KLa Determination Using the Effectiveness-NTU Method: Application to Countercurrent Absorbers in Operation Using Viscous Solvents for VOCs Mass Transfer
Abstract
:1. Introduction
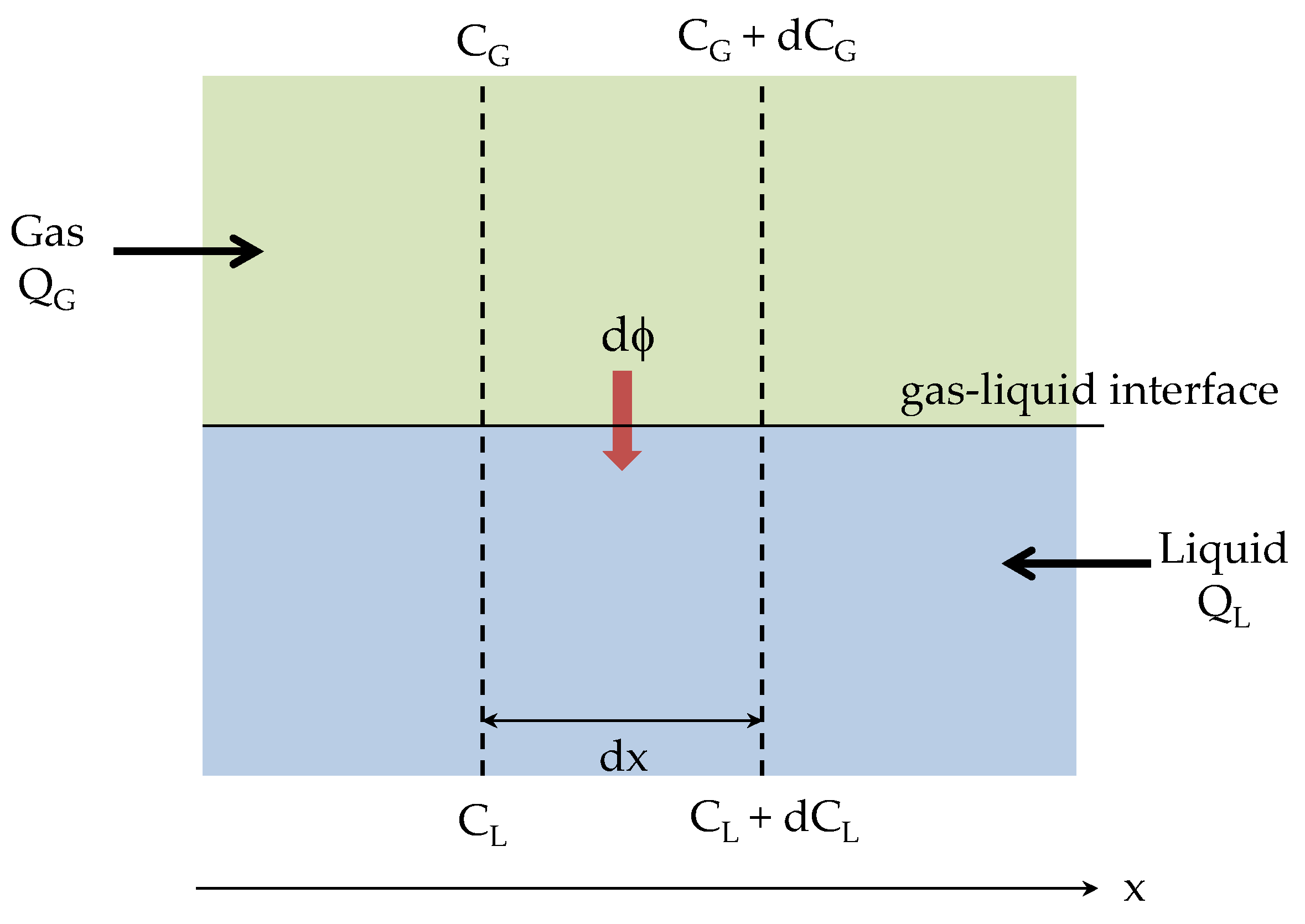
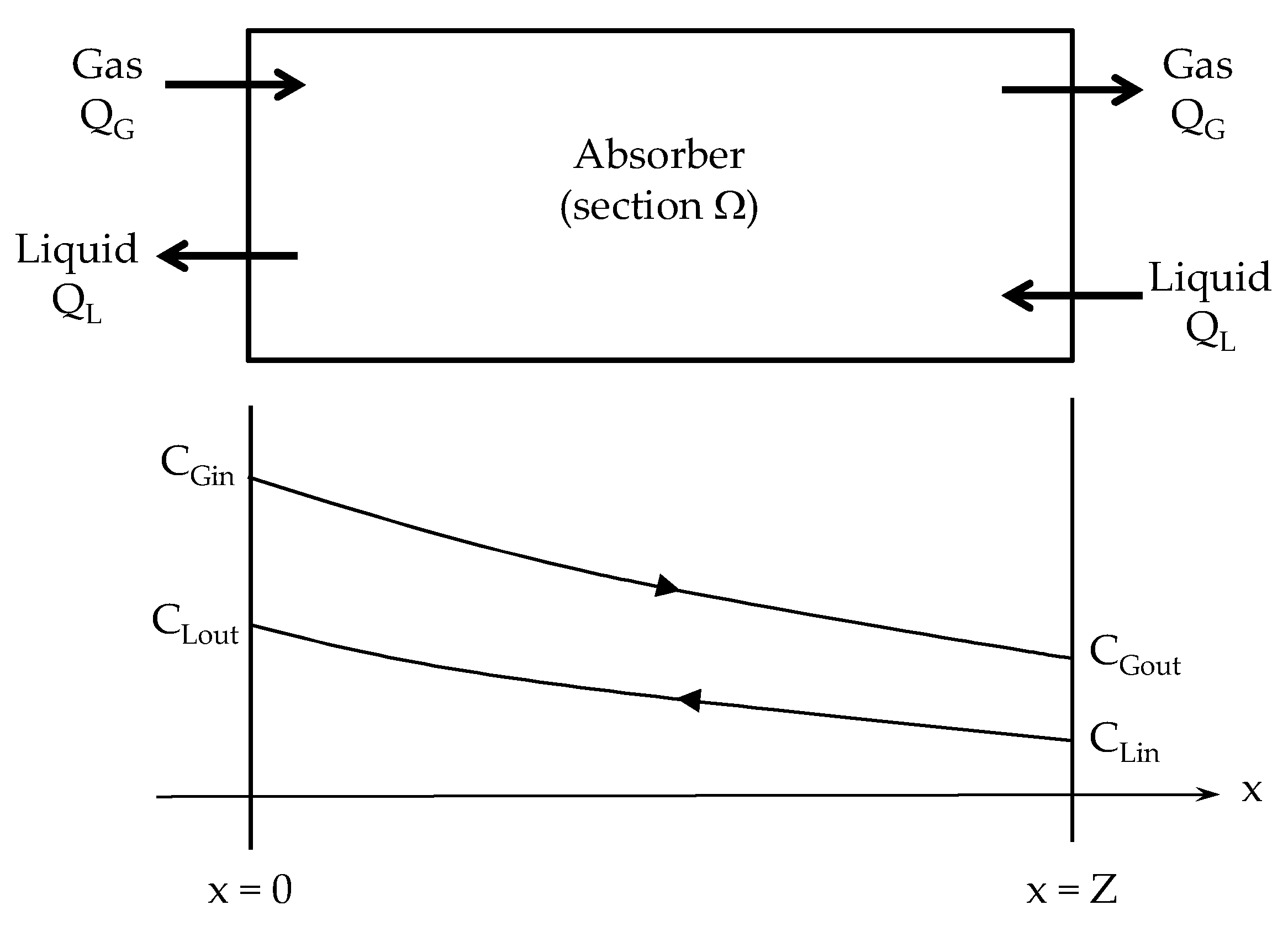
2. Gas–Liquid Absorber Analysis
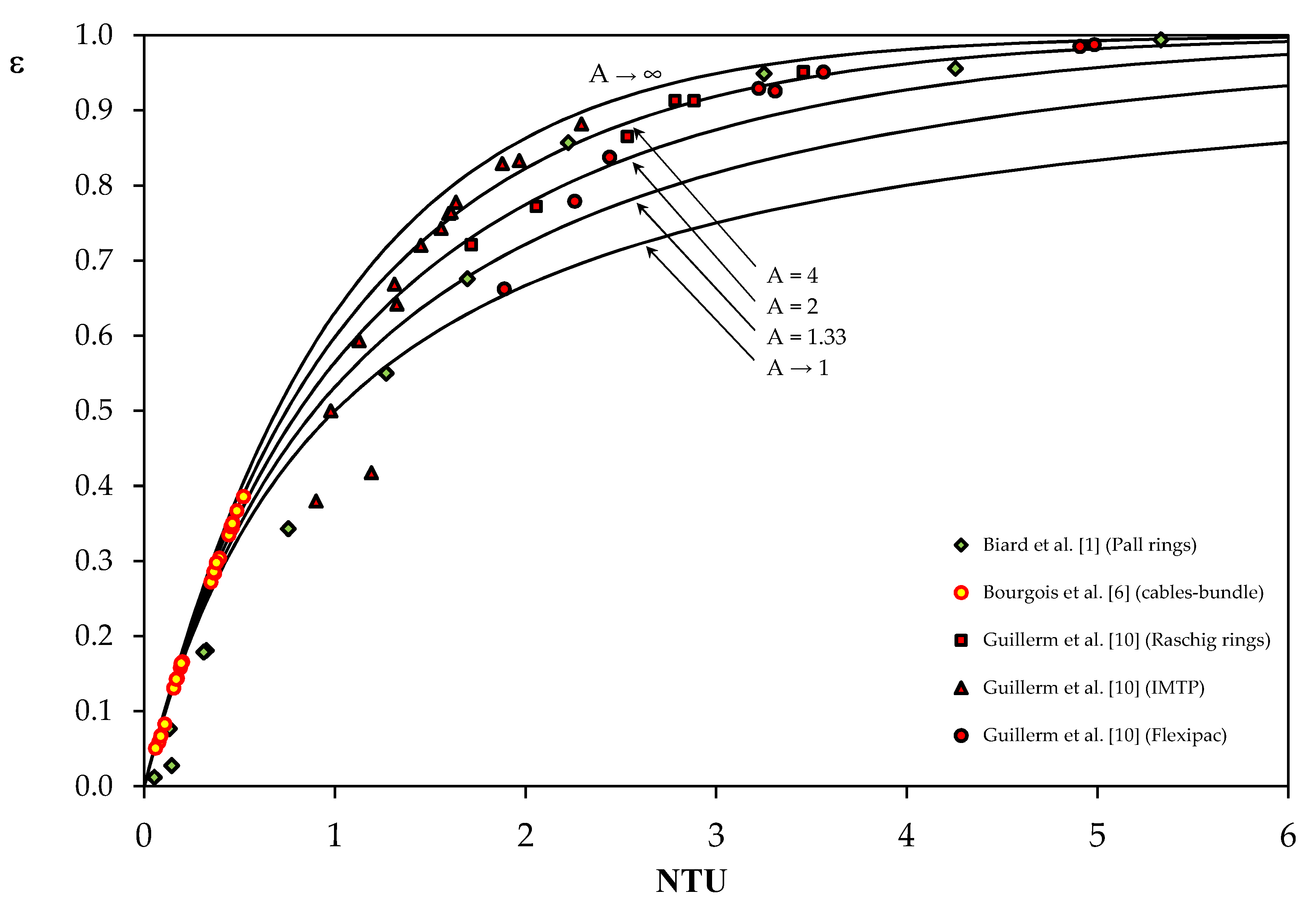
3. Validation of the ε-NTU Method from the Data Reported in the Literature
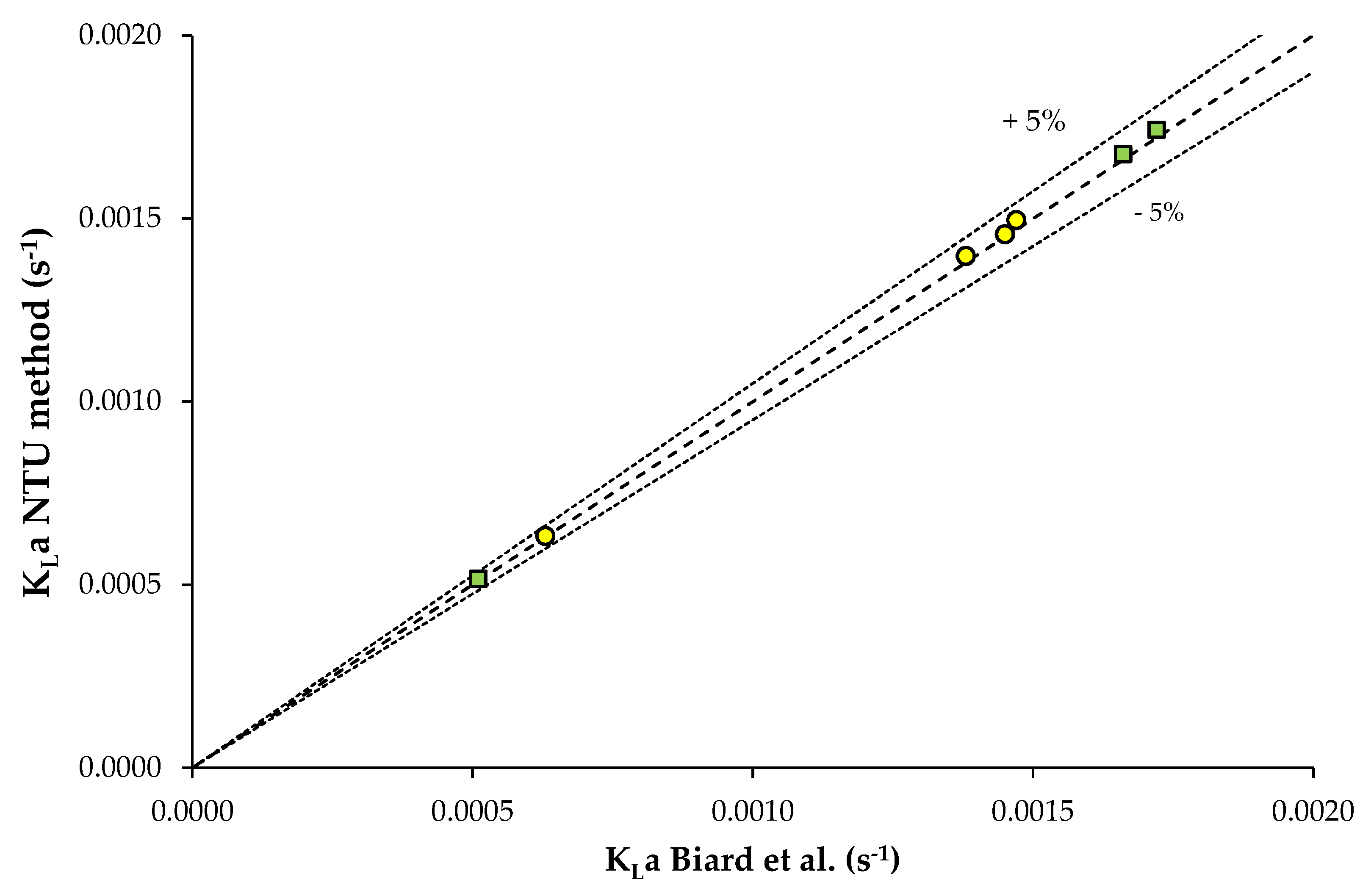
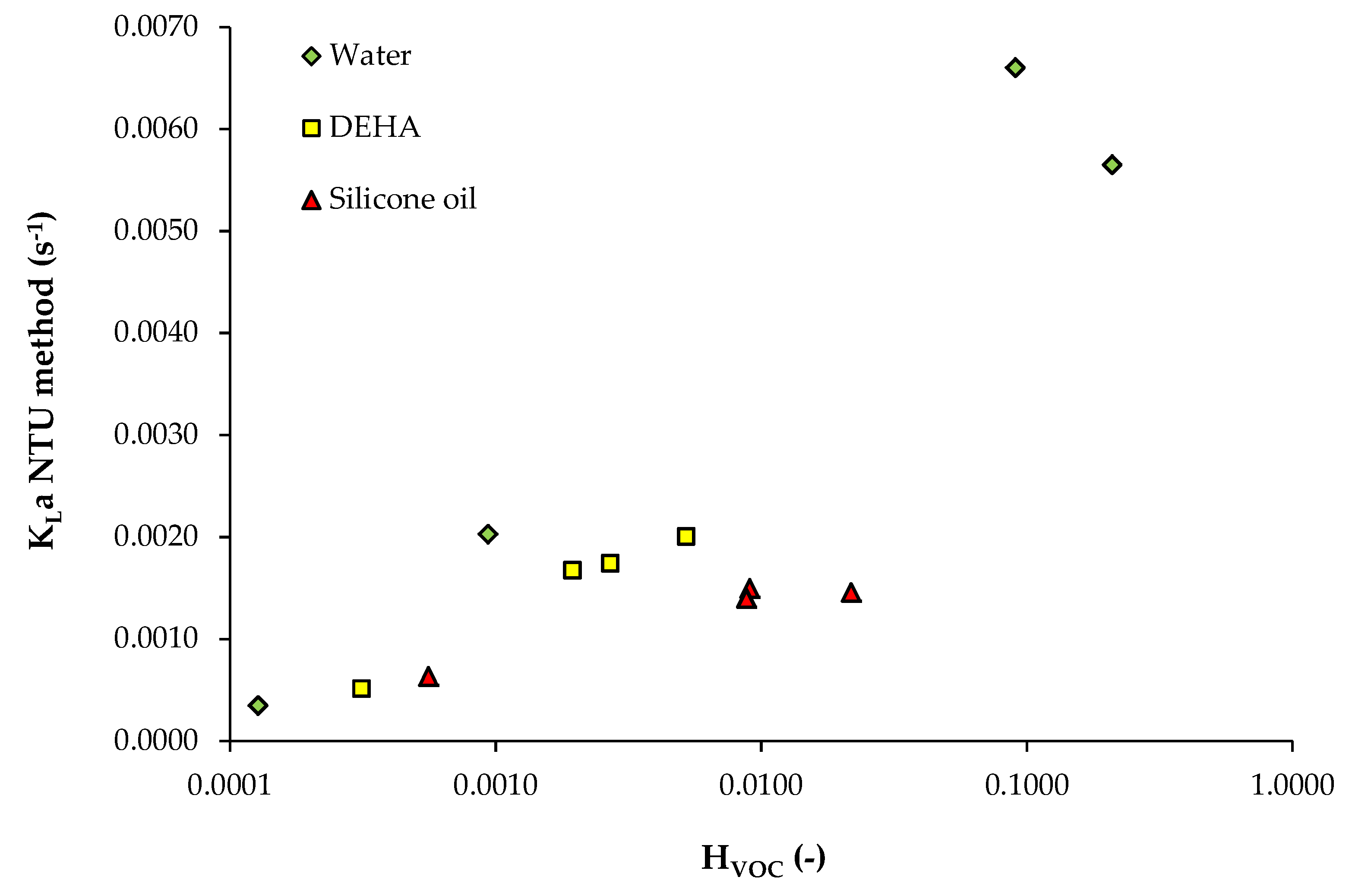
3.1. Data from Study n°1
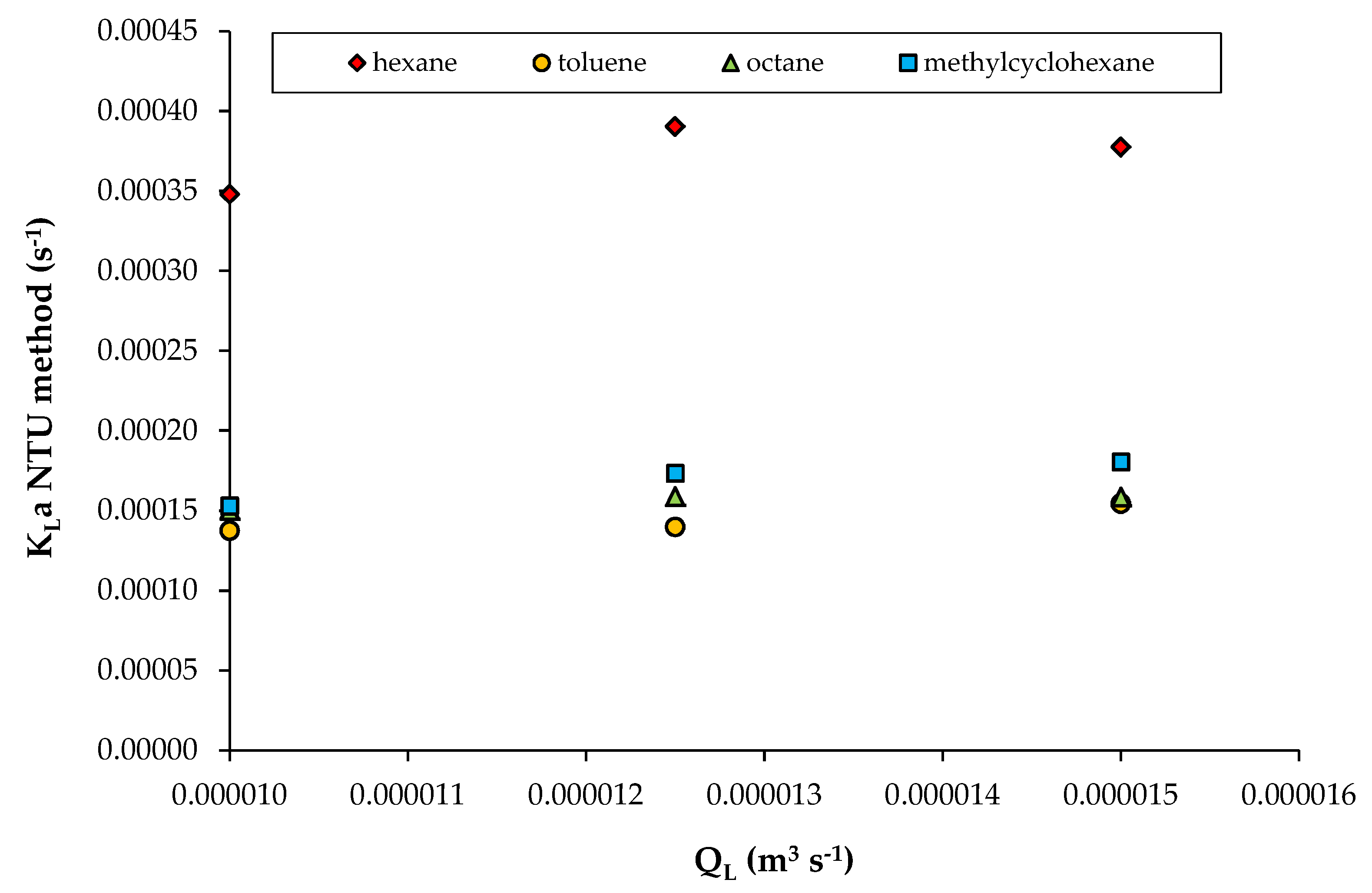
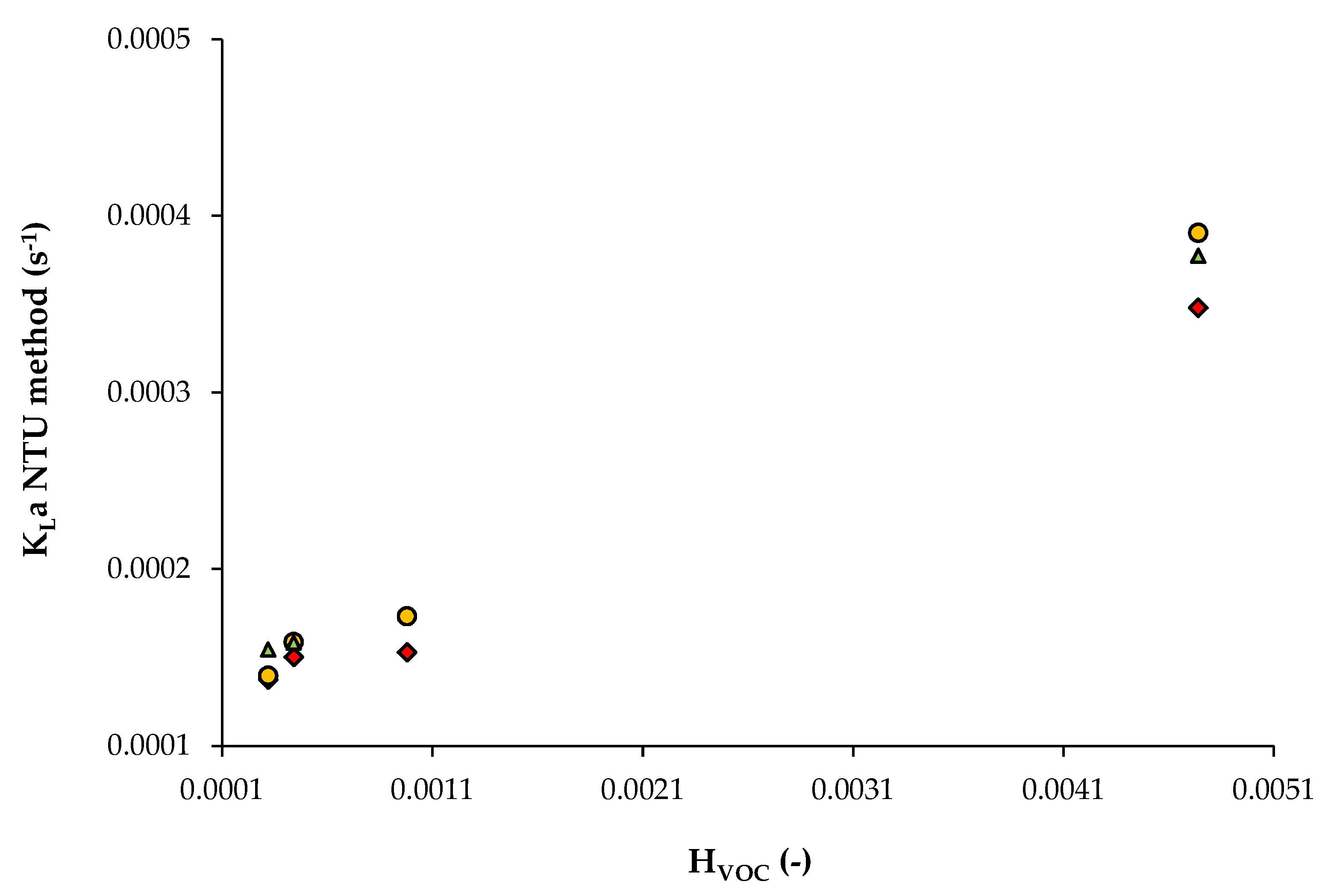
3.2. Data from Study n°2
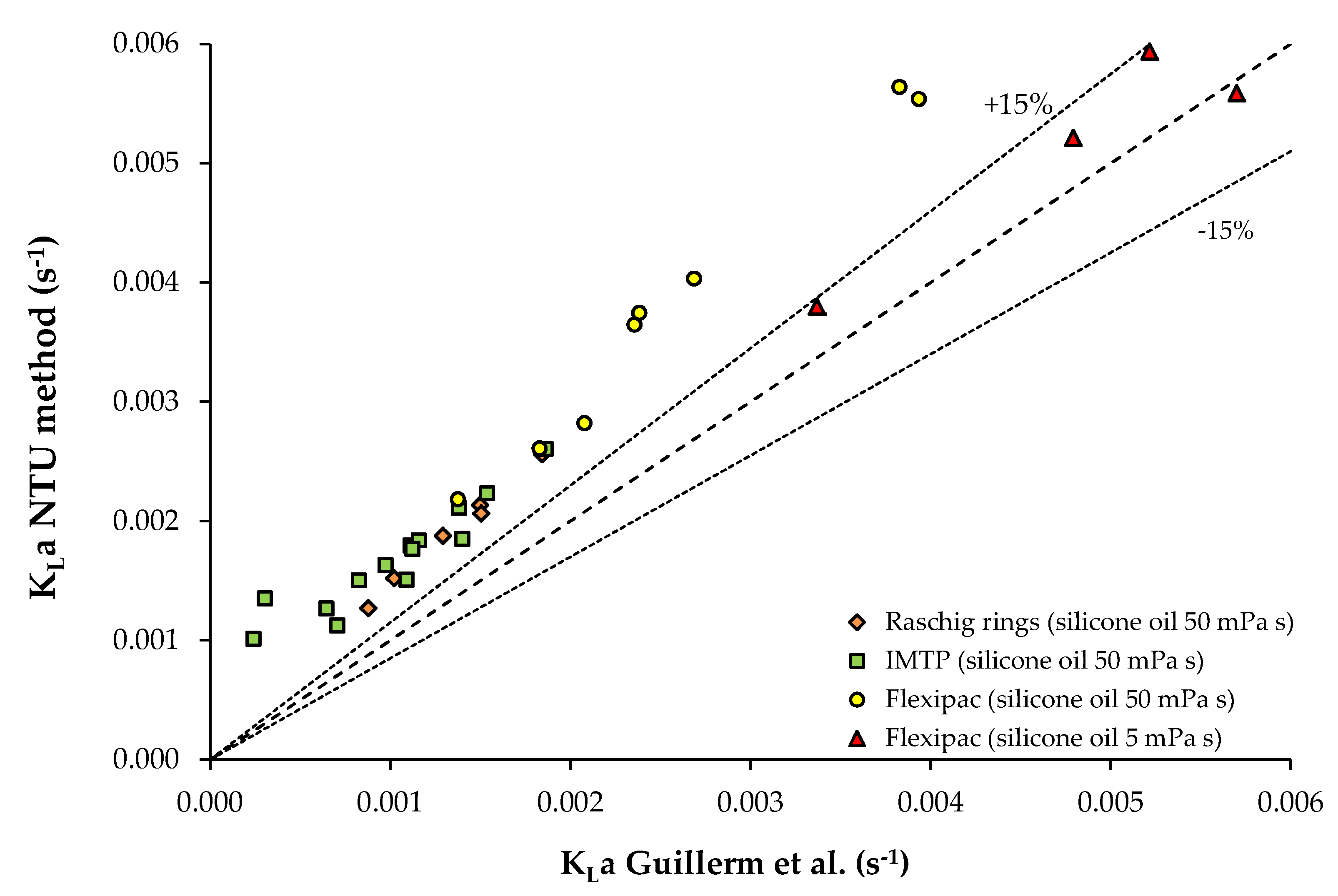
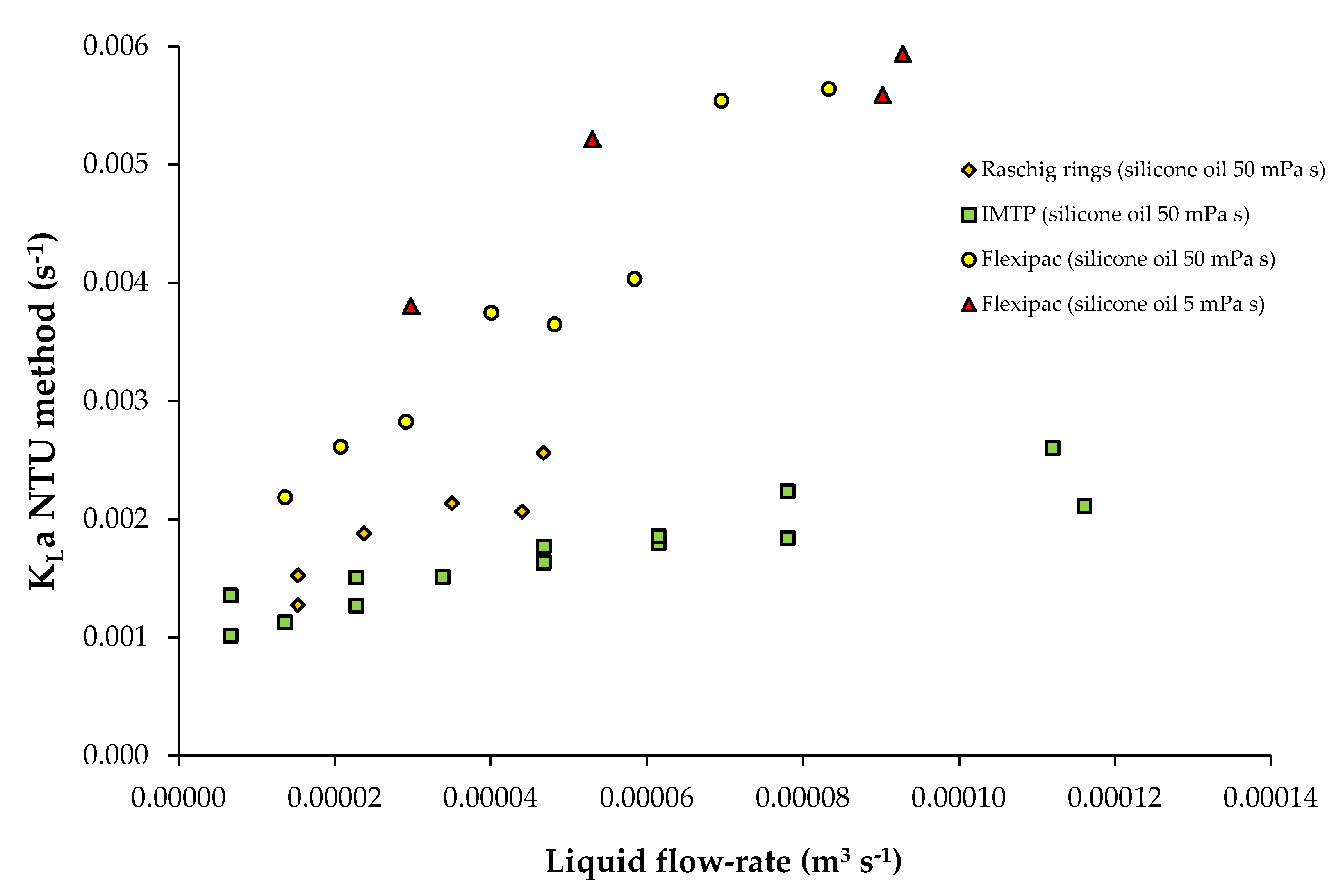
3.3. Data from Study n°3
4. Conclusions
Funding
Conflicts of Interest
Nomenclature
| absorber | |
| a | specific interfacial area (m−1) |
| A | absorption factor |
| C | concentration (mol m−3) |
| EBRT | Empty Bed Residence Time (s) |
| H | Henry coefficient |
| kG, kL | local mass transfer coefficients (m s−1) |
| KGa, KLa | overall mass transfer coefficients (s−1) |
| NTU | Number of Transfer Units |
| Q | flow-rate (m3 s−1) |
| V | packing volume (m3) |
| Z | packing height (m) |
| Greek letters | |
| δ | film thickness (m) |
| ε | effectiveness |
| Ω | cross sectional area (m2) |
| ϕ | molar flux (mol m−3 s−1) |
| Subscripts | |
| G | gas |
| in | inlet |
| L | liquid |
| Max | maximum |
