Optimization of Adsorption Parameters for Removal of Cationic Dyes on Lignocellulosic Agricultural Waste Modified by Citric Acid: Central Composite Design
Abstract
1. Introduction
| Cationic Dye | Adsorbent | Factors | Optimum Conditions | Optimum Response Removal Efficiency (%) or Adsorption Capacity (mg g−1) | References | |
|---|---|---|---|---|---|---|
| Experimental | Predicted | |||||
| Methylene blue | Activated carbon prepared from cashew nutshell | pH Adsorbent dose (m) Initial dye concentration (C) Time (t) | pH = 10 m = 2.18 g L−1 C = 50 mg L−1 t = 63 min | 99.97% | 100% | [11] |
| Methylene blue | Orange tree sawdust modified using alkaline | Concentration of NaOH (C1) Adsorbent dose (m) Time (t) Initial dye concentration (C2) | C1 = 0.14 M m = 50 g L−1 t = 60 min C2 = 69.5 mg L−1 | 95.34% | 100% | [13] |
| Crystal violet | Polyphenol-extracted coffee grounds | pH Adsorbent dose (m) Initial dye concentration (C) Time (t1) Time of the adsorbent microwave activation (t2) | pH = 8.53 m = 14.8 g L−1 C = 242.38 mg L−1 t1 = 7 min t2 = 31.97 s | 99.63% | 100% | [14] |
| Methylene blue | Modified oak waste residues | pH Adsorbent dose (m) Initial dye concentrations (C) Time (t) | pH = 6.2 m = 2 g L−1 C = 70 mg L−1 t = 160 min | 85.36% | 84.15% | [15] |
| Methyl violet | Raw date pits | pH Initial dye concentration (C) Temperature (T) | pH = 7.28 C = 60.25 mg L−1 T = 37.96 °C | 100% | 100% | [16] |
| Basic red 2 | Raw date pits | pH Initial dye concentration (C) Temperature (T) | pH = 7.70 C = 59.77mg L−1 T = 38.75 °C | 96.66% | 100% | [16] |
| Acridine orange | A. esculentus seeds powder | pH Adsorbent dose (m) Initial dye concentrations (C) Time (t) | pH = 8.96 m = 1.89 g L−1 C = 867.71 mg L−1 t = 32.06 min | 312.1 mg g−1 | 313.4 mg g−1 | [17] |
| Methylene blue | Banana leaves ash | Adsorbent dose (m) Time (t) Shaking speed (s) | m = 0.239 g L−1 t = 180 min s = 356 rpm | 93.75% | 100% | [18] |
| Basic yellow 2 | Montmorillonite | Adsorbent dose (m) Initial dye concentration (C) Time (t) Temperature (T) | m = 0.6 g L−1 C = 60 mg L−1 t = 10 min T = 25 °C | 97.32% | 100% | [19] |
| Malachite green | Chrysanthemum indicum flowers | pH Adsorbent dose (m) Time (t) Shaking speed (s) | pH = 11 m = 3 g L−1 t = 75 min s = 150 rpm | 99.3% | 100% | [20] |
| Malachite green | Sodium alginate/ NaOH treated activated sugarcane bagasse charcoal composite beads | pH Adsorbent dose (m) Time (t) | pH = 8 m = 0.3 g L−1 t = 115.43 min | 97.78% | 100% | [21] |
