However, as soon as the catalyst is hydrated at the lower temperatures (20 °C), polymeric chains become swelled, and chains become free, leading to the activation of the catalyst. These conformational changes provide an effective way to modulate catalytic efficacy using the diffusional barrier of the pollutants for fabricated HMGs.
Chang et al. [34] developed Au NPs@modified PNIPAM polymeric shell-based HMGs and documented that the VPTTs, modulated by the temperature, were utilized as a switch for controllable CRR of the 4NP. The authors identified that the simple Au NPs followed the Arrhenius equation, where the increase in temperature increased the catalytic reduction potential of the catalyst, whereas, in the case of the Au NPs@modified PNIPAM polymeric shell, the reaction followed the Arrhenius equation until the attainment of the VPTT. However, as soon as the shell was contracted with the increase in the temperature, the catalytic efficacy decreased rapidly. The study affirms that the capping of the polymeric shell can be utilized as an ON–OFF switch for modulating the catalytic efficacy of HMGs. The disadvantage of the catalyst being ineffective at higher temperatures was observed in the case of rigid core@polymeric shell HMGs, which was quite similar to the homogenous HMGs. Han et al. [32], Minier et al. [49], and Li et al. [26] observed a similar trend of the reduction in the catalytic efficacy of HMGs owing to steric hindrance. The synthesis mechanism associated with the fabrication of the rigid core@polymeric shell is presented in Figure 11.
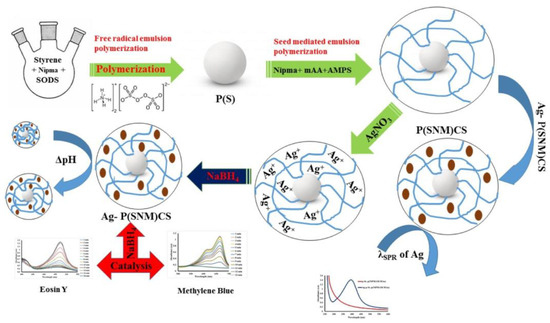
Figure 11.
Synthesis of the rigid core@polymeric shell for CRRs of MB and Eosin Y. Figure adapted with the permission from Ref. [50]—copyright 2023 Taylor and Francis.
Polymeric Core@Polymeric Shell/NM-Based HMGs
As explained in the above section, the cores of HMGs can be made rigid using inorganic NMs as a core in the core@shell morphology of HMGs. Another type of morphology that can be utilized for HMGs is the development of a polymeric core@polymeric shell to achieve a soft polymeric catalyst. Typically, the polymeric core is made up of one material, while the polymeric shell is made up of other materials using the fabricated core as the seed. NMs are then introduced into the assembly (mostly in the shell) using the in situ reduction of salts. The shells can be differentiated based on the differences in the crosslinking density of both materials. Generally, the synthesis of such HMGs is achieved in three steps, namely the synthesis of the core via emulsion polymerization, the synthesis of the shell via polymerization, and then the synthesis of NMs for HMG fabrication. Naseem et al. [51] synthesized the polymeric core@polymeric shell using a similar three-step methodology. The polystyrene (PS) core was first developed using emulsion polymerization, followed by the development of the poly(N-isopropylmethacrylamide) (PNIPMAM)-AAc polymeric shell by seed-mediated polymerization. In the third step, Ag NPs were fabricated inside the sieves of the polymeric shell using the in situ reduction methodology to develop HMGs. Besold et al. [22] also developed Ag NPs fabricated in the PNIPAM shell of PS@PNIPAM to develop HMGs using the same abovementioned synthetic route. The fabricated HMGs were used for the CRR of the 4NP. The study is interesting in that it explores two different types of reduction mechanisms (i.e., condensation route and direct reduction route) associated with the 4NP reduction carried out in the presence of HMGs.
An interesting fabrication methodology to achieve the polymeric core@polymeric shell morphology was reported by Mustafa et al. [52]. The authors reported a novel methodology with a reduced number of steps (two steps rather than three steps) for the fabrication of such an assembly using the polymerization rates of the added monomers or comonomers. The authors added the N-isopropyl acrylamide (first monomer) and 2-hydroxyethyl methacrylate (HEMA; second monomer) in a single pot alongside the crosslinker and free-radical initiator for the radical polymerization reaction. As the HEMA polymerization rate is higher in comparison to the NIPAM, the HEMA core with high crosslinking density was developed first, acting as a polymeric core of the assembly. After the consumption of the HEMA, the remaining NIPAM monomer was then polymerized to develop the PNIPAM polymeric shell around the PHEMA core. This is followed by the second step, where the precursor salt is introduced along with the reductant in the medium to develop the Ag NPs in the sieves of the polymeric shell. The soft core of PHEMA provided much-needed mechanical strength owing to its high crosslinking density and hydrophobic polymeric nature, while the smart PNIPAM polymeric shell was used to develop the stimuli-responsive catalyst for the CRRs of the pollutants. The authors implemented synthesized HMGs for the reduction of 4-NP [52].
Ullah et al. [53] developed cadmium (Cd) NPs-PNIPAM-AAc@poly acrylic acid (PAAc) HMGs (containing the core of PAAc and the shell of PNIPAM-AAc) by utilizing a similar approach. However, it should be mentioned that the reactivity difference between the monomers (NIPAM and AAc) was not that much, leading to the presence of AAc in the core as well as in the smart polymeric shell. Moreover, the presence of AAc in the core and the shell further facilitated the swelling of the overall assembly owing to the presence of a negative charge under basic conditions. Consequently, Cd NPs were fabricated in both the shell and core regions of HMGs. The fabricated assembly was utilized as a catalyst for the CRRs of 4-nitroanisole (4NAs), 4NP, 3-nitrophenol (3NP), MB, Congo red (CR), methyl red (MB), reactive black 5 (RB5), 2,4-dinitro phenol (DNPO), crystal violet (CV), and 4-nitrobenzoic acid (4NBA). This study documented the excellent protocol for performing comparative analysis of CRRs of numerous dyes and nitroaromatic pollutants carried out under similar conditions.
