Space and Time Crystal Engineering in Developing Futuristic Chemical Technology
Abstract
:1. Introduction
2. Crystal Engineering in Designing MOF Based Catalyst
2.1. Supramolecular Role
2.2. Designing Ligands
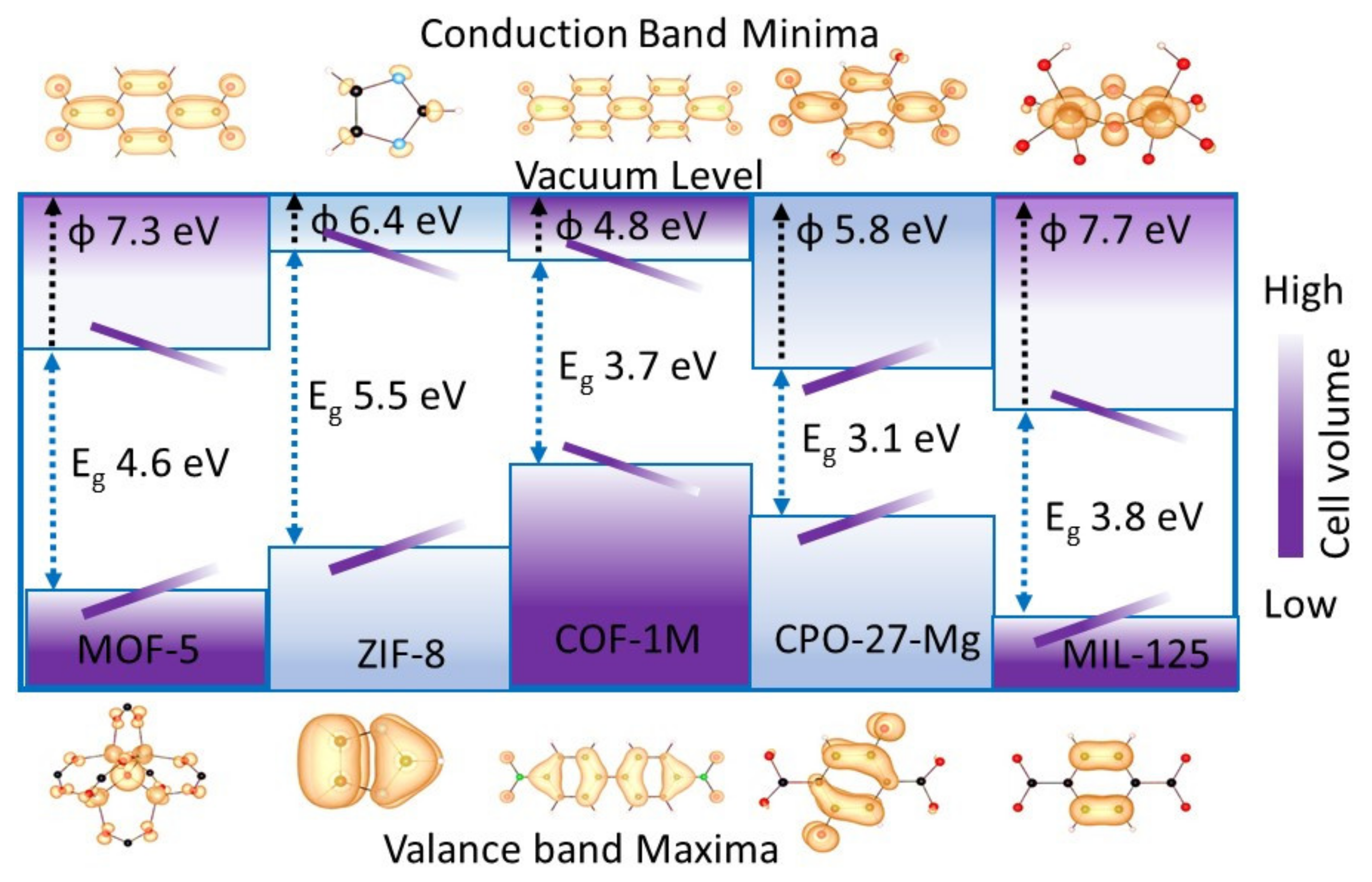
2.3. Secondary Building Unit
2.4. Decorating MOFs with Nanoparticles
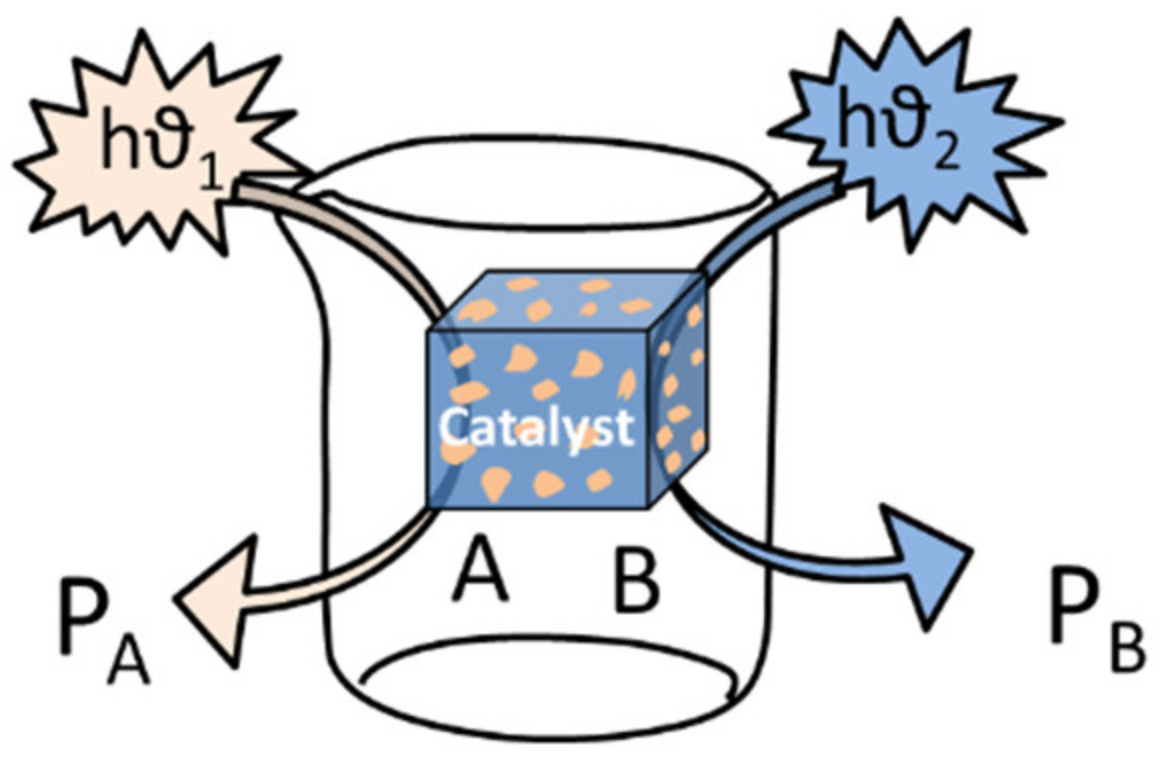
3. Designing Multipurpose Catalyst
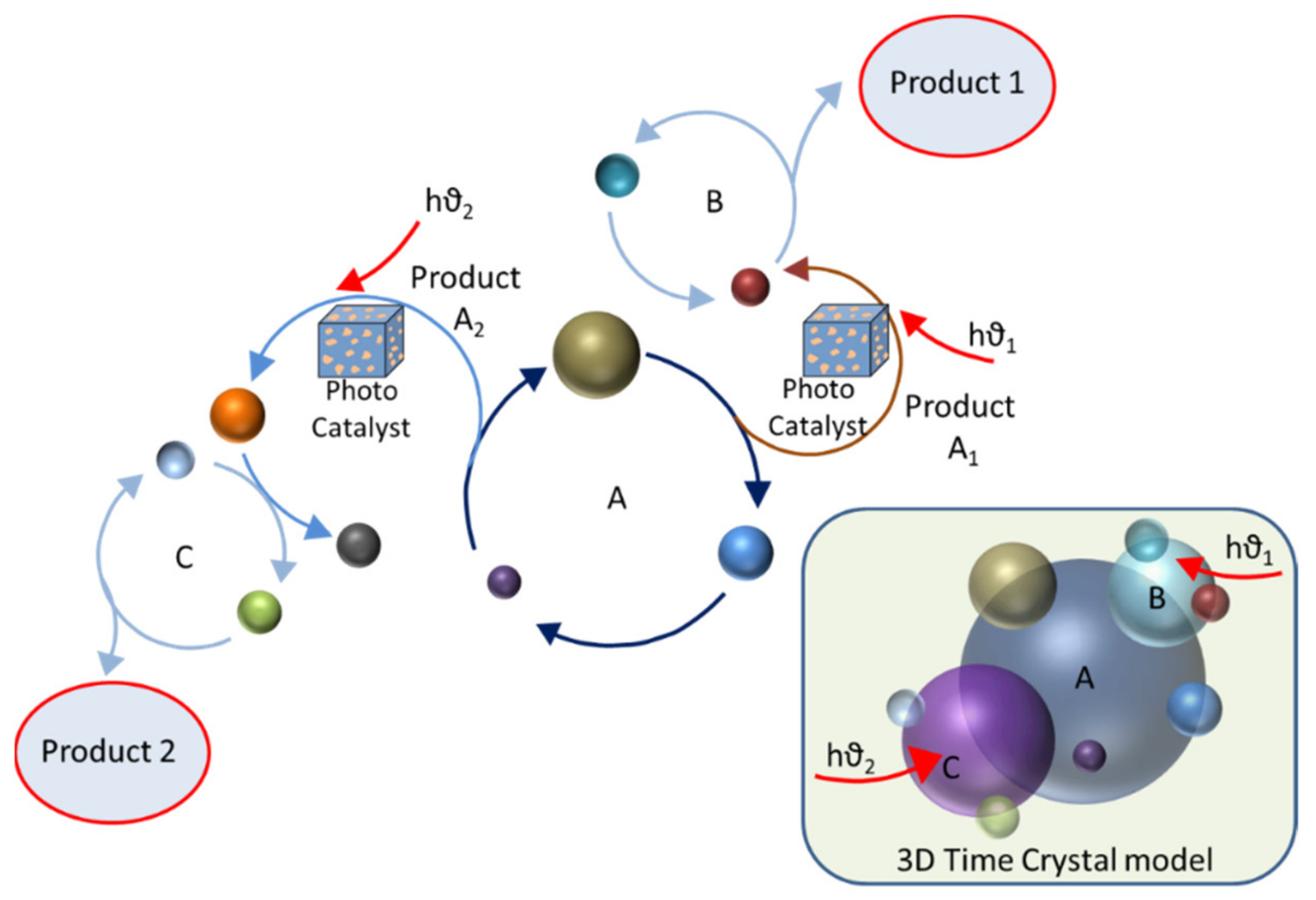
4. Time Crystal Engineering in Selecting One of the Competitive Products
4.1. Time Crystals in Nested Catalytic Cycles
4.2. Time Breathing in Tuning the Reaction Rate
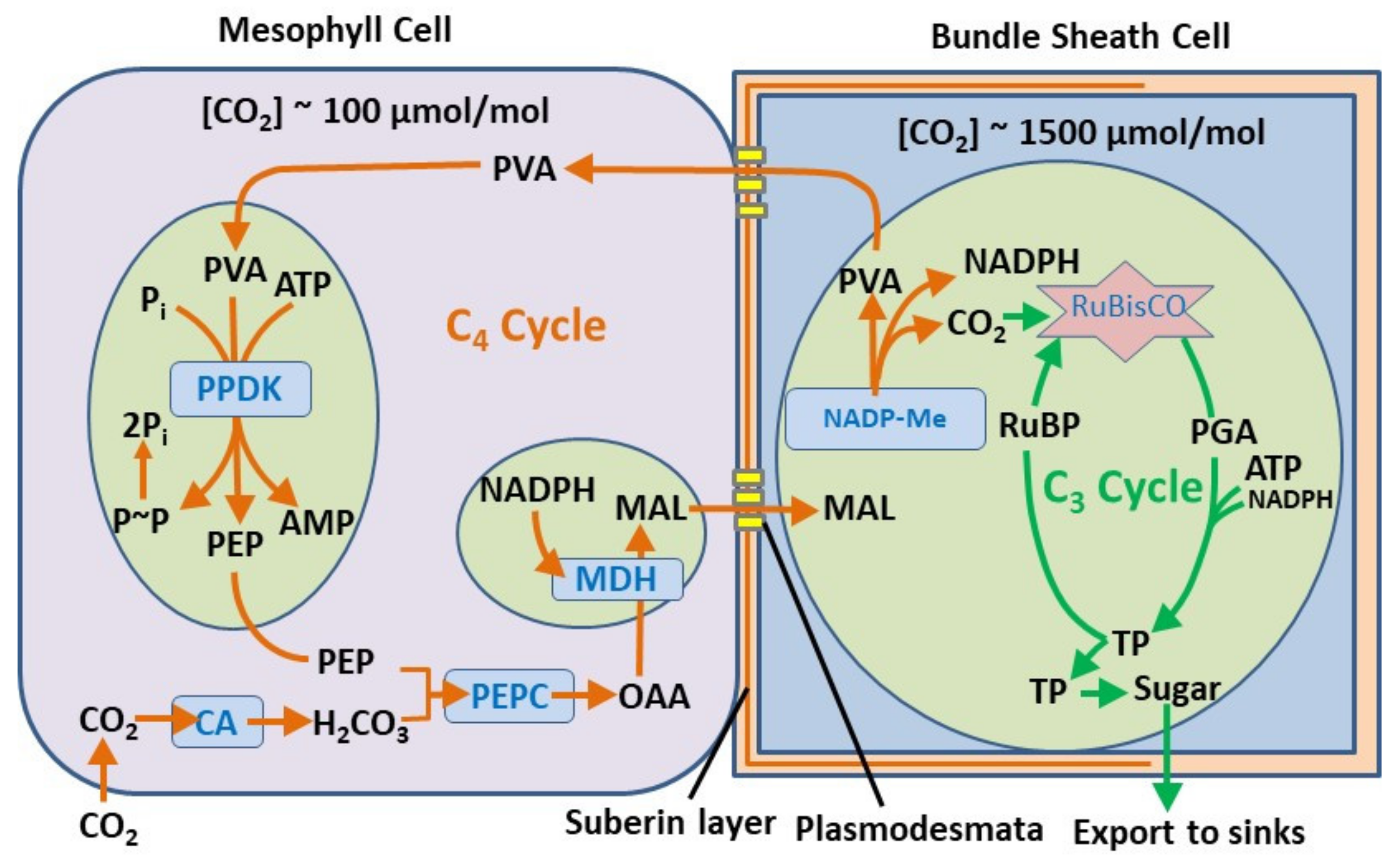
4.3. Time Crystal Engineering in Nature
5. Future Chemical Engineering
5.1. Engineering Technologies
5.1.1. Crystal Engineering
5.1.2. Time Crystal Engineering
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Schwarz, P.S.; Laha, S.; Janssen, J.; Huss, T.; Boekhoven, J.; Weber, C.A. Parasitic behavior in competing chemically fueled reaction cycles. Chem. Sci. 2021, 12, 7554–7560. [Google Scholar] [CrossRef] [PubMed]
- Orgel, L.E. Self-organizing biochemical cycles. Proc. Natl. Acad. Sci. USA 2000, 97, 12503–12507. [Google Scholar] [CrossRef] [Green Version]
- Bizzarri, M.; Giuliani, A.; Cucina, A.; Anselmi, F.D.; Soto, A.M.; Sonnenschein, C. Fractal analysis in a Systems Biology approach to cancer. Semin. Cancer Biol. 2011, 21, 175–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsuda, N.; Ohme-Takagi, M. Functional Analysis of Transcription Factors in Arabidopsis. Plant Cell Physiol. 2009, 50, 1232–1248. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Oshima, Y.; Yamamura, T.; Sugiyama, M.; Mitsuda, N.; Ohtsubo, N.; Ohme-Takagi, M.; Terakawa, T. Multi-petal cyclamen flowers produced by AGAMOUS chimeric repressor expression. Sci. Rep. 2013, 3, 2641. [Google Scholar] [CrossRef]
