Synthesis of Uniform Mesoporous Zeolite ZSM-5 Catalyst for Friedel-Crafts Acylation
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Synthesis of Mesoporous ZSM-5 Catalyst
2.3. Characterization Methods
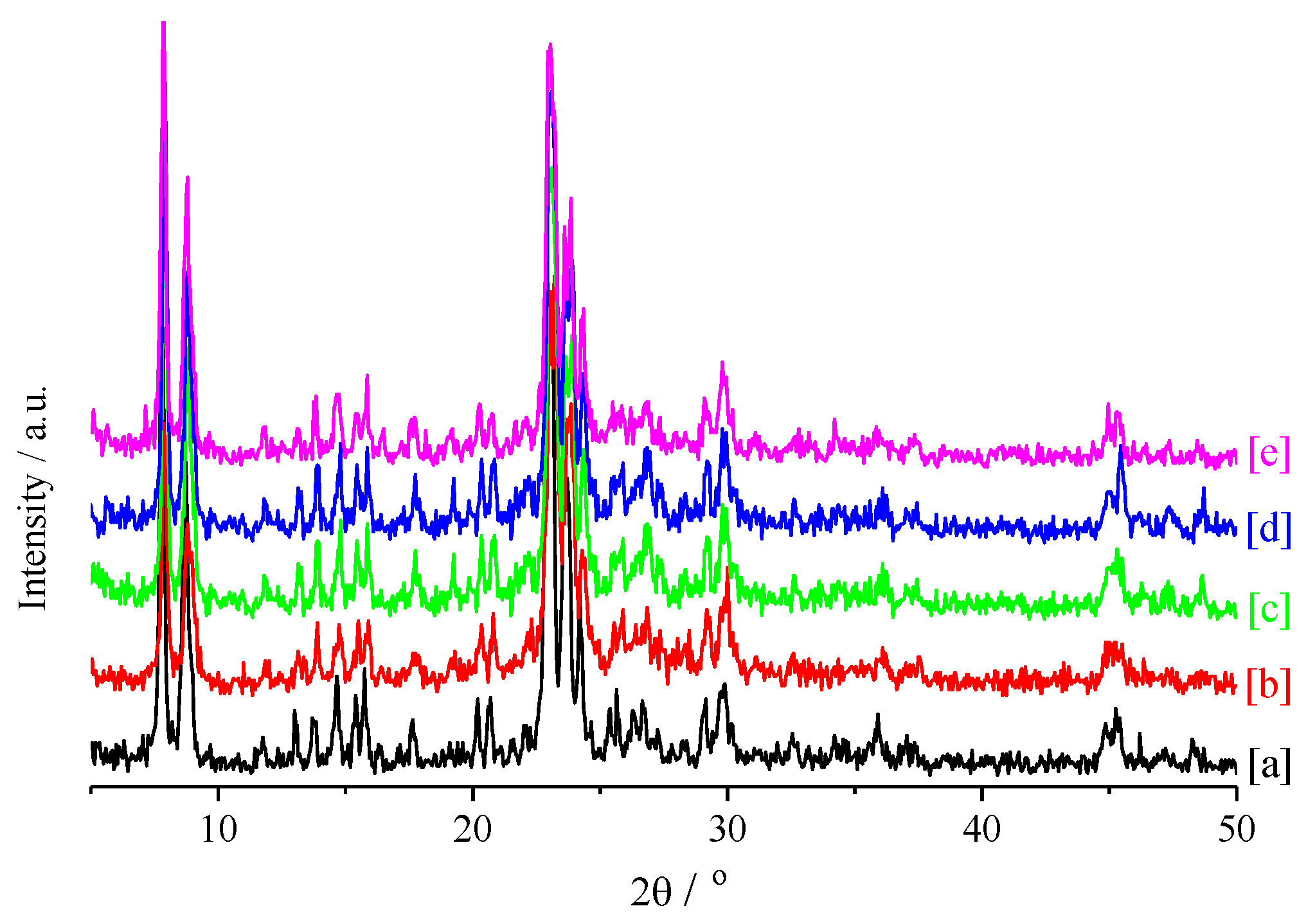
2.3.1. X-Ray Diffraction (XRD)
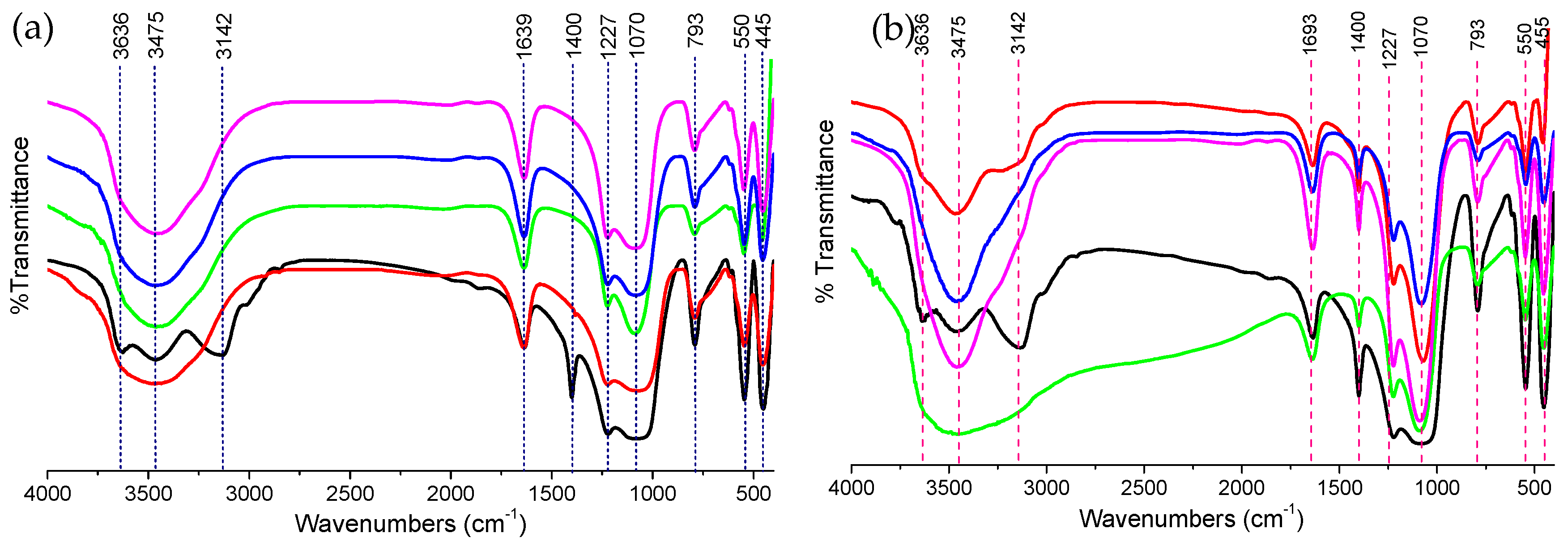
2.3.2. Fourier Transform Infrared (FT-IR) Spectroscopy
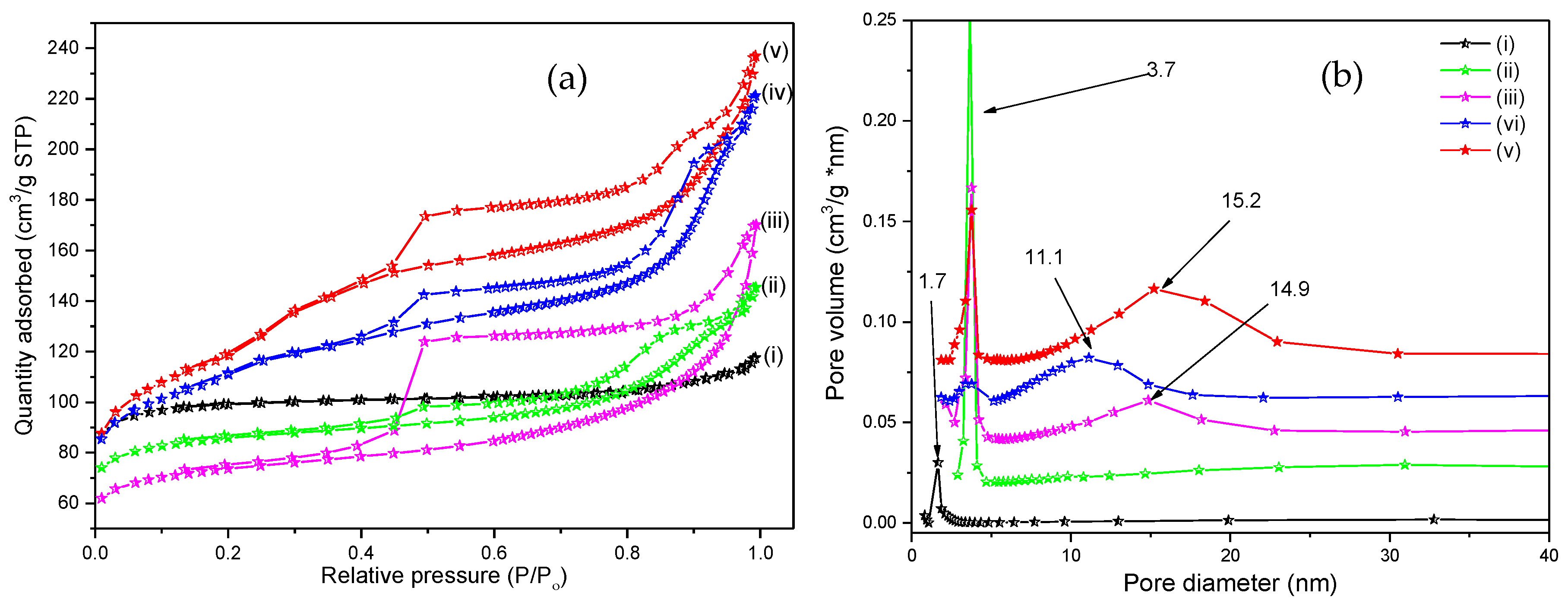
2.3.3. Nitrogen Porosimetry
2.3.4. Morphology
2.3.5. Surface Acidity
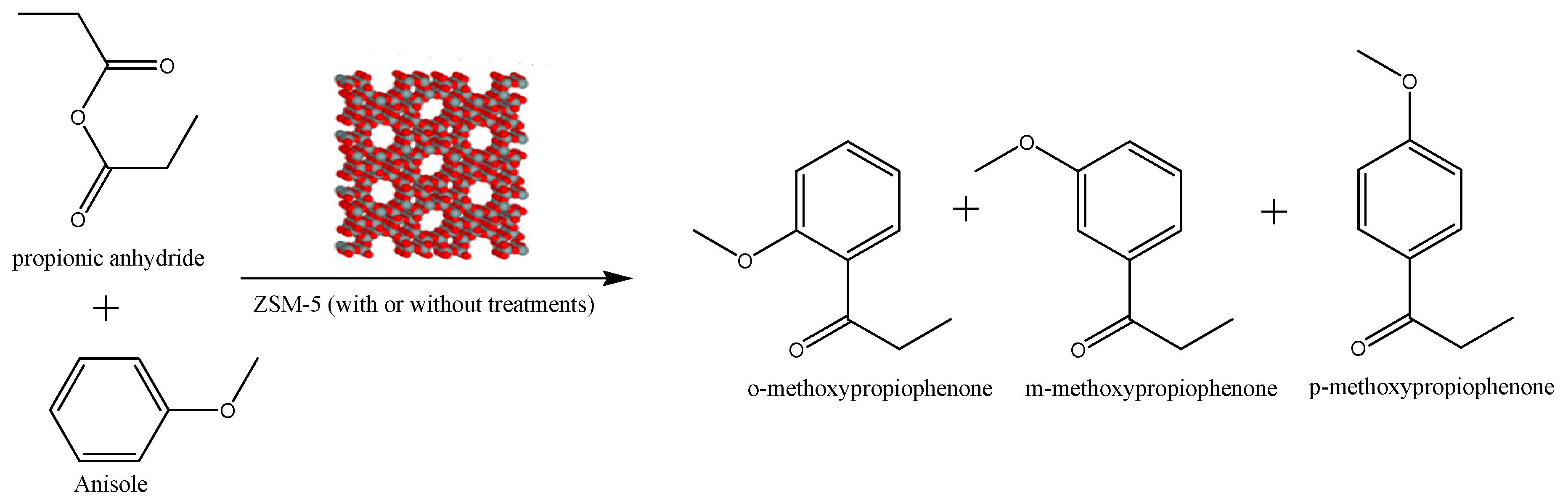
2.4. Synthesis of p-Methoxypropiophenone “Friedel-Crafts Acylation”
3. Results and Discussion
3.1. Zeolite Catalyst Characterizations
3.2. Catalytic Performance
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Olah, G.A.; Donovan, D.J.; Lin, H.C. Friedel-Crafts Chemistry. 1. Chem. Informationsdienst 1976, 98, 2661–2663. [Google Scholar]
- Clark, J.H. Solid acids for green chemistry. Acc. Chem. Res. 2002, 35, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; JoséCliment, M.; García, H.; Primo, J. Design of synthetic zeolites as catalysts in organic reactions: Acylation of anisole by acyl chlorides or carboxylic acids over acid zeolites. Appl. Catal. 1989, 49, 109–123. [Google Scholar] [CrossRef]