In terms of the modulation of the catalytic efficacy, the polymeric core@polymeric shell presents even more options in comparison to the rigid core@polymeric shell. This sort of assembly exhibits even higher catalytic rates at extremely high temperatures. The impact of catalytic efficacy with respect to the variation in the temperature for Ag NP-based PS@PNIPMAM-AAc HMGs was studied by Naseem et al. [51]. The authors observed that the four types of regions or trends can be observed for these materials. Below 40 °C, swollen HMGs were present, and the reactants could reach fabricated Ag NPs without facing any substantial diffusional barrier. Accordingly, HMGs exhibited the Arrhenius equation-like trend where the catalytic efficacy was found to be increased by increasing the temperature from 30 °C to 40 °C. With the increment in the temperature from 40 °C to 50 °C, the catalytic efficacy was found to be decreased. This decrease is ascribed to the fact that the microgels contracted after increasing the temperature from the VPTT. Therefore, the reactants cannot reach NPs owing to the contracted state of the polymeric shells, leading to decreased catalytic efficacy. In the third region, the sharp increment in the values was documented by increasing the temperature up to 65 °C. This sharp increment in the catalytic efficacy was attributed to the complete collapse of the polymeric shell, which resulted in NPs becoming exposed to the reactants present in the medium. After the collapse of the polymeric shell, the responsiveness associated with the polymeric shell is lost, and the NPs remain exposed to the dye molecules. Consequently, the becomes independent of the variations in the temperature above 65 °C. Several studies have documented the observance of a similar trend in the case of polymeric core@polymeric shell HMGs [22,23,51,52,54]. The synthesis mechanism associated with the fabrication of the polymeric core@polymeric shell is presented in Figure 12. A literature survey associated with heterogeneous HMGs for CRRs of the pollutants is presented in Table 3.
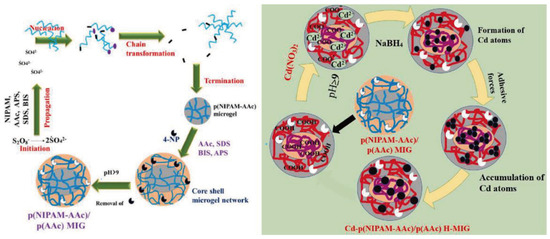
Figure 12.
Synthesis of the polymeric core@polymeric shell (PNIPAM-AAc)@polyAAc MGs and PNIPAM-AAc@polyAAc containing Cd NMs HMGs. Figure adapted with the permission from Ref. [53]—copyright 2020 Elsevier.

Table 3.
Literature survey of PNIPAM-based heterogeneous HMGs for CRRs.
4.2. Advancements in Terms of NMs in HMGs
Apart from the modification in the morphology, the advancement in NMs has also been carried out in recent times to improve the catalytic efficacy of HMGs. Typically, HMGs are divided into three broad categories, namely monometallic/metallic NMs, bimetallic/multi-metallic NMs, and combination/nanocomposites-based HMGs based on the type of fabricated NMs present in HMGs. A literature survey associated with the monometallic, bimetallic NMs, and nanocomposite-based HMGs is provided in Table 4. It should also be noted that the literature associated with bimetallic and nanocomposites-based HMGs is scarce as these are the recent approaches of modifications used for HMGs.
4.2.1. Monometallic NM-Based HMGs
Most of the studies discussed above fall into the category of monometallic NM-based HMGs, as the synthesized HMGs only contain one type of the NMs fabricated inside the sieves of the MGs. For instance, Chang et al. [34] developed a rigid core@polymeric shell catalyst containing Au NPs as the core. Since this assembly contained only one type of nanomaterial, it can be termed a monometallic-based HMG assembly. Cao et al. [62] developed core@shell@shell HMGs containing ZnO NPs as a core, followed by the shell of polydopamine (PDA) and PNIPAM. Begum et al. [1] synthesized polymeric core@polymeric shell MGs assembly (containing PNIPAM-HEMA-AAc as the shell and PHEMA as a core) and fabricated Ag NPs inside the sieves of these particles. HMGs were utilized for carrying out the CRR of the 4NA. The kinetics study performed for the reaction revealed it to be following the PFO kinetic pathway. Complete degradation of 4NA was achieved using this catalyst. The development of monometallic HMGs is the pre-existing approach for the development of HMGs.
4.2.2. Bimetallic/Muli-Metallic NM-Based HMGs
A literature survey of the reviews published before 2020 highlights the development of bimetallic/multi-metallic assembly as one of the major future directions remaining to be explored for the HMGs [63,64,65,66]. This approach has been the point of discussion for the researchers exploiting the research domain of HMGs in 2020–2023 for CRRs of pollutants. Kakar et al. [67] documented the development of a bimetallic HMG assembly containing copper (Cu)/Pd NPs fabricated inside thiol-functionalized PNIPAM-AAc and utilized this assembly for performing the catalytic reduction of MB and 4NP. The bimetallic assembly exhibited better reduction rates in comparison to their monometallic counterparts owing to the synergistic electrochemical effects of Cu and Pd NPs with each other. Just like HMGs, the bimetallic assemblies incorporate the functionalities of both the NMs within a single assembly. Arif et al. [15] also engineered a bimetallic HMG assembly containing Ag/Co NPs encapsulated in PNIPAM MGs and used this assembly as a catalyst for the CRR of methyl orange (MO). Comparison with the academic literature reveals that the bimetallic assembly exhibited better reduction results in comparison to their monometallic counterparts.
The synthesis of bimetallic assembly is not much different in comparison to monometallic assembly. Bimetallic assembly can be prepared in the same pot as monometallic assembly, but instead of adding only one precursor salt in the medium along with the reductant, two or more precursor salts are introduced into the assembly in the case of bimetallic assembly. The added reductant reduces both the precursor ions separately to generate NPs in the medium via the in situ reduction of the salt methodology. Arif [57] developed a bimetallic assembly containing Ag NPs and nickel (Ni) NPs by utilizing a similar approach. The author first synthesized PNIPMAM-MAC copolymerized MGs assembly using the emulsion polymerization and then fabricated Ag and Ni NPs inside the sieves of the MGs by introducing reductant (NaBH4) and precursor salts (silver nitrate for Ag NPs and nickel nitrate for Ni NPs) in a single pot. The reductant reduces the added ions into NPs to perform in situ chemical reduction of the metal ions of the salts. Raza et al. [39] also engineered the bimetallic assembly containing Ag NPs and titanium (Ti) NPs fabricated inside PNIPAM-MAC MGs using a similar approach. The authors utilized the engineered assembly for the CRRs of 4NP, MO, CR, MR, 2NP, CV, EBT, MB, and DNP. The pictorial description of the bimetallic assembly synthesized via in situ reduction methodology is presented in Figure 13.
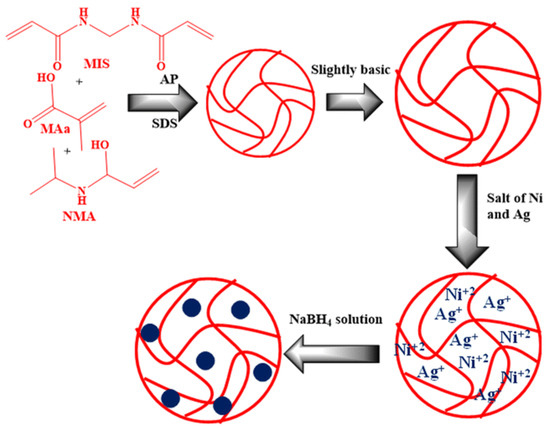
Figure 13.
