Preparation and Spectroscopic Characterization of Ternary Inclusion Complexes of Ascorbyl Palmitate and Urea with γ-Cyclodextrin
Abstract
1. Introduction
2. Materials and Methods
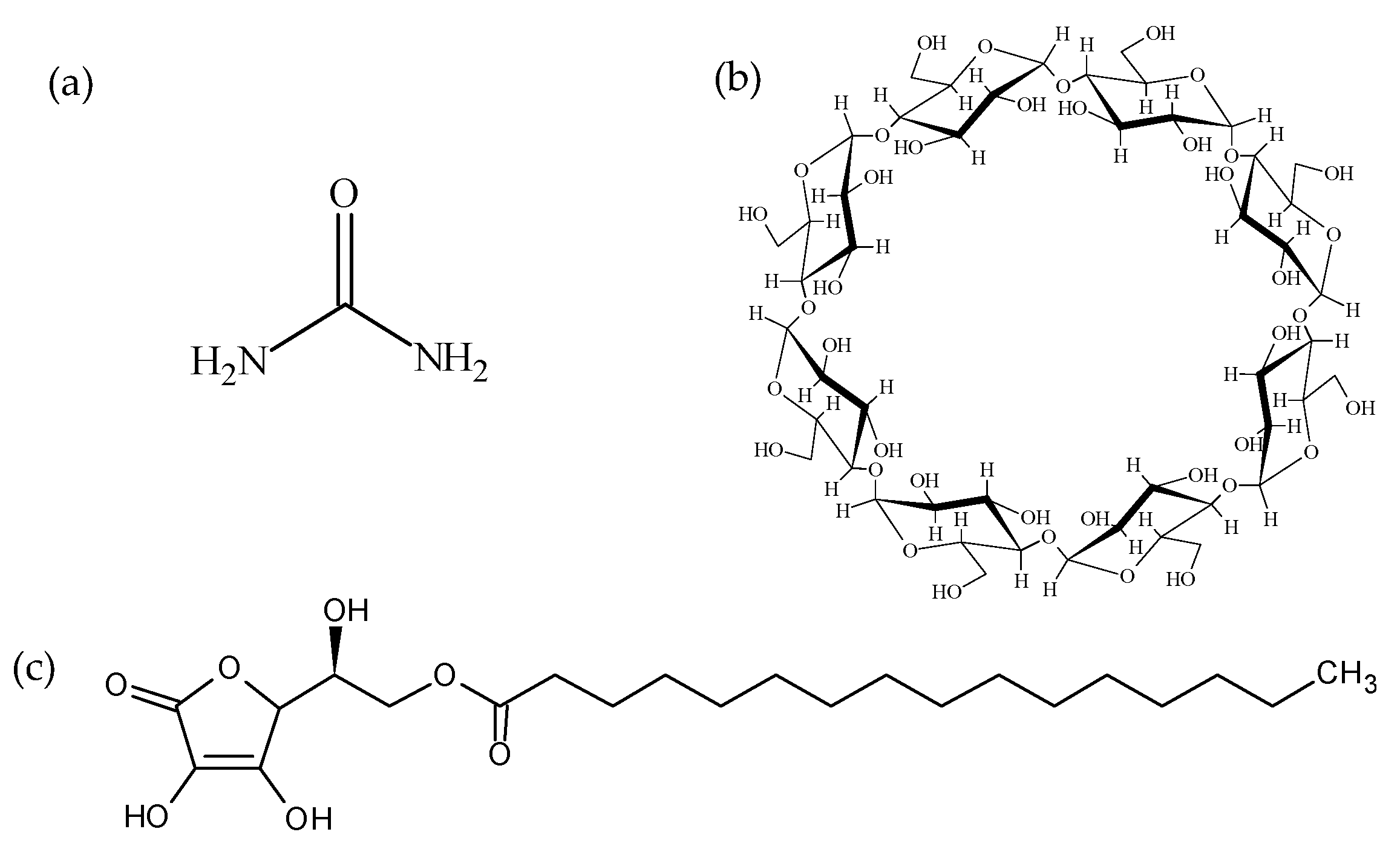
2.1. Materials
2.2. Preparation Methods
2.2.1. Preparation of Samples by Physical Mixture (PM)
2.2.2. Preparation of Samples by the Evaporation Method
2.2.3. Preparation of Samples by Ground Mixture (GM)
2.3. Characterization Methods
2.3.1. Powder X-ray Diffraction (PXRD)
2.3.2. Differential Scanning Calorimeter (DSC)
2.3.3. Infrared (IR) Absorption Spectrum Measurement
2.3.4. Near Infrared (NIR) Spectroscopy
2.3.5. ASCP Solubility Studies
3. Results and Discussion
3.1. Investigation of Intermolecular Interaction in a Mixture of ASCP/UR = 1/12 and γCD
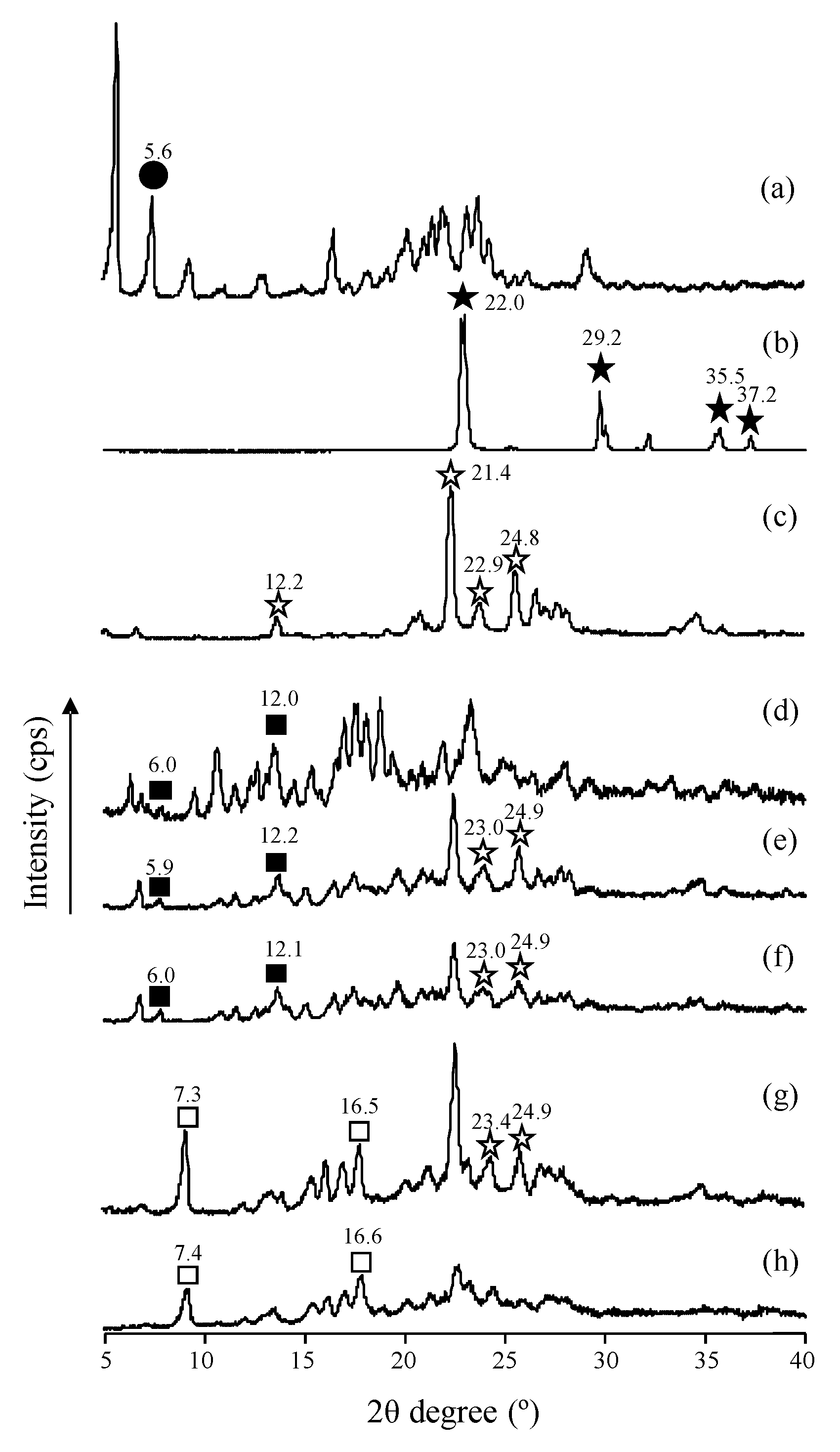
3.1.1. Powder X-ray Diffraction (PXRD) Measurement
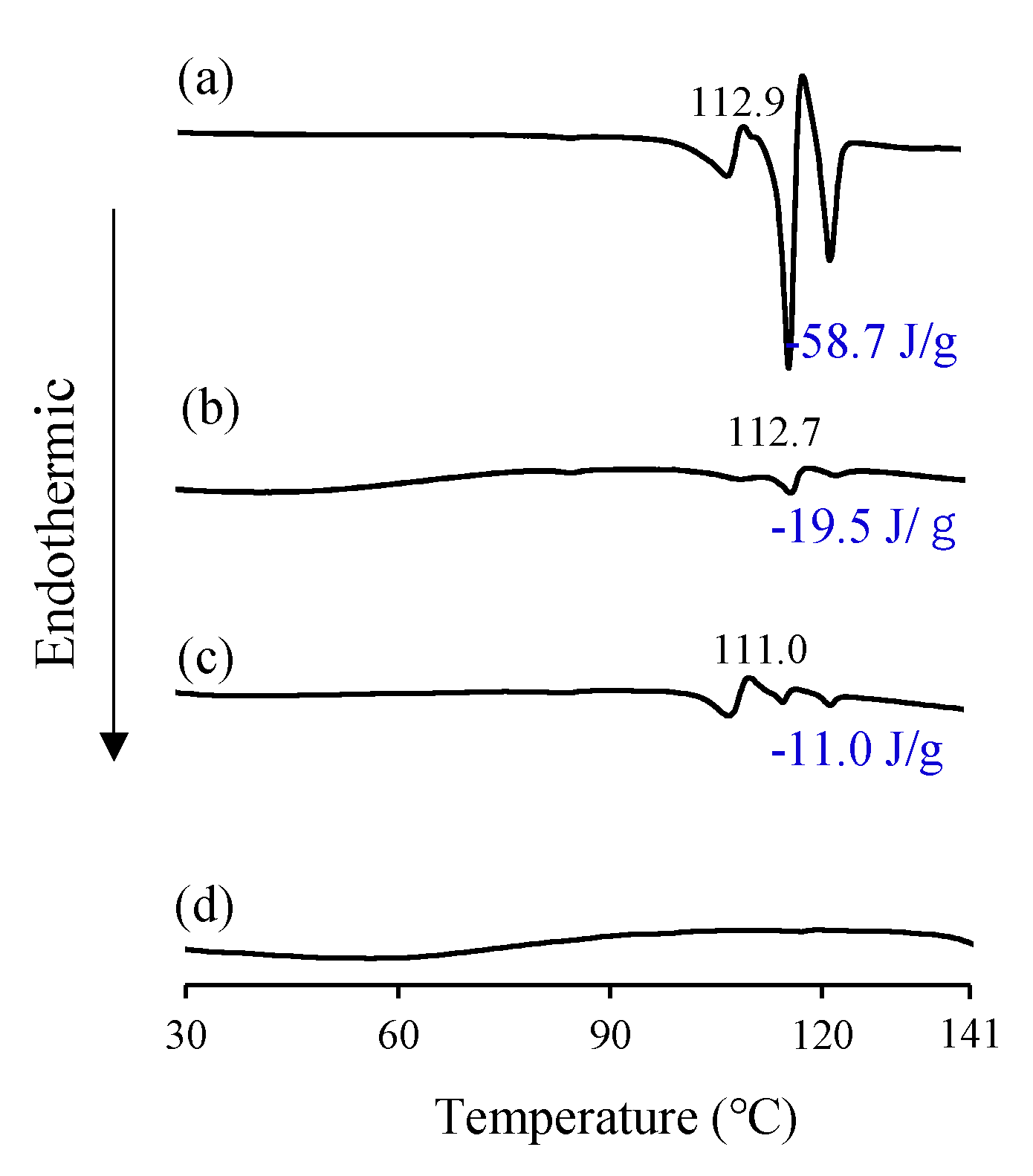
3.1.2. Differential Scanning Calorimetry (DSC) Measurement
3.2. Inclusion of the Molar Ratio of ASCP/UR = 1/12 and γCD
3.3. Examination of the Molecular State in the Solid State
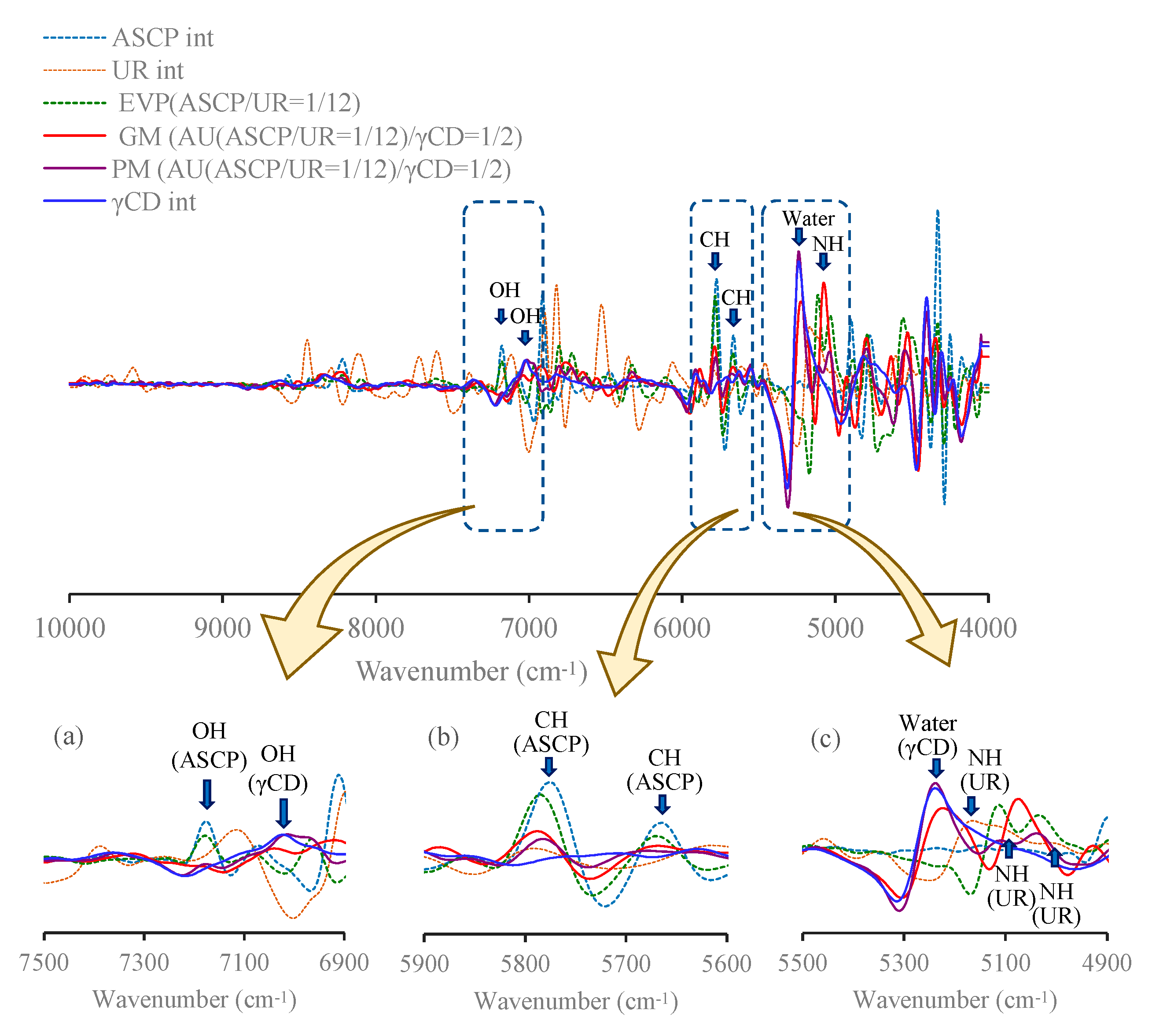
3.3.1. Infrared (IR) Absorption Spectra
3.3.2. Near-Infrared (NIR) Absorption Spectra
3.4. Solubility Study of ASCP/UR = 1/12 and γCD Complex
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niki, E.; Saito, T.; Kawakami, A.; Kamiya, Y. Inhibition of oxidation of methyl linoleate in solution by vitamin E and vitamin C. J. Biol. Chem. 1984, 259, 4177–4182. [Google Scholar] [CrossRef] [PubMed]