- Gaare, K.; Akporiaye, D. Modified zeolites as catalysts in the Friedel-Crafts acylation. J. Mol. Catal. A Chem. 1996, 109, 177–187. [Google Scholar] [CrossRef]
- Freese, U.; Heinrich, F.; Roessner, F. Acylation of aromatic compounds on H-Beta zeolites. Catal. Today 1999, 49, 237–244. [Google Scholar] [CrossRef]
- Smith, K.; Zhenhua, Z.; Hodgson, P.K. Synthesis of aromatic ketones by acylation of aryl ethers with carboxylic anhydrides in the presence of zeolite H-β (H-BEA) in the absence of solvent. J. Mol. Catal. A Chem. 1998, 134, 121–128. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Jiang, W.; Zuo, B. Friedal-Crafts acylation of anisole over zeolite catalysts. Appl. Catal. A Gen. 1997, 165, 199–206. [Google Scholar] [CrossRef]
- Selkirk, J.; Challiss, R.; Price, G.; Nahorski, S. Differential coupling to endogenous G protein subpopulations in CHO and BHK cell lines recombinantly expressing mGlu1 receptors. Br. J. Pharmacol. 1999, 128, 32. [Google Scholar]
- Zaarour, M.; Dong, B.; Naydenova, I.; Retoux, R.; Mintova, S. Progress in zeolite synthesis promotes advanced applications. Microporous Mesoporous Mater. 2014, 189, 11–21. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts. Nature 2009, 461, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Petushkov, A.; Yoon, S.; Larsen, S.C. Synthesis of hierarchical nanocrystalline ZSM-5 with controlled particle size and mesoporosity. Microporous Mesoporous Mater. 2011, 137, 92–100. [Google Scholar] [CrossRef]
