Desulfurization of Biogas from a Closed Landfill under Acidic Conditions Deploying an Iron-Redox Biological Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
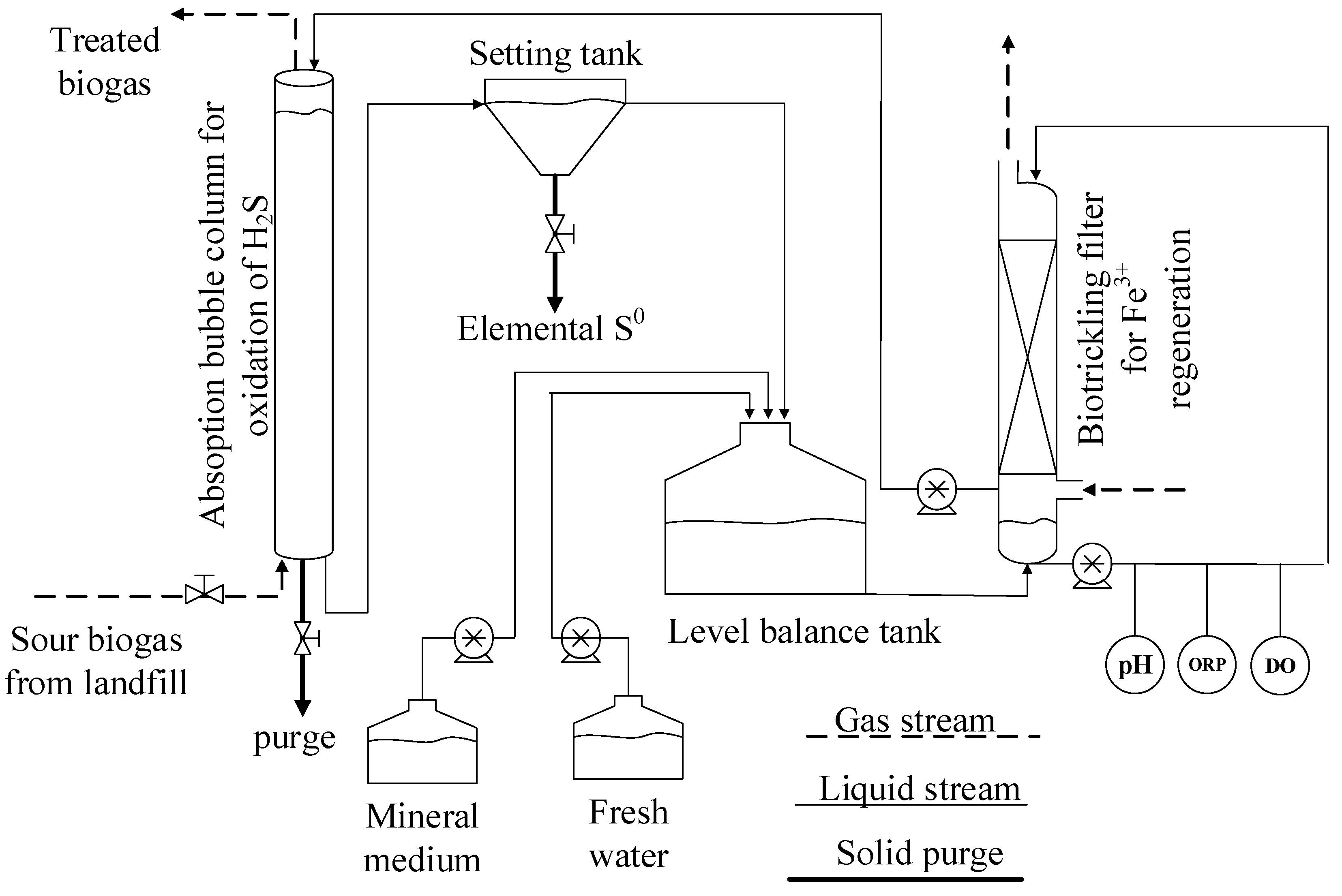
2.2. Prototype Experimental System
2.3. Analytical Methods
3. Results and Discussion
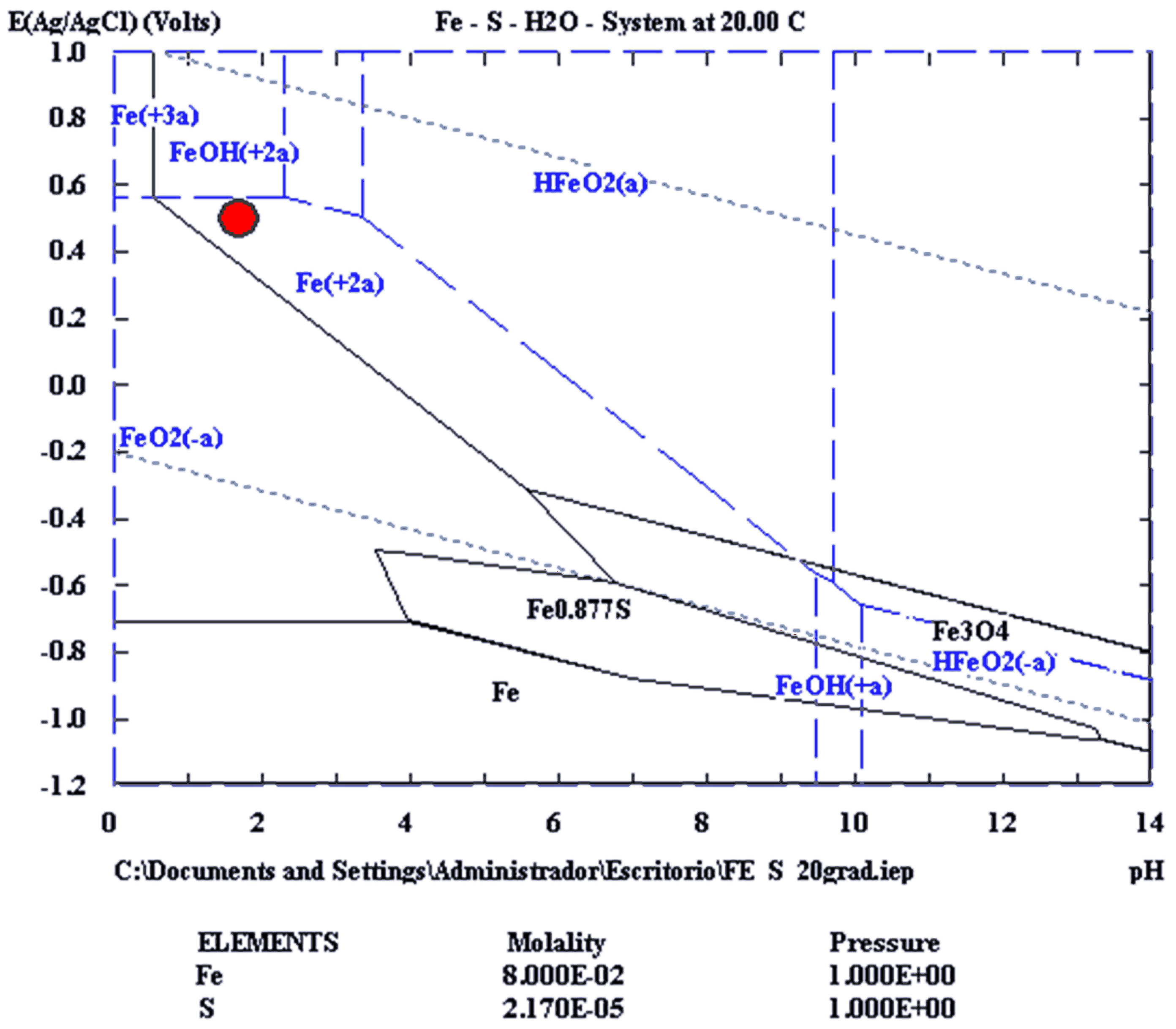
3.1. Oxidation of Ferrous Iron in the BTF
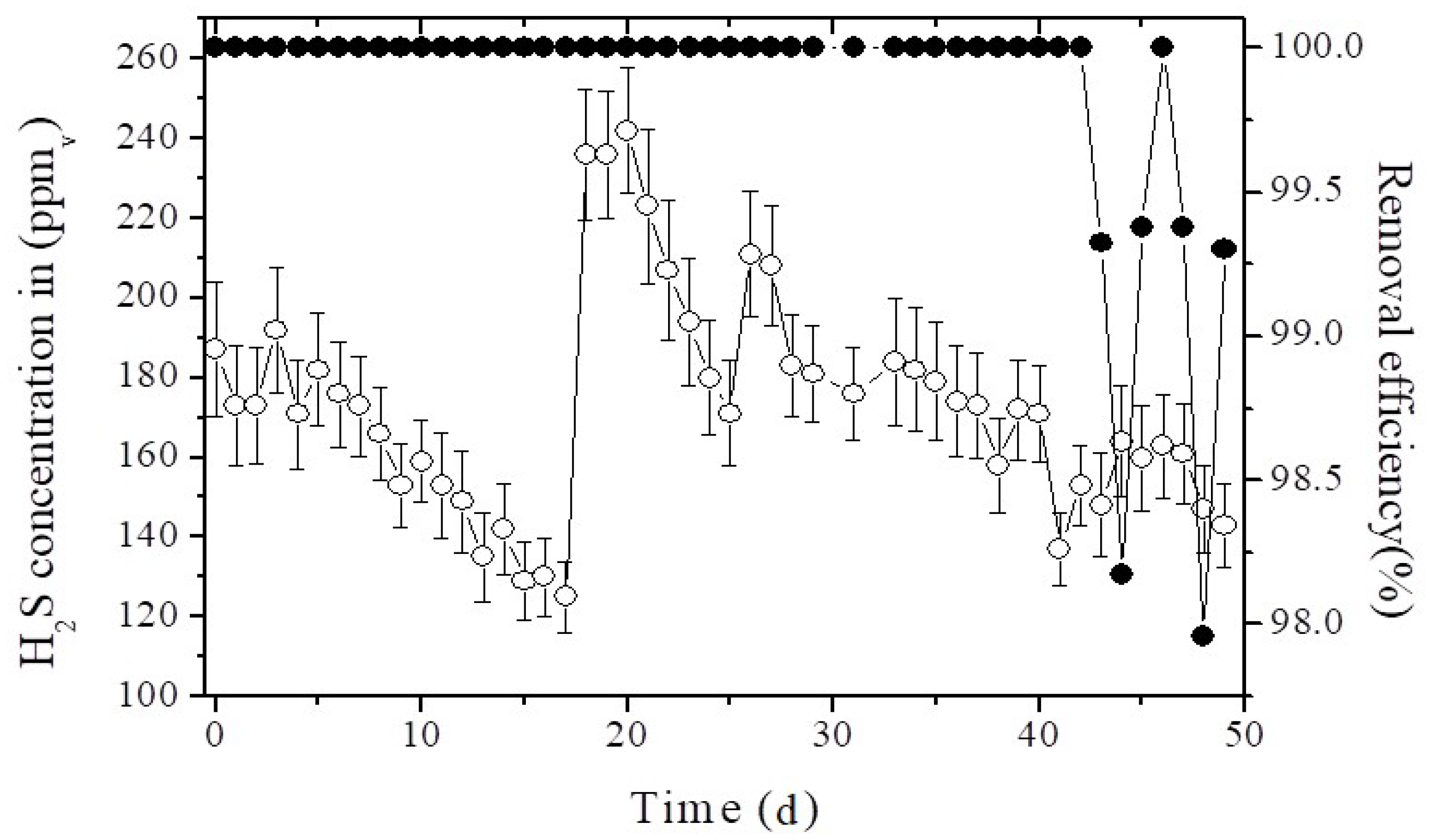
3.2. Removal of H2S in the Prototype Hybrid System under Lab Conditions
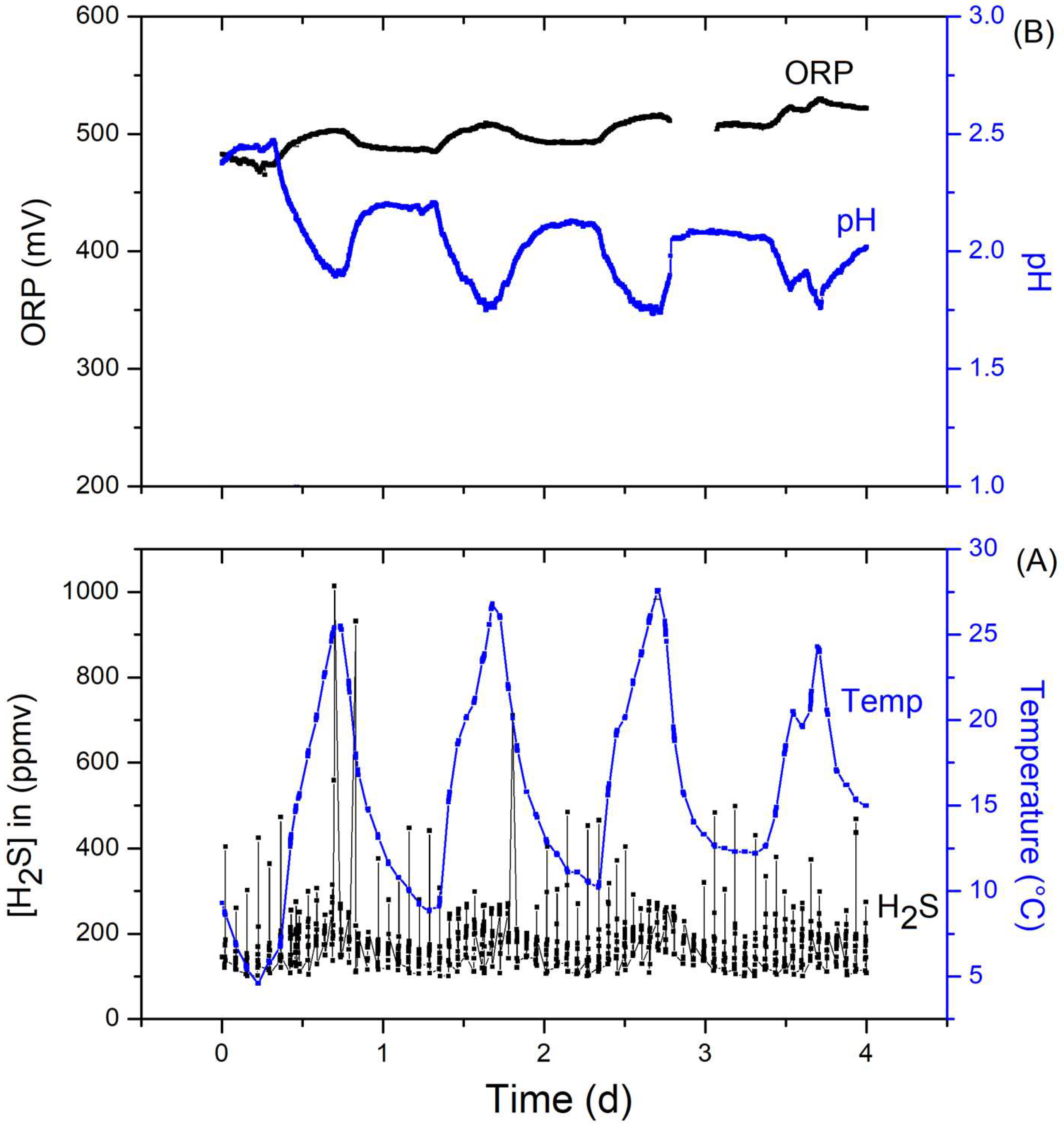
3.3. Performance of the Hybrid System at Pilot Scale (HSPS) in the Landfill
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kigozi, R.; Muzenda, E.; Aboyade, A.O. Biogas technology: Current trends, opportunities and challanges. In Proceedings of the 6th International Conference GREEN Technology, Renewable Energy & Environmental Engineering, Cape Town, South Africa, 27–28 November 2014; pp. 311–317. [Google Scholar]
- Kuhn, J.N.; Elwell, A.C.; Elsayed, N.H.; Joseph, B. Requirements, techniques, and costs for contaminant removal from landfill gas. Waste Manag. 