| out | outlet |
| VOC | Volatile Organic Compound |
| heat exchanger, Table 1 | |
| A | heat-transfer area (m2) |
| C | heat capacity rate of the fluid = M Cp (W K−1) |
| Cp | specific heat capacity (J kg−1 K−1) |
| M | flow rate (kg s−1) |
| NTU | Number of Transfer Units |
| T | temperature (K) |
| U | heat transfer coefficient (W m−2 K−1) |
| Greek letters | |
| ΔTML | log mean temperature difference (K) |
| ε | effectiveness |
| ϕ | power, heat transferred per unit time (W) |
| Subscripts | |
| c | cold |
| h | hot |
| in | inlet |
| Max | maximum |
| Min | minimum |
| out | outlet |
Appendix A. ε-NTU Relationship for a Gas-Liquid Absorber
Appendix B. Gas-Liquid Stirred Tank Reactor (STR)
References
- Biard, P.-F.; Couvert, A.; Giraudet, S. Volatile organic compounds absorption in packed column: Theoretical assessment of water, DEHA and PDMS 50 as absorbents. J. Ind. Eng. Chem. 2018, 59, 70–78. [Google Scholar] [CrossRef]
- Dumont, E.; Delmas, H. Mass transfer enhancement of gas absorption in oil-in-water systems: A review. Chem. Eng. Process. Process Intensif. 2003, 42, 419–438. [Google Scholar] [CrossRef]
- Mackowiak, J. Fluid Dynamics of Packed Columns: Principles of the Fluid Dynamic Design of Columns for Gas/Liquid and Liquid/Liquid Systems; Chemische Technik/Verfahrenstechnik; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-540-88780-5. [Google Scholar]