| Methylene blue | Fe-modified banana peel | Temperature (T) Initial dye concentration (C) Adsorbent dose (m) Time (t) | T = 45 °C C = 5 mg L−1 m = 2.5 g L−1 t = 50 min | 91.89% | 100% | [22] |
| Malachite Green | Sepiolite clay | Adsorbent dose (m) Initial dye concentration (C) Time (t) | m = 26 g L−1 C = 77 mg L−1 t = 42 min | 99% | 100% | [23] |
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Cationic Dyes Solution Preparation
2.2.2. Batch Adsorption Procedure
2.3. Experimental Design
3. Results and Discussion
3.1. Statistical Analysis
3.2. ANOVA
3.3. Optimization of the Proposed Mathematical Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Pavithra, K.G.; Kumar, P.S.; Jaikumar, V.; Rajan, P.S. Removal of colorants from wastewater: A review on sources and treatment strategies. J. Ind. Eng. Chem. 2019, 75, 1–19. [Google Scholar] [CrossRef]
- Bushra, R.; Mohanad, S.; Alias, Y.; Jin, Y.; Ahmad, M. Current approaches and methodologies to explore the perceptive adsorption mechanism of dyes on low-cost agricultural waste: A review. Microporous Mesoporous Mater. 2021, 319, 111040. [Google Scholar] [CrossRef]
- Gong, R.; Zhua, S.; Zhang, D.; Chen, J.; Ni, S.; Guan, R. Adsorption behavior of cationic dyes on citric acid esterifying wheat straw: Kinetic and thermodynamic profile. Desalination 2008, 230, 220–228. [Google Scholar] [CrossRef]
- Baldikova, E.; Safarikova, M.; Safarik, I. Organic dyes removal using magnetically modified rye straw. J. Magn. Magn. Mat. 2015, 380, 181–185. [Google Scholar] [CrossRef]
- Soldatkina, L.M.; Zavrichko, M.A. Application of agriculture waste as biosorbents for dye removal from aqueous solution. Him. Fiz. Ta Tehnol. Poverhni 2013, 4, 99–104. [Google Scholar] [CrossRef]
- Gong, R.; Jin, Y.; Chen, F.; Chen, J.; Liu, Z. Enhanced Malachite Green removal from aqueous solution by citric acid modified rice straw. J. Hazard. Mater. 2006, 137, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ma, Q.; Hu, D.; Wang, L. Adsorption of Methylene Blue by a low-cost biosorbent: Citric acid modified peanut shell. Desalin. Water Treat. 2016, 57, 10261–10269. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, Y.; Lü, Q.; Cheng, X. Biosorption of Methylene Blue by chemically modified cellulose waste. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2014, 29, 817–823. [Google Scholar] [CrossRef]
- Subramaniam, R.; Ponnusamy, S.K. Novel adsorbent from agricultural waste (cashew NUT shell) for methylene blue dye removal: Optimization by response surface methodology. Water Resour. Ind. 2015, 11, 64–70. [Google Scholar] [CrossRef]