- Telang, P.S. Vitamin C in dermatology. Ind. Dermatol. Online J. 2013, 4, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Liston, L.S.; Rivas, P.L.; Sakdiset, P.; See, G.L.; Arce, F.A.J. Chemical permeation enhancers for topically-applied vitamin C and its derivatives: A systematic review. Cosmetics 2022, 9, 85. [Google Scholar] [CrossRef]
- Ahmad, I.; Sheraz, M.A.; Ahmed, S.; Shaikh, R.H.; Vaid, F.H.; ur Khattak, R.S.; Ansari, S.A. Photostability and interaction of ascorbic acid in cream formulations. AAPS PharmSciTech 2011, 12, 917–923. [Google Scholar] [CrossRef]
- Ochiai, Y.; Kaburagi, S.; Obayashi, K.; Ujiie, N.; Hashimoto, S.; Okano, Y.; Masaki, H.; Ichihashi, M.; Sakurai, H. A new lipophilic pro-vitamin C, tetra-isopalmitoyl ascorbic acid (VC-IP), prevents UV-induced skin pigmentation through its anti-oxidative properties. J. Dermatol. Sci. 2006, 44, 37–44. [Google Scholar] [CrossRef]
- Huang, W.Y.; Lee, P.C.; Huang, L.K.; Lu, L.P.; Liao, W.C. Stability studies of ascorbic acid 2-glucoside in cosmetic lotion using surface response methodology. Bioorg. Med. Chem. Lett. 2013, 23, 1583–1587. [Google Scholar] [CrossRef]
- Segall, A.I.; Moyano, M.A. Stability of vitamin C derivatives in topical formulations containing lipoic acid, vitamins A and E. Int. J. Cosmet. Sci. 2008, 30, 453–458. [Google Scholar] [CrossRef]
- Trommer, H.; Neubert, R.H.H. Overcoming the stratum corneum: The modulation of skin penetration. Skin Pharmacol. Physiol. 2006, 19, 106–121. [Google Scholar] [CrossRef]