- Jin, H.; Ansari, M.B.; Park, S.-E. Mesoporous MFI zeolites by microwave induced assembly between sulfonic acid functionalized MFI zeolite nanoparticles and alkyltrimethylammonium cationic surfactants. Chem. Commun. 2011, 47, 7482–7484. [Google Scholar] [CrossRef] [PubMed]
- Galarneau, A.; Iapichella, J.; Bonhomme, K.; Di Renzo, F.; Kooyman, P.; Terasaki, O.; Fajula, F. Controlling the morphology of mesostructured silicas by pseudomorphic transformation: A route towards applications. Adv. Funct. Mater. 2006, 16, 1657–1667. [Google Scholar] [CrossRef]
- Gonçalves, M.L.; Dimitrov, L.D.; Jordão, M.H.; Wallau, M.; Urquieta-González, E.A. Synthesis of mesoporous ZSM-5 by crystallisation of aged gels in the presence of cetyltrimethylammonium cations. Catal. Today 2008, 133, 69–79. [Google Scholar] [CrossRef]
- Wang, L.; Yin, C.; Shan, Z.; Liu, S.; Du, Y.; Xiao, F.-S. Bread-template synthesis of hierarchical mesoporous ZSM-5 zeolite with hydrothermally stable mesoporosity. Colloids Surf. A Physicochem. Eng. Asp. 2009, 340, 126–130. [Google Scholar] [CrossRef]
- Shanbhag, G.V.; Choi, M.; Kim, J.; Ryoo, R. Mesoporous sodalite: A novel, stable solid catalyst for base-catalyzed organic transformations. J. Catal. 2009, 264, 88–92. [Google Scholar] [CrossRef]
