Comparison of Extractive and Heteroazeotropic Distillation of High-Boiling Aqueous Mixtures
Abstract
1. Introduction
2. Methods
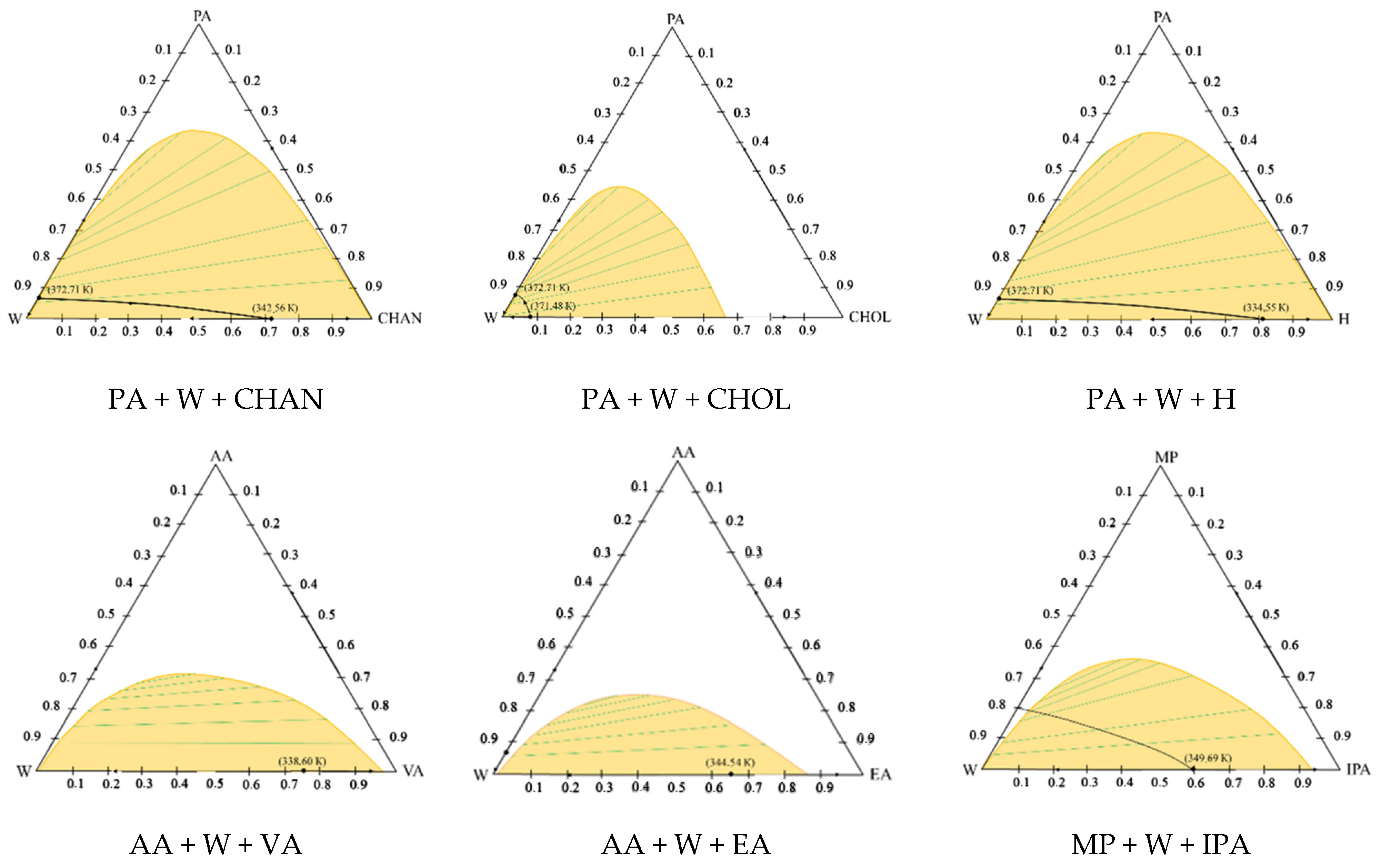
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Timofeev, V.S.; Serafimov, L.A.; Tymoshenko, A.V. Principles of the Technology of Basic Organic and Petrochemical Synthesis. Textbook for Universities, 3rd ed.; Higher School: Moscow, Russia, 2010; p. 408. [Google Scholar]
- Zhigang, L.; Chengyue, L.; Biaohua, C. Extractive Distillation: A Review. Sep. Purif. Rev. 2003, 32, 121–213. [Google Scholar] [CrossRef]
- Gerbaud, V.; Rodriguez-donis, I.; Hegely, L.; Lang, P.; Denes, F.; You, X. Review of Extractive Distillation. Process design, operation, optimization and control. Chem. Eng. Res. Des. 2019, 141, 229–271. [Google Scholar] [CrossRef]
- Ziyatdinov, N.N.; Emelyanov, I.I.; Ryzhova, A.A.; Chernakov, P.S. Algorithm and software for the optimal technological design of a system of simple distillation columns. Fine Chem. Technol. 2021, 16, 379–389. [Google Scholar] [CrossRef]
- Luyben, W.L. Comparison of extractive distillation and Pressure-Swing Distillation for acetone-methanol separation. Ind. Eng. Chem. Res. 2008, 47, 2696–2707. [Google Scholar] [CrossRef]
- Ghuge, P.D.; Mali, N.A.; Joshi, S.S. Comparative Analysis of Extractive and Pressure Swing Distillation for Separation of THF-Water Separation. Comput. Chem. Eng. 2017, 103, 188–200. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, J.; Jia, J.; Bu, G.; Zhu, Z.; Wang, Y. Comparison of pressure-swing distillation and extractive distillation with varied-diameter column in economics and dynamic control. J. Process Control 2017, 49, 9–25. [Google Scholar] [CrossRef]
- Lladosa, E.; Montón, J.B.; Burguet, M. Separation of di-n-propyl ether and n-propyl alcohol by extractive distillation and pressure-swing distillation: Computer simulation and economic optimization. Chem. Eng. Process. 2011, 50, 1266–1274. [Google Scholar] [CrossRef]
- Muñoz, R.; Montón, J.B.; Burguet, M.C.; Torre, J. Separation of isobutyl alcohol and isobutyl acetate by extractive distillation and pressure-swing distillation: Simulation and optimization. Sep. Purif. Technol. 2006, 50, 175–183. [Google Scholar] [CrossRef]
- Wang, X.; Xie, L.; Tian, P.; Tian, G. Design and control of extractive dividing wall column and pressure-swing distillation for separating azeotropic mixture of acetonitrile/N-propanol. Chem. Eng. Process. 2016, 110, 172–187. [Google Scholar] [CrossRef]