Synthesis scheme of P(NIPAM-MAC) and Ag/Ni-P(NIPAM-MAC). Figure adapted with the permission from Ref. [57]—copyright 2023 Royal Society of Chemistry. Creative Commons License.
4.2.3. Nanocomposites (NCs) Based HMGs
Apart from incorporating metal NPs inside the meshes of MGs, the other nanometer-ranged inorganic assemblies, such as graphene [44], carbon nanotubes [68], etc. can also be utilized to enhance the catalytic efficacy of the engineered assembly, as indicated in Figure 14. Carbon-based inorganic materials have gained much attention in recent times for this purpose as not only does the added material improve the fabrication capacities of the MGs, but it also improves the electronic conduction capabilities of the overall assembly. Consequently, the electrons can be easily transferred from the reductant to the pollutant molecules, resulting in an increment in the catalytic efficacy of the assembly [44]. Dan et al. [69] developed a thermal switchable catalyst containing palladium (Pd) NP fabricated on graphene sheets, followed by the imprinting of PNIPAM MGs to develop a highly sensitive electrochemical sensor for the detection of 4NP. Ali et al. [5] engineered NCs-based HMGs containing zirconium (Zr) NPs fabricated inside the PNIPAM-MAC MGs supplemented with graphene sheets for carrying out the CRRs of the number of pollutants. The authors identified the factors of type of MG, type of NP used, type of comonomer used, dyes studied, and type of inorganic material incorporated to be the major factors responsible for the modulation of the catalytic efficacy of these reactions.
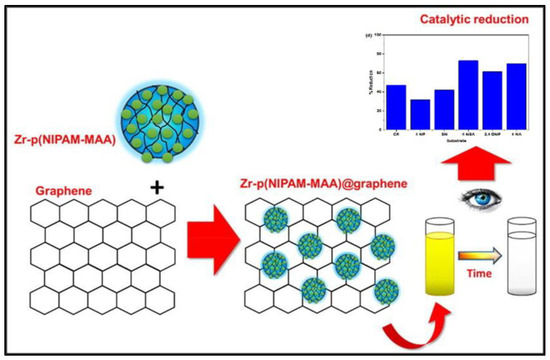
Figure 14.
Synthesis of the NCs-based HMGs (i.e., Graphene-containing HMGs). Figure adapted with the permission from Ref. [5]—copyright 2021 Elsevier.
Of note here is the comparison of the catalytic efficacies of the bimetallic and NC-based HMGs. As indicated by Table 4, almost complete or complete reduction percentages with high reduction rates were acquired using bimetallic HMGs [39,57,67]. However, incomplete reduction with lower percentage degradation was observed for similar dyes when the NC-based monometallic HMG assemblies were used for the catalytic degradation [5,44]. This point requires further exploration and can be utilized by the upcoming researchers as the research gap. However, it is expected that in the case of bimetallic assemblies, surface area associated with the NPs was directly increased, while in the case of NCs-based HMGs, the synergistic effects of both organic and inorganic materials might not be as compatible as claimed by the authors. Consequently, more research is required.

Table 4.
Comparative analysis of nonmetallic, bimetallic, and nanocomposite-based HMGs assembly for the CRRs of pollutants.
Table 4.
Comparative analysis of nonmetallic, bimetallic, and nanocomposite-based HMGs assembly for the CRRs of pollutants.
| Catalytic Assembly | Type of HMGs | Pollutant | Pollutant Dose | Catalyst Dose | Reductant Dose | Rate Constant | Reduction Time | Percentage Reduction | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Thiol-functionalized PNIPAM-AAc/Cu NPs | Monometallic | 4NP | 0.1 mM (1 mL) | 0.06 mg/mL (0.2 mL) | 10 mM (1 mL) NaBH4 | 0.0223 min−1 | 18 min | 100% | [67] |
| Thiol-functionalized PNIPAM-AAc/(Cu/Pd NPs) | Bimetallic | 0.223 min−1 | 120 s | 100% | |||||
| Thiol-functionalized PNIPAM-AAc/Cu NPs | Monometallic | MB | 0.1 mM (1 mL) | 0.06 mg/mL (0.2 mL) | 10 mM (1 mL) NaBH4 | -- | 24 min | 100% | |
| Thiol-functionalized PNIPAM-AAc/(Cu/Pd NPs) | Bimetallic | 0.173 min−1 | 165 s | 100% | |||||
| PNIPMAM-MAC/(Ag/Ni NPs) | Bimetallic | MO | 0.80 mM | 85.71 g/mL | 9.65 mM NaBH4 | 0.925 min−1 | 10 min | 100% | [57] |
| MR | 0.525 min−1 | 9 min | 100% | ||||||
| EBT | 0.540 min−1 | 9 min | 100% | ||||||
| CR | 0.486 min−1 | 10 min | 100% | ||||||
| PNIPAM-MAC/Zr NPs@GS | NCs | 4NP | 0.085 mM | 0.2 mL | 0.1 g NaBH4 | 0.010 min−1 | 68 min | 36% | [5] |
| SN | 0.012 min−1 | 82 min | 41.8% | ||||||
| CR | 0.015 min−1 | 50 min | 48.6% | ||||||
| 4NAs | 0.0210 min−1 | 42 min | 70% | ||||||
| DNPO | 0.023 min−1 | 44 min | 60% | ||||||
| 4NBA | 0.010 min−1 | 48 min | 72% | ||||||
| CNTs@PNIPAM-VBDB@Pd NPs | NCs | NB | 1 mM | 0.01 g | 1 bar H2 gas at 40 | -- | 120 min | 98% | [70] |
| TA-HNTs@PNIPAM/AuNPs | NCs | 4NP | 1 mM (75 L) | 0.05 mL | 133 mM (0.5 mL) NaBH4 | 0.345 min−1 | 10 min | 100% | [71] |
| Perlite glass@PNIPAM-AAm@Pd/NPs | NCs | NB | 1 mM | 0.03 g | 1 atm H2 gas at 40 | -- | 90 min | 98% | [72] |
5. Conclusions
This review presents an overview of the recent academic literature associated with the advanced research domains of HMGs employed as nanocatalysts for the CRRs of aqueous pollutants. To introduce new researchers to this domain, this review introduces the readers to the fundamentals affiliated with HMGs and CRRs. It has been observed that free-radical emulsion polymerization is the most common technique used for the development of the MGs, and for the incorporation of NPs in MGs, the in situ reduction reaction is followed. The combination of these strategies has been found to be an effective way to develop homogenous HMGs. Apart from synthesis techniques, characterization techniques and properties associated with HMGs have also been studied. It has been found that the comonomer and functional moiety available in the HMGs are essential for achieving pH responsiveness and temperature responsiveness of HMGs, while the NMs present in HMGs have been found to be responsible for the optical responsiveness of HMGs. In terms of recent advancements, the morphology of HMGs exhibits the most modification. Broadly, the morphology of HMGs is divided into homogenous and heterogeneous, where it has been identified that all these morphologies play a significant role in the modulation of the catalytic efficacies of HMGs concerning variations in temperature. The types and nature of the NMs incorporated also play a significant role in determining the catalytic efficacy of the synthesized assemblies. The bimetallic assembly exhibited the highest catalytic efficacy for several pollutants. To conclude, HMGs for CRRs are advanced materials that have been given extreme attention in the past three years, and we believe that this review will serve as a tutorial as well as a research-gap identification study for this particular domain.
