Abstract
This study exploited the application of pulsed electric field (PEF) on the recovery of polyphenols from aerial parts of Sideritis scardica, tepals of Crocus sativus, and fruits of Vitis vinifera. Short pulses of 10 μs in a period of 1 ms were applied to the plant material, while different electric field intensities, 1.2 to 2.0 kV/cm were tested to optimize the procedure. The content in total polyphenols and the polyphenolic profile of the plant extracts were evaluated. Along with PEF samples, control samples were prepared for comparison. PEF treatment enhanced the recovery in total polyphenols for all the three plants examined. A significant increase was noticed in each plant tested and PEF condition applied, though lower electric field intensities up to 1.4 kV/cm proved to be more effective. Under the optimum electric field intensities, 1.4 kV/cm for V. vinifera and 1.2 kV/cm for S. scardica and C. sativus, increases of 49.15%, 35.25%, and 44.36% in total polyphenol content, respectively, were achieved. Additionally, an 85% increase of quercetin 3-rutinoside for V. vinifera, a 56% of apigenin 7-O-glucoside for S. scardica, and a 64% increase for kaempferol 3-O-glucoside for C. sativus were obtained.
1. Introduction
Since antiquity, herbal medicines have been used to maintain health and treat various diseases. Nowadays herbal formulations, being safer, less expensive, and often more biocompatible than their synthetic analogs, have gained an important position and great demand globally [1,2]. According to the World Health Organization (WHO) [1], 60% of the world’s population still rely on herbal medicine and about 80% of the population in developing countries depend almost totally on it for their primary health care needs.
Vitis vinifera, Crocus sativus, and Sideritis scardica are medicinal plants, well known for their beneficial bioactivities. The pharmacological properties of V. vinifera fruit, grape, as well as the active compounds in different parts of the fruit, including skin, seeds, pomace, and stems, have been extensively described in the literature [3,4,5]. Among grapes’ pharmacological effects, skin protection, antioxidant, antibacterial, anticancer, anti-inflammatory, and antidiabetic activities, as well as hepatoprotective, cardioprotective, and neuroprotective effects are included. C. sativus is one of the most studied species from the Crocus genus due to the production of the world’s most expensive spice, saffron, from its stigmas. Several parts of the plant, specifically tepals, stigmas, leaves, and corns, possess biologically active constituents that have shown health-promoting effects. Therapeutic potential of C. sativus L. and its main constituents have been evaluated against different diseases like cancer, Alzheimer’s, erectile dysfunction, diabetes, and cardiovascular disease [6,7]. S. scardica, known as “mountain tea”, has been used in traditional medicine of the Balkan countries against a broad spectrum of disorders, including coughs, asthma, emphysema, and bronchitis. Nowadays, pharmacological investigations attribute to the plant, among others, anti-inflammatory, gastroprotective, antiglioma, cytotoxic, antimicrobial, and antioxidant activities [8,9].
Aforementioned bioactivities are nowadays attributed, to a significant extent, to the bioactive phytoconstituents that belong to the chemical group of polyphenols [10,11,12,13]. Despite their beneficial properties, polyphenols are not systematically isolated nowadays from natural sources, due to significant drawbacks of conventional extraction methods. The major drawbacks of conventional extraction methods include the use of expensive and toxic organic solvents in a large quantity, thermal energy and/or substantial mechanical force, the application of long extraction times, the low extraction selectivity, and the decomposition of thermo labile phytochemicals [14,15,16,17,18].
Pulse electric field (PEF) extraction is a green, non-thermal, and selective extraction technique that does not require substantial energy demand and has proven to be effective in short extraction durations [14,19]. The applied electric field causes electroporation to the cell membranes, leading to the formation of pores in weak areas of the cell membrane. As a result, cell membrane permeability is increased, resulting in a more efficient diffusion of the bioactive compounds from the plant tissue. The main factors implicated in PEF’s treatment efficiency are the electric field strength, pulse shape, pulse width, number of pulses, pulse specific energy, and frequency [15,20,21]. These factors can be optimized to release only the desired compounds from the cell, adjusting extraction selectivity in addition to extraction efficiency. PEF effectiveness in ambient temperatures, short extraction durations, and minimized quantities of cheap green solvents leads to reduced energy consumption, financial costs, and environmental impact, as well as reduced degradation of heat-sensitive compounds. The above-mentioned properties, in combination with its selectivity, make PEF a promising green alternative extraction technique, which could enhance the recovery of bioactive compounds from plants and food industry waste, leading to the production of new drugs precursors, bio-functional foods, and food supplements [14,22].