- Fair, J.; Steinmeyer, D.; Penney, W.; Corcker, B. Gas absorption and gas-liquid system design. In Perry’s Chemical Engineers’ Handbook, 8th ed.; Perry, R.H., Green, D.W., Eds.; McGraw-Hill: New York, NY, USA, 2008; pp. 14-1–14-98. [Google Scholar]
- Sinnott, R. Coulson and Richardson’s Chemical Engineering Volume 6 (Design); Pergamon Press: Oxford, UK, 1994; Volume 6, ISBN 0-08-041865-1. [Google Scholar]
- Bourgois, D.; Vanderschuren, J.; Thomas, D. Study of mass transfer of VOCs into viscous solvents in a pilot-scale cables-bundle scrubber. Chem. Eng. J. 2009, 145, 446–452. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Gomez, E. Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview. Biotechnol. Adv. 2009, 27, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Russell, T.W.F.; Robinson, A.S.; Wagner, N.J. Mass and Heat Transfer: Analysis of Mass Contactors and Heat Exchangers; Cambridge Series in Chemical Engineering; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2008; ISBN 978-0-521-88670-3. [Google Scholar]
- Staudinger, J.; Roberts, P.V. A critical compilation of Henry’s law constant temperature dependence relations for organic compounds in dilute aqueous solutions. Chemosphere 2001, 44, 561–576. [Google Scholar] [CrossRef]
- Guillerm, M.; Couvert, A.; Amrane, A.; Norrant, E.; Lesage, N.; Dumont, É. Absorption of toluene in silicone oil: Effect of the solvent viscosity on hydrodynamics and mass transfer. Chem. Eng. Res. Des. 2016, 109, 32–40. [Google Scholar] [CrossRef]