6. Future Perspectives
The following future perspectives were identified while performing the above critical review of HMGs utilized for CRRs of organic pollutants:
- Thermodynamic parameters should be given as much significance as kinetic analysis because thermodynamic studies provide critical insights regarding underlying reactions.
- There is a recent trend in the use of the response surface methodology (RSM) as a means for the optimization of the catalytic reactions [73]. RSM is an advanced computational and statistical tool that includes the interaction coefficients in the modeling of the underlying reaction [74]. To the best of our knowledge, this approach has not yet been implemented for MGs or HMGs, making it one of the prospective fields for exploration.
- The implementation of the RSM approach for photocatalytic reactions is just starting to be documented [35], indicating that this field should be implemented for catalytic reduction-based reactions as well.
- Data-driven approaches, particularly machine learning, are also finding use in almost every research domain [75]. These approaches can be implemented for the modeling of HMGs for CRRs as well.
- The use of novel types of nanocomposite materials, comonomers, or inorganic substances can develop appropriate and novel nanocatalysts.
- The morphological features of HMGs can be used as a key switch in wastewater treatment plants for simultaneous biomedical (antibacterial, antifungal, antioxidant activities, etc.) and CRR reactions.
Funding
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [Project No: GRANT4544].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Abbreviations
| DPE | 1:1-Diphenylethane |
| 1B2NB | 1-bromo-2-nitrobenzene |
| 1B3NB | 1-bromo-3-nitrobenzene |
| 1C2NB | 1-chloro-2-nitrobenzene |
| 1C4NB | 1-chloro-4-nitrobenzene |
| CEB | 1-chloro-4-ethylbenzene |
| 1M2NB | 1-methyl-2-nitrobenzene |
| 1M22DNB | 1-methyl-2,2-dinitrobenzene |
| 1M24DNB | 1-methyl-2,4-dinitrobenzene |
| VIM | 1-vinylimidazole |
| VBDB | 1-vinyl-3-butylimidazolium bromide |
| 24Dc1NB | 2,4-dichloro-1-nitrobenzene |
| DNPO | 2,4-Dinitrophenol |
| AMPS | 2-acrylamido-2-methylpropane sulfate |
| AMPSA | 2-acrylamido-2-methyl-propane sulfonic acid |
| HMABP | 2-hydroxy-4-(methacryloyloxy)-benzophenone |
| 2MO4NA | 2-methoxy-4-nitroaniline |
| 2NA | 2-nitroaniline |
| 2NPM | (2-nitrophenyl) methanol |
| 3MSi | 3-methacryloxypropyltrimethoxysilane |
| 3MO4NA | 3-methoxy-4-nitroaniline |
| 4CANI | 4-chloroaniline |
| 4NA | 4-nitroaniline |
| 4C12DNB | 4-chloro-1,2-dinitrobenzene |
| 4B4NB | 4-Bromo-4-nitrobenzene |
| 4NAs | 4-nitroanisole |
| 4NBA | 4-nitrobenzoic acid |
| 4NP | 4-nitrophenol |
| 5NB13DA | 5-nitrobenzene-1,3-diamine |
| AA | Acrylamide |
| AAc | Acrylic acid |
| AAm | Allylamine |
| ANI | Aniline |
| AB | Azobenzene |
| BCH | Bicyclo[2.2.1]heptane |
| CNTs | Carbon nanotubes |
| CRR | Catalytic reduction reaction |
| CFI | Confocal imaging |
| CR | Congo red |
| COP | Copolymerized |
| CV | Crystal violet |
| CD | Cyclodextrin |
| DSPB | Diblock spherical polymer brushes |
| DEAEMA | Diethylaminoethyl methacrylate |
| DSC | Differential scanning calorimetry |
| DHC | Dihydrochalcone |
| DLS | Dynamic light scattering |
| EDX | Energy-dispersive X-ray spectrometer |
| EY | Eosin Y |
| EBT | Eriochrome Black T |
| EDTA | Ethylenediaminetetraacetic acid |
| EB | Ethylbenzene |
| FTIR | Fourier transform infrared spectroscopy |
| FEPol | Free-radical emulsion polymerization |
| Au | Gold |
| GO | Graphene oxide |
| GS | Graphene sheets |
| t1/2 | Half-life time |
| HMGs | Hybrid microgels |
| HEMA | Hydroxyethyl methylacrylate |
| ICP | Inductively coupled plasma |
| Fe3O4 | Iron oxide |
| MG | Malachite green |
| MS | Mass spectrometry |
| MAC | Methacrylic acid |
| MB | Methylene blue |
| MO | Methyl orange |
| MR | Methyl red |
| DM4NA | N,N-dimethyl-4-nitroaniline |
| NCs | Nanocomposites |
| NMs | Nanomaterials |
| NPs | Nanoparticles |
| NRs | Nanorods |
| Ni | Nickel |
| NB | Nitrobenzene |
| NMR | Nuclear magnetic resonance |
| OVAT | One-variable-at-a-time |
| TOL | p-Toluidine |
| Pd | Palladium |
| PIMP | Photoiniferter-mediated polymerization |
| Pt | Platinum |
| PAMP | Poly (2-acrylamide-2-methylpropanesulfonic acid) |
| PMETAC | Poly 2-(methacryloyloxy)ethyl-trimethyl-ammonium chloride |
| PNIPAM | Poly (N-isopropyl acrylamide) |
| Bpnipam | Poly (N-isopropylacrylamide) brushes |
| PNIPMAM | Poly (N-isopropyl methacrylic acid) |
| PS | Polystyrene |
| PSV | Polystyrene coated with 4-vinylbenzyl N,N-diethyldithiocarbamate |
| PSS | Polystyrene sulfonate |
| PFO | Pseudo-first-order kinetics |
| RB5 | Reactive black 5 |
| SN | Safranin |
| SEM | Scanning electron microscopy |
| SiO2 | Silica |
| Ag | Silver |
| NaBH4 | Sodium borohydride |
| SPR | Surface plasmonic resonance |
| TA-HNTs | Tannin-aminopropyltriethoxysilane-coated halloysite nanotubes |
| TEOS | Tetraethoxysilane |
| TGA | Thermal gravimetric analysis |
| TChPILs | Thiol-functionalized chitosan poly(protic ionic liquids) |
| TEM | Transmission electron microscopy |
| TOF | Turnover frequency |
| UV–VIS | Ultraviolet–visible spectroscopy |
| VPTT | Volume phase transition temperature |
| XRD | X-ray Diffraction |
| XPS | X-ray photoelectron spectroscopy |
