Hydrogen and Oxygen Evolution in a Membrane Photoreactor Using Suspended Nanosized Au/TiO2 and Au/CeO2
Abstract
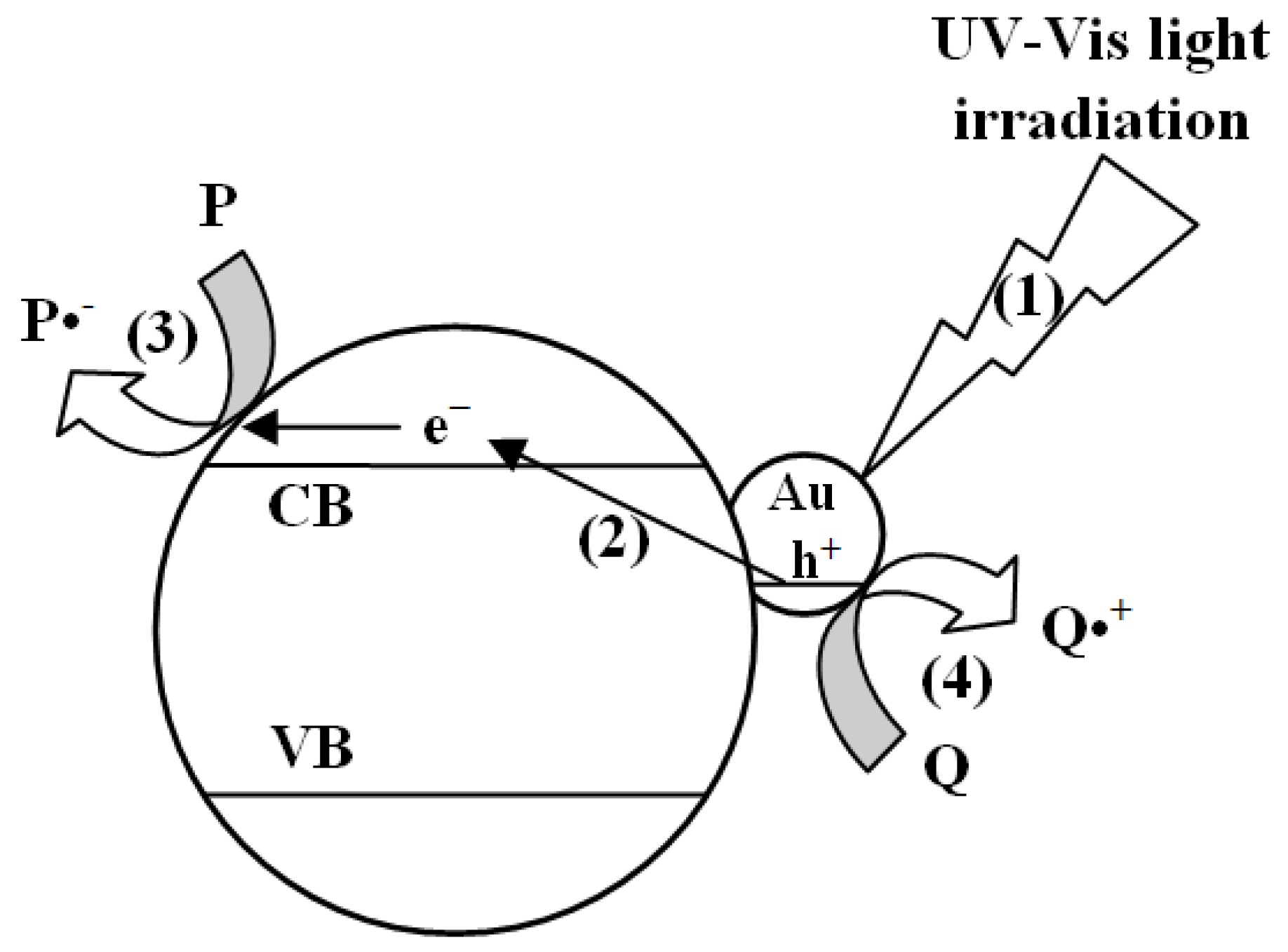
1. Introduction
2. Materials and Methods
2.1. Photocataytic Tests
2.2. Membrane Modification
2.3. Iron Spectrophotometric Determination