- Karimifard, S.; Moghaddam, M.R.A. Application of response surface methodology in physicochemical removal of dyes from wastewater: A critical review. Sci. Total Environ. 2018, 640, 772–797. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Jellali, S.; Acrout, H.; Assadi, A.A.; Bousselmi, L. Optimization of a cationic dye removal by a chemically modified agriculture by-product using response surface methodology: Biomasses characterization and adsorption properties. Environ. Sci. Pollut. Res. 2017, 24, 9831–9846. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, M.D.; Buntić, A.B.; Mihajlovski, K.R.; Šiler-Marinković, S.S.; Antonović, D.J.; Radovanović, Ž.; Dimitrijević-Branković, S.I. Rapid cationic dye adsorption on polyphenol-extracted coffee grounds—A response surface methodology approach. J. Taiwan Inst. Chem. Eng. 2014, 45, 1691–1699. [Google Scholar] [CrossRef]
- Samarbaf, S.; Tahmasebi, Y.; Yazdani, M.; Babaei, A.A. A comparative removal of two dyes from aqueous solution using modified oak waste residues: Process optimization using response surface methodology. J. Ind. Eng. Chem. 2019, 73, 67–77. [Google Scholar] [CrossRef]
- Wakkel, M.; Khiari, B.; Zagrouba, F. Basic red 2 and methyl violet adsorption by date pits: Adsorbent characterization, optimization by RSM and CCD, equilibrium and kinetic studies. Environ. Sci. Pollut. Res. 2019, 26, 18942–18960. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, A. Rapid and high-performance adsorptive removal of hazardous acridine orange from aqueous environment using Abelmoschus esculentus seed powder: Single- and multi-parameter optimization studie. J. Environ. Manag. 2018, 217, 573–591. [Google Scholar] [CrossRef]
- Alam, M.Z.; Bari, M.N.; Kawsari, S. Statistical optimization of Methylene Blue dye removal from a synthetic textile wastewater using indigenous adsorbents. Environ. Sustain. Indic. 2022, 14, 100176. [Google Scholar] [CrossRef]
- Hassani, A.; Kiransan, M.; Darvishi Cheshmeh Soltani, R.; Khataee, A.; Karaca, S. Optimization of the adsorption of a textile dye onto nanoclay using a central composite design. Turk. J. Chem. 2015, 39, 734–749. [Google Scholar] [CrossRef]
- Chukki, J.; Shanthakumar, S. Optimization of malachite green dye removal by chrysanthemum indicum using response surface methodology. Environ. Prog. Sustain. Energy 2016, 35, 1415–1419. [Google Scholar] [CrossRef]
