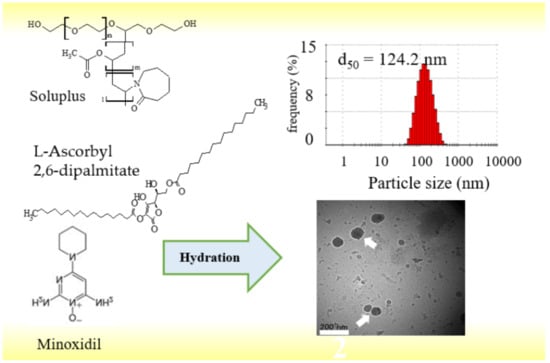
Characterization of Soluplus/ASC-DP Nanoparticles Encapsulated with Minoxidil for Skin Targeting
Abstract
1. Introduction
2. Materials and Methods
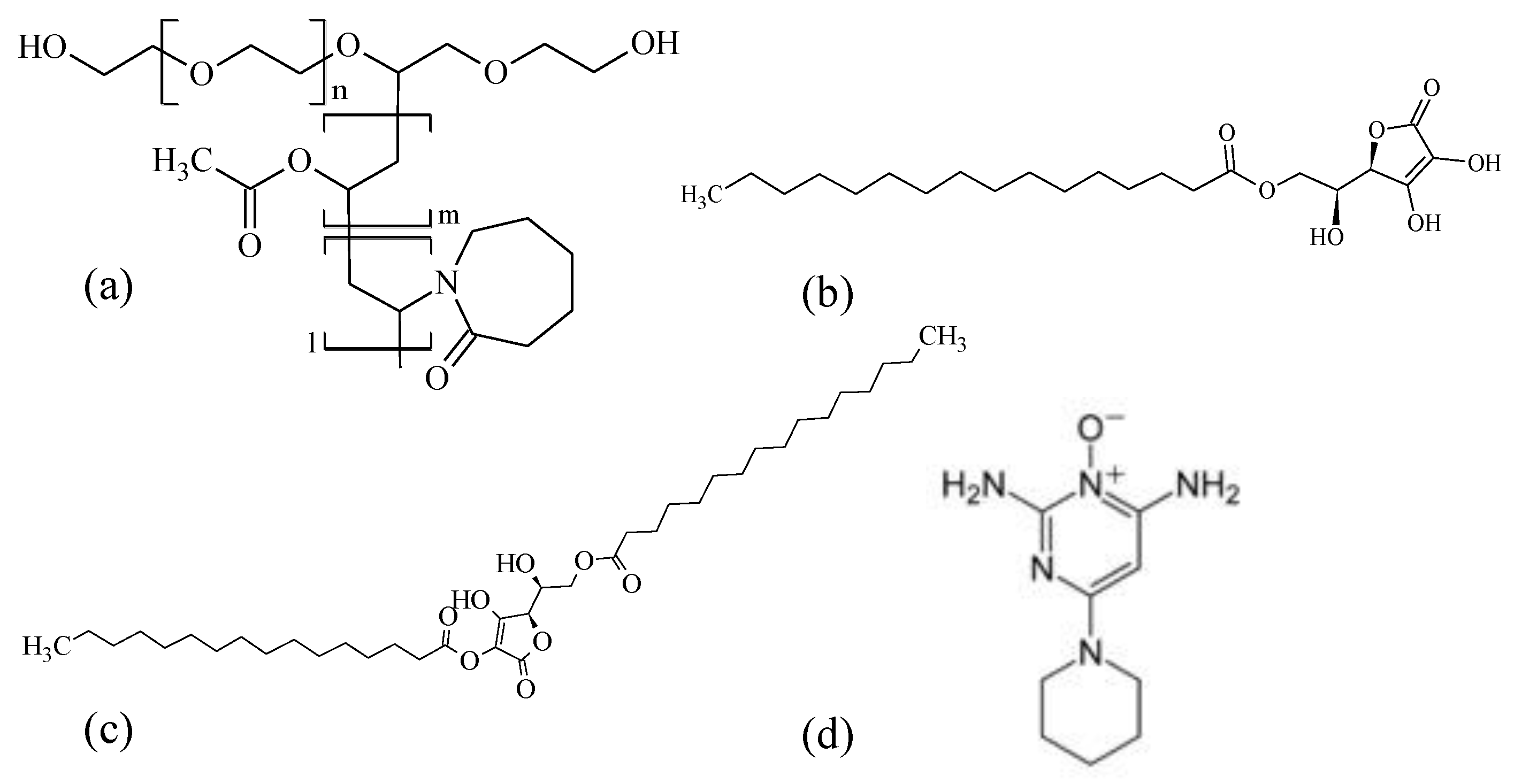
2.1. Materials
2.2. Sample Preparation
2.2.1. Physical Mixture (PM)
2.2.2. EVP Microparticles
2.3. Measurement Methods
2.3.1. Particle Size Measurement Using DLS (Dynamic Light Scattering)
2.3.2. Zeta Potential Measurement
2.3.3. Stability Test
2.3.4. TEM Measurement
2.3.5. PXRD Measurement
2.3.6. DSC
2.3.7. IR Measurements
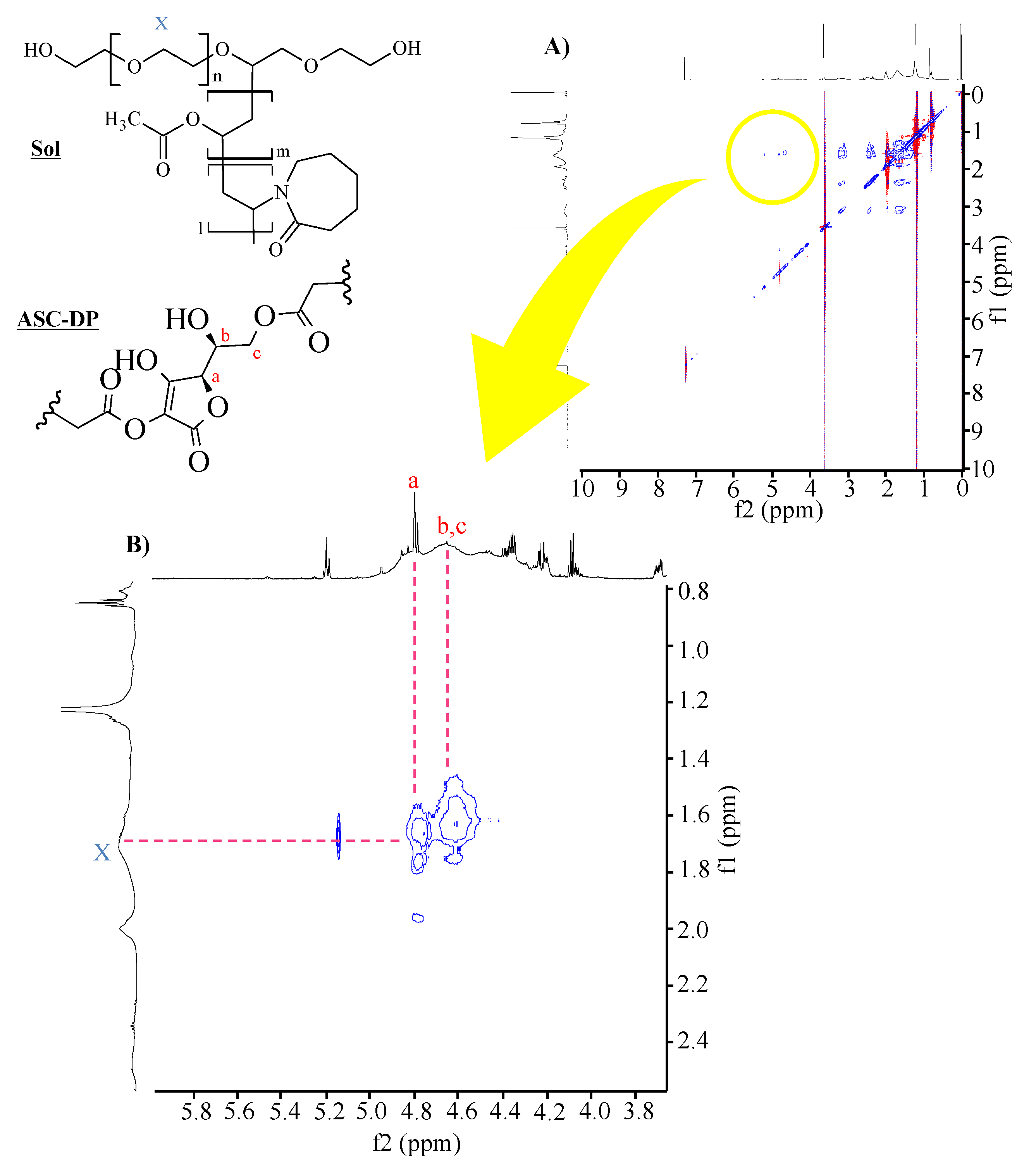
2.3.8. 1H-1H NOESY NMR Spectroscopy
2.3.9. Skin Permeability Test
3. Results and Discussion
3.1. Evaluation of Sol/ASC-DP Particles
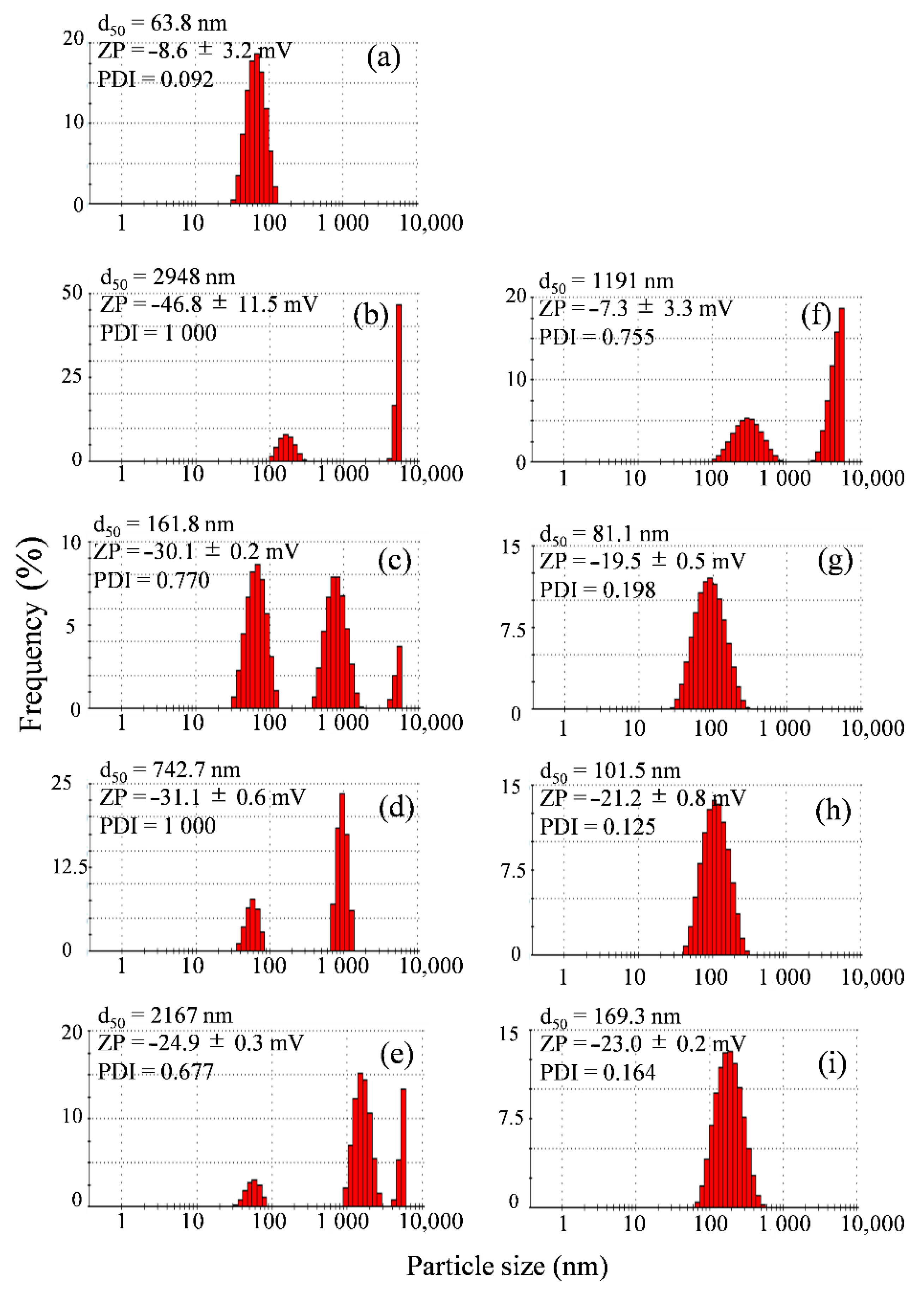
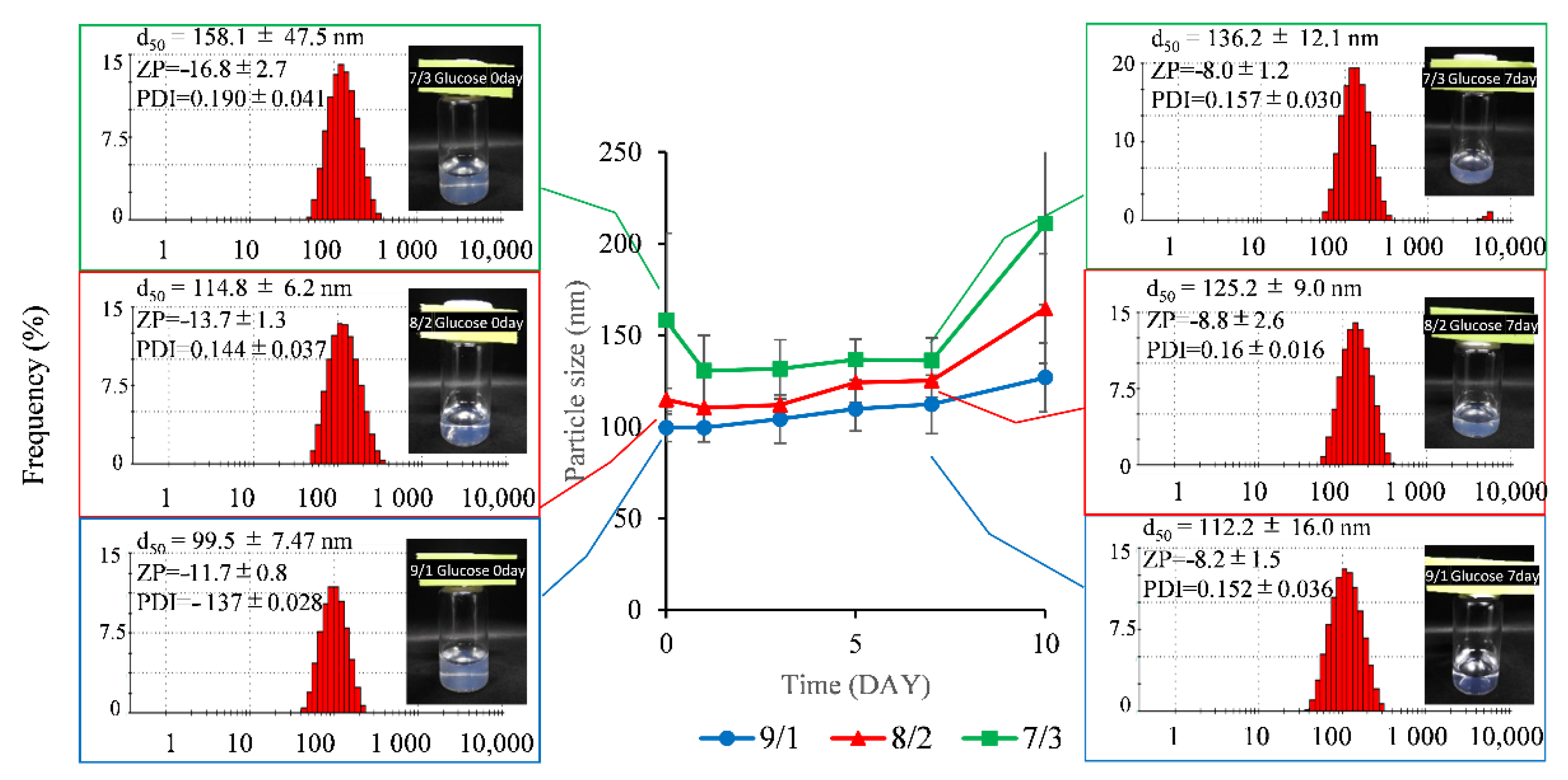
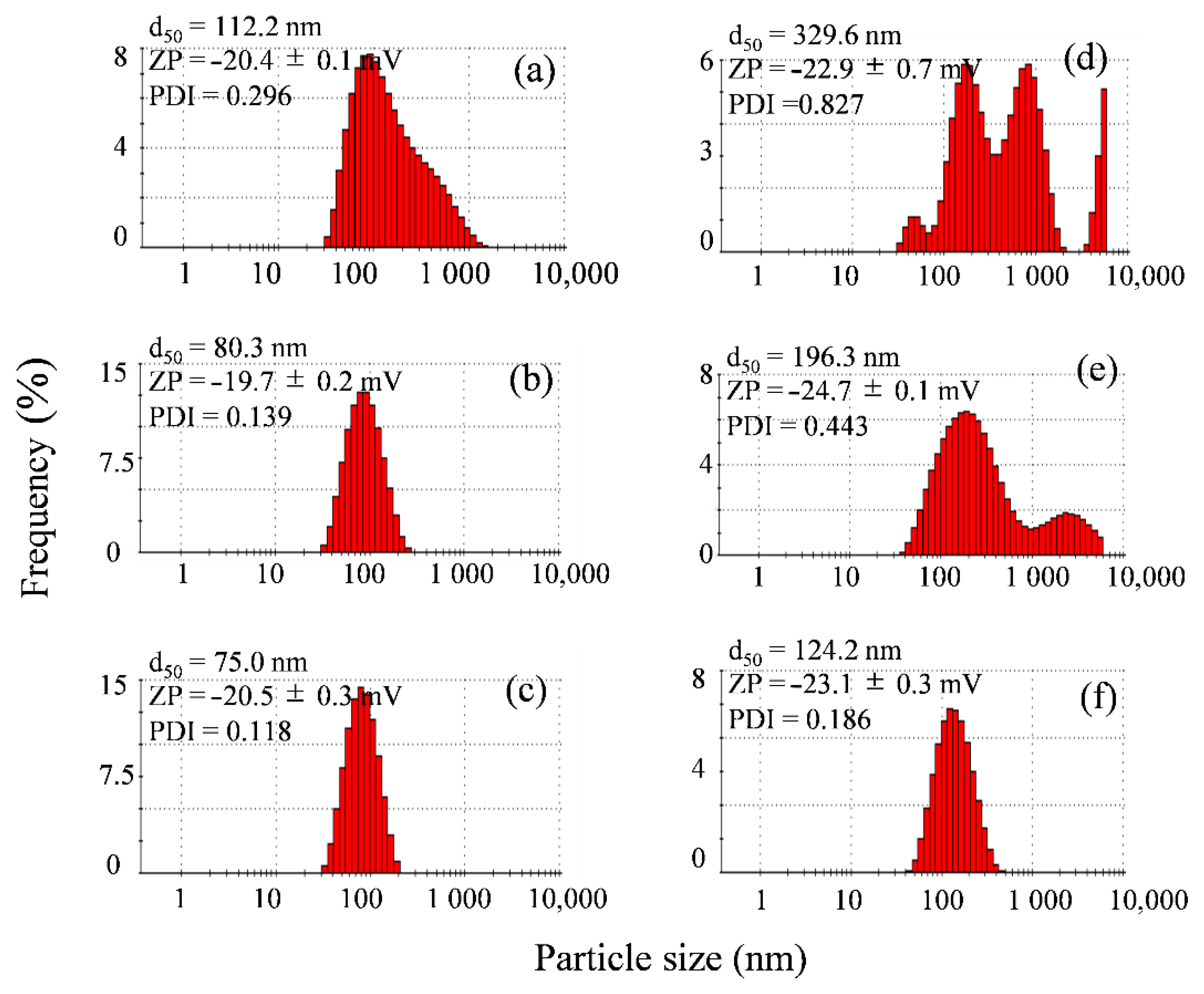
3.1.1. Particle Size and Zeta Potential Measurement
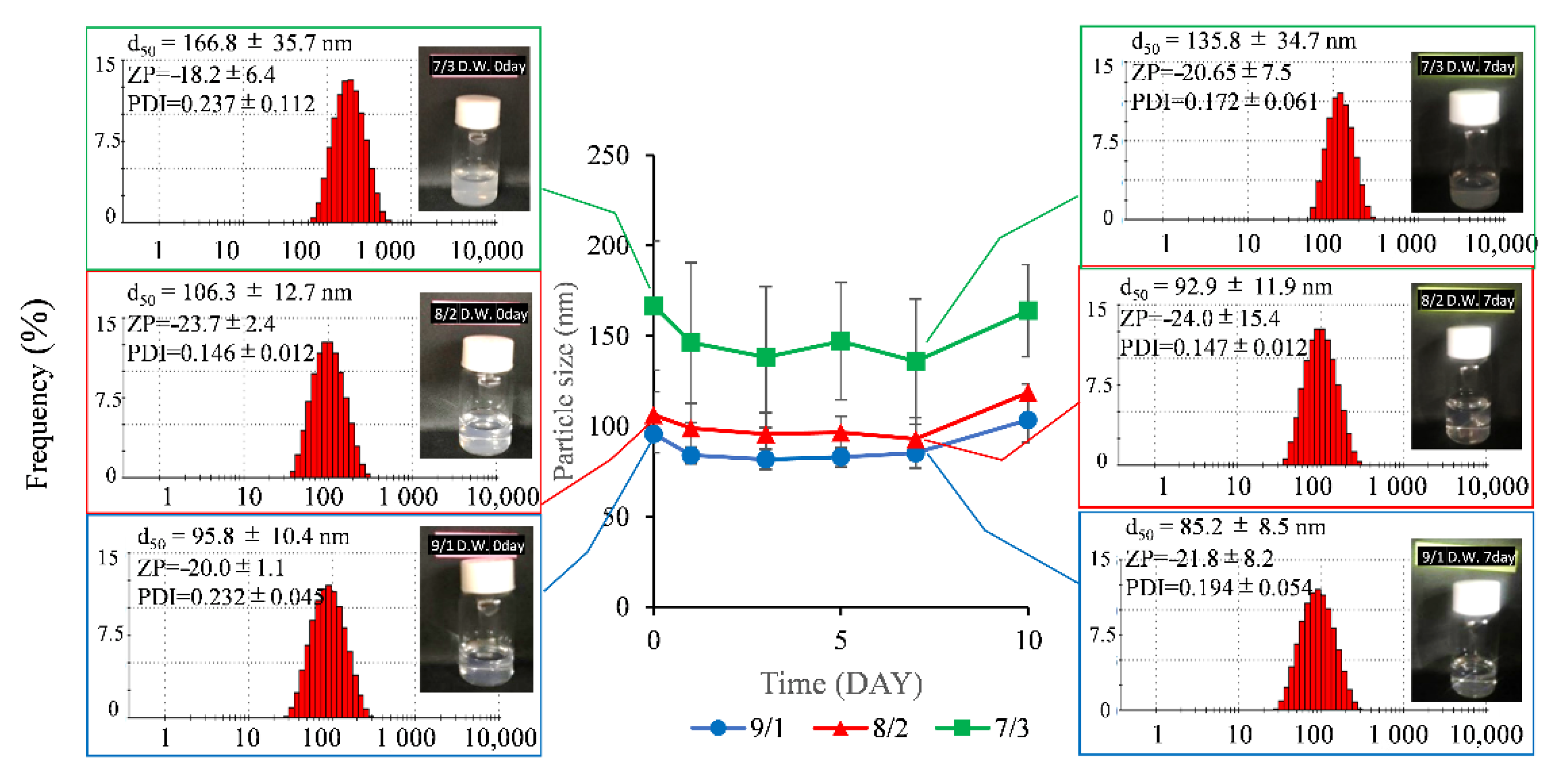
3.1.2. Stability Test
3.1.3. Transmission Electron Microscopy (TEM) Measurement
3.2. Evaluation in Solid State