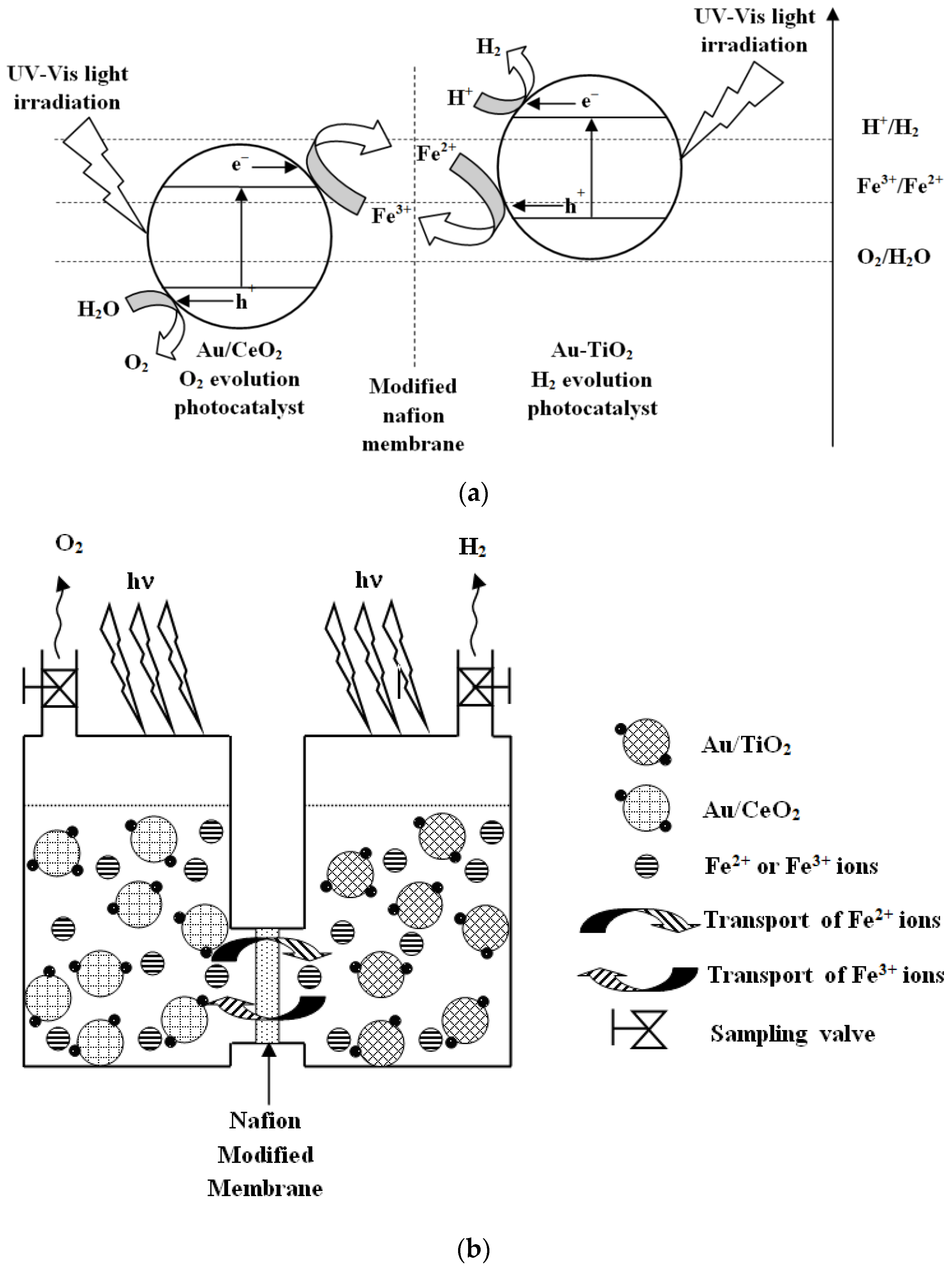
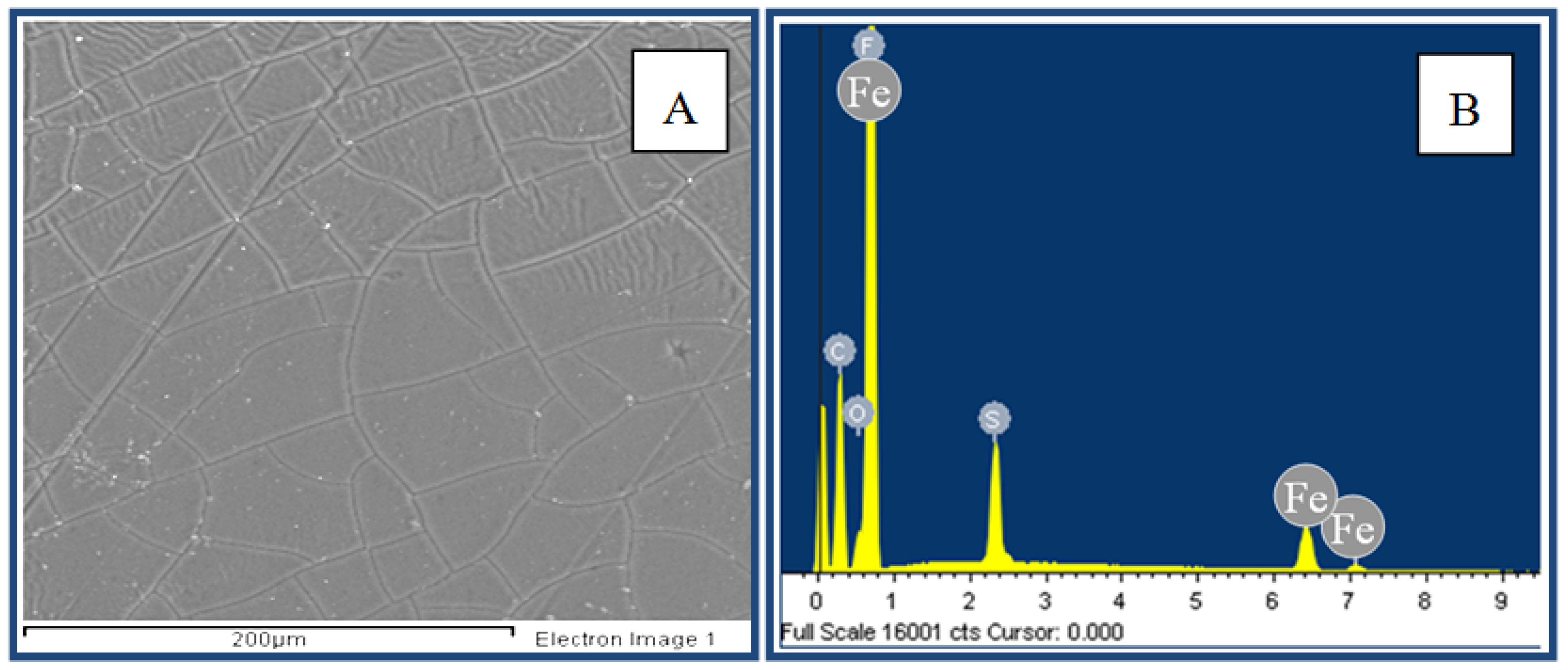
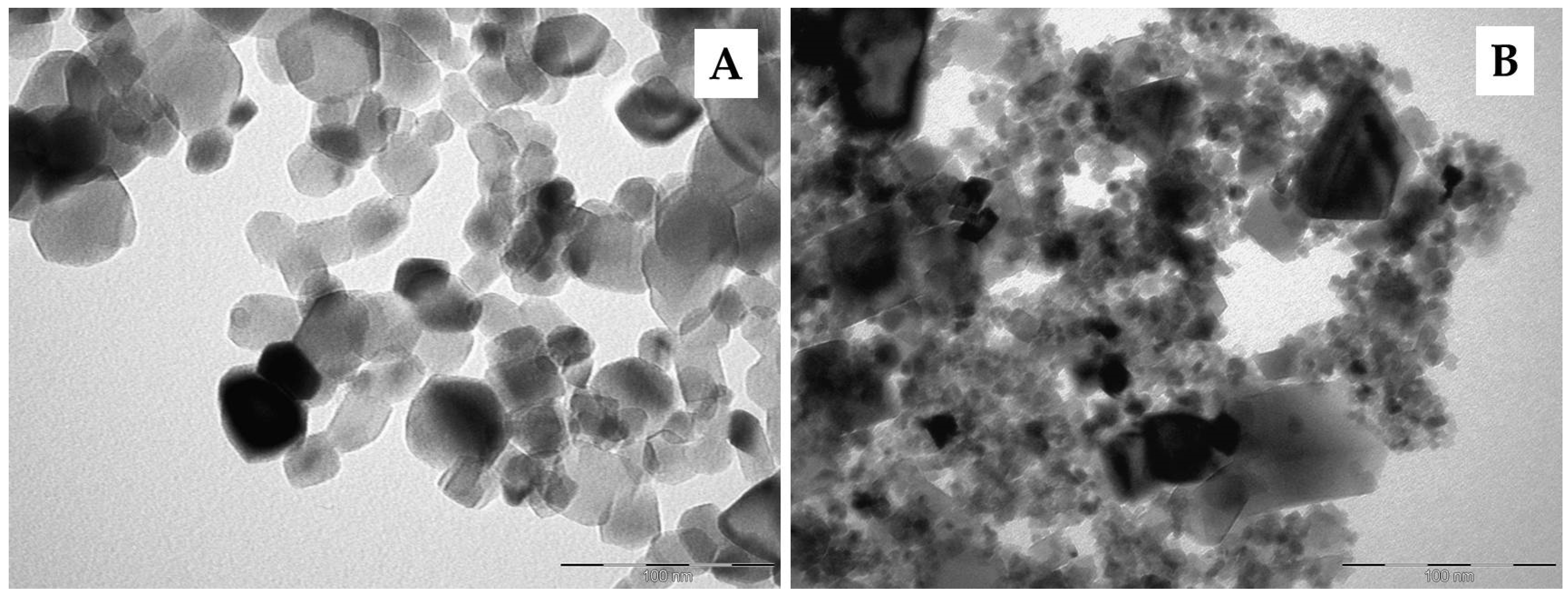
3. Results and Discussion
3.1. Diffusion Test
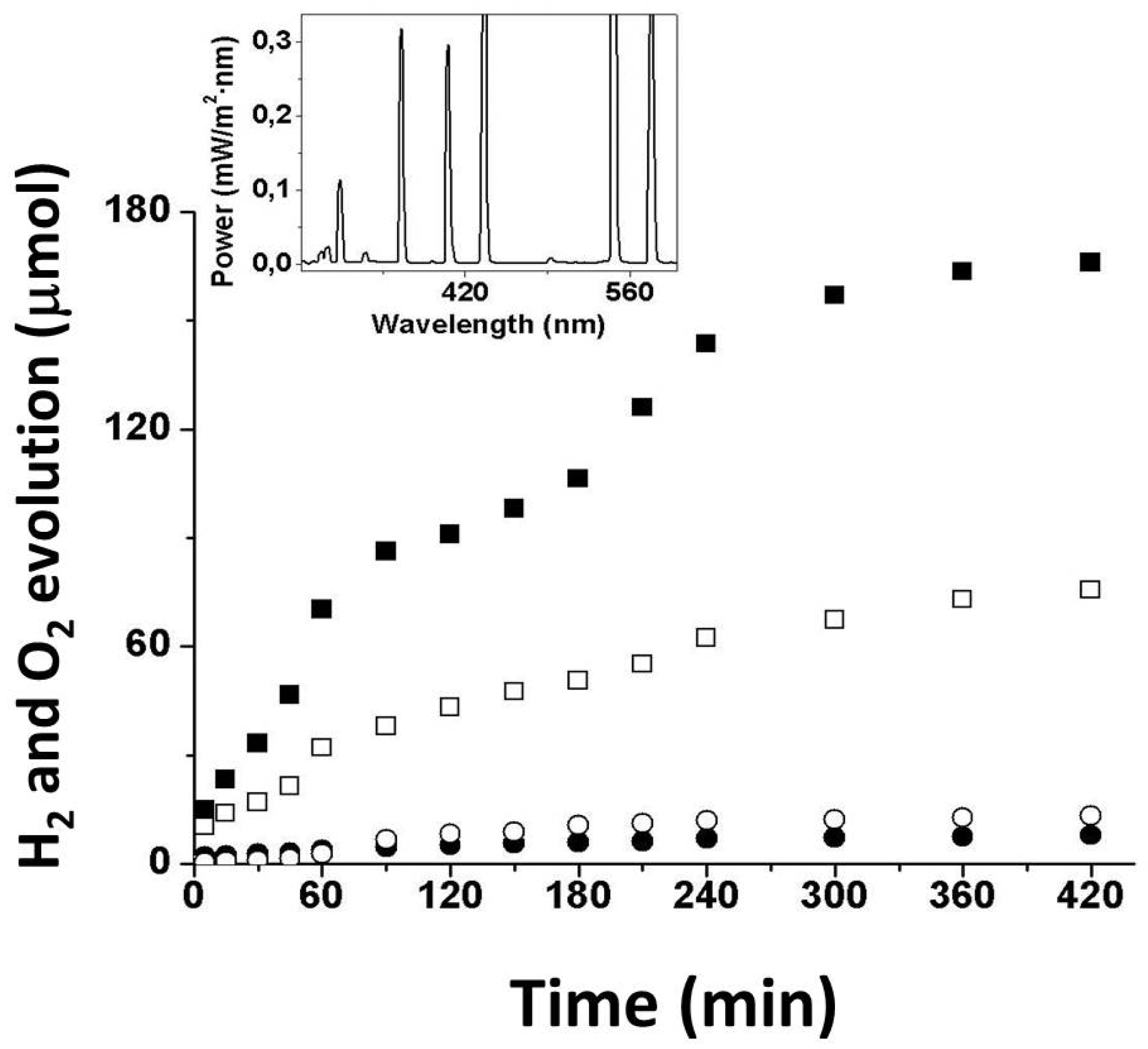
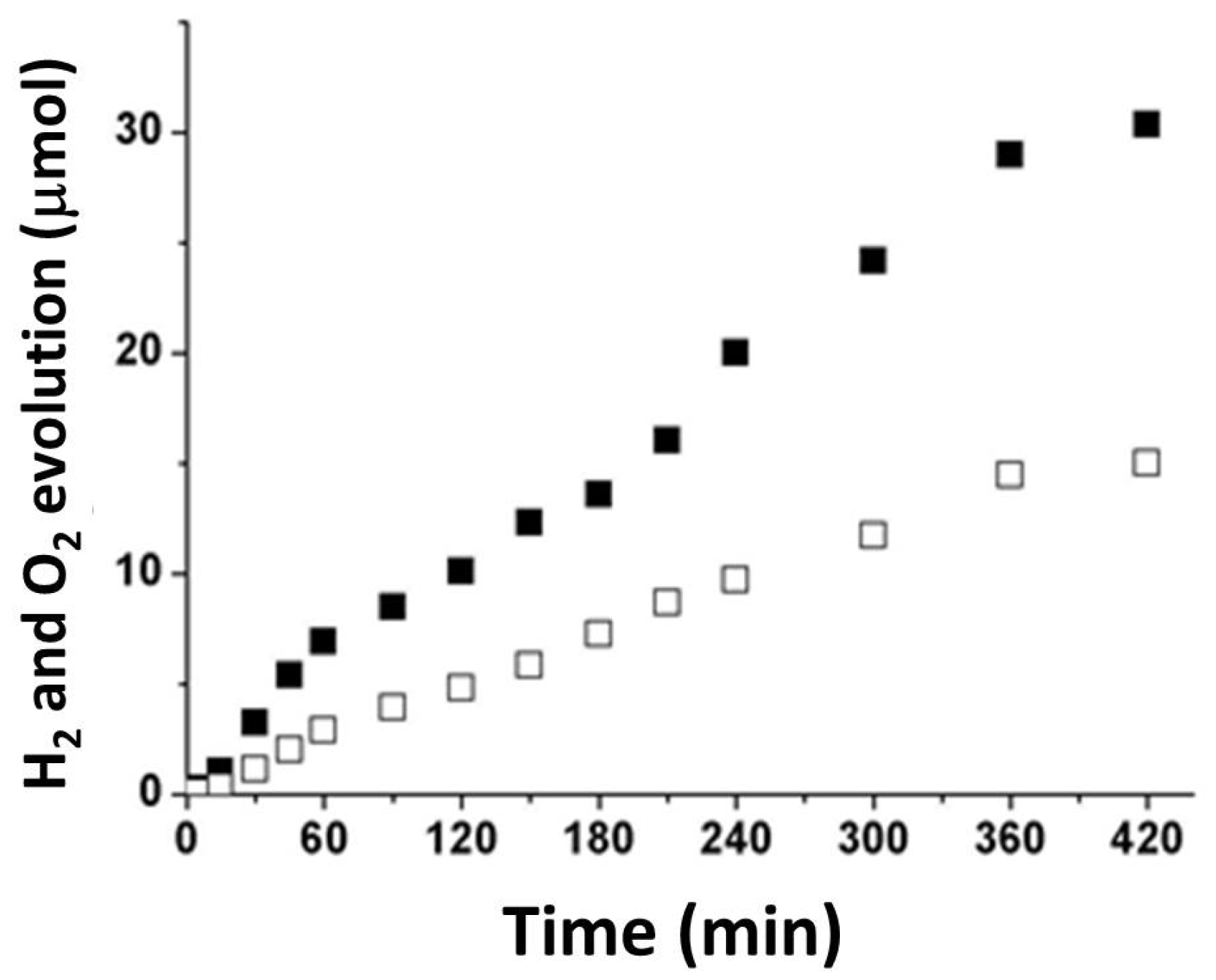
3.2. Photocatalytic Tests
4. Conclusions
- —
- The employed photocatalysts promoted simultaneous hydrogen and oxygen generation;
- —
- The optimal content of ferric ions in the Au/CeO2 compartment was 5 mM;
- —
- Gold operated as a photosensitizer allowing photocatalytic hydrogen and oxygen formation under visible light;
- —
- A gold loading of 0.25 wt% led to the best results in terms of hydrogen and oxygen evolution (166.1 and 75.6 μmol, respectively, after 7 h of UV-visible-light irradiation);
- —
- Hydrogen and oxygen were produced in stoichiometric amounts, i.e., 30.36 and 14.89 μmol, respectively, after 7 h of irradiation with visible light;
- —
- The decrease in permeation rate of iron ions through the Nafion membrane affected the photocatalytic performance, slowing the generation rates of both hydrogen and oxygen.
