Application of Sodium Dodecyl Sulfate/Activated Carbon onto the Preconcentration of Cadmium Ions in Solid-Phase Extraction Flow System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Apparatus
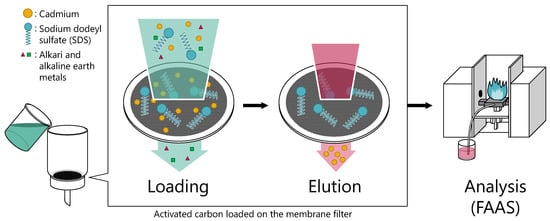
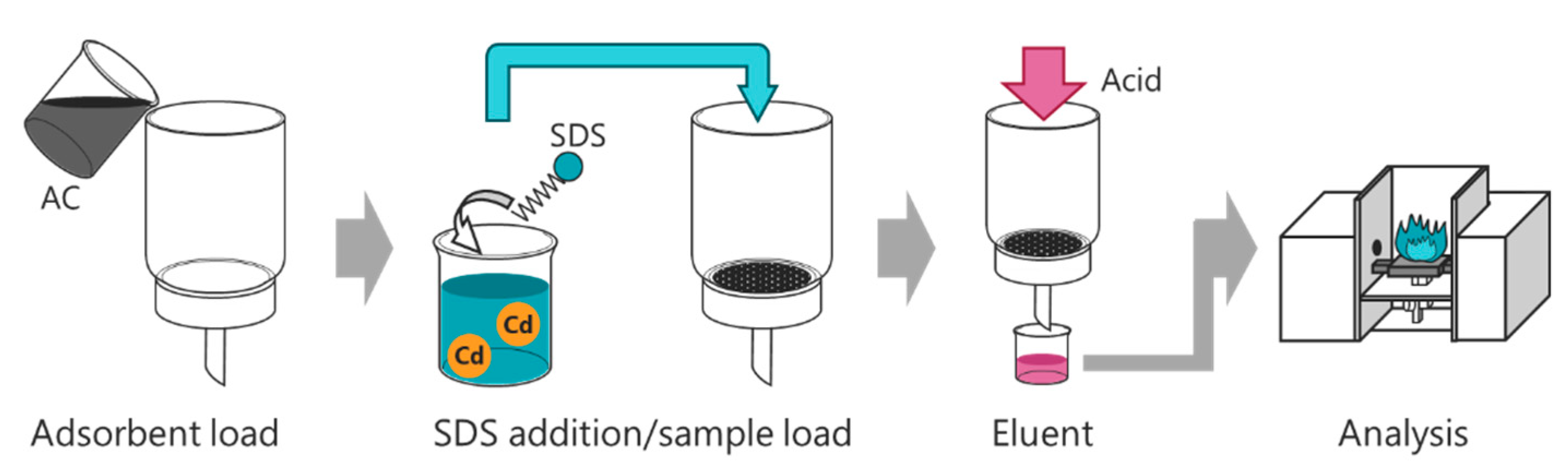
2.3. Preconcentration Procedure
3. Results
3.1. Effect of Surfactants
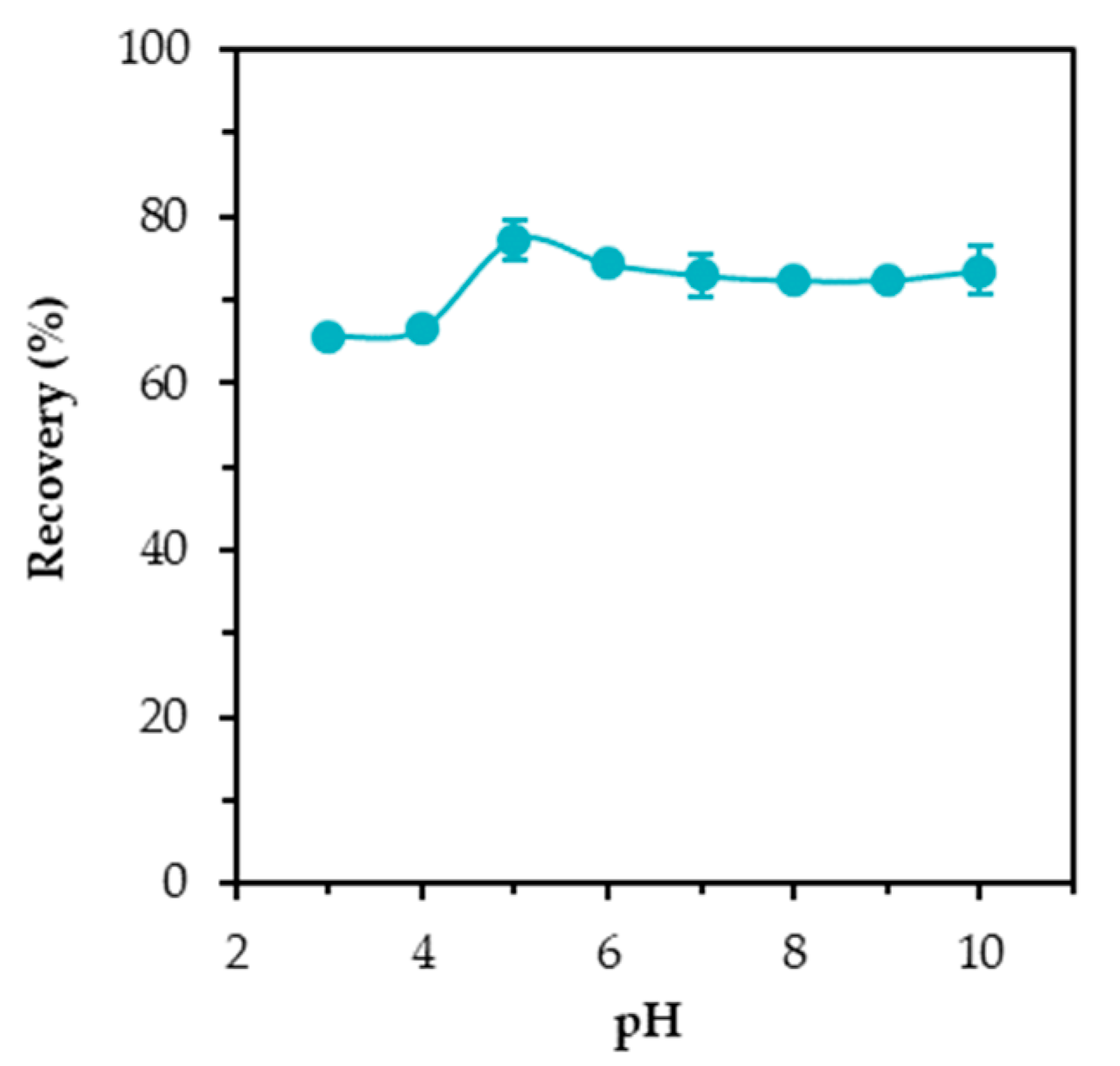
3.2. Effect of Initial PH
3.3. Optimization of Elution Conditions
3.4. Interferences
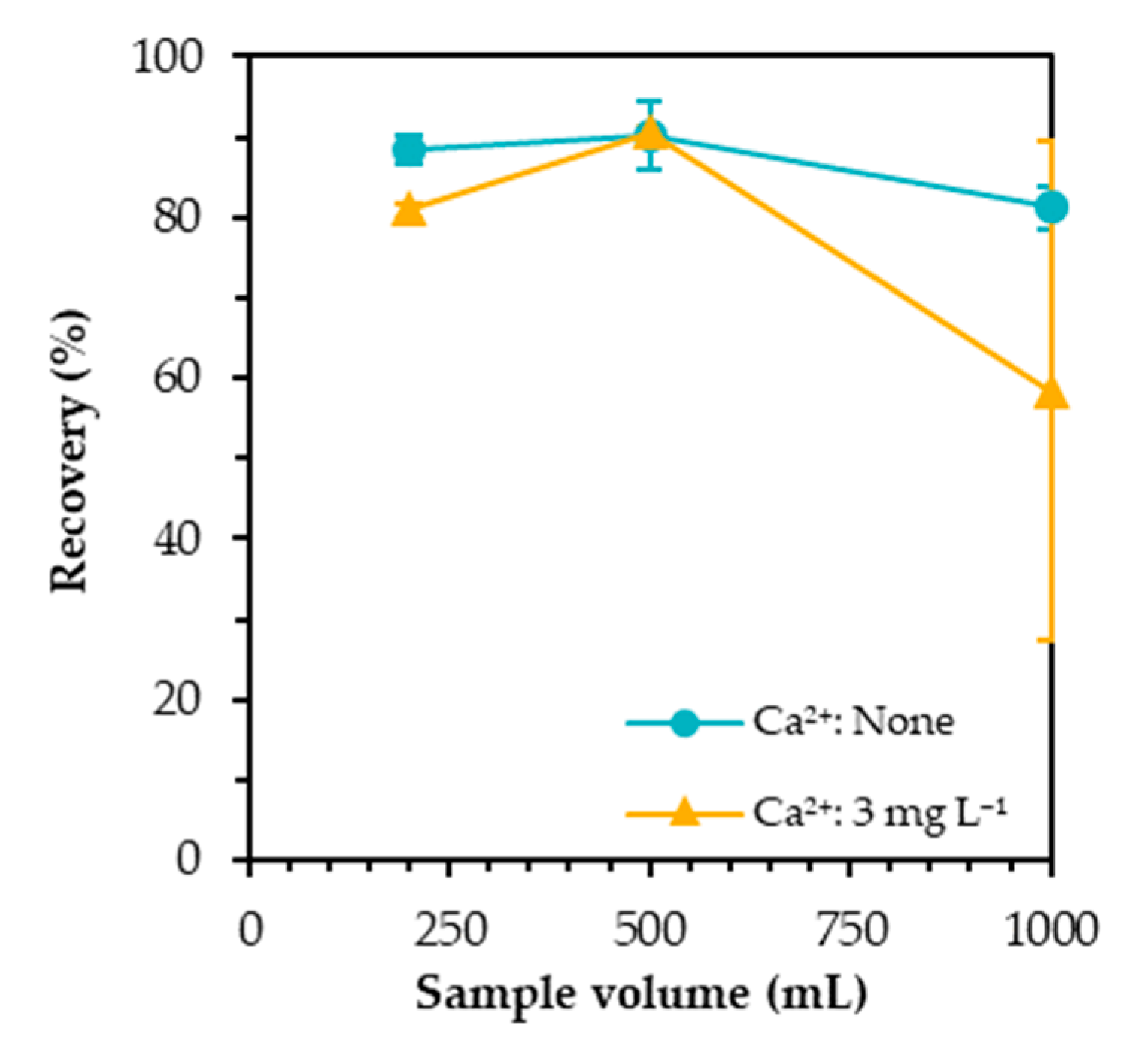
3.5. Effect of Sample Volume
3.6. Analytical Performance
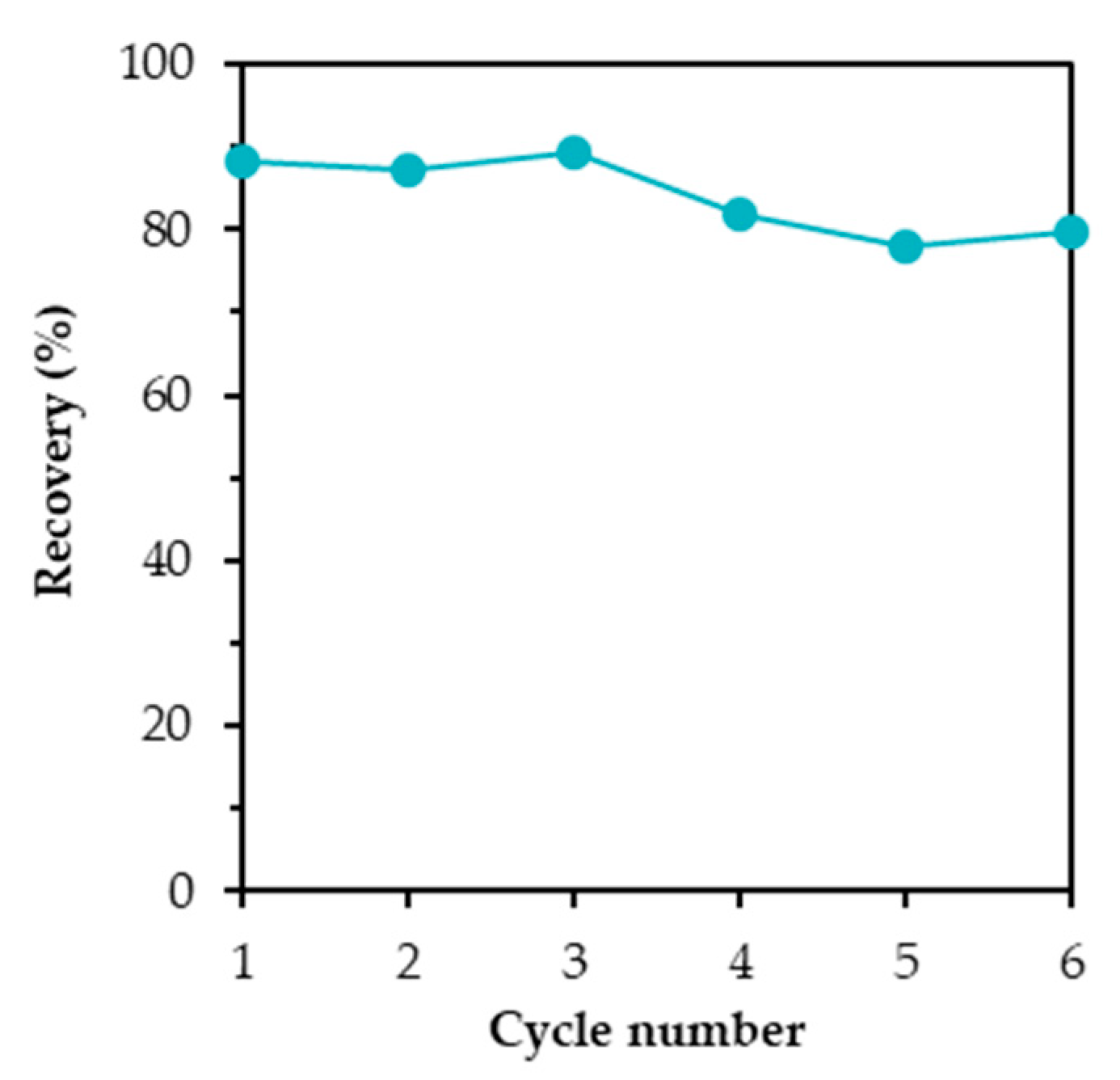
3.7. Reusability
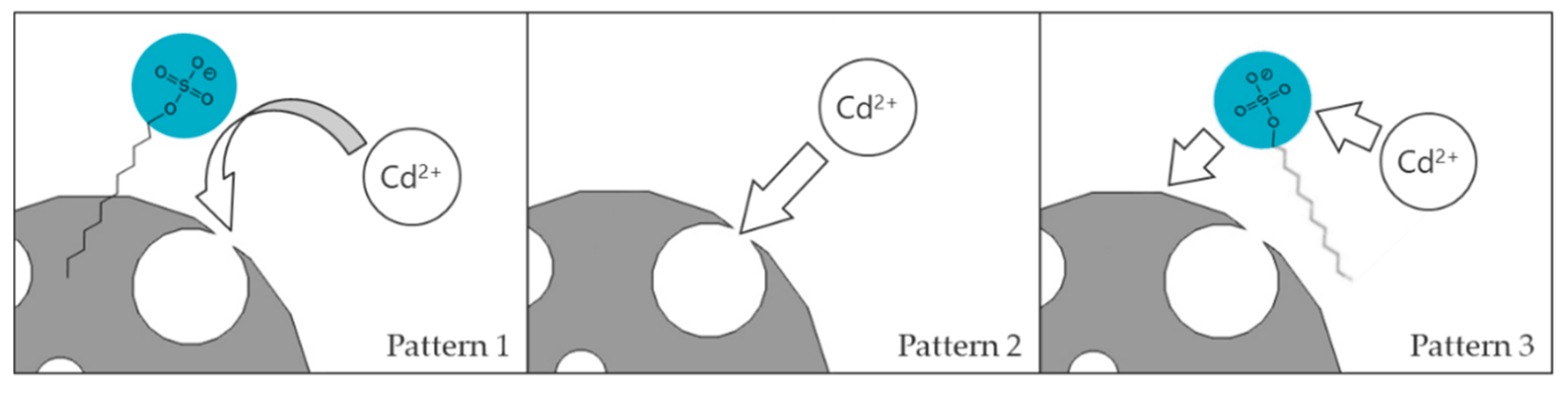
3.8. Reaction Mechanism
3.9. Analysis of Environmental Samples
3.10. Comparative Analysis
4. Conclusions
Author Contributions
Funding
Notes
Conflicts of Interest
References
- Satarug, S.; Ujjin, P.; Vanavanitkun, Y.; Nishijo, M.; Baker, J.R.; Moore, M.R. Effects of cigarette smoking and exposure to cadmium and lead on phenotypic variability of hepatic CYP2A6 and renal function biomarkers in men. Toxicology 2004, 204, 161–173. [Google Scholar] [CrossRef]
- Sasaki, Y.; Yamamoto, N.; Suzuki, Y.; Ueta, I.; Kawakubo, S. Kinetic spectrophotometric determination of trace cadmium in environmental fresh water with 5,10,15,20-tetraphenyl-21H,23H-porphinetetrasulfonic acid. Anal. Sci. 2015, 31, 551–555. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th ed. Incorporating the 1st Addendum. Available online: https://www.who.int/water_sanitation_health/publications/drinking-water-quality-guidelines-4-including-1st-addendum/en/ (accessed on 15 April 2019).