- Mueller, J.; Oliverira, J.S.; Barker, R.; Trapp, M.; Schroeter, A.; Brezesinski, G.; Neubert, R.H. The effect of urea and taurine as hydrophilic penetration enhancers on stratum corneum lipid models. Biochim. Biophys. Acta 2016, 1858, 2006–2018. [Google Scholar] [CrossRef]
- Sakurai, H.; Suzuki, M.; Itakura, S.; Todo, H.; Arce, F., Jr.; See, G.L.; Tanikawa, T.; Inoue, Y. Preparation, Characterization, Solubility, and Antioxidant Capacity of Ellagic Acid-Urea Complex. Materials 2022, 15, 2836. [Google Scholar] [CrossRef]
- Lamelas, F.J.; Dreger, Z.A.; Gupta, Y.M. Raman and X-ray scattering studies of high-pressure phases of urea. J. Phys. Chem. B 2005, 109, 8206–8215. [Google Scholar] [CrossRef]
- Olejniczak, A.; Ostrowska, K.; Katrusiak, A. H-bond breaking in high-pressure urea. J. Phys. Chem. C 2009, 113, 15761–15767. [Google Scholar] [CrossRef]
- Dziubek, K.; Citroni, M.; Fanetti, S.; Cairns, A.B.; Bini, R. High-pressure high-temperature structural properties of urea. J. Phys. Chem. C 2017, 121, 2380–2387. [Google Scholar] [CrossRef]
- Dhall, M.; Madan, A.K. Studies on urea co-inclusion complexes of simvastatin for improvement of pharmaceutical characteristics. J. Incl. Phenom. Macrocycl. Chem. 2015, 81, 105–120. [Google Scholar] [CrossRef]
- White, M.A. Origins of thermodynamic stability of urea: Alkane inclusion compounds. Can. J. Chem. 1998, 76, 1695–1698. [Google Scholar] [CrossRef]
- Arima, H.; Motoyama, K.; Higashi, T. Potential Use of Cyclodextrins as Drug Carriers and Active Pharmaceutical Ingredients. Chem. Pharm. Bull. 2017, 65, 341–348. [Google Scholar] [CrossRef]