- Heymes, F.; Manno Demoustier, P.; Charbit, F.; Louis Fanlo, J.; Moulin, P. Hydrodynamics and mass transfer in a packed column: Case of toluene absorption with a viscous absorbent. Chem. Eng. Sci. 2006, 61, 5094–5106. [Google Scholar] [CrossRef]
- Dumont, E.; Darracq, G.; Couvert, A.; Couriol, C.; Amrane, A.; Thomas, D.; Andrès, Y.; Le Cloirec, P. Determination of partition coefficients of three volatile organic compounds (dimethylsulphide, dimethyldisulphide and toluene) in water/silicone oil mixtures. Chem. Eng. J. 2010, 162, 927–934. [Google Scholar] [CrossRef]
- Dumont, E.; Darracq, G.; Couvert, A.; Couriol, C.; Amrane, A.; Thomas, D.; Andrès, Y.; Le Cloirec, P. VOC absorption in a countercurrent packed-bed column using water/silicone oil mixtures: Influence of silicone oil volume fraction. Chem. Eng. J. 2011, 168, 241–248. [Google Scholar] [CrossRef]
- Dumont, É. Mass Transfer in Multiphasic Gas/Liquid/Liquid Systems. KLa Determination Using the Effectiveness-Number of Transfer Unit Method. Processes 2018, 6, 156. [Google Scholar] [CrossRef]
| Mass Absorber | Heat Exchanger | |
|---|---|---|
| Physical parameter | Concentration C (mol m−3) | Temperature T (K) |
| Transfer coefficient | KL (m s−1) | U (W m−2 K−1) |
| Transfer surface area | a V (m2) | A (m2) |