- Environmental Protection Agency (EPA). 2018 Drinking Water Standards and Health Advisories Tables. Available online: https://www.epa.gov/dwstandardsregulations/2018-drinking-water-standards-and-advisory-tables (accessed on 15 April 2019).
- Ministry of the Environment, Government of Japan. National Effluent Standards. Available online: https://www.env.go.jp/en/water/wq/nes.html (accessed on 15 April 2019).
- Ministry of the Environment, Government of Japan. Environmental Quality Standards for Groundwater Pollution. Available online: https://www.env.go.jp/en/water/gw/gwp.html (accessed on 15 April 2019).
- Thongsaw, A.; Chaiyasith, W.C.; Sananmuang, R.; Ross, G.M.; Ampiah-Bonney, R.J. Determination of cadmium in herbs by SFODME with ETAAS detection. Food Chem. 2017, 219, 453–458. [Google Scholar] [CrossRef]
- Feist, B.; Mikula, B. Preconcentration of heavy metals on activated carbon and their determination in fruits by inductively coupled plasma optical emission spectrometry. Food Chem. 2014, 147, 302–306. [Google Scholar] [CrossRef]
- Cervantes, A.; Rodríguez, R.; Ferrer, L.; Cerdá, V.; Leal, L.O. Automatic solid phase extraction of cadmium exploiting a multicommutated flow system previous ICP-MS detection: Application to tobacco samples. Microchem. J. 2017, 132, 107–111. [Google Scholar] [CrossRef]
- Gonzáles, A.P.S.; Firmino, M.A.; Nomura, C.S.; Rocha, F.R.P.; Oliveira, P.V.; Gaubeur, I. Peat as a natural solid-phase for copper preconcentration and determination in a multicommuted flow system coupled to flame atomic absorption spectrometry. Anal. Chim. Acta 2009, 636, 198–204. [Google Scholar] [CrossRef]
- Duran, A.; Tuzen, M.; Soylak, M. Preconcentration of some trace elements via using multiwalled carbon nanotubes as solid phase extraction adsorbent. J. Hazard. Mater. 2009, 169, 466–471. [Google Scholar] [CrossRef]