| Zr | Zirconium |
References
- Begum, R.; Ahmad, G.; Najeeb, J.; Wu, W.; Irfan, A.; Azam, M.; Nisar, J.; Farooqi, Z.H. Stabilization of silver nanoparticles in crosslinked polymer colloids through chelation for catalytic degradation of p-nitroaniline in aqueous medium. Chem. Phys. Lett. 2021, 763, 138263. [Google Scholar] [CrossRef]
- Naseem, K.; Farooqi, Z.H.; Begum, R.; Ur Rehman, M.Z.; Ghufran, M.; Wu, W.; Najeeb, J.; Irfan, A. Synthesis and characterization of poly (N-isopropylmethacrylamide-acrylic acid) smart polymer microgels for adsorptive extraction of copper (II) and cobalt (II) from aqueous medium: Kinetic and thermodynamic aspects. Environ. Sci. Pollut. Res. 2020, 27, 28169–28182. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Hussain, Z.; Hussain, T.; Mujahid, A.; Najeeb, J.; Izhar, F. Nanocatalytic assemblies for catalytic reduction of nitrophenols: A critical review. Crit. Rev. Anal. Chem. 2020, 50, 322–338. [Google Scholar] [CrossRef]
- Haleem, A.; Syaal, S.B.; Ajmal, M.; Ambreen, J.; Rauf, S.; Ali, N.; Muhammad, S.; Shah, A.; Zia, M.A.; Siddiq, M. Silver and palladium nanoparticle embedded poly (n-isopropylacrylamide-co-2-acrylamido-2-methylpropane sulfonic acid) hybrid microgel catalyst with pH and temperature dependent catalytic activity. Korean J. Chem. Eng. 2020, 37, 614–622. [Google Scholar] [CrossRef]
- Ali, S.; Shah, S.J.U.H.; Jamil, S.; Bibi, S.; Shah, M.U.; Aqib, A.I.; Zaheer, T.; Khan, S.R.; Janjua, M.R.S.A. Zirconium nanoparticles-poly (N-isopropylacrylamide-methacrylic acid) hybrid microgels decorated graphene sheets for catalytic reduction of organic pollutants. Chem. Phys. Lett. 2021, 780, 138915. [Google Scholar] [CrossRef]
- Begum, R.; Farooqi, Z.H.; Xiao, J.; Ahmed, E.; Sharif, A.; Irfan, A. Crosslinked polymer encapsulated palladium nanoparticles for catalytic reduction and Suzuki reactions in aqueous medium. J. Mol. Liq. 2021, 338, 116780. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Hussain, Z. Novel in-situ synthesis of copper oxide nanoparticle in smart polymer microgel for catalytic reduction of methylene blue. J. Mol. Liq. 2022, 358, 119181. [Google Scholar] [CrossRef]
- Zahid, S.; Alzahrani, A.K.; Kizilbash, N.; Ambreen, J.; Ajmal, M.; Farooqi, Z.H.; Siddiq, M. Preparation of stimuli responsive microgel with silver nanoparticles for biosensing and catalytic reduction of water pollutants. RSC Adv. 2022, 12, 33215–33228. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, Z.H.; Sultana, H.; Begum, R.; Usman, M.; Ajmal, M.; Nisar, J.; Irfan, A.; Azam, M. Catalytic degradation of malachite green using a crosslinked colloidal polymeric system loaded with silver nanoparticles. Int. J. Environ. Anal. Chem. 2022, 102, 4104–4120. [Google Scholar] [CrossRef]
- Moon, H.; Kim, Y. Photothermal-mediated catalytic reduction of 4-nitrophenol using poly (N-isopropylacrylamide-acrylamide) and hollow gold nanoparticles. ACS Appl. Polym. Mater. 2021, 3, 2768–2775. [Google Scholar] [CrossRef]
- Rahimkhoei, V.; Akbari, A.; Zirak, M.; Eftekhari-Sis, B. Ag nanoparticles stabilized on cubic polyhedral oligomeric silsesquioxane cross-linked poly (N-isopropyl acrylamide-co-itaconic acid): An efficient catalyst for 4-nitrophenol reduction. Funct. Mater. Lett. 2020, 13, 2051040. [Google Scholar] [CrossRef]
- Agnihotri, P.; Dan, A. Temperature-and pH-Responsive Hydrogel Nanoparticles with Embedded Au Nanoparticles as Catalysts for the Reduction of Dyes. ACS Appl. Nano Mater. 2022, 5, 10504–10515. [Google Scholar] [CrossRef]
- Zhu, L.; Song, Q.; Ma, H. Synthesis of hyperbranched polysiloxane/poly (N-isopropylacrylamide) microgel, its stimulus responsive behavior and study for drug release. J. Macromol. Sci. Part A 2023, 60, 18–28. [Google Scholar] [CrossRef]
- Naz, S.; Bibi, G.; Jamil, S.; UrRehman, S.; Bibi, S.; Ali, S.; Khan, T.; Khan, S.R.; Janjua, M.R.S.A. Preparation of manganese-doped tin oxide nanoparticles for catalytic reduction of organic dyes. Chem. Phys. Lett. 2022, 802, 139768. [Google Scholar] [CrossRef]
- Arif, M.; Tahir, F.; Fatima, U.; Begum, R.; Farooqi, Z.H.; Shahid, M.; Ahmad, T.; Faizan, M.; Naseem, K.; Ali, Z. Catalytic degradation of methyl orange using bimetallic nanoparticles loaded into poly (N-isopropylmethacrylamide) microgels. Mater. Today Commun. 2022, 33, 104700. [Google Scholar] [CrossRef]
- Du, J.-T.; Qiao, M.; Pu, Y.; Wang, J.-X.; Chen, J.-F. Aqueous dispersions of monodisperse Pt, Pd, and Au nanoparticles stabilized by thermosensitive polymer for the efficient reduction of nitroarenes. Appl. Catal. A Gen. 2021, 624, 118323. [Google Scholar] [CrossRef]
- Hussain, I.; Farooqi, Z.H.; Ali, F.; Begum, R.; Irfan, A.; Wu, W.; Wang, X.; Shahid, M.; Nisar, J. Poly (styrene@ N-isopropylmethacrylamide-co-methacrylic acid)@ Ag hybrid particles with excellent catalytic potential. J. Mol. Liq. 2021, 335, 116106. [Google Scholar] [CrossRef]
- Egemole, F.O.; Eyimegwu, F.M.; Yun, J.; Jang, W.; Byun, H.; Hou, J.; Kim, J.-H. Effects of crosslinking density on the in situ formation of gold-polymer composite particles and their catalytic properties. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128409. [Google Scholar] [CrossRef]