PEF has been applied mostly in the food industry for the reduction of microbial growth/pasteurization and for the enhanced extraction of phytochemicals from fruit and vegetable industry wastes [15,17,18,20,23,24,25,26,27]. Bobinaitė et al. (2015) [23] investigated the influence of PEF pretreatment on the recovery of bioactive compounds from by-products (press cake) of blueberry fruits (Vaccinium myrtillus L.). PEF treatments carried out at field strengths of 1, 3, and 5 kV/cm and an energy input of 10 kJ/kg led to higher amounts of total phenolics (+63%), total anthocyanins (+78%), and antioxidant activity (+65%). Pataro et al. (2019) [18] applied PEF pretreatment at different field strengths (E = 0.5–5 kV/cm) and total specific energy input (WT = 0.5–20 kJ/kg) to recover carotenoids from tomato peels. Peels pretreated with PEF at 5 kV/cm showed significantly higher total carotenoid content (47.3%) and antioxidant power (68%).
Fewer are the reports on the use of PEF as a primary extraction method for the recovery of bioactive compounds from food and vegetable industry wastes, while sporadic but promising are the very recent reports that speak for the use of PEF in the direct extraction of bioactive compounds from plants. Bozinou et al. (2019) [14] enhanced the extraction of polyphenols from the leaves of the Moringa oleifera tree by using PEF as the primary and only extraction method. PEF of 7 kV/cm proved to be effective, using water as extraction medium for a total extraction time of 40 min. Ntourtoglou et al. (2020) [28] also reached a 20% increase in the extraction rate of the alpha acids of bitter hop using a pulsed electric field of 1.15 to 2.5 kV/cm as the primary extraction method, while Tsapou et al. (2020) [29] achieved phenolic flavor enhancement in beer with a production efficiency in 4-vinylguaiacol (target compound) up to 120% (mg L−1) by applying PEF extraction of 1 kV/cm during a 15 min treatment.
To further evaluate the PEF’s potential as a green extraction method of bioactive compounds from plant material, the present study dealt with its application in the recovery of polyphenols from the plants V. vinifera, C. sativus, and S. scardica. Such an extraction process of these plants has not been previously reported (to our knowledge). Water was used as extraction medium, in order to produce edible extracts (no need for solvent evaporation) and minimize the cost and environmental impact. Among aforementioned PEF processing factors (electric field intensity, pulse shape, pulse width, number of pulses, pulse-specific energy, and frequency) electric field intensity was chosen to be varied as a preliminary effort to optimize the procedure. The content of produced extracts in total polyphenols was evaluated using the Folin–Ciocalteu method and the polyphenolic profile of the produced extracts was additionally determined using high performance liquid chromatography (HPLC).
2. Materials and Methods
2.1. Chemicals
Acetonitrile, HPLC grade, used for chromatography and formic acid (99%) was from Carlo Erba (Val de Reuil, France). Sodium carbonate anhydrous (99%) and gallic acid monohydrate were from Penta (Prague, Czech Republic). The standard reference compounds used for chromatographic characterization were from Sigma Aldrich (St. Louis, MO, USA). Folin–Ciocalteu reagent was from Panreac (Barcelona, Spain).
2.2. Plant Material
Aerial parts of S. scardica, tepals (not separated petals and sepals) of C. sativus and fruits of V. vinifera, each collected in different seasons of 2019, were air-dried in the dark, then grounded using a mixer and extracted immediately.
2.3. PEF Apparatus
The PEF system used was the static bench-scale apparatus previously reported by Bozinou et al. [14]. A PEF generator that could provide a maximum voltage of 25 kV (Leybold, LD Didactic GmbH, Huerth, Germany); a digital oscilloscope (Rigol DS1052E, Rigol Technologies, Inc, Beaverton, OR, USA) to monitor the signals of the voltage, the current frequency, and pulse waveform; a pulse generator (UPG100, ELV Elektronik AG, Leer, Germany); and a treatment chamber (Val-Electronic, Athens, Greece) were included. The electric field strength, E, was calculated as E = U/d, where U is the applied voltage and d is the distance between the two electrodes (d = 1 cm).