- Luo, H.; Liang, K.; Li, W.; Ming, X.; Xu, C. Comparison of Pressure Swing Distillation and Extractive Distillation Methods for Isopropyl Alcohol/Diisopropyl Ether Separation. Ind. Eng. Chem. Res. 2008, 53, 15167–15182. [Google Scholar] [CrossRef]
- Guang, C.; Shi, X.; Zhang, Z.; Wang, C.; Gao, J. Comparison of heterogeneous azeotropic and pressure-swing distillations for separating the diisopropylether/isopropanol/water mixtures. Chem. Eng. Res. Des. 2019, 143, 249–260. [Google Scholar] [CrossRef]
- Cui, Y.; Shi, X.; Guang, C.; Zhang, Z.; Wang, C.; Wang, C. Comparison of pressure-swing distillation and heterogenous azeotropic distillation for recovering benzene and isopropanol from wastewater. Process Saf. Environ. Prot. 2019, 122, 1–12. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, Q.; Li, X.; Zhong, X.; Huaxue, G. Comparison of heterogeneous azeotropic distillation and extractive methods for ternary azeotrope ethanol/toluene/water separation. Comput. Chem. Eng. 2017, 100, 27–37. [Google Scholar] [CrossRef]
- Kiss, A.A.; David, J.; Suszwalak, P. Enhanced bioethanol dehydratation by extractive and azeotropic distillation in dividing-wall columns. Sep. Purif. Technol. 2012, 86, 70–78. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Yu, B.-Y.; Hsu, C.-C.; Chien, I.-L. Comparison of heteroazeotropic and extractive distillation for the dehydration of propylene glycol methyl ether. Chem. Eng. Res. Des. 2016, 111, 184–195. [Google Scholar] [CrossRef]
- Abdel-Rahman, Z.A.; Mahmood, A.M.; Ali, A.J. Ethanol-Water Separation by Pressure Swing Adsorption (PSA). Iraqi J. Chem. Pet. Eng. 2014, 15, 1–7. [Google Scholar]
- López Núñez, A.R.; Rumbo Morales, J.Y.; Salas Villalobos, A.U.; De La Cruz-Soto, J.; Ortiz Torres, G.; Rodríguez Cerda, J.C.; Calixto-Rodriguez, M.; Brizuela Mendoza, J.A.; Aguilar Molina, Y.; Zatarain Durán, O.A.; et al. Optimization and Recovery of a Pressure Swing Adsorption Process for the Purification and Production of Bioethanol. Fermentation 2022, 8, 293. [Google Scholar] [CrossRef]
- Tajallipour, M.; Niu, C.; Dalai, A. Ethanol Dehydration in a Pressure Swing Adsorption Process Using Canola Meal. Energy Fuels 2013, 27, 6655–6664. [Google Scholar] [CrossRef]
- Serafimov, L.A.; Frolkova, A.K. Fundamental principle of concentration-field redistribution between separation regions as a basis for the design of technological systems. Theor. Found. Chem. Eng. 1997, 31, 159–166. [Google Scholar]