- Das, L.; Das, P.; Bhowal, A.; Bhattachariee, C. Treatment of malachite green dye containing solution using bio-degradable sodium alginate/NaOH treated activated sugarcane baggsse charcoal beads: Batch, optimization using response surface methodology and continuous fixed bed column study. J. Environ. Manag. 2020, 276, 111272. [Google Scholar] [CrossRef] [PubMed]
- Çatlıoglu, F.; Akay, S.; Turunç, E.; Gozmen, B.; Anastopoulos, I.; Kayan, B.; Kalderis, D. Preparation and application of fe-modified banana peel in the adsorption of methylene blue: Process optimization using response surface methodology. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100517. [Google Scholar] [CrossRef]
- Coruh, S.; Elevli, S. Optimization of malachite green dye removal by sepiolite clay using a central composite design. Glob. NEST J. 2014, 16, 339–347. [Google Scholar] [CrossRef]
- Tanaydin, M.K.; Goksu, A. Optimization of the adsorption of methyl green dye on almond shells using central composite design. Desalination Water Treat. 2021, 227, 425–439. [Google Scholar] [CrossRef]
- Low, L.W.; Teng, T.T.; Rafatullah, M.; Morad, N.; Azahari, B. Adsorption studies of methylene blue and malachite green from aqueous solutions by pretreated lignocellulosic materials. Sep. Sci. Technol. 2013, 48, 1688–1698. [Google Scholar] [CrossRef]
- Soldatkina, L.M.; Zavrichko, M.A. Obtaining of adsorbents using citric acid modification of plant waste. Odesa Natl. Univ. Herald. Chem. 2019, 18, 47–59. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Erden, G.; Flibeli, A. Effects of Fenton Pre-Treatment on Waste Activated Sludge Properties. Clean–Soil Air Water 2011, 39, 626–632. [Google Scholar] [CrossRef]
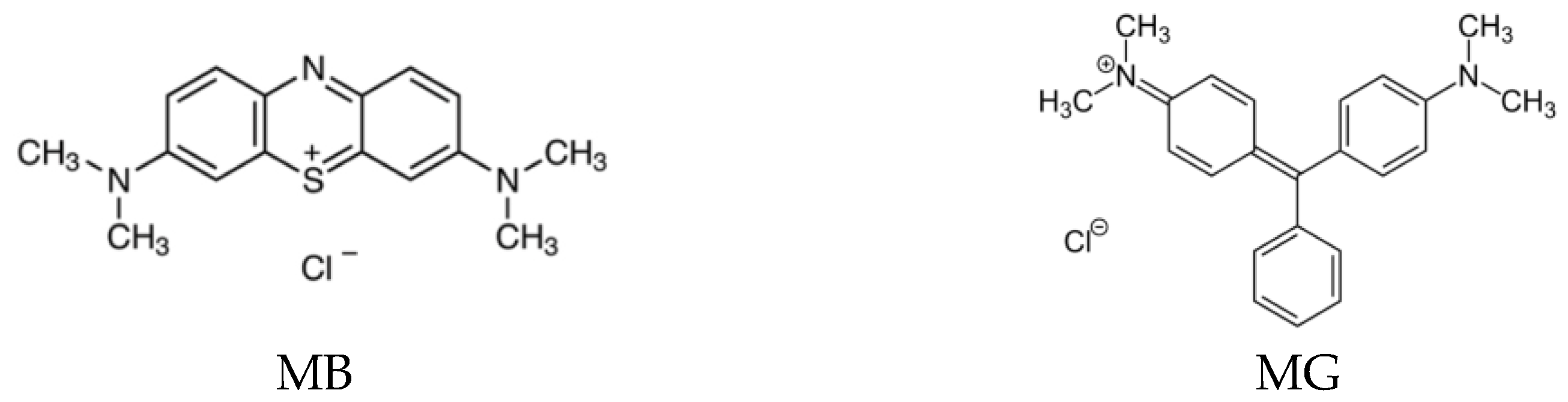

| Adsorbent | Specific Surface Area (m2 g−1) | pHpzc | COOH (mmol g−1) |
|---|---|---|---|
| BS-C | 43.4 | 3.5 | 3.4 |
| CS-C | 45.3 | 3.3 | 3.5 |
| Factors | Unit | Symbols | Coded Levels | ||||
|---|---|---|---|---|---|---|---|
| −2 (−α) | −1 | 0 | +1 | +2 (+α) | |||
| Original Levels | |||||||
| pH | - | x1 | 4 | 5 | 6 | 7 | 8 |
| Time (t) | min | x2 | 20 | 30 | 40 | 50 | 60 |
| Dye concentration (C) | mg L−1 | x3 | 5 | 15 | 25 | 35 | 45 |
| Adsorbent dose (m) | g L−1 | x4 | 6 | 8 | 10 | 12 | 14 |
| Run | Coded Factors | BS-C | CS-C | BS-C | CS-C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x1 (pH) | x2 (t) | x3 (C) | x4 (m) | MB | MG | |||||||
| α, % | ||||||||||||
| Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | |||||
| 1 | −1 | −1 | −1 | −1 | 67 | 69 | 70 | 71 | 57 | 55 | 60 | 59 |
