1. Introduction
Fluoropolymers are high-tech materials with extraordinary properties (non-flammable, chemically stable, high dielectric strength, and operating temperatures up to 280 °C), and thus are not substitutable in numerous technical applications, for example, in the semiconductor/electronic industry, seal- and corrosion-resistant applications, or in future technologies for energy conversion like fuel cells [
1]. The worldwide consumption of fluoropolymers was about 270,000 t per year in 2015 [
2]. The most common polymer is polytetrafluoroethylene (PTFE) with 140,000 t a
−1. The complete fluoropolymer production requires about 162,000 t of tetrafluoroethylene (TFE) and 41,000 t of hexafluoropropylene (HFP) per year [
2]. In addition, partly fluorinated monomers like vinylidene fluoride (VDF) (43,000 t a
−1) and vinyl fluoride (VF) are necessary for polyvinylidene fluoride (PVDF) production, but also for different applications, such as fluorinated membranes or new binding systems in electrodes for lithium ion batteries [
3,
4].
Industrial production: In contrast to “simple” monomers such as ethylene or propylene, which are produced in a one-step process (steamcracking), the production of fluoromonomers follows an expensive, multistage chemical chlorine-synthesis, which is called the R22 route [
5,
6]. The R22 route was first reported by Downing and Benning in 1945 [
7]. This synthesis, as depicted in
Table 1 for TFE, starts with the partial chlorination of methane to trichloromethane (
Table 1, Equation (1)). In this reaction step, not only trichloromethane, but also mono- and dichloromethane are produced, which can only finally be converted into trichloromethane by a respective recycle to the reactor. The other synthesized by-product, tetrachloromethane, cannot be used in further reactions and must be incinerated and disposed of. Further, large amounts of HCl (three mol HCl per mol methane) are formed in this reaction step. In the second synthesis step (
Table 1, Equation (2)), trichloromethane and hydrogen fluoride, which is produced by the reaction of calcium fluoride and sulphuric acid, are converted to difluorochloromethane (R22) through the use of an antimony chloride catalyst. The third reaction step (
Table 1, Equation (3)) consists of the pyrolysis of R22 to difluorocarbene and HCl at temperatures between 800 °C and 900 °C [
8]. The gained difluorocarbene reacts immediately to tetrafluoroethylene (TFE) by dimerization (
Table 1, Equation (4)).
A closer look at the reaction steps in
Table 1 illustrates that current TFE production leads to high amounts of unwanted by-products and waste acids, especially hydrochloric acid (HCl) and hydrofluoric acid (HF). A drawback is also the contamination of HCl with low amounts of HF (around 1500 ppm), which makes further use or recycling of the acid impossible. In addition, current TFE production has a high energy demand for the separation, purification, recycling, or incineration of by-products. It is thus highly desirable to develop a chlorine-free and more energy efficient process to produce fluoromonomers.
Alternative reaction routes: The first invention of a chlorine-free synthesis of TFE was made in 1957 and involved an electric arc or a plasma process for the reaction of CF
4 with coal particles to TFE. However, both processes need huge amounts of energy and achieve only low yields of TFE, and thus could not compete with the conventional R22 route [
9,
10].
Another approach for the production of fluoromonomers is the pyrolysis of fluoroform (R23), which is a by-product through over fluorination of trichloromethane (Equation (2)) in the R22 route. The annual amount of this waste stream is about several millions of kg per year [
11]. Pyrolysis of CHF
3 is conducted by Hauptschein and Fainberg [
12] at temperatures of 700 to 960 °C, residence times about 0.6 s to about 0.08 s, and atmospheric pressure. They reached high conversion rates of about 80% and yields of C
2F
4 and C
3F
6 of about 58.1%. Additionally, Gelblum et al. [
11] tries to utilize CHF
3 waste streams by co-pyrolysing R23/R22 mixtures in the range of 690 to 775 °C and contact times less than 2 s. In the sole feed pyrolysis of R23 at 775 °C, Gelblum et al. measured conversion rates of CHF
3 below 1% and detected TFE as only product gas.
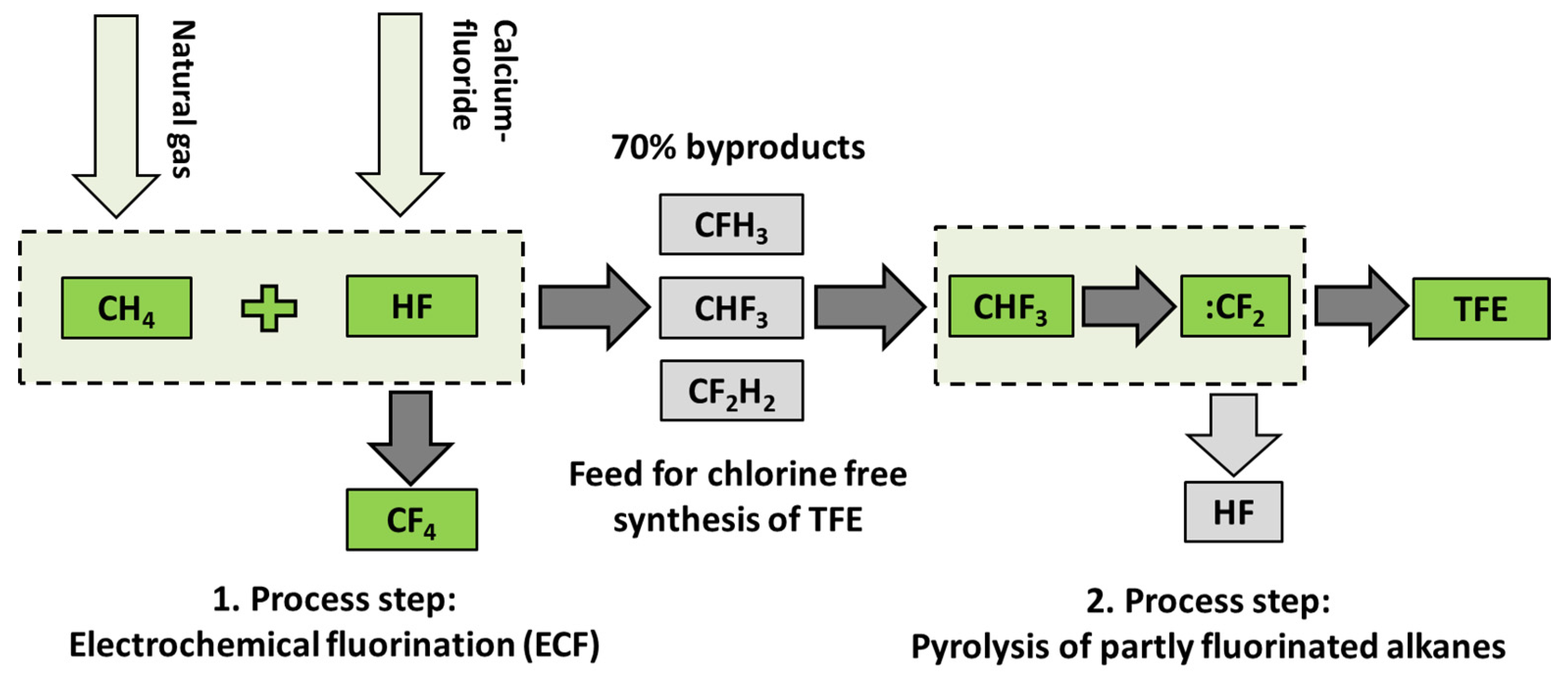
In contrast to R22, which was economically favorable earlier because of its availability as a general-purpose refrigerant, a new approach for a complete chlorine-free process is based on electro-chemical fluorination (ECF), where perfluorinated hydrocarbons are formed by reaction of anhydrous hydrofluoric acid (AHF) with the corresponding hydrocarbons. State-of-the-art ECF processes only provide partly- and perfluorinated alkanes, but no alkenes like TFE [
13]. Therefore, an additional process step is necessary. Aschauer et al. [
14] showed that high yields of TFE could be obtained in a microwave plasma, when the feed consists of perfluorinated alkanes like C
2F
6 and C
4F
10, which originated from ECF, as described by Schmeiser et al. or Nagase et al. (
Table 2) [
15,
16].