3.2.1. Powder X-ray Diffraction (PXRD) Measurement
3.2.2. Differential Scanning Calorimetry (DSC) Measurement
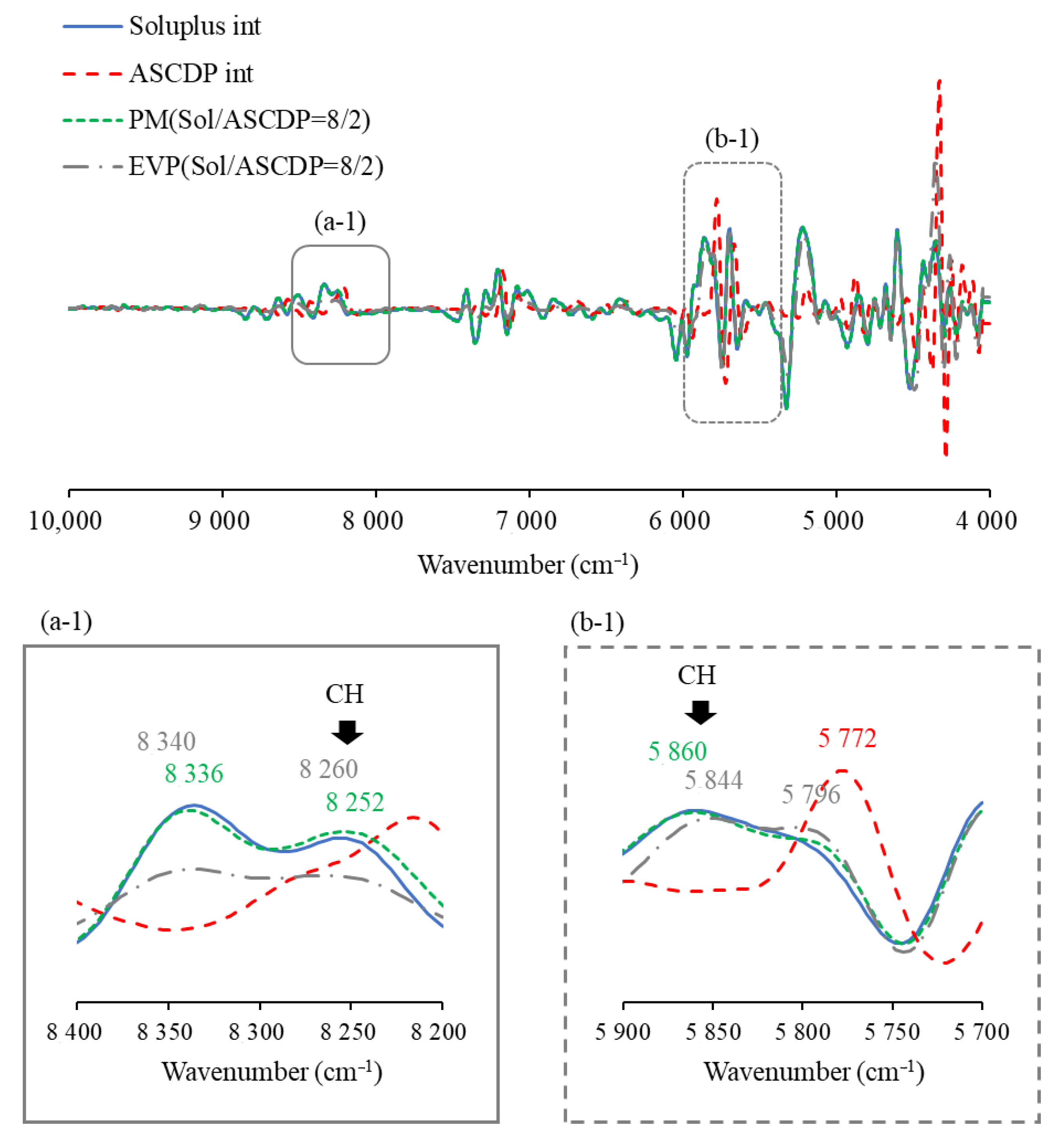
3.2.3. Near-Infrared (NIR) Measurement
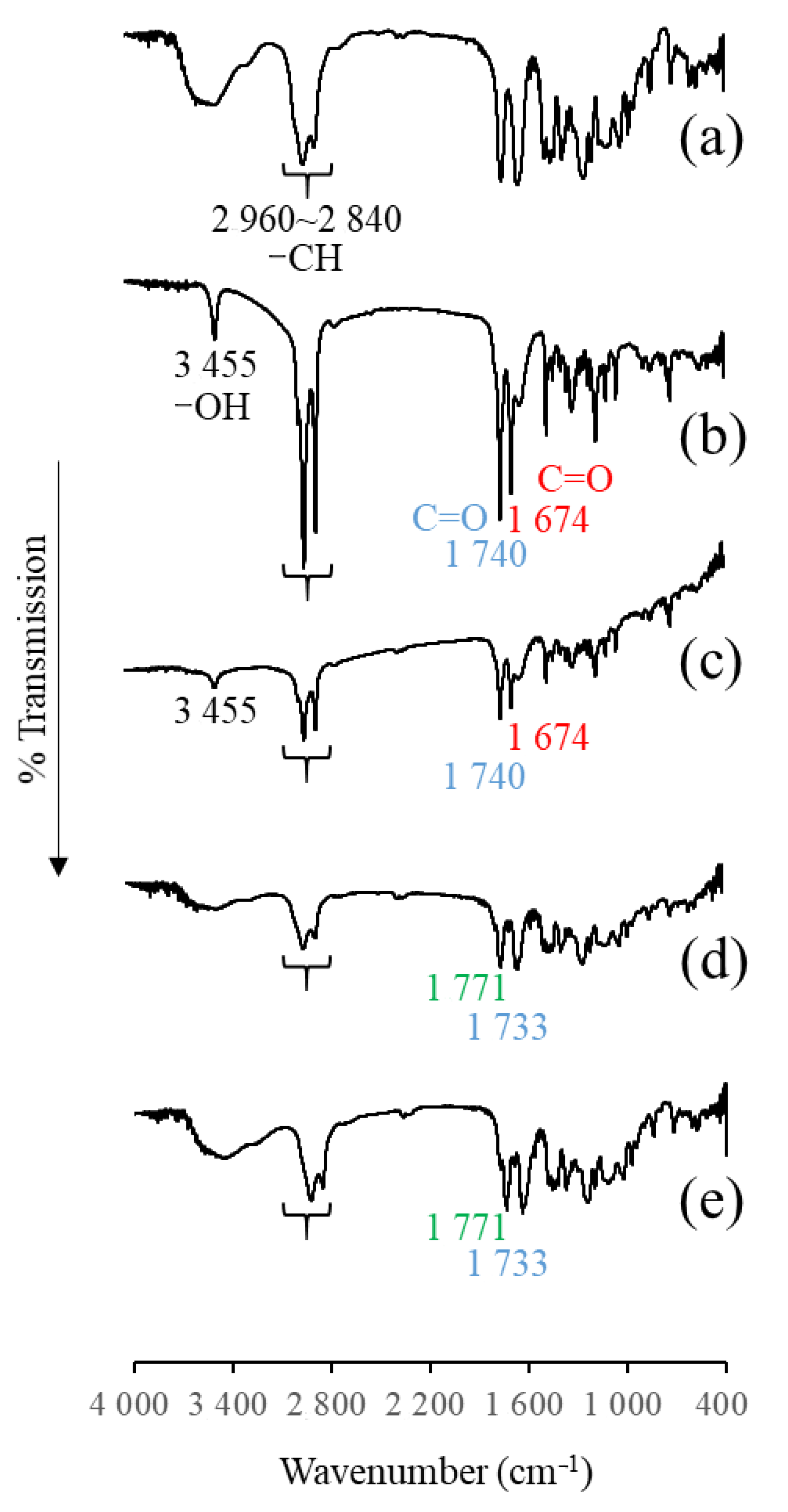
3.2.4. Infrared (IR) Measurement
3.3. Evaluation in Liquid State
NOESY NMR Measurement
3.4. Evaluation of Sol/ASC-DP/Minoxidil Particles
Particle Size and Zeta Potential Measurement
3.5. Evaluation of Skin Adaptation
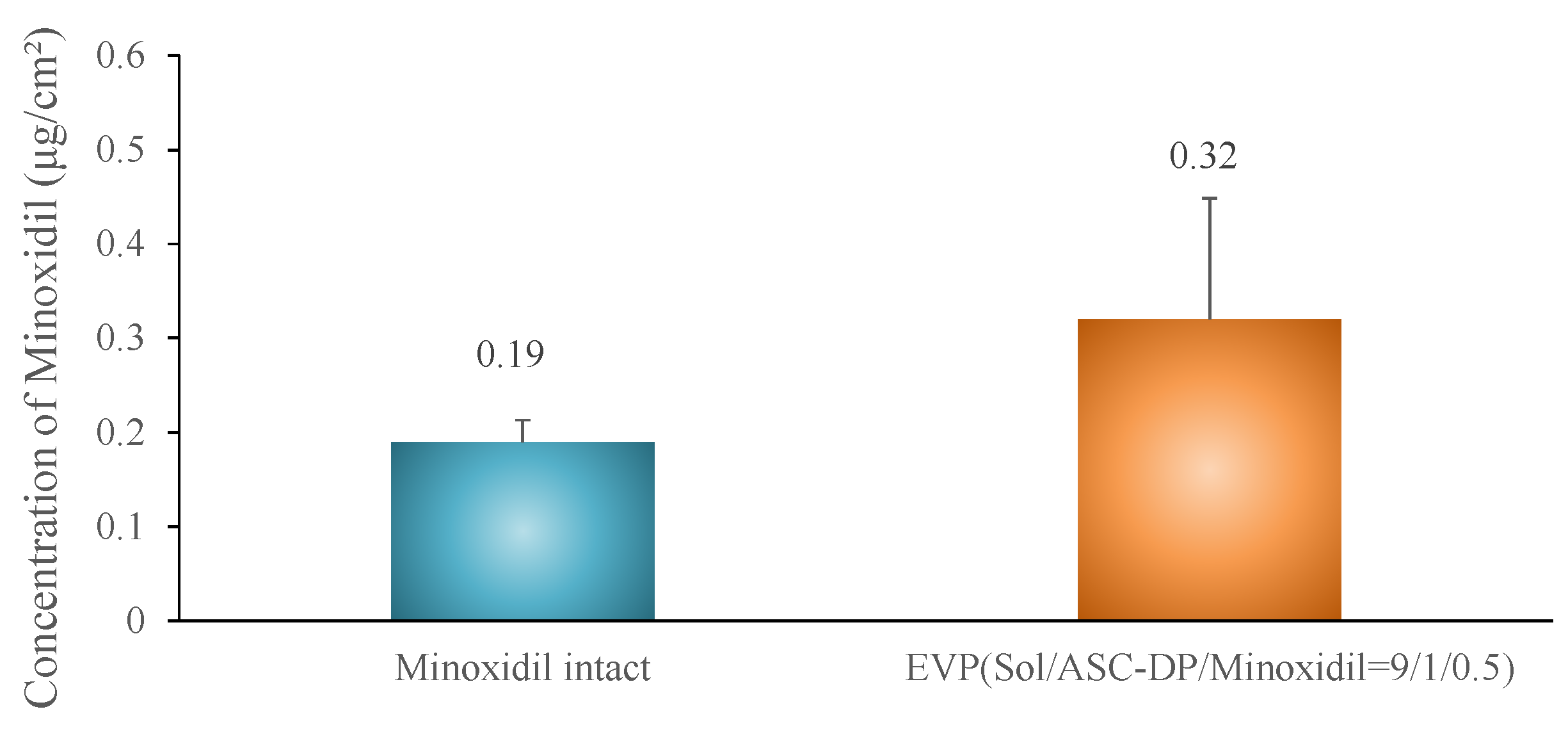
Skin Permeation Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Sample Availability
References
- Clayton, W. Theory of Emulsions, 4th ed.; Blackston Co.: New York, NY, USA, 1943. [Google Scholar]
- Ito, M.; Uehara, M.; Wakui, R.; Shiota, M.; Kuroiwa, T. Preparation Characteristics of Water-in-oil Emulsion Using Olive Oil as a Continuous Phase in Microchannel Emulsifi-cation. Jpn. J. Food Eng. 2017, 18, 103–111. (In Japanese) [Google Scholar] [CrossRef]