| 2 | +1 | −1 | −1 | −1 | 78 | 79 | 82 | 82 | 65 | 64 | 66 | 66 |
| 3 | −1 | +1 | −1 | −1 | 68 | 66 | 71 | 70 | 50 | 56 | 55 | 51 |
| 4 | +1 | +1 | −1 | −1 | 81 | 83 | 85 | 87 | 76 | 75 | 76 | 78 |
| 5 | −1 | −1 | +1 | −1 | 65 | 64 | 69 | 67 | 55 | 53 | 60 | 57 |
| 6 | +1 | −1 | +1 | −1 | 78 | 78 | 81 | 82 | 62 | 61 | 65 | 63 |
| 7 | −1 | +1 | +1 | −1 | 62 | 63 | 66 | 67 | 42 | 44 | 48 | 49 |
| 8 | +1 | +1 | +1 | −1 | 85 | 85 | 89 | 88 | 71 | 73 | 73 | 75 |
| 9 | −1 | −1 | −1 | +1 | 78 | 78 | 82 | 83 | 64 | 66 | 65 | 69 |
| 10 | +1 | −1 | −1 | +1 | 88 | 87 | 91 | 90 | 76 | 75 | 78 | 85 |
| 11 | −1 | +1 | −1 | +1 | 75 | 75 | 83 | 81 | 60 | 63 | 63 | 71 |
| 12 | +1 | +1 | −1 | +1 | 90 | 91 | 93 | 95 | 86 | 87 | 88 | 88 |
| 13 | −1 | −1 | +1 | +1 | 78 | 76 | 82 | 80 | 63 | 64 | 65 | 66 |
| 14 | +1 | −1 | +1 | +1 | 87 | 89 | 91 | 91 | 77 | 81 | 76 | 83 |
| 15 | −1 | +1 | +1 | +1 | 75 | 75 | 79 | 79 | 60 | 60 | 63 | 69 |
| 16 | +1 | +1 | +1 | +1 | 96 | 95 | 99 | 97 | 85 | 84 | 86 | 85 |
| 17 | −2 | 0 | 0 | 0 | 57 | 58 | 60 | 62 | 37 | 34 | 43 | 48 |
| 18 | +2 | 0 | 0 | 0 | 85 | 83 | 89 | 86 | 71 | 73 | 74 | 76 |
| 19 | 0 | −2 | 0 | 0 | 88 | 88 | 91 | 91 | 76 | 74 | 77 | 75 |
| 20 | 0 | +2 | 0 | 0 | 92 | 91 | 95 | 94 | 80 | 81 | 81 | 78 |
| 21 | 0 | 0 | −2 | 0 | 80 | 78 | 84 | 82 | 68 | 71 | 75 | 74 |
| 22 | 0 | 0 | +2 | 0 | 75 | 77 | 78 | 80 | 63 | 68 | 68 | 70 |
| 23 | 0 | 0 | 0 | −2 | 84 | 82 | 88 | 85 | 71 | 70 | 74 | 74 |
| 24 | 0 | 0 | 0 | +2 | 98 | 99 | 99 | 100 | 90 | 89 | 89 | 90 |
| 25 | 0 | 0 | 0 | 0 | 92 | 91 | 95 | 94 | 81 | 80 | 81 | 82 |
| 26 | 0 | 0 | 0 | 0 | 91 | 91 | 94 | 94 | 80 | 81 | 80 | 82 |
| 27 | 0 | 0 | 0 | 0 | 92 | 91 | 95 | 94 | 81 | 81 | 81 | 82 |
| 28 | 0 | 0 | 0 | 0 | 90 | 91 | 93 | 94 | 77 | 81 | 81 | 82 |
| 29 | 0 | 0 | 0 | 0 | 92 | 91 | 95 | 94 | 78 | 81 | 80 | 82 |
| 30 | 0 | 0 | 0 | 0 | 92 | 91 | 94 | 94 | 80 | 81 | 80 | 82 |
| 31 | 0 | 0 | 0 | 0 | 90 | 91 | 95 | 94 | 78 | 81 | 83 | 82 |
| Term | Coefficient | SE | t | p | Coefficient | SE | t | p |
|---|---|---|---|---|---|---|---|---|
| MB on BS-C | MG on BS-C | |||||||
| Constant | 91.29 | 1.14 | 80.09 | 0.000 | 79.29 | 1.06 | 74.90 | 0.000 |
| pH | 7.125 | 0.616 | 11.57 | 0.000 | 8.958 | 0.572 | 15.67 | 0.000 |
| t | 0.875 | 0.616 | 1.42 | 0.174 | 0.792 | 0.572 | 1.38 | 0.185 |
| C | −0.375 | 0.616 | −0.61 | 0.551 | −1.208 | 0.572 | −2.11 | 0.051 |
| m | 4.625 | 0.616 | 7.51 | 0.000 | 5.375 | 0.572 | 9.40 | 0.000 |
| pH2 | −5.769 | 0.564 | −10.23 | 0.000 | −6.957 | 0.524 | −13.28 | 0.000 |