- Marahel, F.; Ghaedi, M.; Montazerozohori, M.; Biyareh, M.N.; Kokhdan, S.N.; Soylak, M. Solid-phase extraction and determination of trace amount of some metal ions on Duolite XAD 761 modified with a new Schiff base as chelating agent in some food samples. Food Chem. Toxicol. 2011, 49, 208–214. [Google Scholar] [CrossRef]
- Ngeontae, W.; Aeungmaitrepirom, W.; Tuntulani, T.; Imyim, A. Highly selective preconcentration of Cu (II) from seawater and water samples using amidoamidoxime silica. Talanta 2009, 78, 1004–1010. [Google Scholar] [CrossRef]
- Swain, S.S.; Nayak, B.; Devi, N.; Das, S.; Swain, N. Liquid–liquid extraction of cadmium(II) from sulfate medium using phosphonium and ammonium based ionic liquids diluted in kerosene. Hydrometallurgy 2016, 162, 63–70. [Google Scholar] [CrossRef]
- Mohammadi, S.Z.; Shamspur, T.; Baghelani, Y.M. Determination of copper, nickel, manganese and cadmium ions in aqueous samples by flame atomic absorption spectrometry after simultaneous coprecipitation with Co(OH)2. Arab. J. Chem. 2014. [Google Scholar] [CrossRef]
- Rezende, H.C.; Nascentes, C.C.; Coelho, M.M. Cloud point extraction for determination of cadmium in soft drinks by thermospray flame furnace atomic absorption spectrometry. Microchem. J. 2011, 97, 118–121. [Google Scholar] [CrossRef]
- Parham, H.; Pourreza, N.; Rahbar, N. Solid phase extraction of lead and cadmium using solid sulfur as a new metal extractor prior to determination by flame atomic absorption spectrometry. J. Hazard. Mater. 2009, 163, 588–592. [Google Scholar] [CrossRef]