- Suzuki, R.; Takizawa, T.; Kuwata, Y.; Mutoh, M.; Ishiguro, N.; Utoguchi, N.; Shinohara, A.; Eriguchi, M.; Yanagie, H.; Maruyama, K. Effective Anti–Tumor Activity of Oxaliplatin Encapsulated in Transferrin–PEG–Liposome. Int. J. Pharm. 2008, 346, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Onoue, S.; Yamada, S.; Chan, H.K. Nanodrugs: Pharmacoki-netics and Safety. Int. J. Nanomed. 2014, 9, 1025–1037. [Google Scholar] [CrossRef]
- Chen, H.; Khemtong, C.; Yang, X.; Chang, X.; Gao, J. Nan-onizasion Strategies for Poorly Water-Soluble Drugs. Drug Discov. 2010, 16, 354–360. [Google Scholar]
- Morita, M.; Katada, M. Preparation of O/W Emulsions by High Pressure Homogenizer. J. Jpn. Oil Chem. Soc. 1991, 40, 58–63. [Google Scholar] [CrossRef]
- Inoue, Y.; Hibino, M.; Murata, I.; Kanamoto, I. A Nanocarri-er Skin-Targeted Drug Delivery System Using an Ascorbic Acid Derivative. Pharm. Res. 2017, 35, 1–14. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block Copolymer Micelles for Drug Delivery: Design, Characterization and Biological Significance. Adv. Drug Deliv. Rev. 2012, 64, 37–48. [Google Scholar] [CrossRef]