| t2 | −1.019 | 0.564 | −1.81 | 0.090 | −0.957 | 0.524 | −1.83 | 0.086 |
| C2 | −4.144 | 0.564 | −7.35 | 0.000 | −4.082 | 0.524 | −7.79 | 0.000 |
| m2 | −0.769 | 0.564 | −1.36 | 0.191 | −0.457 | 0.524 | −0.87 | 0.396 |
| pH·t | 1.813 | 0.754 | 2.40 | 0.029 | 4.062 | 0.700 | 5.80 | 0.000 |
| pH·C | 1.062 | 0.754 | 1.41 | 0.178 | 0.187 | 0.700 | 0.27 | 0.792 |
| pH·m | −0.312 | 0.754 | −0.41 | 0.684 | 0.437 | 0.700 | 0.62 | 0.541 |
| t·C | 0.437 | 0.754 | 0.58 | 0.570 | −0.562 | 0.700 | −0.80 | 0.434 |
| t·m | −0.187 | 0.754 | −0.25 | 0.807 | 0.688 | 0.700 | 0.98 | 0.341 |
| C·m | 0.562 | 0.754 | 0.75 | 0.466 | 1.063 | 0.700 | 1.52 | 0.149 |
| MB on CS-C | MG on CS-C | |||||||
| Constant | 94.43 | 1.11 | 85.08 | 0.000 | 80.86 | 1.09 | 73.85 | 0.000 |
| pH | 6.958 | 0.599 | 11.61 | 0.000 | 7.875 | 0.591 | 13.32 | 0.000 |
| t | 1.042 | 0.599 | 1.74 | 0.101 | 1.042 | 0.591 | 1.76 | 0.097 |
| C | −0.542 | 0.599 | −0.90 | 0.380 | −1.125 | 0.591 | −1.90 | 0.075 |
| m | 4.625 | 0.599 | 7.72 | 0.000 | 4.708 | 0.591 | 7.96 | 0.000 |
| pH2 | −5.576 | 0.549 | −10.15 | 0.000 | −6.433 | 0.542 | −11.87 | 0.000 |
| t2 | −0.951 | 0.549 | −1.73 | 0.103 | −1.183 | 0.542 | −2.18 | 0.044 |
| C2 | −3.951 | 0.549 | −7.19 | 0.000 | −3.183 | 0.542 | −5.88 | 0.000 |
| m2 | −0.701 | 0.549 | −1.28 | 0.220 | −0.683 | 0.542 | −1.26 | 0.225 |
| pH·t | 1.562 | 0.734 | 2.13 | 0.049 | 3.688 | 0.724 | 5.09 | 0.000 |
| pH·C | 1.187 | 0.734 | 1.62 | 0.125 | −0.062 | 0.724 | −0.09 | 0.932 |
| pH·m | −0.813 | 0.734 | −1.11 | 0.285 | 0.937 | 0.724 | 1.29 | 0.214 |
| t·C | 0.187 | 0.734 | 0.26 | 0.802 | −0.562 | 0.724 | −0.78 | 0.449 |
| t·m | −0.062 | 0.734 | −0.09 | 0.933 | 0.938 | 0.724 | 1.29 | 0.214 |
| C·m | 0.312 | 0.734 | 0.43 | 0.676 | 0.438 | 0.724 | 0.60 | 0.554 |
| Term | DF | Adj SS | Adj MS | F | p | DF | Adj SS | Adj MS | F | p |
|---|---|---|---|---|---|---|---|---|---|---|
| MB on BS-C | MG on BS-C | |||||||||
| Model | 14 | 3147.91 | 224.85 | 24.72 | 0.000 | 14 | 4685.58 | 334.68 | 42.66 | 0.000 |
| Linear | 4 | 1753.50 | 438.38 | 48.20 | 0.000 | 4 | 2669.50 | 667.37 | 85.08 | 0.000 |
| Square | 4 | 1313.53 | 328.38 | 36.11 | 0.000 | 4 | 1717.71 | 429.43 | 54.74 | 0.000 |
| Two-Way Interaction | 6 | 80.87 | 13.48 | 1.48 | 0.246 | 6 | 298.37 | 49.73 | 6.34 | 0.001 |
| Error | 16 | 145.51 | 9.09 | 16 | 125.51 | 7.84 | ||||
| Lack-of-Fit | 10 | 140.08 | 14.01 | 15.48 | 0.002 | 10 | 110.08 | 11.01 | 4.28 | 0.044 |
| Pure Error | 6 | 5.43 | 0.90 | 6 | 15.43 | 2.57 | ||||
| Total | 30 | 3293.42 | 30 | 4811.10 | ||||||