| Transfer rate | (mol m−3 s−1) | (W) |
| Maximum possible transfer rate | (mol m−3 s−1) | (W) |
| Effectiveness (dimensionless) | ||
| NTU (dimensionless) |
| Solvent | Solute | HVOC | Operating Conditions | Ref |
|---|---|---|---|---|
| Water 1 mPa s | Toluene Dichloromethane Propanol Acetone | 2.09 × 10−1 9.03 × 10−2 1.27 × 10−4 9.36 × 10−4 | V = 2.36 m3 Packed column (Pall rings) QL = 3.06 × 10−3 m3/s QG: 1.20 m3/s (gas EBRT: 2 s) 20 °C | Study n°1 [1] |
| DEHA (bis(di-2-ethylhexyl)adipate) 12.5 mPa s | Toluene Dichloromethane Propanol Acetone | 3.12 × 10−4 1.94 × 10−3 2.70 × 10−3 5.21 × 10−3 | ||
| Silicone oil 50 mPa s | Toluene Dichloromethane Propanol Acetone | 5.58 × 10−4 9.04 × 10−3 8.79 × 10−3 2.17 × 10−2 | ||
| DEHP (di-2-ethylhexyl phthalate) 76 mPa s | Hexane Toluene Octane Methylcyclohexane | 4.74 × 10−3 3.19 × 10−4 4.40 × 10−4 9.79 × 10−4 | V = 1.46 × 10−2 m3 Cables-bundle contactor QL = 1.00 × 10−5; 1.25 × 10−5; 1.50 × 10−5 m3/s QG: 1.39 × 10−2 m3/s (gas EBRT: 1 s) 20 °C | Study n°2 [6] |
| Silicone oil 50 mPa s | Toluene | 1.17 × 10−3 | V = 1.13 × 10−2 m3 Packed column (Raschig rings) QL = 1.53 × 10−5–4.58 × 10−5 m3/s QG: 7.15 × 10−3 m3/s (gas EBRT: 1.6 s) 25 °C | Study n°3 [10] |
| Silicone oil 50 mPa s | Toluene | 1.17 × 10−3 | V = 1.13 10−2 m3 Packed column (IMTP®) QL = 6.62 × 10−6–1.16 × 10−4 m3/s QG: 1.10 × 10−2 m3/s (gas EBRT: 1 s) 25 °C | Study n°3 [10] |