- Ghosh, S.; Fujita, D.; Bandyopadhyay, A. An organic jelly made fractal logic gate with an infinite truth table. Sci. Rep. 2015, 5, 11265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Dutta, M.; Ray, K.; Fujita, D.; Bandyopadhyay, A. A simultaneous one pot synthesis of two fractal structures via swapping two fractal reaction kinetic states. Phys. Chem. Chem. Phys. 2016, 18, 14772–14775. [Google Scholar] [CrossRef]
- Chandrashekaran, M.K. Phase shifts in the Drosophila pseudoobscura circadian rhythm evoked by temperature pulses of varying durations. J. Interdiscipl. Cycle Res. 1974, 5, 371–380. [Google Scholar] [CrossRef]
- Bag, P.; Sahoo, P. Designing Metal-Organic Frameworks Based Photocatalyst for Specific Photocatalytic Reactions: A Crystal Engineering Approach. In Green Photocatalyst for Energy and Environmental Process; Saravanan, R., Naushad, M., Cornejo, L., Lichtfouse, E., Eds.; Springer: New York, NY, USA, 2020; Chapter 6; Volume 36, pp. 141–186. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, Q.; Al-Enizi, A.M.; Nafady, A.; Ma, S. Recent advances in MOF-based photocatalysis: Environmental remediation under visible light. Inorg. Chem. Front. 2020, 7, 300–339. [Google Scholar] [CrossRef]
- Sahoo, P.; Ghosh, S. Time Crystal Engineering in Catalytic Reaction cycles. In Rhythmic Oscillations in Proteins to Human Cognition; Bandyopadhyay, A., Ray, K., Eds.; Springer Book Series ‘Studies in Rhythm Engineering’; Springer: New York, NY, USA, 2021; Chapter 4; pp. 103–134. [Google Scholar] [CrossRef]