- Yang, H.; Teng, F.; Wang, P.; Tian, B.; Lin, X.; Hu, X.; Zhang, L.; Zhang, K.; Zhang, Y.; Tang, X. Investigation of a Nano-suspension Stabilized by Soluplus® to Improve Bioavaila-bility. Int. J. Pharm. 2014, 477, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.C.; Li, S.; Liu, C.; Gong, T.; Sun, X.; Fu, Y.; Zhang, Z.R. Soluplus Micelles for Improving the Oral Bioavailabil-ity of Scopoletin and their Hypouricemic Effect In Vivo. Acta Pharmacol. Sin. 2017, 38, 424–433. [Google Scholar] [CrossRef]
- Dian, L.; Yu, E.; Chen, X.; Wen, X.; Zhang, Z. Enhancing Oral Bioavailability of Quercetin Using Novel Soluplus Polymeric Micelles Nanoscale. Res. Lett. 2014, 9, 684. [Google Scholar]
- Meister, A. Glutathione-Ascorbic Acid Antioxidant System in Animals. J. Biol. Chem. 1994, 269, 9397–9400. [Google Scholar] [CrossRef]
- Kameyama, K.; Sakai, C.; Kondoh, S. Inhibitory Effect of Magnesium L-Ascorbyl-2-Phosphate (VC-PMG) on Mel-anogenesis In Vitro and In Vivo. J. Am. Acad. Dermatol. 1996, 34, 29–33. [Google Scholar] [CrossRef]