| Silicone oil 50 mPa s Silicone oil 5 mPa s | Toluene Toluene | 1.17 × 10−3 1.09 × 10−3 | V = 1.13 × 10−2 m3 Packed column (Flexipac®) QL = 1.36 × 10−5–9.28 × 10−5 m3/s QG: 1.10 × 10−2 m3/s (gas EBRT: 1 s) 25 °C | Study n°3 [10] |
| Solute | HVOC (-) | A (-) | ε (-) | NTU (-) | KLa (s−1) | |
|---|---|---|---|---|---|---|
| Toluene | Water | 0.20936 | 0.012 | 0.012 | 0.053 | 5.63 × 10−3 |
| DEHA | 0.00031 | 8.162 | 0.949 | 3.251 | 5.17 × 10−4 | |
| PDMS50 | 0.00056 | 4.561 | 0.857 | 2.225 | 6.33 × 10−4 | |
| Dichloromethane | Water | 0.09031 | 0.028 | 0.028 | 0.144 | 6.60 × 10−3 |
| DEHA | 0.00194 | 1.311 | 0.676 | 1.695 | 1.68 × 10−3 | |
| PDMS50 | 0.00904 | 0.282 | 0.181 | 0.325 | 1.50 × 10−3 | |
| Propanol | Water | 0.00013 | 20.009 | 0.994 | 5.331 | 3.46 × 10−4 |
| DEHA | 0.00270 | 0.944 | 0.550 | 1.269 | 1.74 × 10−3 | |
| PDMS50 | 0.00879 | 0.290 | 0.179 | 0.312 | 1.40 × 10−3 | |
| Acetone | Water | 0.00094 | 2.721 | 0.956 | 4.254 | 2.03 × 10−3 |
| DEHA | 0.00521 | 0.488 | 0.343 | 0.756 | 2.01 × 10−3 | |
| PDMS50 | 0.02174 | 0.117 | 0.077 | 0.132 | 1.46 × 10−3 |
| Solute | HVOC (-) | QL (m3/s) | A (-) | ε (-) | NTU (-) | KLa (s−1) | δ (m) |
|---|---|---|---|---|---|---|---|
| Hexane | 4.7 × 10−3 | 1.00 × 10−5 | 0.152 | 0.059 | 0.077 | 3.48 × 10−4 | 4.30 × 10−5 |
| 1.25 × 10−5 | 0.190 | 0.068 | 0.087 | 3.94 × 10−4 | 3.80 × 10−5 | ||
| 1.25 × 10−5 | 0.190 | 0.067 | 0.086 | 3.87 × 10−4 | 3.87 × 10−5 | ||