- Sahoo, P.; Dastidar, P. Secondary Ammonium Dicarboxylate (SAD)—A Supramolecular Synthon in Designing Low Molecular Weight Gelators Derived from Azo-Dicarboxylates. Cryst. Growth Des. 2012, 12, 5917. [Google Scholar] [CrossRef]
- Sahoo, P.; Chakraborty, I.; Dastidar, P. Reverse thermal gelation of aromatic solvents by a series of easily accessible organic salt based gelators. Soft Matter 2012, 8, 2595. [Google Scholar] [CrossRef]
- Sahoo, P.; Sankolli, R.; Lee, H.-Y.; Raghavan, S.R.; Dastidar, P. Gel Sculpture: Moldable, Load-Bearing and Self-Healing Non-Polymeric Supramolecular Gel Derived from a Simple Organic Salt. Chem. Eur. J. 2012, 18, 8057. [Google Scholar] [CrossRef]
- Sahoo, P.; Adarsh, N.N.; Chacko, G.E.; Raghavan, S.R.; Puranik, V.G.; Dastidar, P. Combinatorial Library of Primaryalkylammonium Dicarboxylate Gelators: A Supramolecular Synthon Approach. Langmuir 2009, 25, 8742–8750. [Google Scholar] [CrossRef]
- Bag, P.P.; Kothur, R.R.; Reddy, C.M. Tautomeric preference in polymorphs and pseudopolymorphs of succinylsulfathiazole: Fast evaporation screening and thermal studies. CrystEngComm 2014, 16, 4706–4714. [Google Scholar] [CrossRef] [Green Version]
- Adarsh, N.N.; Sahoo, P.; Dastidar, P. Is a Crystal Engineering Approach Useful in Designing Metallogels? A Case Study. Cryst. Growth Des. 2010, 10, 4976. [Google Scholar] [CrossRef]
- Butler, K.T.; Hendon, C.H.; Walsh, A. Electronic Chemical Potentials of Porous Metal–Organic Frameworks. J. Am. Chem. Soc. 2014, 136, 2703–2706. [Google Scholar] [CrossRef] [Green Version]