| R-Sq = 95.58% R-Sq(adj) = 91.72% | R-Sq = 97.39% R-Sq(adj) = 95.11% | |||||||||
| MB on CS-C | MG on CS-C | |||||||||
| Model | 14 | 2999.46 | 214.25 | 24.85 | 0.000 | 14 | 3698.69 | 264.19 | 31.48 | 0.000 |
| Linear | 4 | 1708.50 | 427.13 | 49.53 | 0.000 | 4 | 2076.83 | 519.21 | 61.87 | 0.000 |
| Square | 4 | 1216.58 | 304.15 | 35.27 | 0.000 | 4 | 1367.99 | 342.00 | 40.75 | 0.000 |
| Two-Way Interaction | 6 | 74.37 | 12.40 | 1.44 | 0.261 | 6 | 253.87 | 42.31 | 5.04 | 0.004 |
| Error | 16 | 137.96 | 8.62 | 16 | 134.27 | 8.39 | ||||
| Lack-of-Fit | 10 | 134.25 | 13.43 | 21.69 | 0.001 | 10 | 127.42 | 12.74 | 11.15 | 0.004 |
| Pure Error | 6 | 3.71 | 0.62 | 6 | 6.86 | 1.14 | ||||
| Total | 30 | 3137.42 | 30 | 3832.97 | ||||||
| R2 = 95.60% R2 (adj) = 91.75% | R2 = 96.50% R2 (adj) = 93.43% | |||||||||
| Adsorption Scenarios | Factors | BS-C | CS-C | ||
|---|---|---|---|---|---|
| MB | MG | MB | MG | ||
| Scenario 1 | pH | 6.5 | 7.2 | 6.5 | 6.6 |
| t, min | 50 | 60 | 45 | 50 | |
| m, g L−1 | 12 | 14 | 11 | 12 | |
| C, mg L−1 | 25 | 24 | 20 | 24 | |
| αexp, % | 97 | 95 | 97 | 94 | |
| αpred, % | 100 | 98 | 98 | 96 | |
| Scenario 2 | pH | 6.0 | 6.0 | 6.5 | 6.0 |
| t, min | 50 | 40 | 45 | 40 | |
| m, g L−1 | 10 | 14 | 11 | 12 | |
| C, mg L−1 | 25 | 25 | 30 | 45 | |
| αexp, % | 97 | 89 | 96 | 88 | |
| αpred, % | 99 | 90 | 97 | 90 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soldatkina, L.; Yanar, M. Optimization of Adsorption Parameters for Removal of Cationic Dyes on Lignocellulosic Agricultural Waste Modified by Citric Acid: Central Composite Design. ChemEngineering 2023, 7, 6. https://doi.org/10.3390/chemengineering7010006
Soldatkina L, Yanar M. Optimization of Adsorption Parameters for Removal of Cationic Dyes on Lignocellulosic Agricultural Waste Modified by Citric Acid: Central Composite Design. ChemEngineering. 2023; 7(1):6. https://doi.org/10.3390/chemengineering7010006
Chicago/Turabian StyleSoldatkina, Liudmyla, and Marianna Yanar. 2023. "Optimization of Adsorption Parameters for Removal of Cationic Dyes on Lignocellulosic Agricultural Waste Modified by Citric Acid: Central Composite Design" ChemEngineering 7, no. 1: 6. https://doi.org/10.3390/chemengineering7010006
APA StyleSoldatkina, L., & Yanar, M. (2023). Optimization of Adsorption Parameters for Removal of Cationic Dyes on Lignocellulosic Agricultural Waste Modified by Citric Acid: Central Composite Design. ChemEngineering, 7(1), 6. https://doi.org/10.3390/chemengineering7010006