2017, 63, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Awe, O.W.; Zhao, Y.; Nzihou, A.; Minh, D.P.; Lyczko, N. A Review of Biogas Utilisation, Purification and Upgrading Technologies. Waste Biomass Valoriz. 2017, 8, 267–283. [Google Scholar] [CrossRef] [Green Version]
- Bailon, A.L.; Hinge, J. Biogas Upgrading Evaluation of Methods for H2S Removal; Danish Technological Institute: Taastrup, Denmark, 2014. [Google Scholar]
- Latosov, E.; Loorits, M.; Maaten, B.; Volkova, A.; Soosaar, S. Corrosive effects of H2S and NH3 on natural gas piping systems manufactured of carbon steel. Energy Procedia 2017, 128, 316–323. [Google Scholar] [CrossRef]
- González-Sánchez, A.; Revah, S.; Deshusses, M.A. Alkaline biofiltration of H2S odors. Sci. Technol. 2008, 42, 7398–7404. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, A.; Bellussi, G.; Pollesel, P.; Perego, C. New method for H2S removal in acid solutions. ChemSusChem 2010, 3, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Devinny, J.S.; Deshusses, M.A.; Webster, T.S. Biofiltration for Air Pollution Control, 1st ed.; Lewis Publishers: Boca Raton, FL, USA, 2017. [Google Scholar]