- Benyounes, H.; Frolkova, A.K. Investigation of the distribution of temperatures and concentrations during the distillation of real mixtures in complex columns. Sci. Notes M.V. Lomonosov MITHT 2002, 5, 50–53. (In Russian) [Google Scholar]
- Frolkova, A.K. Theoretical Foundations of Separation of Multicomponent Multiphase Systems Using Functional Complexes. Ph.D. Thesis, Moscow State University of Fine Chemical Technologies, Moscow, Russia, 2000. [Google Scholar]
- Berg, L.; Szabados, R.J.; Wendt, K.M.; Yeh, A.-I. The dehydration of the lower fatty acids by extractive distillation. Chem. Eng. Commun. 1990, 89, 113–131. [Google Scholar] [CrossRef]
- Cohen, L.R. Method for Separating Carboxylic Acids from Mixtures with Non-Acids. US Patent 4,576,683, 6 June 1986. Available online: https://patents.google.com/patent/US4576683A/en (accessed on 18 September 2022).
- Huang, H.J.; Chien, I.-L. Choice of suitable entrainer in heteroazeotropic batch distillation system for acetic acid dehydration. Chin. J. Chem. Eng. 2008, 39, 503–517. [Google Scholar] [CrossRef]
- Li, X.; Tang, C.; Guan, G. Mechanism of NMA as entrainer in separating acetic acid from water with extractive distillation. Chin. J. Chem. Eng. 2007, 58, 141–144. [Google Scholar]
- Raeva, V.M.; Gromova, O.V. Separation of water—formic acid—acetic acid mixtures in the presence of sulfolane. Fine Chem. Technol. 2019, 14, 24–32. [Google Scholar] [CrossRef]
- Lebedeva, N.D. Heat of Combustion of a Series of Monocarboxylic Acids. Russ. J. Phys. Chem. 1964, 38, 1435–1437. [Google Scholar]
- Sokolov, N.M.; Sevryugova, N.N.; Zhavoronkov, N.M. Liquid-vapor phase equilibrium in the systems acrylonitrile + water and acrolein + water at various pressures. Theor. Found. Chem. Eng. 1969, 3, 128–135. [Google Scholar]
- Aldrich Chemical Company Inc. Catalog Handbook of Fine Chemicals; Milwaukee Wis: Milwaukee, WI, USA, 1990. [Google Scholar]
- Weast, R.C.; Grasselli, J.G. CRC Handbook of Data on Organic Compounds; CRC Press: Boca Raton, FL, USA, 1989. [Google Scholar]
- Lee, F.-M. Use of Organic Sulfones as the Extractive Distillation Solvent for Aromatic Recovery. Ind. Eng. Chem. Process Des. Dev. 1986, 25, 949–957. [Google Scholar] [CrossRef]
- Rivenq, F. Vapor-liquid data for the system water-propionic acid. Bull. Soc. Chim. Fr. 1961, 1392–1395. Available online: http://pure-oai.bham.ac.uk/ws/files/18560974/Roman_Ramirez_et_al_Vapour_liquid_equilibrium_Fluid_Phase_Equilibria_2015.pdf14.10.2022 (accessed on 18 September 2022).