Author Contributions
Funding
Conflicts of Interest
References
- Bamwenda, G.R.; Tsubota, S.; Nakamura, T.; Haruta, M. Photoassisted Hydrogen-Production from a water-ethanol solution—A Comparison of activities of Au-TiO2 and Pt-TiO2. J. Photochem. Photobiol. A-Chem. 1995, 89, 177–189. [Google Scholar] [CrossRef]
- Khan, S.U.M.; Al-Shahry, M.; Ingler, W.B. Efficient photochemical water splitting by a chemically modified n-TiO2. Science 2002, 297, 2243–2245. [Google Scholar] [CrossRef] [PubMed]
- Loddo, V.; Palmisano, L.; Marino, T.; Molinari, R. Membranes for photocatalysis in water and wastewater treatment. In Advanced Membrane Science and Technology for Sustainable Energy and Environmental Applications; Basile, A., Nunes, S.P., Eds.; Woodhead Publishing: Sawston, UK, 2011; pp. 746–768. [Google Scholar]
- Maeda, K.; Domen, K. New non-oxide photocatalysts designed for overall water splitting under visible light. J. Phys. Chem. C 2007, 111, 7851–7861. [Google Scholar] [CrossRef]
- Li, D.; Yu, J.C.; Nguyen, V.; Wu, C.S.; Wang, X. A dual-function photocatalytic system for simultaneous separating hydrogen from water splitting and photocatalytic degradation of phenol in a twin-reactor. Appl. Catal. B Environ. 2018, 239, 268–279. [Google Scholar] [CrossRef]
- Argurio, P.; Fontananova, E.; Molinari, R.; Drioli, E. Photocatalytic Membranes in Photocatalytic Membrane Reactors. Processes 2018, 6, 162. [Google Scholar] [CrossRef]
- Iglesias, O.; Rivero, M.J.; Urtiaga, A.M.; Ortiz, I. Membrane-based photocatalytic systems for process intensification. Chem. Eng. J. 2016, 305, 136–148. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Mahmoodi, L. Performance of Reactors With PMs; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128135495. [Google Scholar]
- Molino, A.; Migliori, M.; Blasi, A.; Davoli, M.; Marino, T.; Chianese, S.; Catizzone, E.; Giordano, G. Municipal waste leachate conversion via catalytic supercritical water gasification process. Fuel 2017, 206, 155–161. [Google Scholar] [CrossRef]
- Marbán, G.; Valdés-Solís, T. Towards the hydrogen economy? Int. J. Hydrog. Energy 2007, 32, 1625–1637. [Google Scholar] [CrossRef]
- Armaroli, N.; Balzani, V. The hydrogen issue. ChemSusChem 2011, 4, 21–36. [Google Scholar] [CrossRef]
- Ball, M.; Wietschel, M. The future of hydrogen—Opportunities and challenges. Int. J. Hydrog. Energy 2009, 34, 615–627. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.G.; Juárez, R.; Marino, T.; Molinari, R.; García, H. Influence of excitation wavelength (UV or visible light) on the photocatalytic activity of titania containing gold nanoparticles for the generation of hydrogen or oxygen from water. J. Am. Chem. Soc. 2011, 133, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Galiano, F.; Song, X.; Marino, T.; Boerrigter, M.; Saoncella, O.; Simone, S.; Faccini, M.; Chaumette, C.; Drioli, E.; Figoli, A. Novel Photocatalytic PVDF/Nano-TiO2 Hollow Fibers for Environmental Remediation. Polymers 2018, 10, 1134. [Google Scholar] [CrossRef]
- Molinari, R.; Caruso, A.; Palmisano, L. Photocatalytic Processes in Membrane Reactors. In Comprehensive Membrane Science and Engineering; Drioli, E., Giorno, L., Eds.; Elsevier Science B.V.: Oxford, UK, 2010; Volume 3, pp. 165–193. ISBN 9780080932507. [Google Scholar]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Marino, T.; Boerigter, M.; Faccini, M.; Chaumette, C.; Arockiasamy, L.; Bundschuh, J.; Figoli, A. Photocatalytic activity and synthesis procedures of TiO2 nanoparticles for potential applications in membranes. In Application of Nanotechnology in Membranes for Water Treatment; Hoinkis, J., Figoli, A., Altinkaya, S.A., Bundschuh, J., Eds.; CRC, Taylor and Francis Group: Boca Raton, FL, USA, 2017; ISBN 9781138896581. [Google Scholar]