- Palma, S.; Manzo, R.; Nostro, P.; Allemandi, D. Nanostruc-tures from Alkyl Vitamin C Derivatives (ASCn): Properties and Potential Platform for Drug Delivery. Int. J. Pharm. 2007, 345, 26–34. [Google Scholar] [CrossRef]
- Lan, H.H.; Yukitaka, K.; Shuji, A. Discoloration Kinetics of L-Ascorbyl 0-Palmitate Powders with Various Water Contents. Food Sci. 2007, 13, 7–12. [Google Scholar]
- Moribe, K.; Maruyama, S.; Inoue, Y.; Suzuki, T.; Fukami, T.; Tomono, K.; Higashi, K.; Tozuka, Y.; Yamamoto, K. Ascor-byl Dipalmitate/PEG-Lipid Nanoparticles as a Novel Carrier for Hydrophobic Drugs. Int. J. Pharm. 2010, 387, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Takayama, R.; Inoue, Y.; Murata, I.; Kanamoto, I. Character-ization of Nanoparticles Using DSPE-PEG2000 and Solup-lus. J. Colloids Interfaces 2020, 4, 28. [Google Scholar] [CrossRef]
- Dargie, H.J.; Dollery, C.T.; Daniel, J. Minoxidil in resistant hypertension. Lancet 1977, 2, 515–518. (In Japanese) [Google Scholar] [CrossRef]