- Ngeontae, W.; Aeungmaitrepirom, W.; Tuntulani, T. Chemically modified silica gel with aminothioamidoanthraquinone for solid phase extraction and preconcentration of Pb (II), Cu (II), Ni (II), Co (II) and Cd (II). Talanta 2007, 71, 1075–1082. [Google Scholar] [CrossRef]
- Omidi, F.; Behbahani, M.; Bojdi, M.K.; Shahtaheri, S.J. Solid phase extraction and trace monitoring of cadmium ions in environmental water and food samples based on modified magnetic nanoporous silica. J. Magn. Magn. Mater. 2015, 395, 213–220. [Google Scholar] [CrossRef]
- Sanchez-polo, M.; Rivera-utrilla, J. Adsorbent-Adsorbate Interactions in the adsorption of Cd (II) and Hg (II) on ozonized activated carbons. Environ. Sci. Technol. 2002, 36, 3850–3854. [Google Scholar] [CrossRef]
- Channarong, B.; Lee, S.H.; Bade, R.; Shipin, O.V. Simultaneous removal of nickel and zinc from aqueous solution by micellar-enhanced ultrafiltration and activated carbon fiber hybrid process. Desalination 2010, 262, 221–227. [Google Scholar] [CrossRef]
- Moreno-Pirajan, J.C.; Giraldo, L.; Liliana, G. Heavy metal ions adsorption from wastewater using activated carbon from orange peel. J. Chem. 2012, 9, 926–937. [Google Scholar] [CrossRef]
- Tavakkoli, N.; Habibollahi, S.; Tehrani, S.A. Separation and preconcentration of Arsenic (III) ions from aqueous media by adsorption on MWCNTs. Arab. J. Chem. 2014. [Google Scholar] [CrossRef]
- Tsoi, Y.K.; Leung, K.S.Y. Toward the use of surface modified activated carbon in speciation: Selective preconcentration of selenite and selenate in environmental waters. J. Chromatogr. A 2011, 1218, 2160–2164. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Wu, Y.W.; Liu, J.F.; Xia, X.Q.; Wang, D.L. Ammonium pyrrolidinedithiocarbamate-modified activated carbon micro-column extraction for the determination of as (III) in water by graphite furnace atomic absorption spectrometry. Microchim. Acta 2008, 161, 137–142. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Shiraz, A.Z. On-line separation and preconcentration of lead (II) by solid-phase extraction using activated carbon loaded with xylenol orange and its determination by flame atomic absorption spectrometry. J. Hazard. Mater. 2008, 150, 554–559. [Google Scholar] [CrossRef]
- Fu, W.; Qu, F.; Yu, G.; You, J. High selectivity for sodium dodecyl sulphate by polymer nanoparticles and detection of proteins based on the polymer nanoparticles-sodium dodecyl sulphate system. Sens. Actuators B Chem. 2017, 245, 774–779. [Google Scholar] [CrossRef]
- Shawabkeh, R.A.; Abu-Nameh, E.S.M. Absorption of phenol and methylene blue by activated carbon from pecan shells. Colloid J. 2007, 69, 355–359. [Google Scholar] [CrossRef]
- Manbohi, A.; Ahmadi, S.H. In-tube magnetic solid phase microextraction of some fluoroquinolones based on the use of sodium dodecyl sulfate coated Fe3O4 nanoparticles packed tube. Anal. Chim. Acta 2015, 885, 114–121. [Google Scholar] [CrossRef]
- Marahel, F.; Ghaedi, M.; Shokrollahi, A.; Montazerozohori, M.; Davoodi, S. Sodium dodecyl sulfate coated poly (vinyl) chloride: An alternative support for solid phase extraction of some transition and heavy metals. Chemosphere 2009, 74, 583–589. [Google Scholar] [CrossRef]
- Ahn, C.K.; Kim, Y.M.; Woo, S.H.; Park, J.M. Removal of cadmium using acid-treated activated carbon in the presence of nonionic and/or anionic surfactants. Hydrometallurgy 2009, 99, 209–213. [Google Scholar] [CrossRef]