The problem of utilizing feed gases from ECF is the low selectivity to perfluorinated products obtained in this process. Depending on the hydrocarbon used as feed in the ECF, up to 70% of the product spectrum consists of only partly fluorinated alkanes (
Table 2) [
15]. Production of 1000 t perfluorinated compounds results in waste streams of about 400 t of partly fluorinated residues. Further, hydrofluorocarbon by-products, for example, CHF
3, C
2HF
5, and C
2H
2F
4, are produced, which are not allowed to be released into the atmosphere, because of their high global warming potential (GWP) (
Table 2) [
17].
Table 2.
Product distribution of electro-chemical fluorination (ECF) [
16,
18] and global warming potential (GWP) of fluorocarbons [
17].
Table 2.
Product distribution of electro-chemical fluorination (ECF) [
16,
18] and global warming potential (GWP) of fluorocarbons [
17].
| Feed | Reaction | Total Current Eff. a [%] | Product Comp. [mol%] | 100 yr GWP [kg CO2/kg] |
|---|
| CH4 | | 48.2 | CF4 | 24.1 | 6500 |
| CHF3 | 11.0 | 11,700 |
| CF2H2 | 8.3 | 650 |
| CFH3 | 56.6 | 150 |
| C2H6 | | 65.0 | C2F6 | 56.2 | 9200 |
| C2F5H | 14.2 | 2800 |
| C2F4H2 | 12.1 | 1000 |
| CF3CFH2 | 10.3 | 1300 |
| C2F3H3 | 7.2 | 300 |
Currently, all partly fluorinated compounds must be incinerated (
Table 3) under very high expenditure of energy. Another drawback of incineration is HF formation, which requires neutralization by bases like NaOH or Ca(OH)
2 to receive depositable salts (NaF, CaF
2) (
Table 3).
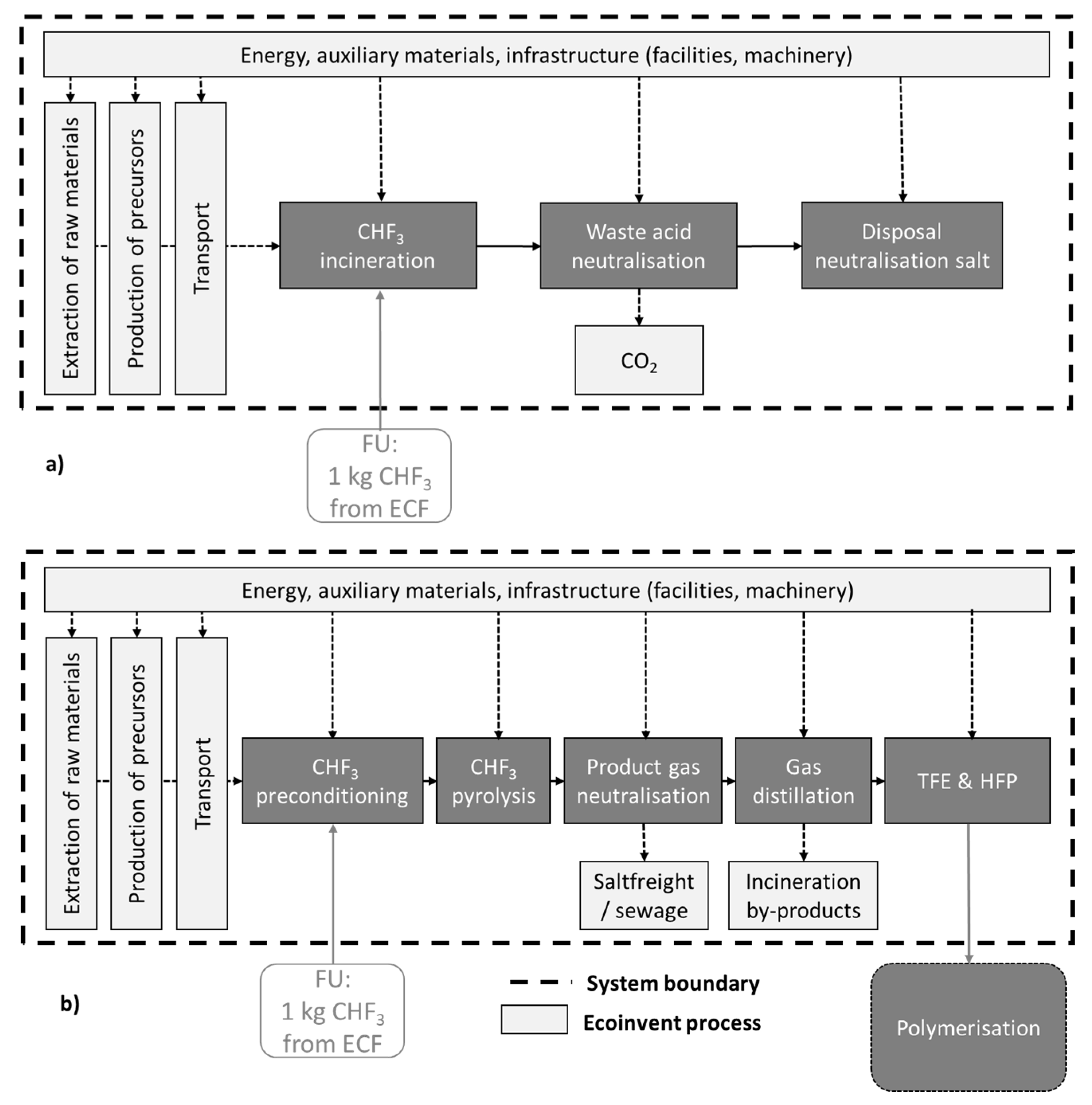
To counteract the disadvantages of thermal utilization and to utilize the complete product spectrum of the ECF, in this study, partly fluorinated alkanes were proven regarding their suitability as starting material for fluoromonomer production. The respective process route of a utilization of partly fluorinated compounds for the production of TFE and HFP is illustrated in
Figure 1. The synthesis starts with the supply of feed gas as by-products of ECF followed by pyrolysis for generation of monomers like TFE and HFP. The main advantages of this process are the utilization of cheap starting materials (currently waste streams) and the prevention of HCl waste acids.