- Ogorodnikov, S.K.; Lesteva, T.M.; Kogan, V.B. Azeotropic Mixtures (in Russian); Chemistry: Moscow, Russia, 1971; p. 848. [Google Scholar]
- Tochigi, K.; Takahara, H.; Shiga, Y.; Kawase, Y. Isobaric vapor–liquid equilibria for water + propylene glycol monomethyl ether (PGME), water + propyleneglycol monomethyl ether acetate (PGMEA), and PGME + PGMEA at reduced pressures. Fluid Phase Equilib. 2007, 260, 65–69. [Google Scholar] [CrossRef]
- Lecat, M. L’Azeotropisme. Monograph; Brussel: Maurice Lamerti, 1918. [Google Scholar]
- Zharikov, L.K.; Krylova, K.S.; Kopylevich, G.M.; Tikhonova, N.K.; Oparina, G.K.; Serafimov, L.A. Phase equilibria in water-aniline, water-cyclohexanol, ethanol-aniline, ethanol-cyclohexanol systems. Zhournal Prikladnoi Khimii 1975, 48, 1249–1250. [Google Scholar]
- Marinichev, A.N.; Susarev, M.P. Concentration ranges of ternary hetero-azeotropes. Russ. J. Phys. Chem. 1969, 43, 631–634. [Google Scholar]
- Zhao, Q.; Li, X.; Zhong, X. Phase Equilibrium of Ternary System of VAc-TBA-Water. Huaxue Gongcheng 1992, 20, 61–65. [Google Scholar]
- Kato, M.; Konishi, H.; Hirata, M.J. New apparatus for isobaric dew and bubble point method methanol + water, ethyl acetate + ethanol, water + 1-butanol, and ethyl acetate + water systems. J. Chem. Eng. Data 1970, 15, 435–439. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Tu, C.-H. Isobaric vapor-liquid equilibria for the binary and ternary mixtures of 2-propanol, water, and 1,3-propanediol at p = 101.3 kPa: Effect of the 1,3-propanediol addition. Fluid Phase Equilib. 2014, 368, 104–111. [Google Scholar] [CrossRef]
- Budantseva, L.S.; Lesteva, T.M.; Nemtsov, M.S. Liquid–liquid equilibria in methanol–water–paraffin hydrocarbons C7, C8 systems. Russ. J. Phys. Chem. 1976, 50, 1344–1345. [Google Scholar]
- Senol, A.; Cehreli, S.; Ozmen, D. Phase Equilibria for the Ternary Liquid Systems of (Water + Tetrahydrofurfuryl Alcohol + Cyclic Solvent) at 298.2 K. J. Chem. Eng. Data 2005, 50, 688–691. [Google Scholar] [CrossRef]
- Glover, S.T. Liquid-liquid extraction: Removal of acetone and acetaldehyde from vinyl acetate with water in packed column. Transac. Inst. Chem. Eng. 1946, 24, 54–55. [Google Scholar]
- Stephenson, R.M.; Stuart, J.J. Mutual binary solubilities: Water-alcohols and water-esters. J. Chem. Eng. Data 1986, 31, 56–57. [Google Scholar] [CrossRef]