| 1.50 × 10−5 | 0.228 | 0.051 | 0.059 | 2.67 × 10−4 | 5.59 × 10−5 | ||
| 1.50 × 10−5 | 0.228 | 0.083 | 0.108 | 4.87 × 10−4 | 3.07 × 10−5 | ||
| Toluene | 3.2 × 10−4 | 1.00 × 10−5 | 2.257 | 0.345 | 0.462 | 1.40 × 10−4 | 5.07 × 10−5 |
| 1.00 × 10−5 | 2.257 | 0.335 | 0.444 | 1.35 × 10−4 | 5.27 × 10−5 | ||
| 1.25 × 10−5 | 2.821 | 0.346 | 0.455 | 1.38 × 10−4 | 5.15 × 10−5 | ||
| 1.25 × 10−5 | 2.821 | 0.351 | 0.464 | 1.41 × 10−4 | 5.05 × 10−5 | ||
| 1.25 × 10−5 | 2.821 | 0.35 | 0.462 | 1.40 × 10−4 | 5.07 × 10−5 | ||
| 1.50 × 10−5 | 3.386 | 0.367 | 0.486 | 1.48 × 10−4 | 4.82 × 10−5 | ||
| 1.50 × 10−5 | 3.386 | 0.386 | 0.520 | 1.58 × 10−4 | 4.50 × 10−5 | ||
| 1.50 × 10−5 | 3.386 | 0.386 | 0.520 | 1.58 × 10−4 | 4.50 × 10−5 | ||
| Octane | 4.4 × 10−4 | 1.00 × 10−5 | 1.636 | 0.272 | 0.349 | 1.46 × 10−4 | 5.50 × 10−5 |
| 1.00 × 10−5 | 1.636 | 0.284 | 0.369 | 1.54 × 10−4 | 5.21 × 10−5 | ||
| 1.25 × 10−5 | 2.045 | 0.304 | 0.394 | 1.65 × 10−4 | 4.87 × 10−5 | ||
| 1.25 × 10−5 | 2.045 | 0.286 | 0.364 | 1.53 × 10−4 | 5.27 × 10−5 | ||
| 1.50 × 10−5 | 2.455 | 0.298 | 0.379 | 1.58 × 10−4 | 5.07 × 10−5 | ||
| 1.50 × 10−5 | 2.455 | 0.298 | 0.379 | 1.58 × 10−4 | 5.07 × 10−5 | ||
| Methylcyclo-hexane | 9.8 × 10−4 | 1.00 × 10−5 | 0.735 | 0.144 | 0.174 | 1.62 × 10−4 | 3.38 × 10−5 |
| 1.00 × 10−5 | 0.735 | 0.131 | 0.155 | 1.44 × 10−4 | 3.79 × 10−5 | ||