In the field of fluorine chemistry and fluorine materials, the handling and processing of different reactions in microreactors are of growing relevance. The reasons for this trend are safer handling of hazardous reactants, a reduction in the (potential) exposure to toxic or hazardous chemical species, and higher process safety in terms of thermal runaway by efficient cooling. Moreover, the application of microreactors in fluorine containing reactions is beneficial in terms of reduced reaction volume, optimal process conditions, and thus higher yields of target products [
19,
20]. Scale-up of a microreactor process can be easily done just by numbering up [
21]. Microreactors are also advantageous with regard to kinetic studies of highly exothermic or endothermic reactions, for example, here for the highly endothermic decomposition of fluorinated hydrocarbons. Compared with lab-scale fixed bed reactors, microreactors have a much higher surface-to-volume ratio, which enables very efficient heating or cooling, and thus almost isothermal operation. Hence, the determination of kinetic parameters is not hindered by limitations of heat and mass transfer [
22].
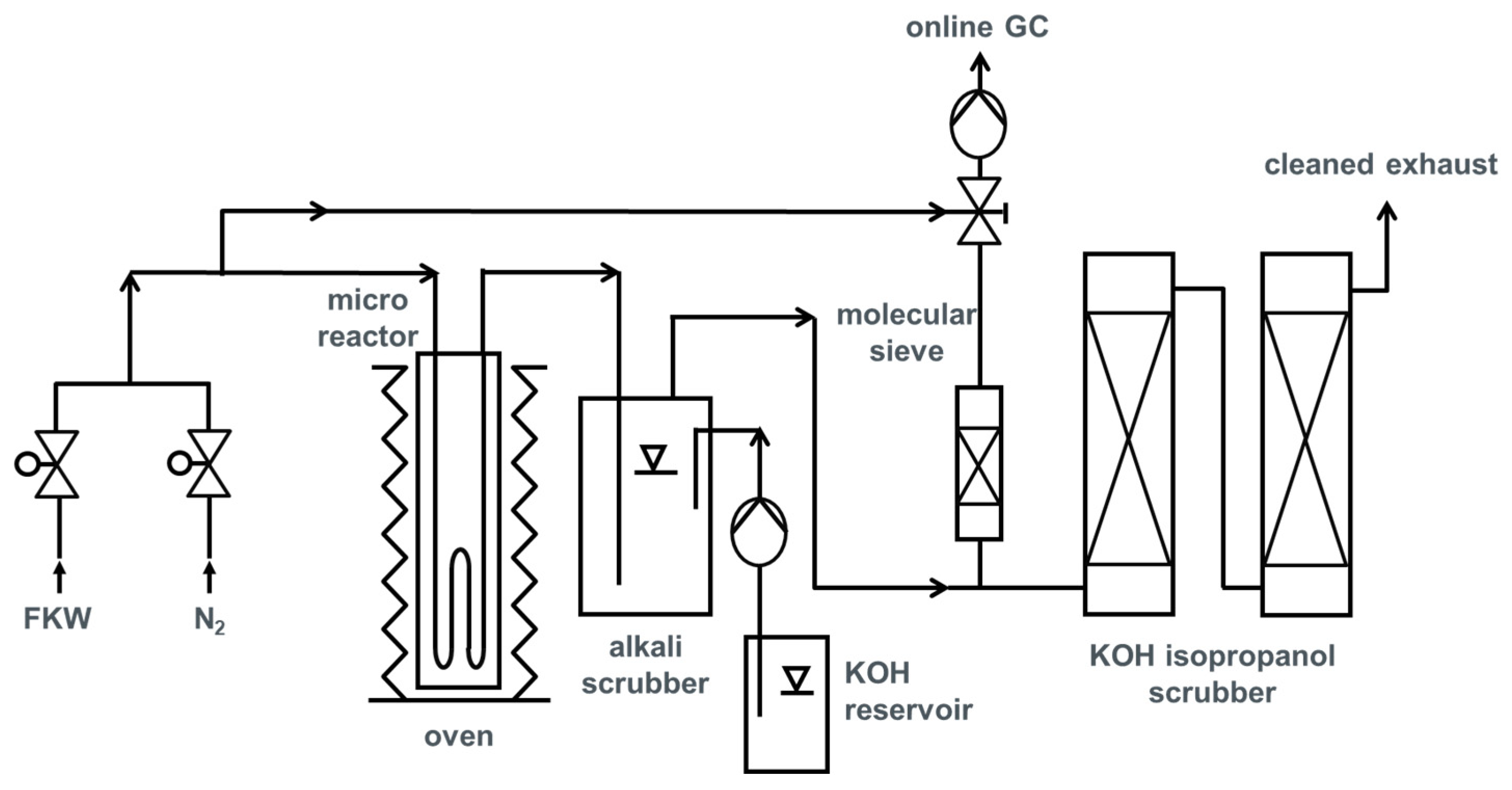
In this work, the experimental results of the pyrolysis of partly fluorinated alkane CHF3 in a high temperature microreactor are presented. On the basis of the rate of CHF3 conversion, the kinetic data of this initial reaction step of pyrolysis (dehydrofluorination of CHF3 to HF and difluorocarbene) were determined. In consecutive reactions, fluorinated products such as tetrafluoroethylene (TFE), hexafluoroproplene (HFP), and perfluoroisobutene (PFiB) are formed. The measured product distribution was compared with a simulation of the pyrolysis using kinetic data from literature for these consecutive steps. The optimum reaction conditions for monomer formation were investigated. Finally, the ecological and economical potential of the process was evaluated by a life cycle assessment (LCA).