- Jang, W.; Yun, J.; Eyimegwu, P.N.; Hou, J.; Byun, H.; Kim, J.-H. Controlling the formation of encapsulated gold nanoparticles for highly reactive catalysts in the homocoupling of phenylboronic acid. Catal. Today 2022, 388, 109–116. [Google Scholar] [CrossRef]
- Castro Issasi, C.S.; Morales Ibarra, R.; Sasaki, M. In situ synthesis of poly (N-isopropylacrylamide) decorated with silver nanoparticles using pulsed electrical discharge in contact with water interface. Nanocomposites 2022, 8, 136–141. [Google Scholar] [CrossRef]
- Sabadasch, V.; Dirksen, M.; Fandrich, P.; Hellweg, T. Multifunctional Core-Shell Microgels as Pd-Nanoparticle Containing Nanoreactors With Enhanced Catalytic Turnover. Front. Chem. 2022, 10, 889521. [Google Scholar] [CrossRef] [PubMed]
- Besold, D.; Risse, S.; Lu, Y.; Dzubiella, J.; Ballauff, M. Kinetics of the reduction of 4-nitrophenol by silver nanoparticles immobilized in thermoresponsive core–shell nanoreactors. Ind. Eng. Chem. Res. 2021, 60, 3922–3935. [Google Scholar] [CrossRef]
- Naseem, K.; Farooqi, Z.H.; Begum, R.; Wu, W.; Irfan, A.; Ajmal, M. Systematic study of catalytic degradation of nitrobenzene derivatives using core@ shell composite micro particles as catalyst. Colloids Surf. A Physicochem. Eng. Asp. 2020, 594, 124646. [Google Scholar] [CrossRef]
- Bera, S.; Pal, J.; Sahoo, S.; Dhara, D. Stimuli-Responsive Polymer Stabilized Iron Oxide Nanoparticle as Green Catalyst for Styrene Oxidation Reaction. ACS Appl. Polym. Mater. 2022, 5, 720–730. [Google Scholar] [CrossRef]
- Yin, Z.; Xiao, Y.; Li, H.; Chen, G.; Feng, N.; Wu, J.; Li, H.; Xu, H.; Cao, S. Metal nanoparticles confined within an inorganic–organic framework enable superior substrate-selective catalysis. ACS Appl. Mater. Interfaces 2020, 12, 42739–42748. [Google Scholar] [CrossRef]
- Li, R.; Cheng, C.; Wang, Z.; Gu, X.; Zhang, C.; Wang, C.; Liang, X.; Hu, D. Conformational stability of poly (N-isopropylacrylamide) anchored on the surface of gold nanoparticles. Materials 2021, 14, 443. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Ajmal, M.; Naseem, A.; Jabeen, N.; Farooqi, Z.H.; Mahmood, K.; Ali, A.; Rasheed, L.; Saqib, A.N.S. Synthesis of poly (N-isopropyl acrylamide-co-2-acrylamido methylpropane sulfonic acid) hydrogel containing copper and nickel nanoparticles with easy recycling and efficient catalytic potential. Z. Phys. Chem. 2022, 236, 1441–1460. [Google Scholar] [CrossRef]
- Anushree, C.; Krishna, D.N.G.; Philip, J. Synthesis of Ni doped iron oxide colloidal nanocrystal clusters using poly (N-isopropylacrylamide) templates for efficient recovery of cefixime and methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129616. [Google Scholar] [CrossRef]
- Jabeen, N.; Farooqi, Z.H.; Shah, A.; Ali, A.; Khurram, M.; Mahmood, K.; Sahiner, N.; Ajmal, M. Synthesis and characterization of cobalt nanoparticles containing anionic polymer hydrogel nanocomposite catalysts for fast reduction of nitrocompounds in water. J. Porous Mater. 2021, 28, 1563–1576. [Google Scholar] [CrossRef]
- Mahdieh, Z.; Holian, A. Electrospun fibers loaded with ball-milled poly (n-isopropylacrylamide) microgel particles for smart delivery applications. J. Appl. Polym. Sci. 2020, 137, 49786. [Google Scholar] [CrossRef]
- Yadav, R.; Kumar, S.; Narang, P.; Venkatesu, P. How does the addition of shape distinct gold nanoparticles influence on the conformational transition of poly (N-isopropylacrylamide)? J. Colloid Interface Sci. 2021, 582, 478–487. [Google Scholar] [CrossRef]
- Han, Y.; Li, J.; Zhang, X.; Xia, F.; Dai, Y. Reversible down-regulation and up-regulation of catalytic activity of poly (N-isopropylacrylamide)-anchored gold nanoparticles. Nanotechnology 2022, 33, 165601. [Google Scholar] [CrossRef]
- Maddahzadeh-Darini, N.; Ghorbanloo, M. Supra-Amphiphilic Porphyrin Based on Thermoresponsive Poly (N-Isopropylacrylamide-co-2-Acrylamido-2-Methylpropane Sulfonic Acid Sodium) Hydrogels: Synthesis, Characterization and Catalytic Applications. Catal. Lett. 2023, 153, 3342–3356. [Google Scholar] [CrossRef]
- Chang, K.; Yan, Y.; Zhang, D.; Xia, Y.; Chen, X.; Lei, L.; Shi, S. Synergistic Bonding of Poly (N-isopropylacrylamide)-Based Hybrid Microgels and Gold Nanoparticles Used for Temperature-Responsive Controllable Catalysis of p-Nitrophenol Reduction. Langmuir 2023, 39, 2408–2421. [Google Scholar] [CrossRef] [PubMed]
- Awais, S.; Munir, H.; Najeeb, J.; Anjum, F.; Naseem, K.; Kausar, N.; Shahid, M.; Irfan, M.; Najeeb, N. Green synthesis of iron oxide nanoparticles using Bombax malabaricum for antioxidant, antimicrobial and photocatalytic applications. J. Clean. Prod. 2023, 406, 136916. [Google Scholar] [CrossRef]
- Sathiyaraj, S.; Suriyakala, G.; Gandhi, A.D.; Babujanarthanam, R.; Almaary, K.S.; Chen, T.-W.; Kaviyarasu, K. Biosynthesis, characterization, and antibacterial activity of gold nanoparticles. J. Infect. Public Health 2021, 14, 1842–1847. [Google Scholar] [CrossRef]
- Ahmad, A.; Roy, P.G.; Zhou, S.; Irfan, A.; Kanwal, F.; Begum, R.; Farooqi, Z.H. Fabrication of silver nanoparticles within chitosan based microgels for catalysis. Int. J. Biol. Macromol. 2023, 240, 124401. [Google Scholar] [CrossRef]
- Hou, J.; Li, B.; Jang, W.; Yun, J.; Eyimegwu, F.M.; Kim, J.-H. Integration of Gold Nanoparticles into Crosslinker-Free Polymer Particles and Their Colloidal Catalytic Property. Nanomaterials 2023, 13, 416. [Google Scholar] [CrossRef]