- Velasco, A.; Morgan-Sagastume, J.M.; González-Sánchez, A. Evaluation of a hybrid physicochemical/biological technology to remove toxic H2S from air with elemental sulfur recovery. Chemosphere 2019, 222, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Meruane, G.; Vargas, T. Bacterial oxidation of ferrous iron by Acidithiobacillus. Hydrometallurgy 2003, 71, 149–158. [Google Scholar] [CrossRef]
- Alemzadeh, I.; Kahrizi, E.; Vossoughi, M. Bio-oxidation of ferrous ions by Acidithioobacillus ferrooxidans in a monolithic bioreactor. J. Chem. Technol. Biotechnol. 2009, 8, 504–510. [Google Scholar] [CrossRef]
- Almenglo, F.; Ramírez, M.; Gómez, J.M.; Cantero, D.; Revah, S.; González-Sánchez, A. Effect of VOCs and methane in the biological oxidation of the ferrous ion by an acidophilic consortium. Environ. Technol. 2012, 335, 531–537. [Google Scholar] [CrossRef]
- Lin, W.-C.; Chen, Y.-P.; Tseng, C.-P. Pilot-scale chemical–biological system for efficient H2S removal from biogas. Bioresour. Technol. 2013, 135, 283–291. [Google Scholar] [CrossRef]