2.4. PEF and Non-PEF Assisted Extraction
An amount of about 1.25 g of ground material of each plant examined was mixed with 25 mL of double distilled water outside the treatment chamber for better homogenization and hydration (liquid to solid ratio 20:1 mL/g) for 10 min. Then, the mixture was added to the treatment chamber where PEF was applied for a total extraction time of 20 min. The processing factors selected to achieve PEF-assisted extraction are described in detail in Table 1. Pulse duration time was set at 10 μs in a period of 1 ms, while electric field intensity varied from 1.2 to 2.0 kV/cm. The period of the phenomena was 1 ms (frequency = 1000 Hz) and the total extraction time of 20 min results in N = 1.2 × 106 total number of pulse cycles. The total PEF treatment time (“tPulse × N”) within the 20 min interval of each extraction trial was 12 s. Produced extracts were tested for possible temperature increase immediately after the end of the extraction using an infrared thermometer (GM300, Benetech, Shenzhen Jumaoyuan Science and Technology Co., Ltd., Shenzhen, China). Significant temperature increase was not observed (Δt < 1 °C). Extraction was followed by centrifugation for 10 min at 4500× g and the supernatant was collected in a suitable Falcon tube for immediate analysis. Control samples for each plant examined were prepared in the same way as PEF samples, but without the application of PEF.

Table 1.
Electric field intensity of extractions.
2.5. Total Polyphenol Content of Extracts
The content of the extracts in total polyphenols was determined by the Folin–Ciocalteu assay, described by Lakka et al. [30]. A calibration curve using gallic acid as standard (10–80 mg L−1) was used to determine the total polyphenol concentration (CTP), and the yield in total polyphenols (YTP) was calculated as mg gallic acid equivalents (GAE) per gram of dry weight (dw) according to the following equation:
where CTP is the total polyphenol concentration of the extract (mg L−1), V is the volume of the extraction medium (L), and w is the dry weight (g) of the plant material.
2.6. High-Performance Liquid Chromatography Diode Array (HPLC-DAD)
A methodology previously described by Kaltsa et al. (2020) [31] was used. Chromatographic analyses were carried out using a Shimadzu CBM-20A liquid chromatograph (Shimadzu Europa GmbH, Duisburg, Germany), coupled to a Shimadzu SPD-M20A detector, and interfaced by Shimadzu LC solution software. The column used was a Phenomenex Luna C18(2) (100 Å, 5 μm, 4.6 × 250 mm) (Phenomenex, Inc., Torrance, CA, USA). In addition, 0.5% aqueous formic acid (A) and 0.5% formic acid in acetonitrile/water (6:4) (B) were used as eluents to perform chromatography according to the following elution program: 100% A to 60% A in 40 min; 60% A to 50% A in 10 min; and 50% A to 30% A in 10 min, which was kept constant for another 10 min. The column temperature was maintained at 40 °C, the flow rate was 1 mL min−1, and the injected volume 20 μL.
Detection was performed by scanning from 190 to 800 nm. Identification of individual polyphenols was obtained by comparing their retention times and spectra with those of known standards, while calibration curves for each standard were constructed for quantification. The results are expressed as milligram per gram of dry weight (mg g−1dw).
2.7. Statistical Analysis
The extraction procedures and all determinations were performed in triplicate. Statistical significance (at p < 0.05) of the differences between mean values was assessed by ANOVA test using SPSS (SPSS Inc., Chicago, IL, USA) software.
3. Results and Discussion
Among PEF’s processing factors that influence efficiency and selectivity, electric field intensity and pulse duration are considered to possess a key role [15,16]. The electroporation of the cell membrane depends on the electric field intensity. Higher electric intensities create more significant levels of electroporation and mass transfer of cellular substance. The required electric intensity is differentiated according to the dimension of the target cell. The pulse duration of the applied high voltage pulses is related to the magnitude of the membranes’ disturbance that affects the cells’ efficiency in disintegrating and expelling intracellular substances. Generally, short pulses (micro or milliseconds) of high electric intensity (E > 1.5 KV/cm) are believed to be the most effective way in extracting phytochemicals using PEF.