- Groen, J.C.; Moulijn, J.A.; Pérez-Ramírez, J. Decoupling mesoporosity formation and acidity modification in ZSM-5 zeolites by sequential desilication-dealumination. Microporous Mesoporous Mater. 2005, 87, 153–161. [Google Scholar] [CrossRef]
- Harding, G. X-ray diffraction imaging—A multi-generational perspective. Appl. Radiat. Isot. 2009, 67, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Gardy, J.; Hassanpour, A.; Lai, X.; Ahmed, M.H. Synthesis of Ti(SO4)O solid acid nano-catalyst and its application for biodiesel production from used cooking oil. Appl. Catal. A Gen. 2016, 527, 81–95. [Google Scholar] [CrossRef]
- Rusu, D.; Rusu, G.; Luca, D. Structural characteristics and optical properties of thermally oxidized zinc films. TC 2011, 100, 101. [Google Scholar] [CrossRef]
- Gardy, J. Biodiesel Production from Used Cooking Oil Using Novel Solid Acid Catalysts. Ph.D. Thesis, University of Leeds, Leeds, UK, 2017. [Google Scholar]
- Byrappa, K.; Kumar, B.S. Characterization of zeolites by infrared spectroscopy. Asian J. Chem. 2007, 19, 4933. [Google Scholar]
- Zhang, Y.; Zhu, K.; Duan, X.; Li, P.; Zhou, X.; Yuan, W. Synthesis of hierarchical ZSM-5 zeolite using CTAB interacting with carboxyl-ended organosilane as a mesotemplate. RSC Adv. 2014, 4, 14471–14474. [Google Scholar] [CrossRef]