- Hou, G.; Li, Y.; Cheng, Y. Activated carbon modified with sodium dodecyl sulfonate as a solid phase sorbent for the separation and preconcentration of trace cadmium in water samples with detection by electrothermal atomic absorption spectrometry. Anal. Lett. 2013, 46, 1978–1990. [Google Scholar] [CrossRef]
- Furukawa, M.; Katsumata, H.; Suzuki, T.; Kaneco, S. Determination of cadmium in environmental samples by flame atomic absorption spectrometry with preconcentration using sodium dodecyl sulfate/activated carbon. Bunseki Kagaku 2016, 65, 419–424. [Google Scholar] [CrossRef]
- Nurul, A.; Satoshi, K.; Yukitake, N.; Hideyuki, K.; Tohru, S.; Kiyohisa, O. Preconcentration of trace lead by adsorption onto a tantalum wire for electrothermal atomization atomic absorption spectrometry with a tungsten tube atomizer. Microchem. J. 2007, 86, 89–93. [Google Scholar] [CrossRef]
- Ahn, C.K.; Park, D.; Woo, S.H.; Park, J.M. Removal of cationic heavy metal from aqueous solution by activated carbon impregnated with anionic surfactants. J. Hazard. Mater. 2009, 164, 1130–1136. [Google Scholar] [CrossRef]
- Choi, J.; Ide, A.; Truong, Y.B.; Kyratzis, I.L.; Caruso, R.A. High surface area mesoporous titanium–zirconium oxide nanofibrous web: A heavy metal ion adsorbent. J. Mater. Chem. A 2013, 1, 5847–5853. [Google Scholar] [CrossRef]
- Martín-González, M.A.; González-Díaz, O.; Susial, P.; Araña, J.; Herrera-Melián, J.A.; Doña-Rodríguez, J.M.; Pérez-Peña, J. Reuse of Phoenix canariensis palm frond mulch as biosorbent and as precursor of activated carbons for the adsorption of Imazalil in aqueous phase. Chem. Eng. J. 2014, 245, 348–358. [Google Scholar] [CrossRef]
- Hiroki, H. Multielement profiling analyses of biological, geochemical, and environmental samples as studied by analytical atomic spectrometry. Bull. Chem. Soc. Jpn. 1999, 72, 1163–1186. [Google Scholar] [CrossRef]