- Chao, J.; Wang, H.; Zhao, W. Investigation of the inclusion behavior of chlorogenic acid with hydroxypropyl-β cyclodextrin. Int. J. Biol. Macromol. 2012, 50, 277–282. [Google Scholar] [CrossRef]
- Pápay, Z.E.; Sebestyén, Z.; Ludányi, K.; Kállai, N.; Balogh, E.; Kósa, A.; Somavarapu, S.; Böddi, B.; Antal, I. Comparative evaluation of the effect of cyclodextrins and pH on aqueous solubility of apigenin. J. Pharm. Biomed. Anal. 2016, 117, 210–216. [Google Scholar] [CrossRef]
- Anselmi, C.; Centini, M.; Ricci, M.; Buonocore, A.; Granata, P.; Tsuno, T.; Facino, R.M. Analytical characterization of a ferulic acid/γ-cyclodextrin inclusion complex. J. Pharm. Biomed. Anal. 2006, 40, 875–881. [Google Scholar] [CrossRef]
- Zoppi, A.; Delrivo, A.; Aiassa, V.; Longhi, M.R. Binding of Sulfamethazine to β-cyclodextrin and Methyl-β-cyclodextrin. AAPS PharmSciTech 2013, 14, 727–735. [Google Scholar] [CrossRef]
- Aigner, Z.; Berkesi, O.; Farkas, G.; Szabó-Révész, P. DSC, X-ray and FT-IR studies of a gemfibrozil/dimethyl-β-cyclodextrin inclusion complex produced by co-grinding. J. Pharm. Biomed. Anal. 2011, 57, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.F.; Nie, H.C.; Shangguang, X.C.; Yin, Z.P.; Zheng, G.D.; Chen, J.G. Aqueous Solubility and Stability Enhancement of Astilbin through Complexation with Cyclodextrins. J. Agric. Food Chem. 2012, 61, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.X.; Fan, M.G.; Liu, Y.Y.; Ma, Y.; Zhang, G.J.; Yao, J.N. Inclusion complex of spironaphthoxazine with γ-cyclodextrin and its photochromism study. Langmuir 2007, 23, 9443–9446. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, N.; Wakao, Y.; Tomono, S.; Tatemichi, M.; Yano, T.; Ohshima, H. The enhancement of the oral bioavailability of γ-tocotrienol in mice by γ-cyclodextrin inclusion. J. Nutr. Biochem. 2011, 22, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Tabary, N.; Leclercq, L.; Junthip, J.; Degoutin, S.; Aubert-Viard, F.; Cazaux, F.; Lyskawa, J.; Janus, L.; Bria, M.; et al. Multilayered textile coating based on a β-cyclodextrin polyelectrolyte for the controlled release of drugs. Carbohydr. Polym. 2012, 93, 718–730. [Google Scholar] [CrossRef]
- Inoue, Y.; Suzuki, R.; Horage, M.; Togano, M.; Kimura, M.; Ogihara, M.; Ando, S.; Kikuchi, J.; Murata, I.; Kanamoto, I. Effects of oxethazaine and gamma-cyclodextrin complex formation on intestinal contractions. World J. Pharm. Sci. 2016, 4, 269–280. [Google Scholar]
- Saokham, P.; Burapapadh, K.; Praphanwittaya, P.; Loftsson, T. Characterization and Evaluation of Ternary Complexes of Ascorbic Acid with γ-Cyclodextrin and Poly(vinyl Alcohol). Int. J. Mol. Sci. 2020, 21, 4399. [Google Scholar] [CrossRef]
- Hu, X.; Wei, B.; Li, H.; Wu, C.; Bai, Y.; Xu, X.; Jin, Z.; Tian, Y. Preparation of the β-cyclodextrin-vitamin C (β-CD-Vc) inclusion complex under high hydrostatic pressure (HHP). Carbohydr. Polym. 2012, 90, 1193–1196. [Google Scholar] [CrossRef]
- Aiassa, V.; Garnero, C.; Longhi, M.R.; Zoppi, A. Cyclodextrin Multicomponent Complexes: Pharmaceutical Applications. Pharmaceutics 2021, 13, 1099. [Google Scholar] [CrossRef]