- Otomo, S. Hair Growth Effect of Minoxidil. Folia Pharmacol. 2002, 119, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Tanida, S.; Kurokawa, T.; Sato, H.; Kadota, K.; Tozuka, Y. Evaluation of the Micellization Mechanism of an Amphi-pathic Graft Copolymer with Enhanced Solubility of Ipri-flavone. Chem. Pharm. Bull. 2016, 64, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Mitsui, T.; Aso, Y.; Sugibayashi, K. Structure–Permeability Relationship Analysis of the Permeation Barrier Properties of the Stratum Corneum and Viable Epidermis/Dermis of Rat Skin. J. Pharm. Sci. 2008, 97, 4391–4403. [Google Scholar] [CrossRef]
- Takeuchi, H.; Terasaka, S.; Sakurai, T.; Furuya, A.; Urano, H.; Sugibayashi, K. Variation Assessment for in Vitro Permeabilities through Yucatan Micropig Skin. Biol. Pharm. Bull. 2011, 34, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Arce, F.J.; Asano, N.; See, G.L.; Oshizaka, T.; Itakura, S.; Todo, H.; Sugibayashi, K. Prediction of skin permeation and concentration of rhododendrol applied as finite dose from complex cosmetic vehicles. Int. J. Pharm. 2020, 578, 119186. [Google Scholar] [CrossRef]