- Butler, K.T.; Hendon, C.H.; Walsh, A. Electronic Structure Modulation of Metal–Organic Frameworks for Hybrid Devices. ACS Appl. Mater. Interfaces 2014, 6, 22044–22050. [Google Scholar] [CrossRef] [Green Version]
- Singhania, A.; Ghosh, I.; Sahoo, P.; Fujita, D.; Ghosh, S.; Bandyopadhyay, A. Radio Waveguide–Double Ratchet Rotors Work in Unison on a Surface to Convert Heat into Power. Nano Lett. 2020, 20, 6891–6898. [Google Scholar] [CrossRef]
- Hendon, C.H.; Tiana, D.; Fontecave, M.; Sanchez, C.; D’arras, L.; Sassoye, C.; Rozes, L.; Mellot-Draznieks, C.; Walsh, A. Engineering the Optical Response of the Titanium-MIL-125 Metal–Organic Framework through Ligand Functionalization. J. Am. Chem. Soc. 2013, 135, 10942–10945. [Google Scholar] [CrossRef] [Green Version]
- Akimov, A.V.; Asahi, R.; Jinnouchi, R.; Prezhdo, O.V. What Makes the Photocatalytic CO2 Reduction on N-Doped Ta2O5 Efficient: Insights from Nonadiabatic Molecular Dynamics. J. Am. Chem. Soc. 2015, 137, 11517–11525. [Google Scholar] [CrossRef]
- Ha, J.; Lee, J.H.; Moon, H.R. Alterations to secondary building units of metal–organic frameworks for the development of new functions. Inorg. Chem. Front. 2020, 7, 12–27. [Google Scholar] [CrossRef]
- Yang, L.-M.; Fang, G.-Y.; Ma, J.; Ganz, E.; Han, S.S. Band Gap Engineering of Paradigm MOF-5. Cryst. Growth Des. 2014, 14, 2532–2541. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Z.; deKrafft, K.E.; Lin, W. Doping Metal–Organic Frameworks for Water Oxidation, Carbon Dioxide Reduction, and Organic Photocatalysis. J. Am. Chem. Soc. 2011, 133, 13445–13454. [Google Scholar] [CrossRef]