- Jash, P.; Meaux, K.; Trenary, M. Transmission infrared spectroscopy of ammonia borane. J. Undergrad. Res. 2012, 5. [Google Scholar] [CrossRef]
- Oh, H.-S.; Kang, K.-K.; Kim, M.-H.; Rhee, H.-K. Synthesis of MFI-type zeolites under atmospheric pressure. Korean J. Chem. Eng. 2001, 18, 113–119. [Google Scholar] [CrossRef]
- Kresge, C.; Leonowicz, M.; Roth, W.; Vartuli, J.; Beck, J. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Wang, B. Zeolite Deactivation during Hydrocarbon Reactions: Characterisation of Coke Precursors and Acidity, Product Distribution. Ph.D. Thesis, UCL (University College London), London, UK, 2008. [Google Scholar]
- Chiche, B.; Finiels, A.; Gauthier, C.; Geneste, P.; Graille, J.; Pioch, D. Friedel-Crafts acylation of toluene and p-xylene with carboxylic acids catalyzed by zeolites. J. Organ. Chem. 1986, 51, 2128–2130. [Google Scholar] [CrossRef]
- Chiche, B.; Finiels, A.; Gauthier, C.; Geneste, P. The effect of structure on reactivity in zeolite catalyzed acylation of aromatic compounds: A ϱ-σ+ relationship. Appl. Catal. 1987, 30, 365–369. [Google Scholar] [CrossRef]
- Topsøe, N.-Y.; Pedersen, K.; Derouane, E.G. Infrared and temperature-programmed desorption study of the acidic properties of ZSM-5-type zeolites. J. Catal. 1981, 70, 41–52. [Google Scholar] [CrossRef]