In this framework, relatively high electric field intensities from 1.2 to 2.0 kV/cm and short pulses of 10 μs (pulse duration) were selected to achieve enhanced polyphenol extraction from plants using PEF as the primary and only extraction method. In order to estimate the procedure, produced plant extracts were evaluated determining their content in total polyphenols and their polyphenolic profile (by HPLC).
3.1. Total Polyphenol Content of the Extracts
The Folin–Ciocalteu method was applied to determine the content of the produced extracts in total polyphenols. According to the results presented in Figure 1, Figure 2 and Figure 3, PEF was always more efficient as a primary extraction technique to achieve direct enhanced recovery of bioactive compounds from the examined plant material. The selection of water as extraction solvent seemed to be suitable while the intervals of 20 min as total extraction time and 10 μs as pulse duration appeared to be also adequate. Applied electric field from 1.2 to 2.0 kV/cm led to different percentages of increase for each plant examined, though, through the results it appeared that lower electric field intensities up to 1.4 kV/cm (conditions PEF 3 and PEF 4) could be more effective.
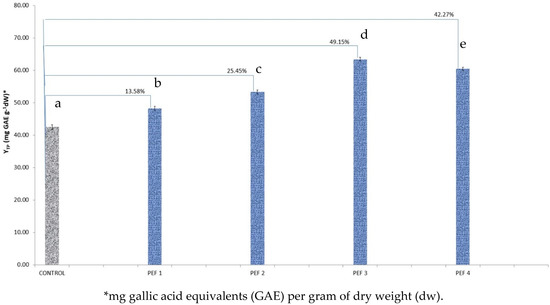
Figure 1.
Yield in total polyphenols (YTP) for Vitis vinifera fruit PEF extracts in four different PEF conditions, in comparison to control extract. The reported percentages indicate the percentage increase in the YTP of the PEF sample in reference to the control sample. Bars designated with different letters indicate statistically different values (p < 0.05).
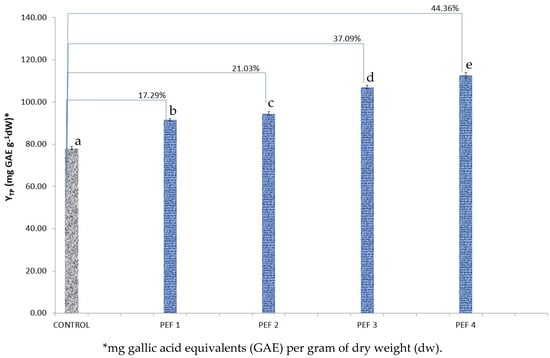
Figure 2.
Yield in total polyphenols (YTP) for Crocus sativus tepal PEF extracts in four different PEF conditions, in comparison to control extract. The reported percentages indicate the percentage increase in the YTP of the PEF sample in reference to the control sample. Bars designated with different letters indicate statistically different values (p < 0.05).
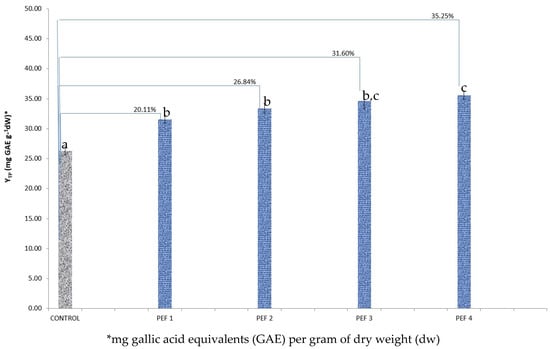
Figure 3.
Yield in total polyphenols (YTP) for Sideritis scardica aerial part PEF extracts in four different PEF conditions, in comparison to control extract. The reported percentages indicate the percentage increase in the YTP of the PEF sample in reference to the control sample. Bars designated with different letters indicate statistically different values (p < 0.05).
More specifically, regarding V. vinifera fruits, the most effective condition (in comparison to the control) was PEF 3 followed by PEF 4 (Figure 1). The two conditions led to significant (p < 0.05) increases of 49.15% and 42.27%, respectively. Conditions PEF 1 and PEF 2 of higher electric intensities brought lower but also significant changes, with condition PEF 2 reaching an increase of 25.45%. Between all electric intensities, the differences in yield of total polyphenols were significant (p < 0.05).
Similar were the results in the case of C. sativus (Figure 2). There was a significant (p < 0.05) difference in all conditions, in comparison to the control. The condition PEF 4 turned out to be the most effective (44.36% increase). Again, between all applied electric intensities, the differences in yield of total polyphenols were significant (p < 0.05).