- Abatzoglou, N.; Boivin, S. A review of biogas purification processes. Biofuels Bioprod. Bioref. 2009, 3, 42–71. [Google Scholar] [CrossRef]
- Silverman, M.P.; Lundgren, D.G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans: I. An improved medium and a harvesting procedure for securing high cell yields. J. Bacteriol. 1959, 77, 642. [Google Scholar] [PubMed]
- Villanueva-Estrada, R.E.; Rocha-Miller, R.; Arvizu-Fernández, J.L.; Castro González, A. Energy production from biogas in a closed landfill: A case study of Prados de la Montaña, Mexico City. Sustain. Energy Technol. Assess. 2019, 31, 236–244. [Google Scholar] [CrossRef]
- Vogel, A.I. A Text-Book of Quantitative Inorganic Analysis-Theory and Practice; Longmans, Green and Co.: London, UK; New York, NY, USA; Toronto, ON, Canada, 2013. [Google Scholar]
- Daoud, J.; Karamanev, D. Formation of jarosite during Fe2+ oxidation by Acidithiobacillus ferrooxidans. Miner. Eng. 2006, 19, 960–967. [Google Scholar] [CrossRef]
- Ho, K.L.; Lin, W.C.; Chung, Y.C.; Chen, Y.P.; Tseng, C.P. Elimination of high concentration hydrogen sulfide and biogas purification by chemical-biological process. Chemosphere 