- Bag, P.P.; Wang, X.; Sahoo, P.; Xiong, J.; Cao, R. Efficient photocatalytic hydrogen evolution under visible light by ternary composite CdS@NU-1000/RGO. Catal. Sci. Technol. 2017, 7, 5113–5119. [Google Scholar] [CrossRef]
- Shen, L.; Luo, M.; Liu, Y.; Liang, R.; Jing, F.; Wu, L. Noble-metal-free MoS2 co-catalyst decorated UiO-66/CdS hybrids for efficient photocatalytic H2 production. Appl. Catal. B 2015, 166, 445–452. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Kong, X.; Yao, K.; Liu, L.; Zhang, J.; Guo, Z.; Xu, W.; Fang, Z.; Liu, Y. Photocatalytic Performance of the MOF-Coating Layer on SPR-Excited Ag Nanowires. ACS Omega 2021, 6, 2882–2889. [Google Scholar] [CrossRef]
- Mori, K.; Matsuo, J.; Yamashita, H. PdAg Nanoparticles Supported on an Amine-functionalized MOF as a Photo-switchable Catalyst for Hydrogen Storage/Delivery Mediated by CO2/Formic Acid. Chem. Lett. 2021, 50, 607–610. [Google Scholar] [CrossRef]
- Sha, Z.; Sun, J.; Chan, H.S.O.; Jaenicke, S.; Wu, J. Enhanced photocatalytic activity of the AgI/UiO66(Zr) composite for rhodamine B degradation under visible-light irradiation. ChemPlusChem 2015, 80, 1321–1328. [Google Scholar] [CrossRef]
- Pi, Y.; Li, X.; Xia, Q.; Wu, J.; Li, Z.; Li, Y.; Xiao, J. Formation of willow leaf-like structures composed of NH2-MIL68(In) on a multifunctional multiwalled carbon nanotube backbone for enhanced photocatalytic reduction of Cr (VI). Nano Res. 2017, 10, 3543–3556. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, H.; Wu, Y.; Zeng, G.; Chen, X.; Leng, L.; Wu, Z.; Li, H. One-pot self-assembly and photoreduction synthesis of silver nanoparti-cle-decorated reduced graphene oxide/MIL-125(Ti) photocatalyst with improved visible light photocatalytic activity. Appl. Organomet. Chem. 2016, 30, 289–296. [Google Scholar] [CrossRef]