Finally, in the case of S. scardica, the maximum increase (significant at p < 0.05) was reached with the condition PEF 4 (35.25%) in comparison to the control (Figure 3). However, for this plant there was no significant difference between PEF3 and PEF4 or PEF1 and PEF2.
3.2. Polyphenol Composition
Further to the content in total polyphenols, produced extracts were also characterized by their composition in individual polyphenols. The metabolites contained were determined both qualitatively and quantitatively using HPLC-DAD. Results concerning qualitative determination were in line with those reported in relevant literature [30,32,33,34]. More specifically in V. vinifera fruits, quercetin 3-glucoside and quercetin 3-glucuronide were identified as the predominant metabolites, followed by kaempferol 3-glucoside, gallic acid, and quercetin 3-rutinoside. Chlorogenic acid, verbascoside, 5-caffeoylquinic acid, and apigenin 7-O-glucoside were the polyphenolic compounds identified in S. scardica aerial part extracts, while analysis of C. sativus tepal extract revealed the existence of kaempferol 3-O-sophoroside-7-glucoside, quercetin 3-O-sophoroside, kaempferol 3-O-sophoroside, and kaempferol 3-O-glucoside. Polyphenols in C. sativus extracts were also tentatively identified in our previous work [30].
The content of examined plants (mg g−1dw) in each metabolite determined, as well as the percentage increase in its recovery, are described in the Table 2, Table 3 and Table 4. Chromatographic results follow phenol content determination results, indicating that electric field intensities up to 1.4 kV/cm were more effective. The optimum condition PEF 3, 1.4 kV/cm for V. vinifera fruit extracts increased the recovery of the predominant glycosides, quercetin 3-glucoside and quercetin 3-glucuronide, at a percentage of about 50%. Higher were corresponding increases for the secondary metabolites, quercetin 3-rutinoside, kaempferol 3-glucoside, and gallic acid, reaching 85%, 66%, and 63%, respectively. The results for the rest of the plants were similar. In S. scardica aerial part extracts, the optimum condition PEF 4, 1.2 kV/cm, led to a percentage increase of 54% for chlorogenic acid, 48% for verbascoside, 45% for 5-caffeoylquinic acid, and 56% for apigenin 7-O-glucoside. In C. sativus tepals, extracts treated under the same condition resulted in an increase of 25% for the main metabolite kaempferol 3-O-sophoroside. Increases for the secondary metabolites of C. sativus ranged from 52% to 64%. Significant increases (p < 0.05) were also found for the rest of the applied conditions for both plants, with condition PEF 3, 1.4 kV/cm, being more efficient compared to conditions PEF 1 and PEF 2.

Table 2.
Polyphenolic profile of Vitis vinifera (fruit) extracts.

Table 3.
Polyphenolic profile of Sideritis scardica (aerial part) extracts.

Table 4.
Polyphenolic profile of Crocus Sativus (tepal) extracts.
Values regarding the content in total polyphenols and individual metabolites, in the optimum for each plant PEF condition, are comparable with those achieved with different extraction methods, using mainly aqueous ethanol or methanol, in a percentage of 50% to 80%. It appears that possible losses in yield, related to the use of water as an extraction solvent, can be earned from the use of PEF. More precisely, in the case of V. vinifera fruits, the content in total polyphenols and the basic metabolites, quercetin 3-glucoside and quercetin 3-glucuronide, reached 63.45, 0.624, and 0.543 mg g−1dw (in optimum condition PEF 3), respectively. Jara-Palacios et al. (2014b) [35] evaluated the total phenolic content, as well as the detailed phenolic composition of white grape pomaces from nine different varieties. They reached up to 31.13, 0.557, and 0.522 mg g−1dw in total polyphenols, quercetin 3-glucoside, and quercetin 3-glucuronide, correspondingly, in 75% methanol extracts. For S. scardica aerial parts we achieved a total polyphenol content of 35.58 mg g−1dw (in the optimum condition PEF 4). Ibraliu et al. (2014) [36] reported the results of the direct phytochemical comparison of samples of S. scardica (among others) from different locations with regards to the polyphenolic total content. They reached a content in total polyphenols up to 21.9 mg g−1dw after extraction with 70% aqueous ethanol. Finally, treatment with condition PEF 4 of C. sativus tepals led to contents in kaempferol 3-O-sophoroside-7-glucoside, quercetin 3-O-sophoroside, and kaempferol 3-O-sophoroside of 3.865, 3.313, and 25.565 mg g−1dw, correspondingly. The above results are in line with Serrano-Diaz et al. (2014) [37], who gave values of 5.601, 4.011, and 30.342 mg g−1dw for kaempferol 3-O-sophoroside-7-glucoside, quercetin 3-O-sophoroside, and kaempferol 3-O-sophoroside, respectively, in water extracts of saffron floral bio-residues.