4. Discussion
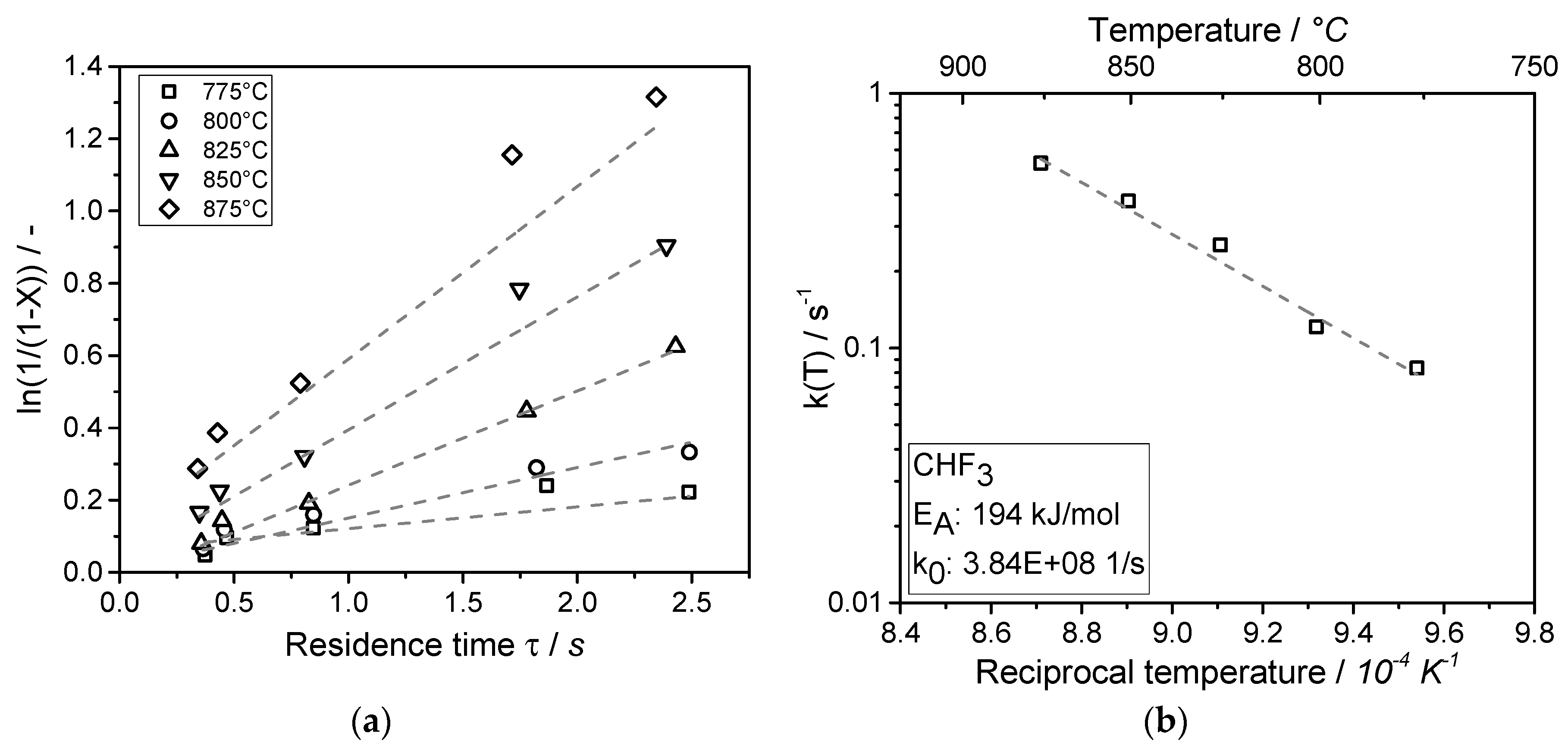
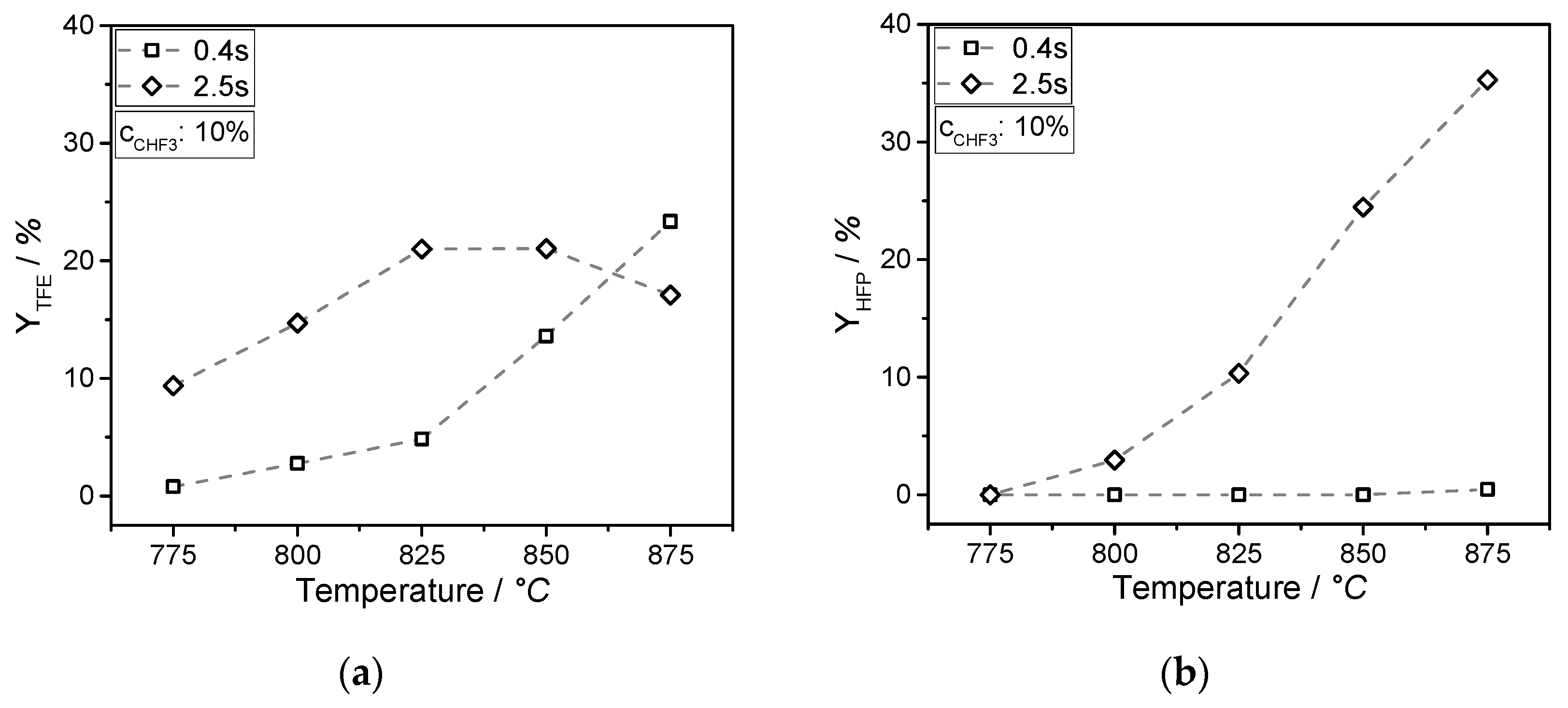
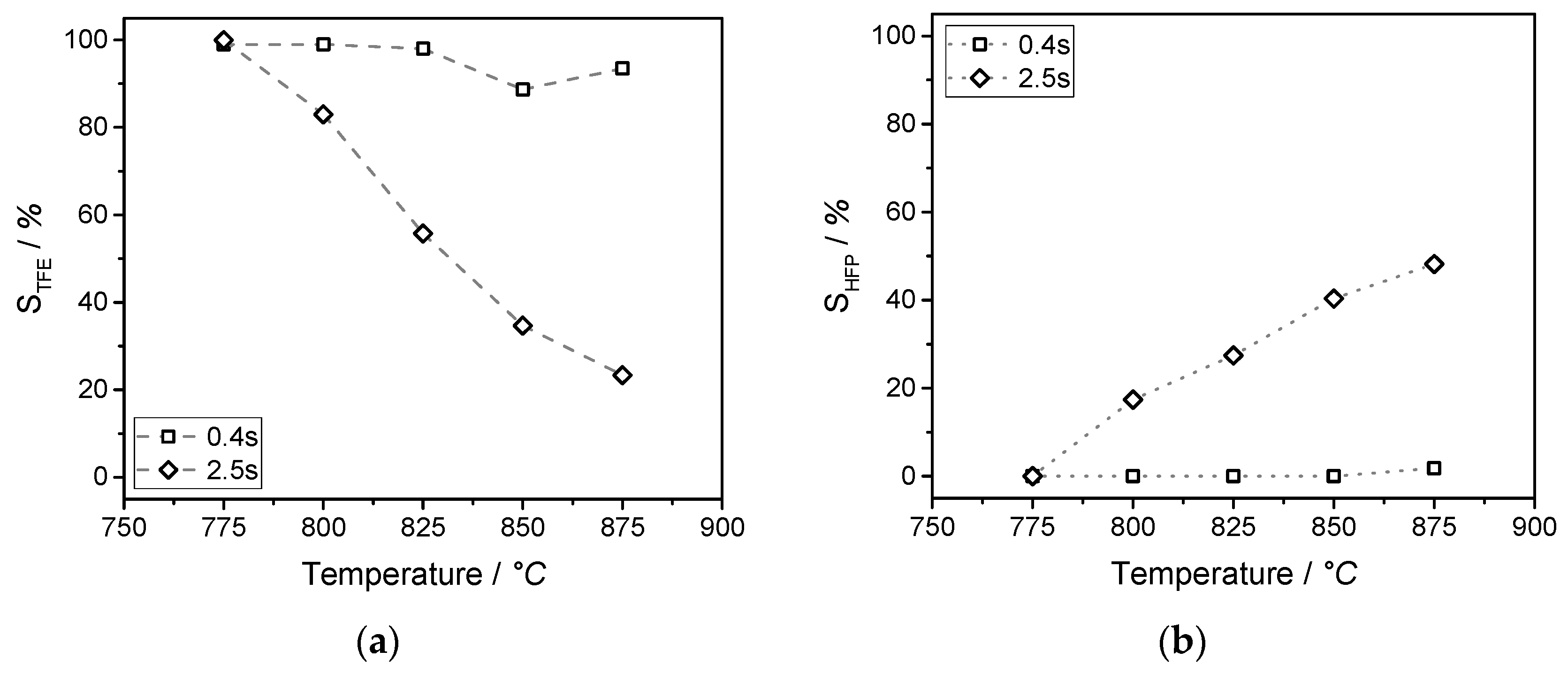
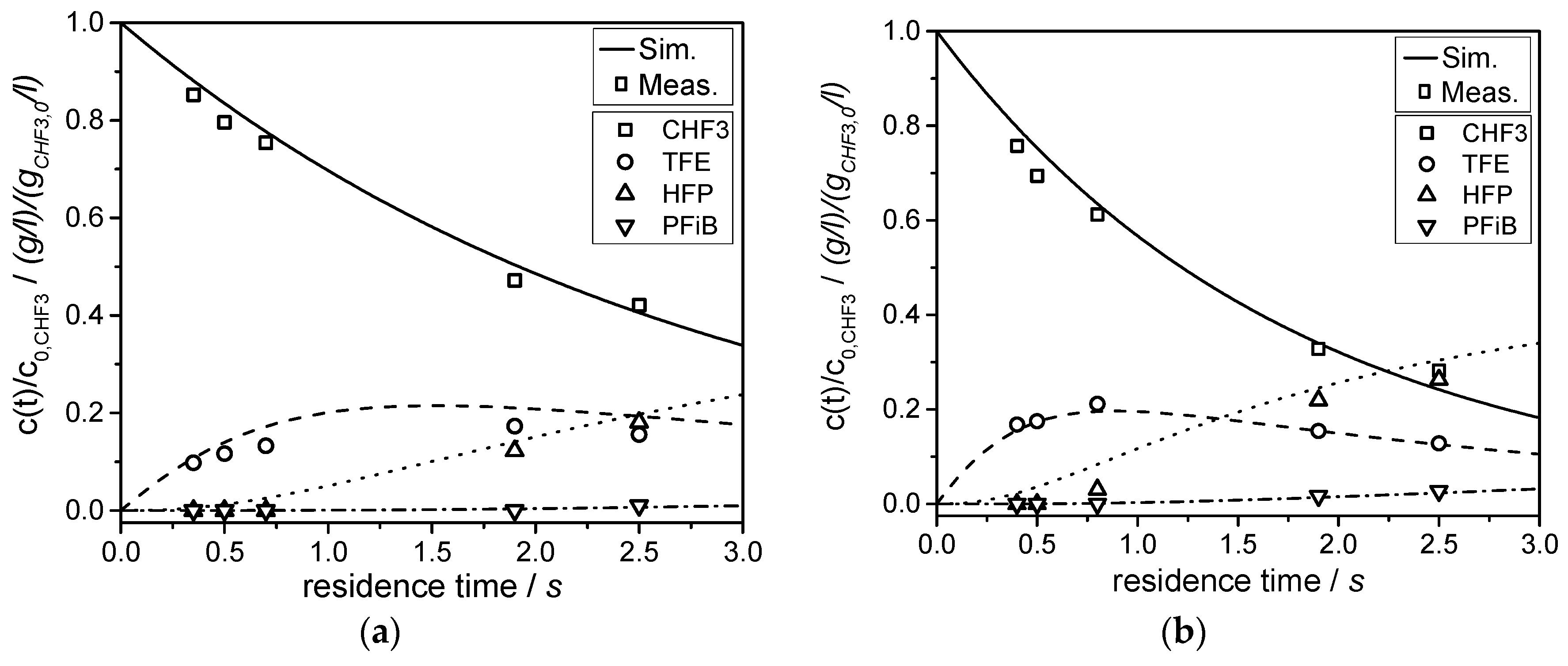
Partly fluorinated compounds (such as CHF3) originate from electrochemical fluorination. In this study, the chlorine-free synthesis of tetrafluoroethylene and hexafluoropropylene by pyrolysis of CHF3 was studied. The pyrolysis was conducted in a microreactor at atmospheric pressure in a temperature range of 775 to 875 °C. Conversion of CHF3 turned out to be a first order reaction. The initial step of the gas phase pyrolysis of CHF3 is formation of HF and CF2 (difluorocarbene), which dimerizes immediately to C2F4. With prolonged residence time, C3F6 is obtained by the reaction of TFE and CF2. The activation energy (EA) and the pre-exponential factor (k0) of the decomposition of CHF3 to HF and CF2 were determined.