- Kazlauskas, R.J. Engineering a multipurpose catalyst. Nat. Chem. Biol. 2006, 2, 514. [Google Scholar] [CrossRef]
- Lind, A.; Vistad, Ø.; Sunding, M.F.; Andreassen, K.A.; Cavka, J.H.; Grande, C.A. Multi-purpose structured catalysts designed and manufactured by 3D printing. Mater. Des. 2020, 187, 108377. [Google Scholar] [CrossRef]
- Racles, C.; Zaltariov, M.-F.; Damoc, M.; Macsim, A.-M.; Iacob, M.; Sacarescu, L. Three Reactions, One Catalyst: A Multi-Purpose Platinum(IV) Complex and its Silica-Supported Homologue for Environmentally Friendly Processes. Appl. Organomet. Chem. 2019, 34, e5422. [Google Scholar] [CrossRef]
- Arena, F. Multipurpose composite MnCeOx catalysts for environmental applications. Catal. Sci. Technol. 2014, 4, 1890. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, C.; Li, X.; Hu, Q.; Wang, C. Ag NPs decorated C-TiO2/Cd0.5Zn0.5S Z-scheme heterojunction for simultaneous RhB degradation and Cr(VI) reduction. Environ. Pollut. 2021, 286, 117305. [Google Scholar] [CrossRef]
- Sahoo, P.; Tan, J.; Zhang, Z.-M.; Singh, S.K.; Lu, T.-B. Engineering Surface Structure of Binary/Ternary Ferrite nanoparticles as High Performance Electrocatalysts for Oxygen Evolution Reaction. ChemCatChem 2018, 10, 1075–1083. [Google Scholar] [CrossRef]
- Sahoo, P.; Das, P. Moisture-Catalyzed Slow Release of Sex Pheromone from Microcrystals in Controlling Phyllophaga Pests. Eng. Sci. 2021. accepted. [Google Scholar] [CrossRef]