- Misikov, G.; Toikka, M.; Samarov, A.; Toikka, A. Phase equilibria liquid-liquid for ternary systems n-amyl alcohol-water-(acetic acid, n-amyl acetate), n-amyl acetate-water-acetic acid at 293.15 K, 303.15 K, 313.15 K and 323.15 K. Fluid Phase Equilib. 2022, 552, 113265. [Google Scholar] [CrossRef]
- Mayevskiy, M.; Frolkova, A.; Frolkova, A. Separation and Purification of Methyl Isobutyl Ketone from Acetone + Isopropanol + Water + Methyl Isobutyl Ketone + Methyl Isobutyl Carbinol + Diisobutyl Ketone Mixture. ACS Omega 2020, 5, 25365–25370. [Google Scholar] [CrossRef]
- Anokhina, E.A.; Shleynikova, E.L.; Timoshenko, A.V. Energy efficiency of complexes with partially coupled thermally and material flows for extractive distillation of methyl acetate—Chloroform mixture. Fine Chem. Technol. 2013, 8, 18–25. (In Russian) [Google Scholar]


| Binary Mixture | Original Mixture Comp. | Entrainer | |
|---|---|---|---|
| X1, Mole Frac. | ED | HAD | |
| PA + W | 0.8 | NMP, NMAA | CHAN, CHOL, H |
| AA + W | 0.8 | NMAA, NMP | VA, EA |
| MP + W | 0.5 | S, NMP | IPA |
| Component/Mixture | Boiling Point, K | ur | X1, Mole Frac | ur | ||
|---|---|---|---|---|---|---|
| Exp. | Calc. | Exp. | Calc. | |||
| PA | 414.05 2.4 × 10−4 | 414.15 | 0.0002 | - | - | - |
| W | 373.20 | 373.15 | 0.0001 | - | - | - |
| MP | 391.70 | 393.15 | 0.0037 | - | - | - |
| AA | 391.30 | 391.25 | 0.0001 | - | - | - |
| NMP | 475.20 | 477.42 | 0.0047 | - | - | - |
| NMAA | 478.20 | 478.15 | 0.0001 | - | - | - |
| CHAN | 353.90 | 353.87 | 0.0001 | - | - | - |
| CHOL | 434.30 | 434.00 | 0.0007 | - | - | - |
| H | 341.90 | 341.88 | 0.0001 | - | - | - |
| VA | 345.50 | 345.65 | 0.0004 | - | - | - |
| S | 558.00 | 560.45 | 0.0044 | - | - | - |
| IPA | 355.60 | 355.30 | 0.0008 | - | - | - |
| EA | 350.20 | 350.21 | 0.0001 | - | - | - |
| W + PA | 372.92 | 372.71 | 0.0006 | 0.9542 | 0.9272 | 0.0283 |
| W + MP | 367.60 | 370.64 | 0.0083 | 0.8250 | 0.8081 | 0.0209 |
| CHAN + W | 342.55 | 342.64 | 0.0003 | 0.7010 | 0.6990 | 0.0028 |
| W + CHOL | 370.92 | 371.48 | 0.0015 | 0.9380 | 0.9313 | 0.0071 |
| H+W | 335.13 | 334.58 | 0.0017 | 0.7810 | 0.7898 | 0.0113 |
| VA + W | 339.15 | 338.65 | 0.0015 | 0.7266 | 0.7452 | 0.0250 |
| EA + W | 343.53 | 344.54 | 2.9 ×10−5 | 0.7000 | 0.6731 | 0.0385 |
| IPA + W | 349.75 | 349.92 | 0.0005 | 0.5980 | 0.5824 | 0.0261 |
| Mixture | Liquid Mole Fraction (Exp. Data) | Liquid Mole Fraction (Calc. Data) | ur | |||
|---|---|---|---|---|---|---|
| X1′ | X2″ | X1′ | X2″ | ΔX1′ | ΔX2″ | |
| W + PA | ||||||
| W + H | 0.9999 | 0.9996 | 0.9999 | 0.9995 | 0.0000 | 0.0001 |