- Tsiatouras, V.A.; Evmiridis, N.P. Study of interactions between ion-exchanged chromium and impregnated vanadium in USY zeolite material. Ind. Eng. Chem. Res. 2003, 42, 1137–1144. [Google Scholar] [CrossRef]
- Hidalgo, C.V.; Itoh, H.; Hattori, T.; Niwa, M.; Murakami, Y. Measurement of the acidity of various zeolites by temperature-programmed desorption of ammonia. J. Catal. 1984, 85, 362–369. [Google Scholar] [CrossRef]
- Karge, H.G. Comparative measurements on acidity of zeolites. Stud. Surf. Sci. Catal. 1991, 65, 133–156. [Google Scholar]
- Botella, P.; Corma, A.; Lopez-Nieto, J.; Valencia, S.; Jacquot, R. Acylation of toluene with acetic anhydride over beta zeolites: Influence of reaction conditions and physicochemical properties of the catalyst. J. Catal. 2000, 195, 161–168. [Google Scholar] [CrossRef]
- Ramanathan, A.; Zhu, H.; Maheswari, R.; Subramaniam, B. Novel zirconium containing cage type silicate (Zr-KIT-5): An efficient Friedel-Crafts alkylation catalyst. Chem. Eng. J. 2015, 278, 113–121. [Google Scholar] [CrossRef]
- Cirujano, F.G.; Stalpaert, M.; De Vos, D.E. Ionic liquids vs. microporous solids as reusable reaction media for the catalytic C–H functionalization of indoles with alcohols. Green Chem. 2018, 20, 2481–2485. [Google Scholar] [CrossRef]