According to the results of the current work, PEF is an alternative and effective technique to be used as a primary extraction method for the direct recovery of bioactive compounds from V. vinifera, S. scardica, and C. sativus, offsetting several of the disadvantages of conventional extraction methods. Future studies could focus on the optimization of PEF processing factors and the application of them in real food and cosmetic preparations. Such an approach would certainly have a significant industrial prospect for large-scale application of PEF.
4. Conclusions
The study presented herein demonstrated the effectiveness of PEF in extracting polyphenols from the medicinal plants V. vinifera, S. scardica, and C. sativus. In order to develop a “green” extraction process and produce edible extracts, water was used as extraction solvent, while the total extraction time was minimized at 20 min. Different electric field intensities from 1.2 to 2.0 kV/cm were tested as a partial optimization of the process. PEF treatment significantly enhanced the recovery in total polyphenols for all the three plants examined. The percentage increase in recovery was important in each case of plant and PEF condition examined, though lower electric field intensities up to 1.4 kV/cm proved to be more effective. Under the optimum electric field intensities, 1.4 kV/cm for V. vinifera and 1.2 kV/cm for S. scardica and C. sativus, increases of 49.15%, 35.25%, and 44.36% in total polyphenol content, respectively, were achieved. Important were also the increases regarding the individual polyphenols of each plant. An 85% increase of quercetin 3-rutinoside for V. vinifera, a 56% increase of apigenin 7-O-glucoside for S. scardica, and a 64% increase for kaempferol 3-O-glucoside for C. sativus were obtained. The choice of water as extraction solvent also seemed to be suitable, which is particularly important as it makes the method less costly and more applicable, compared to methods employing conventional organic solvents. Produced extracts are edible and can be directly applied to food and/or cosmetics as there is no need for additional energy-demanding and time-consuming downstream steps of extract purification.
Author Contributions
Conceptualization, S.I.L. and D.P.M.; methodology, A.L.; validation, A.L. and E.B.; formal analysis, E.B.; investigation, A.L. and E.B.; resources, S.I.L.; data curation, A.L.; writing—original draft preparation, A.L.; writing—review and editing, A.L., E.B., D.P.M. and S.I.L.; supervision, S.I.L. and D.P.M.; project administration, S.I.L.; funding acquisition, S.I.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been co-financed by the European Union and Greek national funds through the Operational Program of Competitiveness, Entrepreneurship, and Innovation, under the call RESEARCH–CREATE–INNOVATE (project code: T1EDK-03762).