| W + CHAN | 0.9999 | 0.9995 | 0.9999 | 0.9995 | 0.0000 | 0.0000 |
| W + CHOL * | 0.9960 | 0.6741 | 0.9964 | 0.6383 | 0.0004 | 0.0531 |
| W + AA | ||||||
| W + VA | 0.9976 | 0.9504 | 0.9970 | 0.9493 | 0.0006 | 0.0012 |
| W + EA | 0.9830 | 0.8733 | 0.9830 | 0.8681 | 0.000 | 0.0060 |
| MP + W | ||||||
| IPA + W | 0.9166 | 0.9950 | 0.9178 | 0.9947 | 0.0013 | 0.0003 |
| Process | E | Column | Stage Number | Feed Stage (Mixture/E) | Reflux Ratio | R1 or F(E), kmol/h | Q, kW |
|---|---|---|---|---|---|---|---|
| PA + W | |||||||
| HAD | CHAN | C1 | 5 | 4/1 | - | 46.74 | 637.01 |
| CHOL | C1 | 18 | 12/3 | 0.32 | 2.42 | 376.80 | |
| H | C1 | 5 | 4/1 | - | 75.42 | 854.41 | |
| ED | NMAA | C1 | 18 | 10/4 | 0.4 | 50.00 | 538.47 |
| C1 | 38 | 4 | 1.5 | - | 1856.16 | ||
| NMP | C1 | 27 | 19/6 | 0.5 | 50.00 | 565.62 | |
| C2 | 13 | 7 | 0.6 | - | 1250.83 | ||
| AA + W | |||||||
| HAD | VA | C1 | 18 | 7/2 | 0.2 | 73.39 | 1069.99 |
| EA | C1 | 16 | 7/3 | 0.4 | 69.00 | 1188.44 | |
| C2 | 5 | 3 | 0.1 | - | 17.99 | ||
| ED | NMAA | C1 | 38 | 11/4 | 3.0 | 25.00 | 999.03 |
| C2 | 50 | 3 | 0.3 | - | 833.33 | ||
| NMP | C1 | 55 | 19/5 | 3.8 | 25.00 | 1176.59 | |
| C2 | 18 | 5 | 0.2 | - | 733.92 | ||
| MP + W | |||||||
| HAD | IPA | C1 | 30 | 25/11 | 0.2 | 92.16 | 1755.94 |
| C2 | 6 | 4 | 0.5 | - | 40.02 | ||
| ED | S | C1 | 46 | 36 | 3 | 100.00 | 1647.55 |
| C2 * | 6 | 4 | 0.2 | - | 623.75 | ||
| NMP | C1 | 23 | 12/6 | 0.4 | 100.00 | 1439.86 | |
| C2 | 35 | 5 | 2.0 | - | 1697.93 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frolkova, A.V.; Frolkova, A.K.; Gaganov, I.S. Comparison of Extractive and Heteroazeotropic Distillation of High-Boiling Aqueous Mixtures. ChemEngineering 2022, 6, 83. https://doi.org/10.3390/chemengineering6050083
Frolkova AV, Frolkova AK, Gaganov IS. Comparison of Extractive and Heteroazeotropic Distillation of High-Boiling Aqueous Mixtures. ChemEngineering. 2022; 6(5):83. https://doi.org/10.3390/chemengineering6050083
Chicago/Turabian StyleFrolkova, Anastasia V., Alla K. Frolkova, and Ivan S. Gaganov. 2022. "Comparison of Extractive and Heteroazeotropic Distillation of High-Boiling Aqueous Mixtures" ChemEngineering 6, no. 5: 83. https://doi.org/10.3390/chemengineering6050083
APA StyleFrolkova, A. V., Frolkova, A. K., & Gaganov, I. S. (2022). Comparison of Extractive and Heteroazeotropic Distillation of High-Boiling Aqueous Mixtures. ChemEngineering, 6(5), 83. https://doi.org/10.3390/chemengineering6050083