- Figoli, A.; Marino, T.; Simone, S.; Boerighter, M.; Faccin, M.; Chaumette, C.; Drioli, E. Application of nano-sized TiO2 in membrane technology. In Application of Nanotechnology in Membranes for Water Treatment; Hoinkis, J., Figoli, A., Altinkaya, S.A., Bundschuh, J., Eds.; CRC, Taylor and Francis Group: Boca Raton, FL, USA, 2017; ISBN 9781138896581. [Google Scholar]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under UV/visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef]
- Primo, A.; Marino, T.; Corma, A.; Molinari, R.; Garcia, H. Efficient visible-light photocatalytic water splitting by minute amounts of gold supported on nanoparticulate CeO2 obtained by a biopolymer templating method. J. Am. Chem. Soc. 2011, 133, 6930–6933. [Google Scholar] [CrossRef]
- Marino, T.; Primo, A.; Corma, A.; Molinari, R.; Garcia, H. Photocatalytic overall water splitting by combining gold nanoparticles supported on TiO2 and CeO2. In Proceedings of the 2nd European Symposium on Photocatalysis, Bordeaux, France, 19 October 2011; p. 76. [Google Scholar]
- Higashi, M.; Abe, R.; Ishikawa, A.; Takata, T.; Ohtani, B.; Domen, K. Z-scheme Overall Water Splitting on Modified-TaON Photocatalysts under Visible Light (λ < 500 nm). Chem. Lett. 2008, 37, 138–139. [Google Scholar] [CrossRef]
- Maeda, K.; Higashi, M.; Lu, D.; Abe, R.; Domen, K. Efficient nonsacrificial water splitting through two-step photoexcitation by visible light using a modified oxynitride as a hydrogen evolution photocatalyst. J. Am. Chem. Soc. 2010, 132, 5858–5868. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, G.L.; Tealdi, C.; Mustarelli, P.; Selli, E. Fabrication of Pt/Ti/TiO2 Photoelectrodes by RF-Magnetron Sputtering for Separate Hydrogen and Oxygen Production. Materials 2016, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Selli, E.; Chiarello, G.L.; Quartarone, E.; Mustarelli, P.; Rossetti, I.; Forni, L. Photocatalytic water splitting device for separate hydrogen and oxygen evolution. Chem. Commun. 2007, 47, 5022–5024. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, L.; Yu, J.; Wageh, S.; Al-ghamdi, A.A.; Jaroniec, M. Direct Z-scheme photocatalysts: Principles, synthesis, and applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]
- Lo, C.; Huang, C.; Liao, C.; Wu, J.C.S. Novel twin reactor for separate evolution of hydrogen and oxygen in photocatalytic water splitting. Int. J. Hydrog. Energy 2010, 35, 1523–1529. [Google Scholar] [CrossRef]
- Seger, B.; Kamat, P.V. Fuel cell geared in reverse: Photocatalytic hydrogen production using a TiO2/Nafion/Pt membrane assembly with no applied bias. J. Phys. Chem. C 2009, 113, 18946–18952. [Google Scholar] [CrossRef]
- Yu, S.-C.; Huang, C.-W.; Liao, C.-H.; Wu, J.C.S.; Chang, S.-T.; Chen, K.-H. A novel membrane reactor for separating hydrogen and oxygen in photocatalytic water splitting. J. Membr. Sci. 2011, 382, 291–299. [Google Scholar] [CrossRef]
- Kitano, M.; Tsujimaru, K.; Anpo, M. Decomposition of water in the separate evolution of hydrogen and oxygen using visible light-responsive TiO2 thin film photocatalysts: Effect of the work function of the substrates on the yield of the reaction. Appl. Catal. A Gen. 2006, 314, 179–183. [Google Scholar] [CrossRef]