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khan, M.S.A.; Ahmad, I.; Chattopadhyay, D. Herbal Medicine: Current Trends and Future Prospects. In New Look to Phytomedicine, 1st ed.; Khan, M.S.A., Ahmad, I., Eds.; Academic Press: London, UK, 2019; pp. 3–13. [Google Scholar] [CrossRef]
- Atmakuri, L.R.; Dathi, S. Current Trends in Herbal Medicines. J. Pharm. Res. 2010, 3, 109–113. [Google Scholar]
- Flamini, R.; Mattivi, F.; Rosso, M.D.; Arapitsas, P.; Bavaresco, L. Advanced Knowledge of Three Important Classes of Grape Phenolics: Anthocyanins, Stilbenes and Flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef] [PubMed]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the Pharmacological Effects of Vitis vinifera (Grape) and its Bioactive Constituents: An Update. Phytother. Res. 2016, 30, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xiao, Y.Y. Grape Phytochemicals and Associated Health Benefits. Crit. Rev. Food Sci. Nutr. 2013, 53, 1202–1225. [Google Scholar] [CrossRef]
- Abu-Izneid, T.; Rauf, A.; Khalil, A.A.; Olatunde, A.; Khalid, A.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Sahab Uddin, M.; Heydari, M.; Khayrullin, M.; et al. Nutritional and health beneficial properties of saffron (Crocus sativus L): A comprehensive review. Crit. Rev. Food Sci. Nutr. 2020, 1–24. [Google Scholar] [CrossRef]
- Razak, S.I.A.; Anwar Hamzah, M.S.; Yee, F.C.; Kadir, M.R.A.; Nayan, N.H.M. A Review on Medicinal Properties of Saffron toward Major Diseases. J. Herbs Spices Med. Plants 2017, 23, 98–116. [Google Scholar] [CrossRef]
- González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Sideritis spp.: Uses, chemical composition and pharmacological activities—A review. J. Ethnopharmacol. 2011, 135, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Todorova, M.; Trendafilova, A. Sideritis scardica Griseb., an endemic species of Balkan peninsula: Traditional uses, cultivation, chemical composition, biological activity. J. Ethnopharmacol. 2014, 152, 256–265. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- George, V.C.; Dellaire, G.; Rupasinghe, H.P.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C. The Role of Phenolic Compounds in the Fight against Cancer—A Review. Anti-Cancer Agents Med. Chem. 2013, 13, 1236–1258. [Google Scholar] [CrossRef] [PubMed]
- Bozinou, E.; Karageorgou, I.; Batra, G.; Dourtoglou, V.; Lalas, S. Pulsed Electric Field Extraction and Antioxidant Activity Determination of Moringa oleifera Dry Leaves: A Comparative Study with Other Extraction Techniques. Beverages 2019, 5, 8. [Google Scholar] [CrossRef]
- Gabrić, D.; Barba, F.; Roohinejad, S.; Gharibzahedi, S.M.T.; Radojčin, M.; Putnik, P.; Bursać Kovačević, D. Pulsed electric fields as an alternative to thermal processing for preservation of nutritive and physicochemical properties of beverages: A review. J. Food Process Eng. 2018, 41. [Google Scholar] [CrossRef]
- Niu, D.; Zeng, X.A.; Ren, E.F.; Xu, F.Y.; Li, J.; Wang, M.S.; Wang, R. Review of the application of pulsed electric fields (PEF) technology for food processing in China. Food Res. Int. 2020, 137, 109715. [Google Scholar] [CrossRef] [PubMed]
- Panja, P. Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Pataro, G.; Carullo, D.; Ferrari, G. Effect of PEF Pre-Treatment and Extraction Temperature on the Recovery of Carotenoids from Tomato Wastes. Chem. Eng. Trans. 2019, 75, 139–144. [Google Scholar] [CrossRef]
- Maza, M.A.; Pereira, C.; Martínez, J.M.; Camargo, A.; Álvarez, I.; Raso, J. PEF treatments of high specific energy permit the reduction of maceration time during vinification of Caladoc and Grenache grapes. Innov. Food Sci. Emerg. Technol. 2020, 63, 102375. [Google Scholar] [CrossRef]
- Martín, B.; Tylewicz, U.; Verardo, V.; Pasini, F.; Caravaca, A.M.G.; Caboni, M.; Dalla Rosa, M. Pulsed electric field (PEF) as pre-treatment to improve the phenolic compounds recovery from brewers’ spent grains. Innov. Food Sci. Emerg. Technol. 2020, 64, 102402. [Google Scholar] [CrossRef]
- Wouters, P.C.; Alvarez, I.; Raso, J. Critical factors determining inactivation kinetics by pulsed electric field food processing. Trends Food Sci. Technol. 2001, 12, 112–121. [Google Scholar] [CrossRef]