- Raza, A.; Khan, S.R.; Ali, S.; Jamil, S.; Bibi, S. Silver/Titanium nanoparticles encapsulated microgel for catalytic reduction. Inorg. Chem. Commun. 2023, 153, 110851. [Google Scholar] [CrossRef]
- Mohan, A.; Peter, J.; Rout, L.; Thomas, A.M.; Nagappan, S.; Parambadath, S.; Zhang, W.; Selvaraj, M.; Ha, C.-S. Facile synthesis of silver nanoparticles stabilized dual responsive silica nanohybrid: A highly active switchable catalyst for oxidation of alcohols in aqueous medium. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125846. [Google Scholar] [CrossRef]
- Yu, J.; Wang, A.C.; Zhang, M.; Lin, Z. Water treatment via non-membrane inorganic nanoparticles/cellulose composites. Mater. Today 2021, 50, 329–357. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, S.; Huang, F.; Li, B.; Li, Y.; Sheng, H.; Hu, L.; Liang, S.; Xie, H. Thiol-functionalized chitosan poly (protic ionic liquids) hydrogel selectively anchors gold nanoparticles for aqueous catalysis. Cellulose 2023, 30, 3837–3852. [Google Scholar] [CrossRef]
- Haleem, A.; Chen, S.; Pan, J.; Weidong, H. Gamma radiation induced synthesis of double network hydrophilic cryogels at low pH loaded with AuNPs for fast and efficient degradation of Congo red. J. Hazard. Mater. Adv. 2023, 10, 100299. [Google Scholar] [CrossRef]
- Hashaam, M.; Ali, S.; Khan, T.; Salman, M.; Khan, S.R.; Aqib, A.I.; Zaheer, T.; Bibi, S.; Jamil, S.; Al-Sharif, M.S. Assembly of Smart Microgels and Hybrid Microgels on Graphene Sheets for Catalytic Reduction of Nitroarenes. Catalysts 2022, 12, 1172. [Google Scholar] [CrossRef]
- Khan, S.R.; Ali, S.; Ullah, B.; Jamil, S.; Zanib, T. Synthesis of iron nanoparticles in poly (N-isopropylacrylamide-acrylic acid) hybrid microgels for catalytic reduction of series of organic pollutants: A first approach. J. Nanopart. Res. 2020, 22, 192. [Google Scholar] [CrossRef]
- Arif, M. A tutorial review on bimetallic nanoparticles loaded in smart organic polymer microgels/hydrogels. J. Mol. Liq. 2023, 375, 121346. [Google Scholar] [CrossRef]
- Hu, J.-H.; Huang, Y.; Redshaw, C.; Tao, Z.; Xiao, X. Cucurbit [n] uril-based supramolecular hydrogels: Synthesis, properties and applications. Coord. Chem. Rev. 2023, 489, 215194. [Google Scholar] [CrossRef]
- Zbonikowski, R.; Iwan, M.; Paczesny, J. Stimuli-Responsive Langmuir Films Composed of Nanoparticles Decorated with Poly (N-isopropyl acrylamide) (PNIPAM) at the Air/Water Interface. ACS Omega 2023, 8, 23706–23719. [Google Scholar] [CrossRef]
- Minier, S.; Kim, H.J.; Zaugg, J.; Mallapragada, S.K.; Vaknin, D.; Wang, W. Poly (N-isopropylacrylamide)-grafted gold nanoparticles at the vapor/water interface. J. Colloid. Interface Sci. 2021, 585, 312–319. [Google Scholar] [CrossRef]
- Hussain, I.; Shahid, M.; Ali, F.; Irfan, A.; Begum, R.; Farooqi, Z.H. Polymer hydrogels for stabilization of inorganic nanoparticles and their application in catalysis for degradation of toxic chemicals. Environ. Technol. 2023, 44, 1679–1689. [Google Scholar] [CrossRef]
- Naseem, K.; Begum, R.; Farooqi, Z.H.; Wu, W.; Irfan, A. Core-shell microgel stabilized silver nanoparticles for catalytic reduction of aryl nitro compounds. Appl. Organomet. Chem. 2020, 34, e5742. [Google Scholar] [CrossRef]
- Mustafa, G.; Roy, P.G.; Zhou, S.; Irfan, A.; Chaudhry, A.R.; Begum, R.; Farooqi, Z.H. Silver-poly (N-isopropylacrylamide-co-2-hydroxyethylmethacrylate) hybrid microgels with excellent catalytic potential. J. Mol. Liq. 2023, 385, 122397. [Google Scholar] [CrossRef]
- Ullah, B.; Khan, S.R.; Ali, S.; Jamil, S.; Janjua, M.R.S.A. 4-Nitrophenol imprinted core-shell poly (N-isopropylacrylamide-acrylic acid)/poly (acrylic acid) microgels loaded with cadmium nanoparticles: A novel catalyst. Mater. Chem. Phys. 2021, 260, 124156. [Google Scholar] [CrossRef]
- Vretik, L.O.; Noskov, Y.V.; Ogurtsov, N.A.; Nikolaeva, O.A.; Shevchenko, A.V.; Marynin, A.I.; Kharchuk, M.S.; Chepurna, O.M.; Ohulchanskyy, T.Y.; Pud, A.A. Thermosensitive ternary core–shell nanocomposites of polystyrene, poly (N-isopropylacrylamide) and polyaniline. Appl. Nanosci. 2020, 10, 4951–4964. [Google Scholar] [CrossRef]
- Paenkaew, S.; Mahanitipong, U.; Rutnakornpituk, M.; Reiser, O. Magnetite Nanoparticles Functionalized with Thermoresponsive Polymers as a Palladium Support for Olefin and Nitroarene Hydrogenation. ACS Omega 2023, 8, 14531–14540. [Google Scholar] [CrossRef]
- Yu, L.; Li, Z.; Hua, C.; Chen, K.; Guo, X. Temperature Responsive Diblock Polymer Brushes as Nanoreactors for Silver Nanoparticles Catalysis. Polymers 2023, 15, 1932. [Google Scholar] [CrossRef] [PubMed]
- Arif, M. Catalytic degradation of azo dyes by bimetallic nanoparticles loaded in smart polymer microgels. RSC Adv. 2023, 13, 3008–3019. [Google Scholar] [CrossRef] [PubMed]
- Kafshboran, H.R.; Ghasemi, S. AU Nanoparticles Decorated on Edta Functionalized Poly (Nipam-Co-Allylamine) Grafted Fe3O4 for Reduction of Nitroarenes. Mater. Chem. Phys. 2023, 308, 128237. [Google Scholar] [CrossRef]
- Li, Z.; Hua, C.; Yu, L.; Zhang, X.; Chang, Y.; Xu, C. Catalytic Degradation of Nitrobenzene Derivatives by Platinum Nanoparticles Stabilized in Diblock Spherical Polymer Brushes. Available online: https://ssrn.com/abstract=4364424 (accessed on 16 October 2023).