2013, 92, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Asai, S.; Yasuhiro, K.; Tadahiro, Y. Kinetics of absorption of hydrogen sulfide into aqueous Fe(III) solutions. AIChE J. 1990, 36, 1331–1338. [Google Scholar] [CrossRef]
- Demmink, J.F.; Beenackers, A.A.C.M. Gas desulfurization with ferric chelates of EDTA and HEDTA. Ind. Eng. Chem. Res. 1998, 37, 1444–1453. [Google Scholar] [CrossRef]
- Wubs, J.H.; Beenackers, A.A.C.M. Kinetics of the Oxidation of Ferrous Chelates of EDTA and HEDTA in Aqueous Solution. Ind. Eng. Chem. Res. 1993, 11, 2580–2594. [Google Scholar] [CrossRef]
- Asai, S.; Nakamura, H.; Aikawa, H. Absorption of hydrogen sulfide into aqueous ferric chloride solutions. J. Chem. Eng. Jpn. 1997, 30, 500–506. [Google Scholar] [CrossRef]
- De La Rosa, D.A.; Velasco, A.; Rosas, A.; Volke-Sepúlveda, T. Total gaseous mercury and volatile organic compounds measurements at five municipal solid waste disposal sites surrounding the Mexico City Metropolitan Area. Atmos. Environ. 2006, 40, 2079–2088. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, L.; Xing, W.; Chen, P.; Zhang, Y.; Wang, W. Acidithiobacillus ferrooxidans and its potential application. Extremophiles 2018, 22, 563–579. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velasco, A.; Franco-Morgado, M.; Revah, S.; Arellano-García, L.A.; Manzano-Zavala, M.; González-Sánchez, A. Desulfurization of Biogas from a Closed Landfill under Acidic Conditions Deploying an Iron-Redox Biological Process. ChemEngineering 2019, 3, 71. https://doi.org/10.3390/chemengineering3030071
Velasco A, Franco-Morgado M, Revah S, Arellano-García LA, Manzano-Zavala M, González-Sánchez A. Desulfurization of Biogas from a Closed Landfill under Acidic Conditions Deploying an Iron-Redox Biological Process. ChemEngineering. 2019; 3(3):71. https://doi.org/10.3390/chemengineering3030071
Chicago/Turabian StyleVelasco, Antonio, Mariana Franco-Morgado, Sergio Revah, Luis Alberto Arellano-García, Matías Manzano-Zavala, and Armando González-Sánchez. 2019. "Desulfurization of Biogas from a Closed Landfill under Acidic Conditions Deploying an Iron-Redox Biological Process" ChemEngineering 3, no. 3: 71. https://doi.org/10.3390/chemengineering3030071