- Inoue, Y.; Niiyama, D.; Murata, I.; Kanamoto, I. Usefulness of Urea as a Means of Improving the Solubility of Poorly Water-Soluble Ascorbyl Palmitate. Int. J. Med. Chem. 2017, 2017, 4391078. [Google Scholar] [CrossRef]
- Inoue, Y.; Horage, M.; Suzuki, R.; Niiyama, D.; Urano, R.; Ando, S.; Kikuchi, J.; Murata, I.; Kanamoto, I. Study on Complexation of Ascorbic Acid Derivatives With γ-Cyclodextrin. Int. J. Pharm. 2017, 7, 9–21. [Google Scholar]
- Higashi, K.; Ideura, S.; Waraya, H.; Limwikrant, W.; Moribe, K.; Yamamoto, K. Simultaneous Dissolution of Naproxen and Flurbiprofen from a Novel Ternary γ-Cyclodextrin Complex. Chem. Pharm. Bull. 2010, 58, 769–772. [Google Scholar] [CrossRef]
- Ikeda, A.; Ishikawa, M.; Aono, R.; Kikuchi, J.; Akiyama, M.; Shinoda, W. Regioselective Recognition of a [60] Fullerene-Bisadduct by Cyclodextrin. J. Org. Chem. 2013, 78, 2534–2541. [Google Scholar] [CrossRef]
- Xie, J.; Yang, F.; Shi, X.; Zhu, X.; Su, W.; Wang, P. Improvement in solubility and bioavailability of puerarin by mechanochemical preparation. Drug Dev. Ind. Pharm. 2013, 39, 826–835. [Google Scholar] [CrossRef]
- Iwata, M.; Fukami, T.; Kawashima, D.; Sakai, M.; Furuishi, T.; Suzuki, T.; Tomono, K.; Ueda, H. Effectiveness of mechanochemical treatment with cyclodextrins on increasing solubility of glimepiride. Die Pharm. Int. J. Pharm. Sci. 2009, 64, 390–394. [Google Scholar]