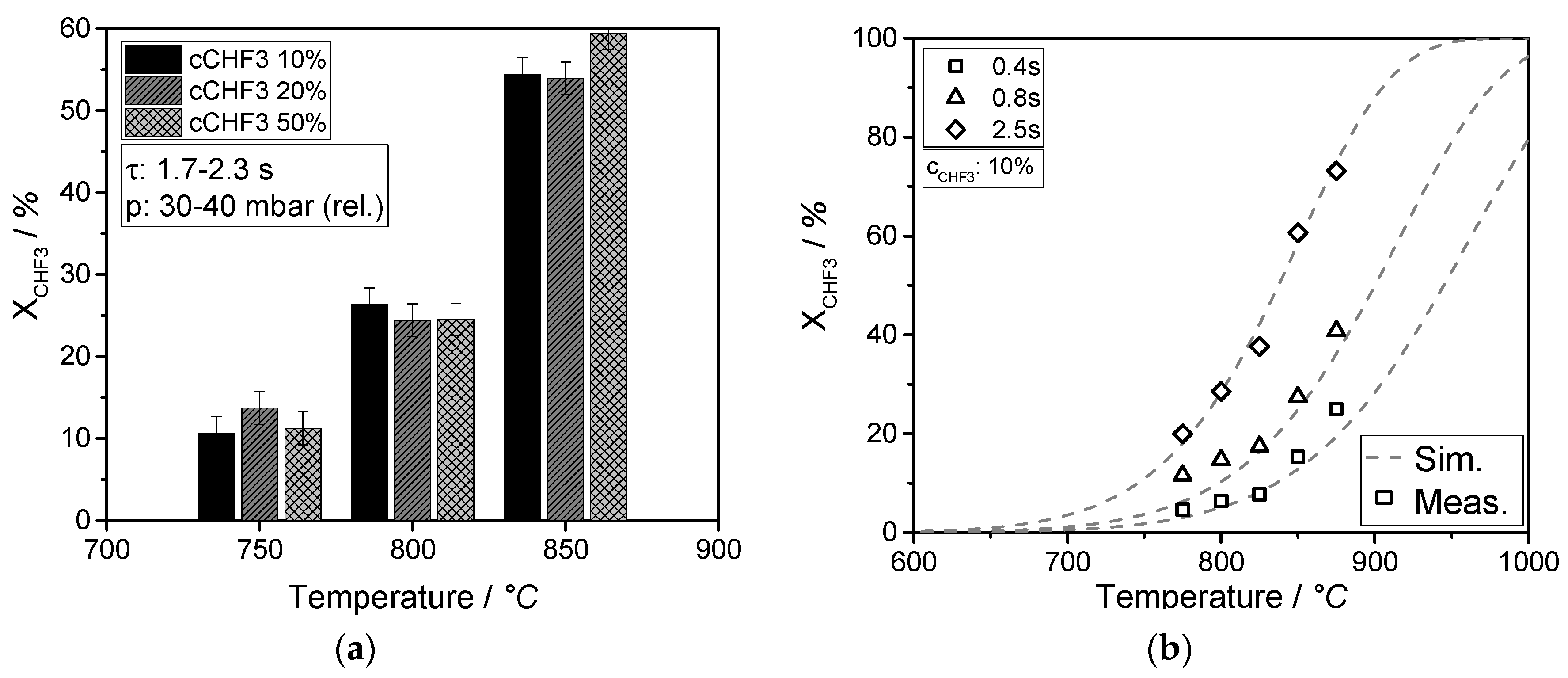
The formation of C2F4, C3F6, and i-C4F8 can be described by a reaction network consisting of six reactions. The simulation is in good agreement with the experimental data. The conversion of CHF3 reaches 73% at a temperature of 875 °C, and a yield of about 52% fluoromonomers is obtained. Short residence times and/or high temperatures, isothermal conditions, and quenching of the product gas to avoid consecutive reactions maximizes the yield of fluoromonomers. These conditions could be realized in the ceramic microreactor used in this study.
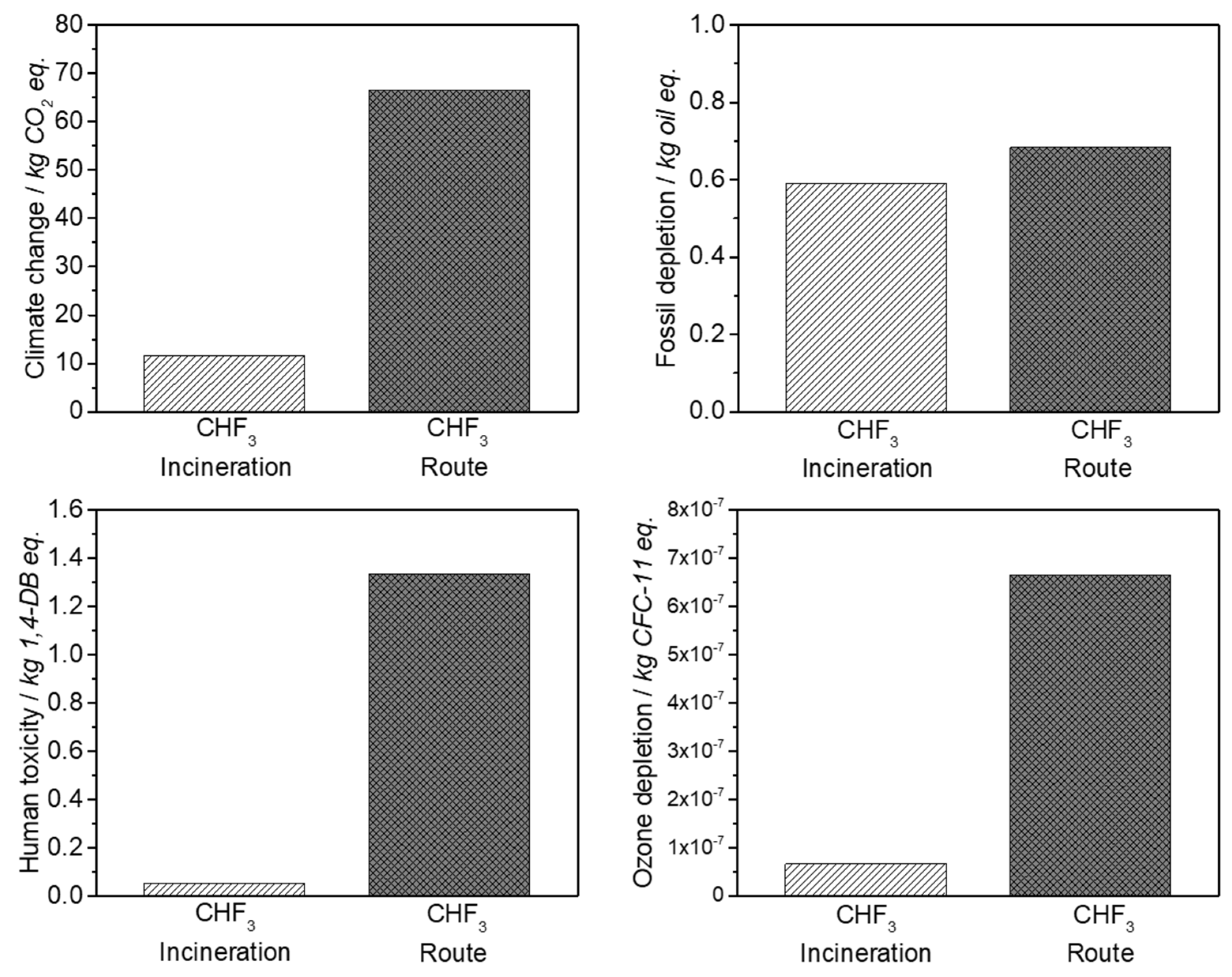
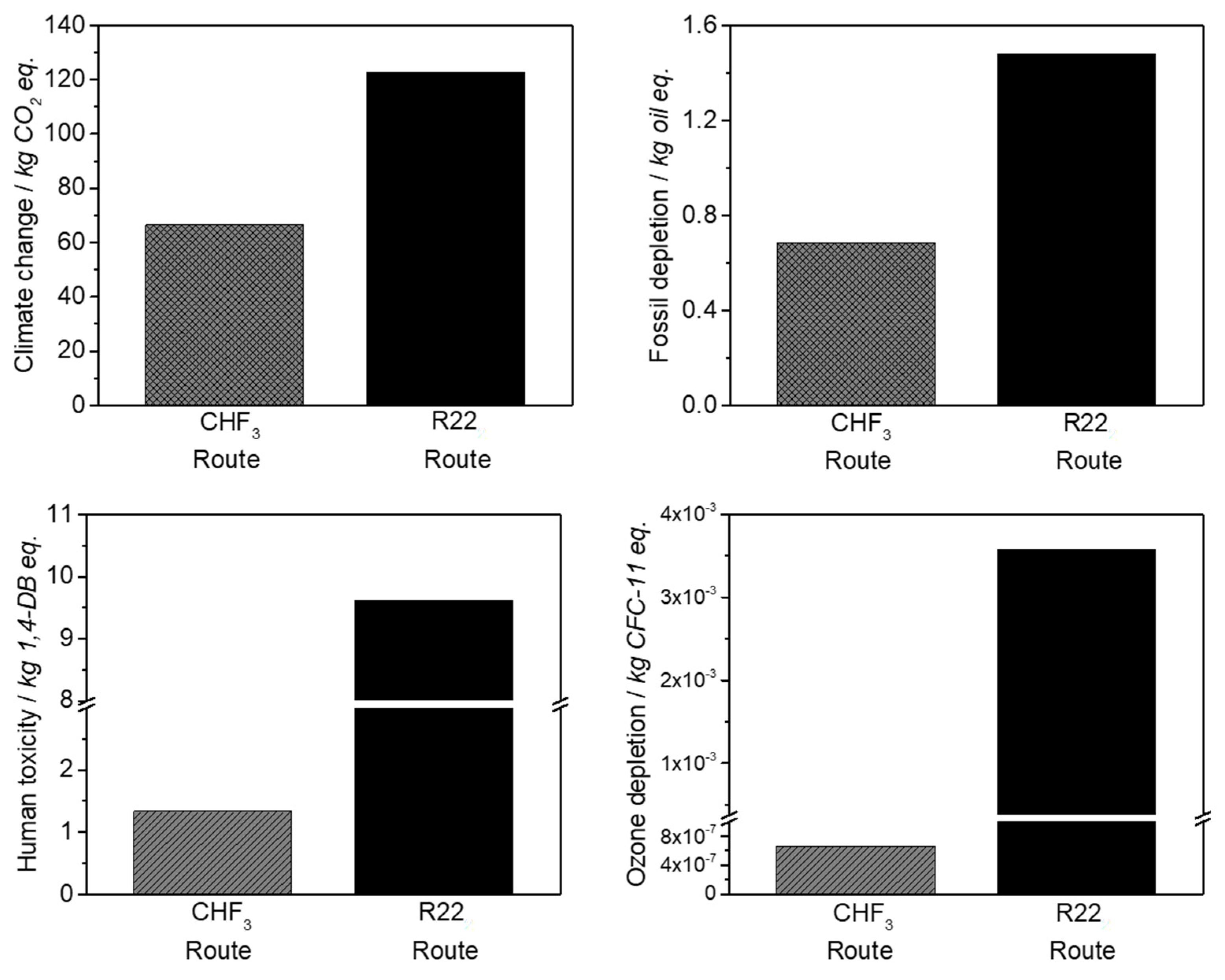
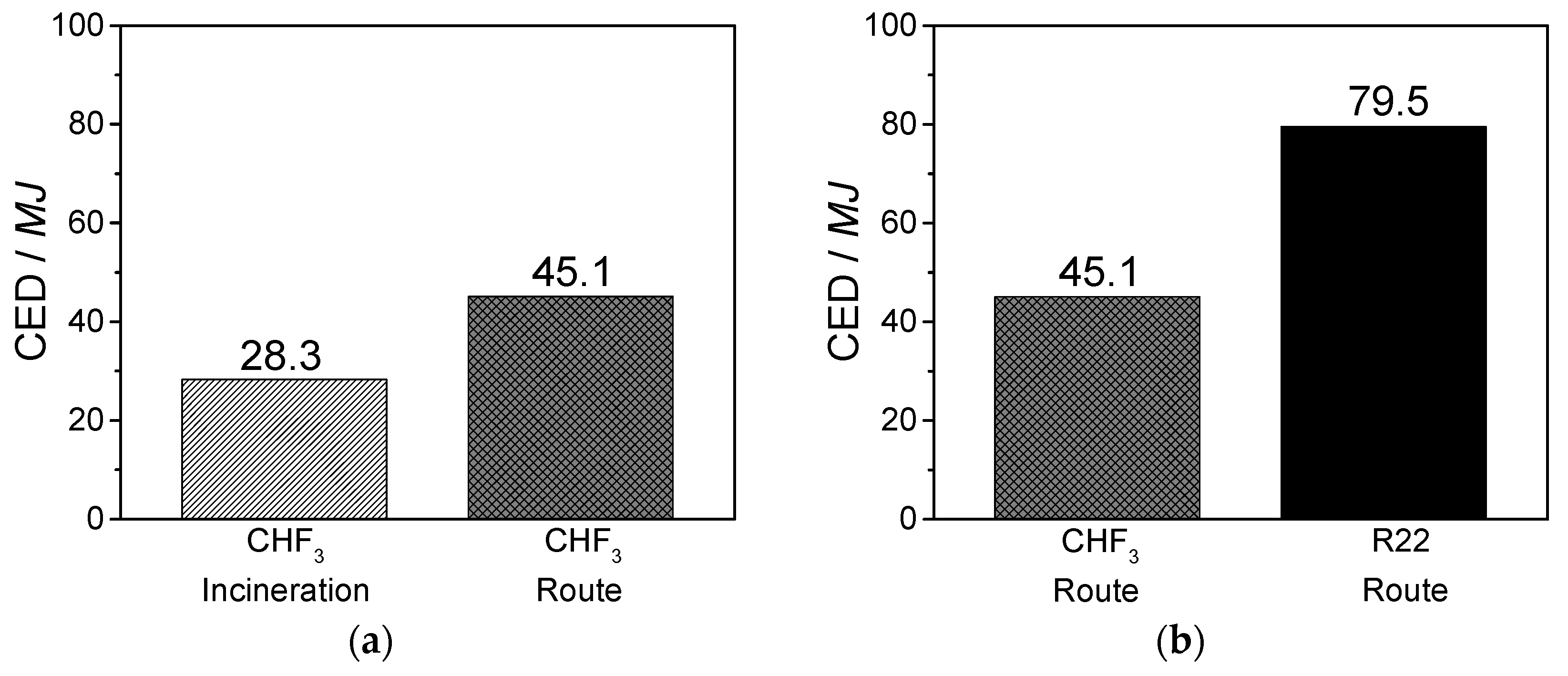
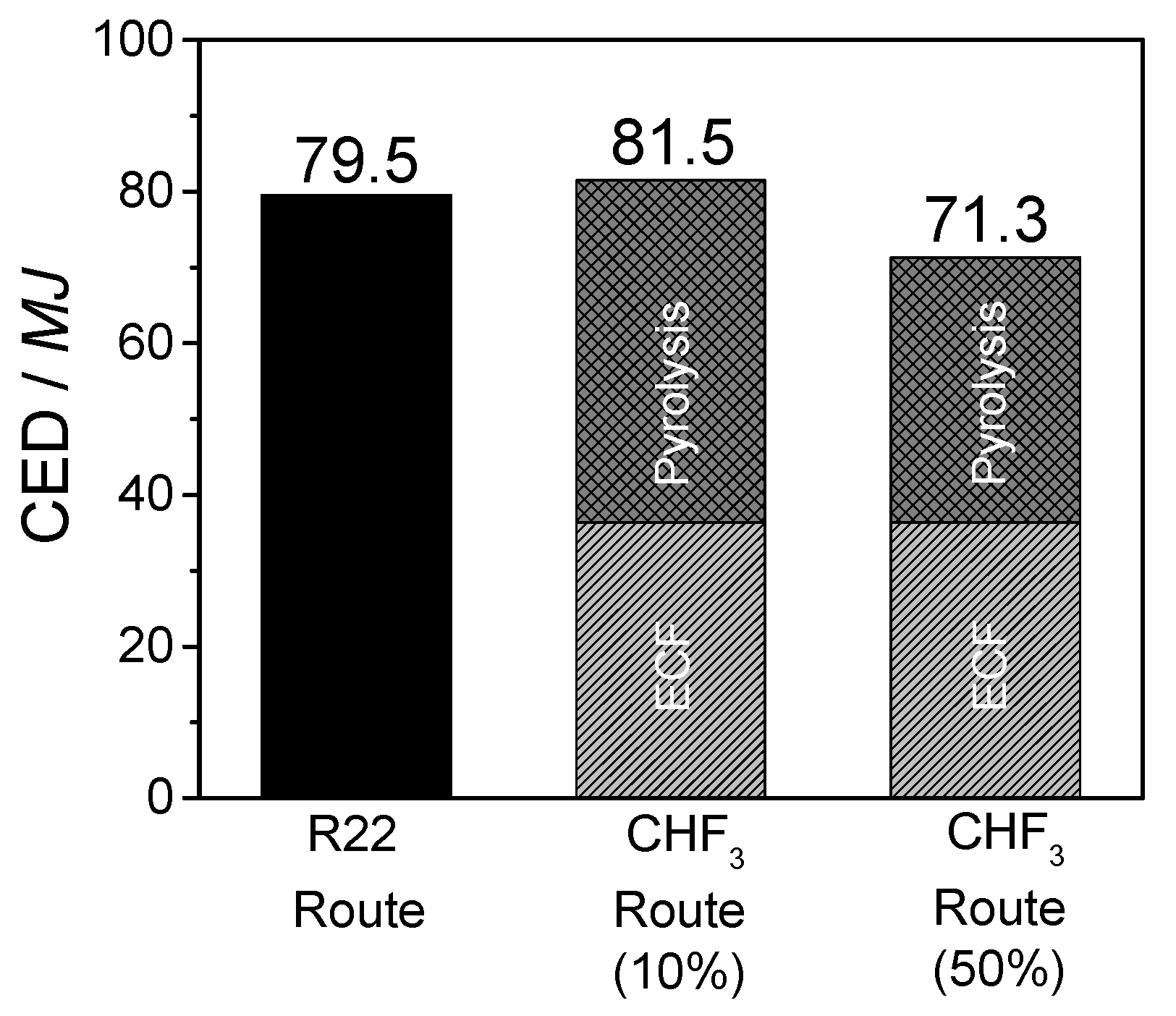