- Pu, L.; Luo, G.; Zhu, M.; Shen, X.; Wei, W.; Li, S. A Trilaminar-Thermosensitive Hydrogel Catalytic Reactor Capable of Single/Tandem Catalytic Switchable Ability. J. Inorg. Organomet. Polym. Mater. 2023, 33, 462–471. [Google Scholar] [CrossRef]
- Naseem, K.; Begum, R.; Wu, W.; Irfan, A.; Nisar, J.; Azam, M.; Farooqi, Z. Core/shell composite microparticles for catalytic reduction of p-nitrophenol: Kinetic and thermodynamic study. Int. J. Environ. Sci. Technol. 2021, 18, 1809–1820. [Google Scholar] [CrossRef]
- Cao, G.; Liang, X.; Wang, D.-C.; Bai, L.; Ni, K.; Guan, Y. Zinc oxide/Polydopamine@ PNIPAm core-shell phase change nanocomposite for samrt NIR-responsive drug delivery systems. J. Drug Deliv. Sci. Technol. 2023, 84, 104522. [Google Scholar] [CrossRef]
- Naseem, K.; Begum, R.; Wu, W.; Irfan, A.; Farooqi, Z.H. Advancement in multi-functional poly (styrene)-poly (N-isopropylacrylamide) based core–shell microgels and their applications. Polym. Rev. 2018, 58, 288–325. [Google Scholar] [CrossRef]
- Naseem, K.; Ur Rehman, M.A.; Huma, R. Review on vinyl acetic acid-based polymer microgels for biomedical and other applications. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 322–332. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Akbar, F.; Ahmad, G.; Najeeb, J.; Nisa Hussain, Z.U. Recent progress of poly (N-isopropylacrylamide) hybrid hydrogels: Synthesis, fundamentals and applications–review. Soft Mater. 2018, 16, 228–247. [Google Scholar] [CrossRef]
- Shahid, M.; Farooqi, Z.H.; Begum, R.; Arif, M.; Wu, W.; Irfan, A. Hybrid microgels for catalytic and photocatalytic removal of nitroarenes and organic dyes from aqueous medium: A review. Crit. Rev. Anal. Chem. 2020, 50, 513–537. [Google Scholar] [CrossRef]
- Kakar, M.U.; Khan, K.; Akram, M.; Sami, R.; Khojah, E.; Iqbal, I.; Helal, M.; Hakeem, A.; Deng, Y.; Dai, R. Synthesis of bimetallic nanoparticles loaded on to PNIPAM hybrid microgel and their catalytic activity. Sci. Rep. 2021, 11, 14759. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.P.; Qiu, J.H.; Yu, S.H. Thermoresponsive poly (N-isopropylacrylamide)/graphene/Au nanocomposite hydrogel for water treatment by a laser-assisted approach. Small 2015, 11, 1165–1170. [Google Scholar] [CrossRef]
- Dan, X.; Ruiyi, L.; Qinsheng, W.; Yongqiang, Y.; Guangli, W.; Zaijun, L. Thermal-switchable sensor based on palladium-graphene composite and poly (N-isopropylacrylamide) for electrochemical detection of 4-nitrophenol. Microchem. J. 2022, 172, 106970. [Google Scholar] [CrossRef]
- Sadjadi, S.; Abedian-Dehaghani, N.; Heravi, M.M. Pd on thermo-responsive composite of silica-coated carbon nanotube and 1-vinyl-3-butylimidazolium-based ionic liquid copolymers as an efficient catalyst for hydrogenation of nitro compounds. Sci. Rep. 2022, 12, 3972. [Google Scholar] [CrossRef]
- Yue, R.; Wen, X.; Mao, Y.; Su, Y.; Shen, Q.; Song, H.; Zhang, H.; Ba, X. Eco-friendly fabrication of Au nanoparticles immobilized on tannin-aminopropyltriethoxysilane-coated halloysite nanotubes for thermally tunable catalysis. J. Mater. Sci. 2020, 55, 17094–17107. [Google Scholar] [CrossRef]
- Abedian-Dehaghani, N.; Heravi, M.M.; Sadjadi, S. Pd on the composite of perlite and Allylamine-N-Isopropylacrylamide copolymer: A thermo-responsive catalyst for hydrogenation of nitroarenes under mild reaction condition. Catalysts 2021, 11, 1334. [Google Scholar] [CrossRef]
- Haider, S.; Khan, S.U.; Najeeb, J.; Naeem, S.; Rafique, H.; Munir, H.; Al-Masry, W.A.; Nazar, M.F. Synthesis of cadmium oxide nanostructures by using Dalbergia sissoo for response surface methodology based photocatalytic degradation of methylene blue. J. Clean. Prod. 2022, 365, 132822. [Google Scholar] [CrossRef]
- Danish, M.; Ayub, H.; Sandhu, Z.A.; Shoaib, A.; Akram, S.; Najeeb, J.; Naeem, S. Synthesis of cerium oxide/cadmium sulfide nanocomposites using inverse microemulsion methodology for photocatalytic degradation of methylene blue. Appl. Nanosci. 2021, 11, 2503–2515. [Google Scholar] [CrossRef]
- Basha, B.; Mubashir, T.; Tahir, M.H.; Najeeb, J.; Naeem, S.; Alrowaili, Z.; Al-Buriahi, M. Designing of novel organic semiconductors materials for organic solar cells: A machine learning assisted proficient pipeline. Inorg. Chem. Commun. 2023, 153, 110818. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).