- Suzuki, R.; Inoue, Y.; Tsunoda, Y.; Murata, I.; Isshiki, Y.; Kondo, S.; Kanamoto, I. Effect of γ-cyclodextrin derivative complexation on the physicochemical properties and antimicrobial activity of hinokitiol. J. Incl. Phenom. Macrocycl. Chem. 2015, 83, 177–186. [Google Scholar] [CrossRef]
- Kitamura, S.; Isuda, H.; Shimada, J.; Takada, T.; Takaha, T.; Okada, S.; Mimura, M.; Kajiwara, K. Conformation of cyclomaltooligosaccharide (“cycloamylose”) of dp21 in aqueous solution. Carbohydr. Res. 1997, 304, 303–314. [Google Scholar] [CrossRef]
- Kitamura, S. Cyclic Oligosaccharides and Polysaccharides. In Cyclic Polymers, 2nd ed.; Semlyen, J.A., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; Chapter 4; pp. 125–160. [Google Scholar]
- Shimada, J.; Handa, S.; Kaneko, H.; Takada, T. Conformation of Novel Cycloamylose: Topological Aspects and Simulations. Macromolecules 1996, 29, 6408–6421. [Google Scholar] [CrossRef]
- Lestander, T.A.; Rudolfsson, M.; Pommer, L.; Nordin, A. NIR provides excellent predictions of properties of biocoal from torrefaction and pyrolysis of biomass. Green Chem. 2014, 16, 4906–4913. [Google Scholar] [CrossRef]
- Gaydou, V.; Kister, J.; Dupuy, N. Evaluation of multiblock NIR/MIR PLS predictive models to detect adulteration of diesel/biodiesel blends by vegetal oil. Chemom. Intell. Lab. Syst. 2011, 106, 190–197. [Google Scholar] [CrossRef]
- Inoue, Y.; Nanri, A.; Murata, I.; Kanamoto, I. Characterization of Inclusion Complex of Coenzyme Q10 with the New Carrier CD-MOF-1 Prepared by Solvent Evaporation. AAPS PharmSciTech 2018, 19, 3048–3056. [Google Scholar] [CrossRef] [PubMed]