The life cycle assessment for the developed CHF3 route demonstrates the ecological and economic benefit of this process. It is shown that the material utilization of CHF3 is preferable to incineration. In contrast to the generation of high amounts of waste acids at CHF3 incineration, the material utilization of CHF3 requires a higher energy expenditure (40%), but generates high yields of the valuable products TFE and HFP. In comparison with the industrial R22 route, the developed CHF3 pyrolysis requires 40% less energy for the production of the same amount of fluoromonomers. Additionally, a reduction in terms of climate change and ozone depletion of 50% is obtained.
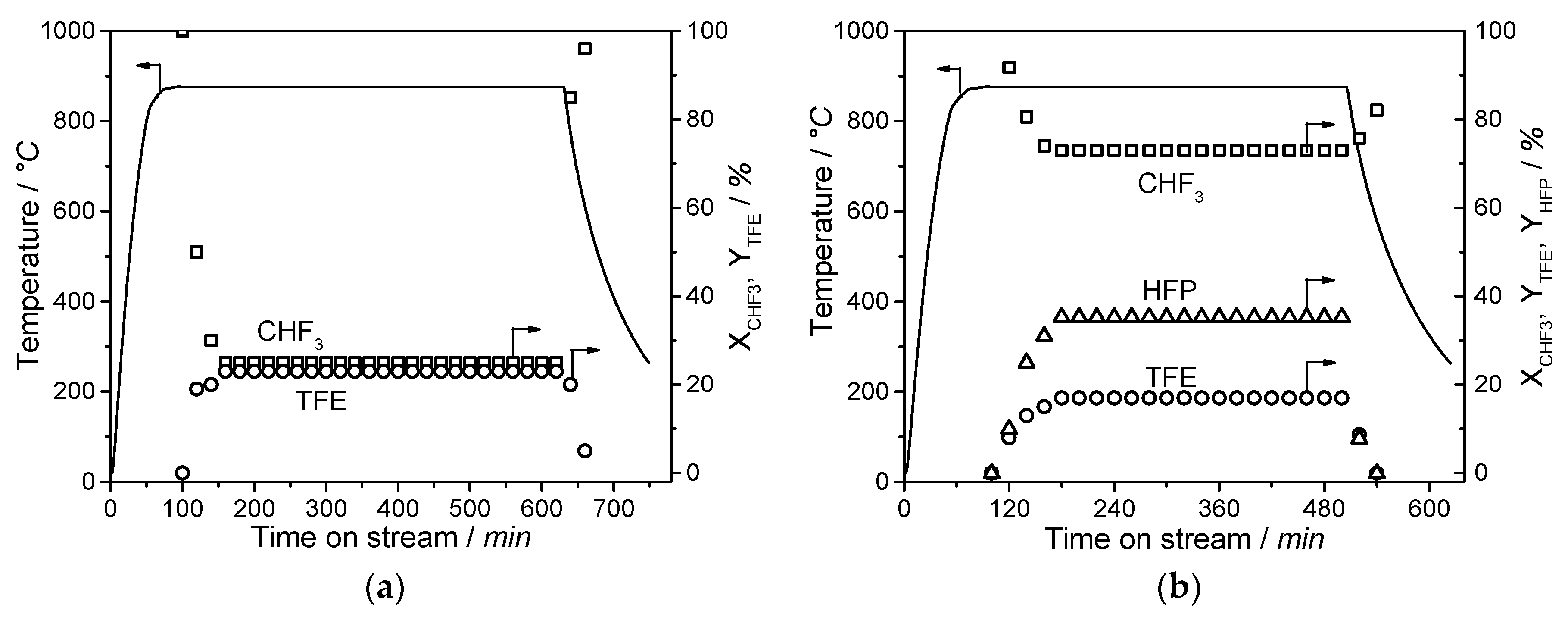
The developed process provides a sustainable synthesis of fluoromonomers with the material utilization of partly fluorinated compounds instead of their incineration. In this study, it is shown that the CHF3 pyrolysis process is stable with a time on stream of about 8 h, shows no significant corrosion on the developed microreactor, and is thus ready for an industrial application. A scale up of the process is possible by employing multiple micro reactors in parallel or by a conventional scale up inspired by the R22 route with a tube bundle reactor, which is adjusted to the higher temperatures. Beneficial for the scale up is the proven first order reaction, which simplifies the reaction engineering. With regard to the tremendous saving potential in energy consumption, undesirable by-products, disposing waste materials, and the complete substitution of chlorine chemistry, the developed process is suitable to be transferred into the industrial scale. Especially, the developed reactor enables the pyrolysis of partly and perfluorinated gases and, therefore, becomes a big step in a complete closing of the fluorine cycle.