- Xiaoxing, Z.; Changle, S.; Li, Z.; Hui, L.; Binxia, C.; Libo, L.; Weimin, G. Adsorption studies of cadmium onto magnetic Fe3O4@FePO4 and its preconcentration with detection by electrothermal atomic absorption spectrometry. Talanta 2018, 181, 352–358. [Google Scholar] [CrossRef]
- Mohammadi, S.Z.; Hamidian, H.; Karimzadeh, L.; Moeinadini, Z. Tween 80 coated alumina: An alternative support for solid phase extraction of copper, nickel, cobalt and cadmium prior to flame atomic absorption spectrometric determination. Arab. J. Chem. 2016, 9, S1290–S1296. [Google Scholar] [CrossRef] [Green Version]
- Teslima, D.; Şerife, S.; Ahmet, Ü.; Şenol, K. A solid phase extraction procedure for the determination of Cd (II) and Pb (II) ions in food and water samples by flame atomic absorption spectrometry. Food Chem. 2015, 174, 591–596. [Google Scholar] [CrossRef]
- Bingshan, Z.; Man, H.; Beibei, C.; Bin, H. Novel ion imprinted magnetic mesoporous silica for selective magnetic solid phase extraction of trace Cd followed by graphite furnace atomic absorption spectrometry detection. Spectrochim. Acta B 2015, 107, 115–124. [Google Scholar] [CrossRef]

| Type | Concentration (mol·L−1) | Vol. (mL) | EF | Rec. (%) | RSD% |
|---|---|---|---|---|---|
| HNO3 | 0.1 | 6 | 26.5 | 79.5 | 5.4 |
| 0.1 | 5 | 35.3 | 88.3 | 2.9 | |
| 0.1 | 3 | 47.3 | 77.3 | 4.9 | |
| 0.1 | 2 + 1 | 58.9 | 88.4 | 1.8 | |
| 1 | 3 | 62.5 | 93.8 | 1.3 | |
| HCl | 0.1 | 3 | 51.6 | 77.5 | 3.9 |
| 1 | 3 | 52.1 | 78.1 | 3.9 | |
| HCl:HNO3 | 0.1:0.1 | 3 | 58.2 | 87.3 | 5.5 |
| Interference | Concentration (mg·L−1) | EFin the matrix/EF × 100 (%) | RSD (%) |
|---|---|---|---|
| K⁺ | 5 | 94.0 | 1.4 |
| Mg2⁺ | 3 | 81.2 | 1.4 |