- Mishra, P.R.; Shaal, L.A.; Müller, R.H.; Keck, C.M. Pro-duction and Characterization of Hesperetin Nanosuspen-sions for Dermal Delivery. Int. J. Pharm. 2009, 371, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Tokudome, Y.; Uchida, R.; Yokote, T.; Todo, H.; Hada, N.; Kon, T.; Yasuda, J.; Hayashi, H.; Hashimoto, F.; Sugibayashi, K. Effect of Topically Applied Sphingomye-lin-Based Liposomes on the Ceramide Level in a Three-Dimensional Cultured Human Skin Model. J. Liposome Res. 2010, 20, 49–54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Inoue, Y.; Yoshimura, S.; Tozuka, Y.; Moribe, K.; Kumamoto, T.; Ishikawa, T.; Yamamoto, K. Application of Ascorbic Acid 2-Glucoside as a Solubilizing Agent for Clarithromy-cin: Solubilization and Nanoparticle Formation. Int. J. Pharm. 2007, 331, 38–45. [Google Scholar] [CrossRef]
- Kurata, T.; Nishikawa, Y. Chemical properties of Oxidized- L-ascorbic acid. Biosci Biotechnol Biochem 2003, 77, 1138–1139. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takayama, R.; Ishizawa, M.; Yamada, M.; Inoue, Y.; Kanamoto, I. Characterization of Soluplus/ASC-DP Nanoparticles Encapsulated with Minoxidil for Skin Targeting. ChemEngineering 2021, 5, 44. https://doi.org/10.3390/chemengineering5030044
Takayama R, Ishizawa M, Yamada M, Inoue Y, Kanamoto I. Characterization of Soluplus/ASC-DP Nanoparticles Encapsulated with Minoxidil for Skin Targeting. ChemEngineering. 2021; 5(3):44. https://doi.org/10.3390/chemengineering5030044
Chicago/Turabian StyleTakayama, Rina, Moe Ishizawa, Miyuki Yamada, Yutaka Inoue, and Ikuo Kanamoto. 2021. "Characterization of Soluplus/ASC-DP Nanoparticles Encapsulated with Minoxidil for Skin Targeting" ChemEngineering 5, no. 3: 44. https://doi.org/10.3390/chemengineering5030044
APA StyleTakayama, R., Ishizawa, M., Yamada, M., Inoue, Y., & Kanamoto, I. (2021). Characterization of Soluplus/ASC-DP Nanoparticles Encapsulated with Minoxidil for Skin Targeting. ChemEngineering, 5(3), 44. https://doi.org/10.3390/chemengineering5030044