- Fujihara, K.; Ohno, T.; Matsumura, M. Splitting of water by electrochemical combination of two photocatalytic reactions on TiO2 particles. J. Chem. Soc. Faraday Trans. 1998, 94, 3705–3709. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Yair, E.-E. Review of Advanced Materials for Proton Exchange Membrane Fuel Cells. Energy Fuels 2014, 28, 7303–7330. [Google Scholar] [CrossRef]
- Ramírez, J.; Godínez, L.A.; Méndez, M.; Meas, Y.; Rodriguez, F.J. Heterogeneous photo-electro-Fenton process using different iron supporting materials. J. Appl. Electrochem. 2010, 40, 1729–1736. [Google Scholar] [CrossRef]
- Feng, J.; Hu, X.; Yue, P.L.; Zhu, H.Y.; Lu, G.Q. Discoloration and mineralization of Reactive Red HE-3B by heterogeneous photo-Fenton reaction. Water Res. 2003, 37, 3776–3784. [Google Scholar] [CrossRef]
- Sabhi, S.; Kiwi, J. Degradation of 2,4-dichlorophenol by immobilized iron catalysts. Water Res. 2001, 35, 1994–2002. [Google Scholar] [CrossRef]
- Kiwi, J. Innovative immobilized Fenton systems useful in the abatement of industrial pollutants. In Proceedings of the Third Asia-Pacific Conference on Sustainable Energy and Environmental Technologies, Hong Kong, China, 3–6 December 2000; p. 562. [Google Scholar]
- Goswami, A.K.; Acharya, A.; Pandey, A.K. Study of self-diffusion of monovalent and divalent cations in Nafion-117 ion-exchange membrane. J. Phys. Chem. B 2001. [Google Scholar] [CrossRef]
- Silva, C.G.; Bouizi, Y.; Fornés, V.; Garcia, H. Layered Double Hydroxides as Highly Efficient Photocatalysts for Visible Light Oxygen Generation from Water. J. Am. Chem. Soc. 2009, 131, 13833–13839. [Google Scholar] [CrossRef] [PubMed]

| C0 Fe3+ (mM) | Evolved H2,7h (μmol) | Evolved O2,7h (μmol) | H2 r0 × 102 (μmol·min−1) | O2 r0 × 102 (μmol·min−1) |
|---|---|---|---|---|
| 2 | 56.2 | 28.1 | 34.2 | 20.7 |
| 5 | 166.1 | 75.6 | 114.0 | 53.4 |
| 10 | 86.2 | 43.0 | 96.3 | 32.5 |
| 20 | 43.2 | 24.2 | 34.8 | 21.1 |
| 50 | 25.5 | 16.3 | 33.4 | 23.5 |
| Au Loading (wt%) | Evolved H2,7h (μmol) | Evolved O2,7h (μmol) | H2 r0 × 102 (μmol·min−1) | O2 r0 × 102 (μmol·min−1) |
|---|---|---|---|---|
| 0.25 | 166.1 | 75.6 | 114.0 | 53.4 |
| 0.6 | 152.0 | 61.3 | 112.1 | 70.8 |
| 1.0 | 71.0 | 25.1 | 67.0 | 32.2 |
| Au Loading (wt%) | Evolved H2,7h (μmol) | Evolved O2,7h (μmol) | H2 r0 × 102 (μmol·min−1) | O2 r0 × 102 (μmol·min−1) |
|---|---|---|---|---|
| 0.25 | 30.36 | 14.89 | 11.0 | 4.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, T.; Figoli, A.; Molino, A.; Argurio, P.; Molinari, R. Hydrogen and Oxygen Evolution in a Membrane Photoreactor Using Suspended Nanosized Au/TiO2 and Au/CeO2. ChemEngineering 2019, 3, 5. https://doi.org/10.3390/chemengineering3010005
Marino T, Figoli A, Molino A, Argurio P, Molinari R. Hydrogen and Oxygen Evolution in a Membrane Photoreactor Using Suspended Nanosized Au/TiO2 and Au/CeO2. ChemEngineering. 2019; 3(1):5. https://doi.org/10.3390/chemengineering3010005
Chicago/Turabian StyleMarino, Tiziana, Alberto Figoli, Antonio Molino, Pietro Argurio, and Raffaele Molinari. 2019. "Hydrogen and Oxygen Evolution in a Membrane Photoreactor Using Suspended Nanosized Au/TiO2 and Au/CeO2" ChemEngineering 3, no. 1: 5. https://doi.org/10.3390/chemengineering3010005
APA StyleMarino, T., Figoli, A., Molino, A., Argurio, P., & Molinari, R. (2019). Hydrogen and Oxygen Evolution in a Membrane Photoreactor Using Suspended Nanosized Au/TiO2 and Au/CeO2. ChemEngineering, 3(1), 5. https://doi.org/10.3390/chemengineering3010005