| Ca2⁺ | 3 | 91.4 | 0.8 |
| Al3⁺ | 2 | 101.9 | 1.5 |
| Fe3⁺ | 1 | 102.7 | 5.7 |
| Ni2⁺ | 5 | 90.0 | 4.7 |
| Cu2⁺ | 5 | 95.8 | 1.5 |
| Zn2⁺ | 5 | 98.2 | 0.4 |
| Sample | Added (μg·L−1) | Found (μg·L−1) | Spike/Recovery (%) |
|---|---|---|---|
| Rain water 1 (Tsu, Mie) | - | N.D. | |
| 5.0 | 5.80 ± 0.02 | 116 | |
| Rain water 2 (Tsu, Mie) | - | N.D. | |
| 5.0 | 5.32 ± 0.10 | 106 | |
| Mineral water | - | N.D. | |
| 5.0 | 5.21 ± 0.09 | 104 | |
| 10.0 | 9.17 ± 0.01 | 92 |
| Adsorbent | EF | Linear Range (µg·L−1) | LOD (ng·L−1) | Detection | Ref. |
|---|---|---|---|---|---|
| Fe3O4@FePO4 | 10 | 0.05–0.5 | 21 | ETAAS | [39] |
| Tween 80 coated alumina | 9.5 | 1.0–400.0 | 200 | FAAS | [40] |
| Dowex Marathon C | 250 | 1–10 | 130 | FAAS | [41] |
| Cd(II)-II-MMS | 50 | 0.01–0.2 | 6.1 | GFAAS | [42] |
| SDS/AC | 150 | 0.1–15 | 17 | FAAS | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furukawa, M.; Tateishi, I.; Katsumata, H.; Kaneco, S. Application of Sodium Dodecyl Sulfate/Activated Carbon onto the Preconcentration of Cadmium Ions in Solid-Phase Extraction Flow System. ChemEngineering 2019, 3, 67. https://doi.org/10.3390/chemengineering3030067
Furukawa M, Tateishi I, Katsumata H, Kaneco S. Application of Sodium Dodecyl Sulfate/Activated Carbon onto the Preconcentration of Cadmium Ions in Solid-Phase Extraction Flow System. ChemEngineering. 2019; 3(3):67. https://doi.org/10.3390/chemengineering3030067
Chicago/Turabian StyleFurukawa, Mai, Ikki Tateishi, Hideyuki Katsumata, and Satoshi Kaneco. 2019. "Application of Sodium Dodecyl Sulfate/Activated Carbon onto the Preconcentration of Cadmium Ions in Solid-Phase Extraction Flow System" ChemEngineering 3, no. 3: 67. https://doi.org/10.3390/chemengineering3030067