- Wilczek, F. Quantum time crystals. Phys. Rev. Lett. 2012, 109, 160401. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Hess, P.W.; Kyprianidis, A.; Becker, P.; Lee, A.; Smith, J.; Pagano, G.; Potirniche, I.-D.; Potter, A.C.; Vishwanath, A.; et al. Observation of a discrete time crystal. Nature 2017, 543, 217. [Google Scholar] [CrossRef]
- Francis, C.D.; Sargent, M.L. Effects of Temperature Perturbations on Circadian Conidiation in Neurospora. Plant Physiol. 1979, 64, 1000–1004. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Sahoo, P.; Saxena, K.; Manna, J.S.; Ray, K.; Ghosh, S.; Bandyopadhyay, A. Cytoskeletal Filaments Deep Inside a Neuron Are not Silent: They Regulate the Precise Timing of Nerve Spikes Using a Pair of Vortices. Symmetry 2021, 13, 821. [Google Scholar] [CrossRef]
- Singh, P.; Saxena, K.; Sahoo, P.; Ghosh, S.; Bandyopadhyay, A. Electrophysiology using coaxial atom probe array: Live imaging reveals hidden circuits of a hippocampal neural network. J. Neurophysiol. 2021, 125, 2107–2116. [Google Scholar] [CrossRef]
- Singh, P.; Saxena, K.; Singhania, A.; Sahoo, P.; Ghosh, S.; Chhajed, R.; Ray, K.; Fujita, D.; Bandyopadhyay, A. A self-operating time crystal model of the human brain: Can we replace entire brain hardware with a 3D fractal architecture of clocks alone? Information 2020, 11, 238. [Google Scholar] [CrossRef]
- Singh, P.; Sahoo, P.; Ray, K.; Ghosh, S.; Bandyopadhyay, A. Building a Non-ionic, Non-electronic, Non-algorithmic Artificial Brain: Cortex and Connectome Interaction in a Humanoid Bot Subject (HBS). In Advances in Intelligent Systems and Computing; Book Series (AISC); Springer: Singapore, 2020; Volume 1309, pp. 245–278. [Google Scholar] [CrossRef]