| Catalysts | Label Code |
|---|---|
| Untreated zeolite catalyst | ZSM-5 |
| Treated zeolite with NaOH | ZSM-5-Na |
| Treated zeolite with CTAB | ZSM-5-C |
| Treated zeolite with TPAOH | ZSM-5-T |
| Treated zeolite with mixed CTAB and TPAOH | ZSM-5-CT |
| Catalysts | Crystallite Size (nm) | Crystallinity (%) |
|---|---|---|
| ZSM-5 | 22 | 100 |
| ZSM-5-Na | 21 | 86 |
| ZSM-5-C | 21 | 93 |
| ZSM-5-T | 20 | 93 |
| ZSM-5-CT | 21 | 95 |
| Catalysts | SBET * (m2·g−1) | Vp ** (mL·g−1) | Dp *** (nm) | t-Plot Values | |
|---|---|---|---|---|---|
| External Surface Area (m2·g−1) | VMicropore (cm3·g−1) | ||||
| ZSM-5 | 279 ± 1.6 | 0.18 | 1.7 ± 0.1 | 55 | 0.13 |
| ZSM-5-Na | 328 ± 1.2 | 0.26 | 3.7 ± 0.1 | 65 | 0.08 |
| ZSM-5-T | 390 ± 1.9 | 0.23 | 11.1 ± 0.1 | 60 | 0.12 |
| ZSM-5-C | 395 ± 2.5 | 0.37 | 14.9 ± 0.1 | 300 | 0.06 |
| ZSM5-CT | 419 ± 2.0 | 0.34 | 15.2 ± 0.1 | 200 | 0.08 |
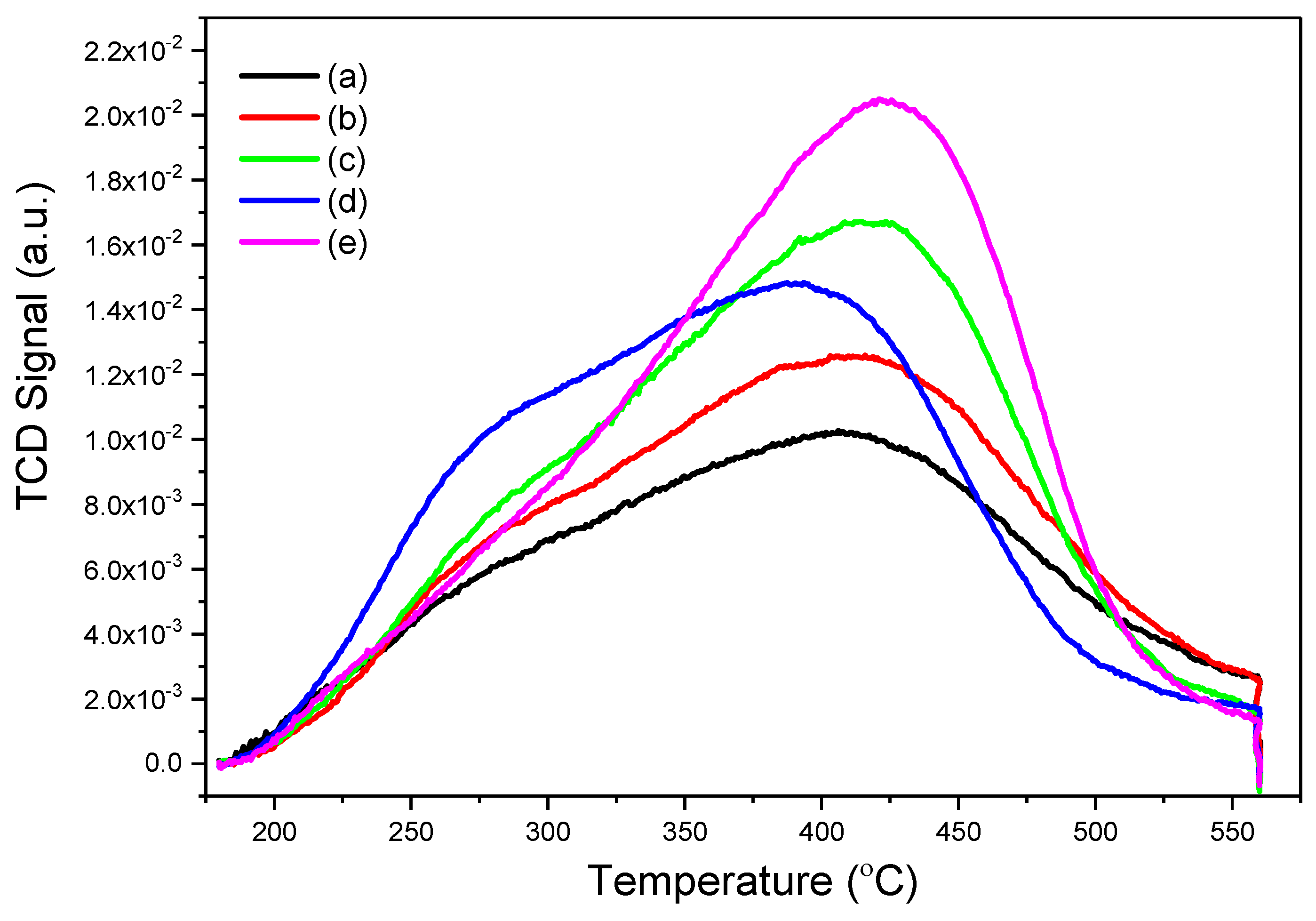
| Catalyst | Desorbed NH3 Amount (mmol·g−1) | Tmax (°C) | |||
|---|---|---|---|---|---|
| First Peak | Second Peak | Total | First Peak | Second Peak | |
| ZSM-5 | 0.45 | 0.49 | 0.94 | 304 | 422 |
| ZSM-5-Na | 0.46 | 0.53 | 0.99 | 306 | 423 |
| ZSM-5-T | 0.74 | 0.49 | 1.23 | 331 | 426 |
| ZSM-5-C | 0.44 | 0.84 | 1.28 | 284 | 388 |
| ZSM-5-CT | 0.47 | 0.86 | 1.33 | 332 | 427 |