- Kandušer, M.; Miklavčič, D. Electrotechnologies for Extraction from Food Plants and Biomaterial: An overview. In Electrotechnologies for Extraction from Food Plants and Biomaterials, 1st ed.; Vorobiev, E., Lebovka, N., Eds.; Springer: New York, NY, USA, 2008; pp. 1–37. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Pataro, G.; Lamanauskas, N.; Šatkauskas, S.; Viškelis, P.; Ferrari, G. Application of pulsed electric field in the production of juice and extraction of bioactive compounds from blueberry fruits and their by-products. J. Food Sci. Technol. 2015, 52, 5898–5905. [Google Scholar] [CrossRef]
- Hendrawan, Y.; Larasati, R.; Wibisono, Y.; Umam, C.; Sutan, S.; Hawa, L. Extraction of Phenol and Antioxidant Compounds from Kepok Banana Skin with PEF Pre-Treatment. IOP Conf. Ser. Earth Environ. Sci. 2019, 305, 012065. [Google Scholar] [CrossRef]
- Luengo, E.; Álvarez, I.; Raso, J. Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2013, 17, 79–84. [Google Scholar] [CrossRef]
- Luengo, E.; Álvarez, I.; Raso, J. Improving Carotenoid Extraction from Tomato Waste by Pulsed Electric Fields. Front. Nutr. 2014, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Pataro, G.; Carullo, D.; Falcone, M.; Ferrari, G. Recovery of lycopene from industrially derived tomato processing by-products by pulsed electric fields-assisted extraction. Innov. Food Sci. Emerg. Technol. 2020, 63, 102369. [Google Scholar] [CrossRef]
- Ntourtoglou, G.; Tsapou, E.A.; Drosou, F.; Bozinou, E.; Lalas, S.; Tataridis, P.; Dourtoglou, V. Pulsed Electric Field Extraction of α and β-Acids from Pellets of Humulus lupulus (Hop). Front. Bioeng. Biotechnol. 2020, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Tsapou, E.A.; Ntourtoglou, G.; Drosou, F.; Tataridis, P.; Dourtoglou, T.; Lalas, S.; Dourtoglou, V. In situ Creation of the Natural Phenolic Aromas of Beer: A Pulsed Electric Field Applied to Wort-Enriched Flax Seeds. Front. Bioeng. Biotechnol. 2020, 8, 1219. [Google Scholar] [CrossRef]
- Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Kaltsa, O.; Bozinou, E.; Lalas, S.; Makris, D.P. Saffron Processing Wastes as a Bioresource of High-Value Added Compounds: Development of a Green Extraction Process for Polyphenol Recovery Using a Natural Deep Eutectic Solvent. Antioxidants 2019, 8, 586. [Google Scholar] [CrossRef]
- Kaltsa, O.; Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. A Green Extraction Process for Polyphenols from Elderberry (Sambucus nigra) Flowers Using Deep Eutectic Solvent and Ultrasound-Assisted Pretreatment. Molecules 2020, 25, 921. [Google Scholar] [CrossRef] [PubMed]
- Jara-Palacios, M.J.; Hernanz, D.; González-Manzano, S.; Santos-Buelga, C.; Escudero-Gilete, M.L.; Heredia, F.J. Detailed phenolic composition of white grape by-products by RRLC/MS and measurement of the antioxidant activity. Talanta 2014, 125, 51–57. [Google Scholar] [CrossRef]
- Petreska, J.; Stefkov, G.; Kulevanova, S.; Alipieva, K.; Bankova, V.; Stefova, M. Phenolic compounds of mountain tea from the Balkans: LC/DAD/ESI/MSn profile and content. Nat. Prod. Commun. 2011, 6, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Vignolini, P.; Heimler, D.; Pinelli, P.; Ieri, F.; Sciullo, A.; Romani, A. Characterization of By-products of Saffron (Crocus Sativus L.) Production. Nat. Prod. Commun. 2008, 3, 1959–1962. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Hernanz, D.; Luisa Escudero-Gilete, M.; Heredia, F.J. Antioxidant potential of white grape pomaces: Phenolic composition and antioxidant capacity measured by spectrophotometric and cyclic voltammetry methods. Food Res. Int. 2014, 66, 150–157. [Google Scholar] [CrossRef]
- Ibraliu, A.B.; Trendafilova, A.D.; Anđelković, B.; Qazimi, B.M.; Gođevac, D.; Shengjergji, D.; Bebeci, E.; Stefkov, G.; Zdunic, G.I.; Aneva, I.; et al. Comparative Study of Balkan Sideritis Species from Albania, Bulgaria and Macedonia. Eur. J. Med. Plants 2014, 5, 328–340. [Google Scholar] [CrossRef]
- Serrano-Díaz, J.; Estevan, C.; Sogorb, M.; Carmona, M.; Alonso, G.L.; Vilanova, E. Cytotoxic effect against 3T3 fibroblasts cells of saffron floral bio-residues extracts. Food Chem. 2014, 147, 55–59. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).