| Samples | ASCP (µg/mL) |
|---|---|
| ASCP intact | 1.49 ± 0.06 |
| EVP AU (ASCP/UR = 1/12) | 1.76 ± 0.05 |
| PM (AU (ASC/UR = 1/12)/γCD = 1/2) | 1.95 ± 0.14 |
| GM (AU (ASC/UR = 1/12)/γCD = 1/2) | 2.77 ± 0.44 |
| GM (ASCP/γCD) [26] | 19.6 ± 3.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, Y.; Nanri, A.; Arce, F.J.; See, G.L.; Tanikawa, T.; Yokogawa, T.; Kitamura, M. Preparation and Spectroscopic Characterization of Ternary Inclusion Complexes of Ascorbyl Palmitate and Urea with γ-Cyclodextrin. ChemEngineering 2023, 7, 29. https://doi.org/10.3390/chemengineering7020029
Inoue Y, Nanri A, Arce FJ, See GL, Tanikawa T, Yokogawa T, Kitamura M. Preparation and Spectroscopic Characterization of Ternary Inclusion Complexes of Ascorbyl Palmitate and Urea with γ-Cyclodextrin. ChemEngineering. 2023; 7(2):29. https://doi.org/10.3390/chemengineering7020029
Chicago/Turabian StyleInoue, Yutaka, Ayumi Nanri, Florencio Jr. Arce, Gerard Lee See, Takashi Tanikawa, Takami Yokogawa, and Masashi Kitamura. 2023. "Preparation and Spectroscopic Characterization of Ternary Inclusion Complexes of Ascorbyl Palmitate and Urea with γ-Cyclodextrin" ChemEngineering 7, no. 2: 29. https://doi.org/10.3390/chemengineering7020029
APA StyleInoue, Y., Nanri, A., Arce, F. J., See, G. L., Tanikawa, T., Yokogawa, T., & Kitamura, M. (2023). Preparation and Spectroscopic Characterization of Ternary Inclusion Complexes of Ascorbyl Palmitate and Urea with γ-Cyclodextrin. ChemEngineering, 7(2), 29. https://doi.org/10.3390/chemengineering7020029