- Saxena, K.; Singh, P.; Sahoo, P.; Sahu, S.; Ghosh, S.; Ray, K.; Fujita, D.; Bandyopadhyay, A. Fractal, scale free electromagnetic resonance of a single brain extracted microtubule nanowire, a single tubulin protein and a single neuron. Fractal Fract. 2020, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.B.; Bercot, E.A.; Rowley, J.M.; Coates, G.W.; Rovis, T. Ligand-dependent catalytic cycle and role of styrene in nickel-catalyzed anhydride cross-coupling: Evidence for turnover -limiting reductive elimination. J. Am. Chem. Soc. 2007, 129, 2718–2725. [Google Scholar] [CrossRef]
- Sage, R.F.; Sage, T.L.; Kocacinar, F. Photorespiration and the Evolution of C4 Photosynthesis. Annu. Rev. Plant. Biol. 2012, 63, 19–47. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahoo, P.; Ghosh, S. Space and Time Crystal Engineering in Developing Futuristic Chemical Technology. ChemEngineering 2021, 5, 67. https://doi.org/10.3390/chemengineering5040067
Sahoo P, Ghosh S. Space and Time Crystal Engineering in Developing Futuristic Chemical Technology. ChemEngineering. 2021; 5(4):67. https://doi.org/10.3390/chemengineering5040067
Chicago/Turabian StyleSahoo, Pathik, and Subrata Ghosh. 2021. "Space and Time Crystal Engineering in Developing Futuristic Chemical Technology" ChemEngineering 5, no. 4: 67. https://doi.org/10.3390/chemengineering5040067
APA StyleSahoo, P., & Ghosh, S. (2021). Space and Time Crystal Engineering in Developing Futuristic Chemical Technology. ChemEngineering, 5(4), 67. https://doi.org/10.3390/chemengineering5040067