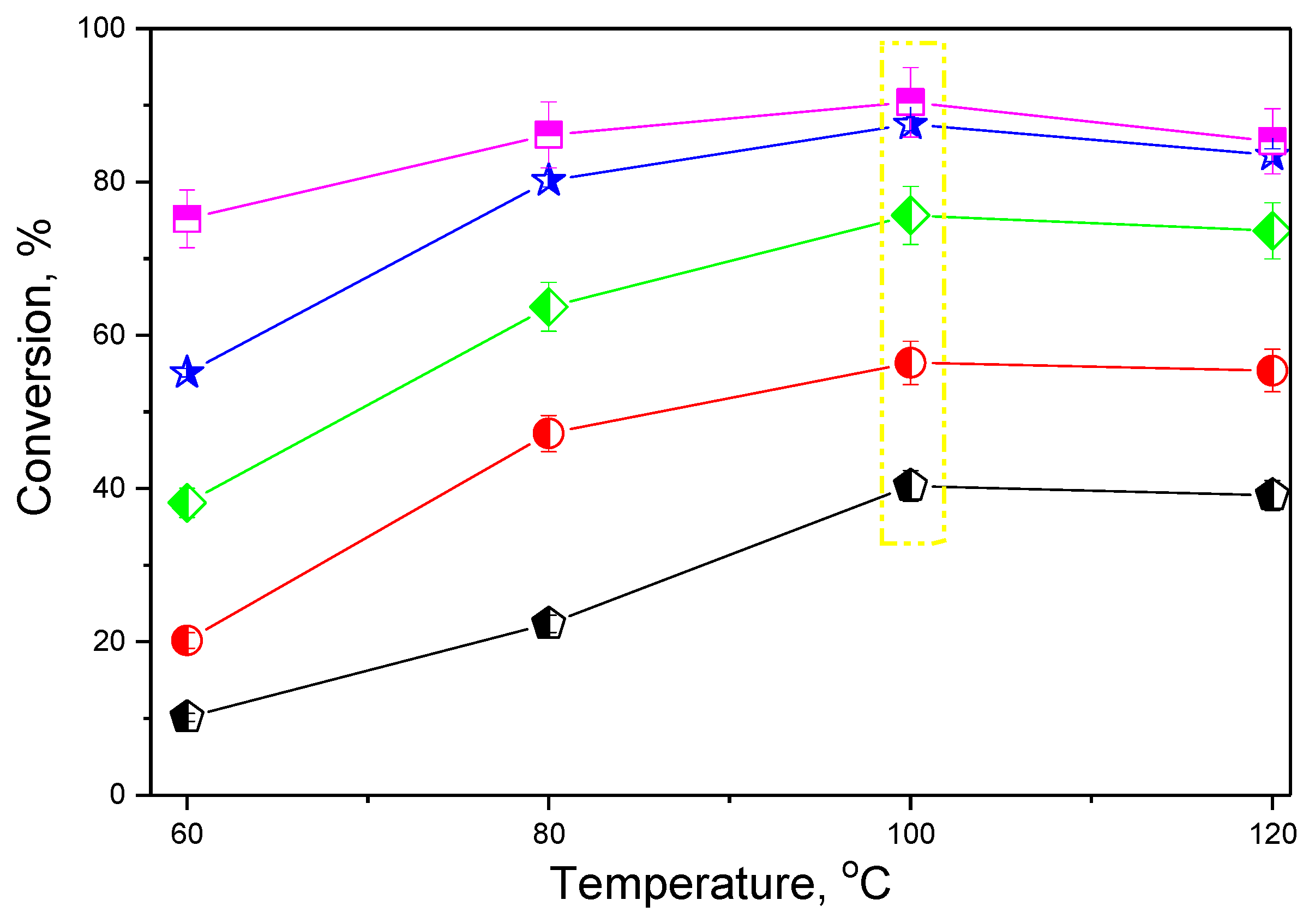
| Catalyst Code | Conversion (%) | Selectivity (%) | Yield (%) |
|---|---|---|---|
| ZSM-5 | 40 | 60 | 24 |
| ZSM-5-Na | 56 | 66 | 37 |
| ZSM-5-T | 76 | 83 | 63 |
| ZSM-5-C | 88 | 94 | 82 |
| ZSM-5-CT | 90 | 96 | 87 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smail, H.A.; Rehan, M.; Shareef, K.M.; Ramli, Z.; Nizami, A.-S.; Gardy, J. Synthesis of Uniform Mesoporous Zeolite ZSM-5 Catalyst for Friedel-Crafts Acylation. ChemEngineering 2019, 3, 35. https://doi.org/10.3390/chemengineering3020035
Smail HA, Rehan M, Shareef KM, Ramli Z, Nizami A-S, Gardy J. Synthesis of Uniform Mesoporous Zeolite ZSM-5 Catalyst for Friedel-Crafts Acylation. ChemEngineering. 2019; 3(2):35. https://doi.org/10.3390/chemengineering3020035
Chicago/Turabian StyleSmail, Heman A., Mohammad Rehan, Kafia M. Shareef, Zainab Ramli, Abdul-Sattar Nizami, and Jabbar Gardy. 2019. "Synthesis of Uniform Mesoporous Zeolite ZSM-5 Catalyst for Friedel-Crafts Acylation" ChemEngineering 3, no. 2: 35. https://doi.org/10.3390/chemengineering3020035
APA StyleSmail, H. A., Rehan, M., Shareef, K. M., Ramli, Z., Nizami, A.-S., & Gardy, J. (2019). Synthesis of Uniform Mesoporous Zeolite ZSM-5 Catalyst for Friedel-Crafts Acylation. ChemEngineering, 3(2), 35. https://doi.org/10.3390/chemengineering3020035