| 1.25 × 10−5 | 0.919 | 0.158 | 0.189 | 1.76 × 10−4 | 3.10 × 10−5 | ||
| 1.25 × 10−5 | 0.919 | 0.143 | 0.168 | 1.57 × 10−4 | 3.49 × 10−5 | ||
| 1.25 10−5 | 0.919 | 0.166 | 0.201 | 1.87 × 10−4 | 2.92 × 10−5 | ||
| 1.50 × 10−5 | 1.103 | 0.163 | 0.193 | 1.80 × 10−4 | 3.04 × 10−5 | ||
| 1.50 × 10−5 | 1.103 | 0.164 | 0.194 | 1.81 × 10−4 | 3.02 × 10−5 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumont, É. KLa Determination Using the Effectiveness-NTU Method: Application to Countercurrent Absorbers in Operation Using Viscous Solvents for VOCs Mass Transfer. ChemEngineering 2019, 3, 57. https://doi.org/10.3390/chemengineering3020057
Dumont É. KLa Determination Using the Effectiveness-NTU Method: Application to Countercurrent Absorbers in Operation Using Viscous Solvents for VOCs Mass Transfer. ChemEngineering. 2019; 3(2):57. https://doi.org/10.3390/chemengineering3020057
Chicago/Turabian StyleDumont, Éric. 2019. "KLa Determination Using the Effectiveness-NTU Method: Application to Countercurrent Absorbers in Operation Using Viscous Solvents for VOCs Mass Transfer" ChemEngineering 3, no. 2: 57. https://doi.org/10.3390/chemengineering3020057
APA StyleDumont, É. (2019). KLa Determination Using the Effectiveness-NTU Method: Application to Countercurrent Absorbers in Operation Using Viscous Solvents for VOCs Mass Transfer. ChemEngineering, 3(2), 57. https://doi.org/10.3390/chemengineering3020057





