1. Introduction
Typical 0Crl7Ni4Cu4Nb stainless steel is characteristic by low carbon (C) and high alloying elements of chromium (Cr), nickel (Ni), and copper (Cu). The material data of this chromium rich GB/T 3280 stainless steel has been provided by Metalinfo, Eagle International Software [
1], with the following mass percent composition (wt %): C 0.04%–0.07%, silicon (Si) 0.3%–1%, manganese (Mn) 0.7%–1%, phosphorum (P) 0.02%–0.04%, sulfur (S) 0.01%–0.03%, Cr 15%–17.5%, Ni 3%–5%, Niobium (Nb) 0.35%–0.45%, Cu 3%–5%, Molybdenum (Mo) 0.3%–0.5%, and Iron (Fe) 72–73.2%. 0Crl7Ni4Cu4Nb is of duplex ferrite-austenite microstructure, and is excellent in corrosion resistance and mechanical stability [
2]. It is widely used in aerospace, turbine blades, the food industry, offshore platforms, and synthetic fiber mold manufacturing. However, the thermal plasticity of 0Crl7Ni4Cu4Nb remains to be improved. Cracks often arise in ingot forming and the hot forging process, which is the major cause of reducing steel plasticity and limiting the service life of 0Crl7Ni4Cu4Nb steel in practice.
The service life of duplex ferrite-austenite steel highly depends on aging factors, such as ferrite formation, chemical composition, thermal history, corrosion, microstructure, and texture [
3]. Among those factors, ferrite formation plays an essential role in steel aging. Ferrite in steel leads to the decrease in ductility, toughness, and impacts corrosion resistance—thus damage and the fracture of steel microstructure—and consequently limits its service life [
3,
4]. Ferrite is a blend of Fe and one or more additional metallic elements, such as Cr, C, Mo, and other alloying elements. It is commonly formed at high temperature in steel forming, thus it is also called high temperature ferrite. Ferrite is susceptible to thermal hardening, which leads to local premature cracking in steel microstructure. The formed cracks eventually become cavities that lead to the final ductile fracture by cavity growth and fusion [
3,
4]. Therefore, controlling ferrite formation is the key technique to improve the mechanical property and to prolong the service life of steel.
Ferrite in steel can be divided into equilibrium and non-equilibrium phase ferrite, according to the mechanism of ferrite body formation [
3,
5]. Ferrite body in the equilibrium state is mainly determined by the chemical composition of steel. The increase of ferrite elements, such as Cr, Mo, vanadium (V), and Si in steel microstructure, can promote the formation of ferrite. The ferrite body, once formed in equilibrium phase, is difficult to be removed by subsequent heat treatment. The ferrite in a non-equilibrium state is generally formed at high temperatures, which is indeed part of the steel tissue not yet transited into austenite in the supercooling processes. The ferrite formed in a non-equilibrium state can be thermally eliminated in subsequent processes. Ferrite also appears at the time of solidification and heat processing. The temporally different ferrites are various in morphology and distribution.
To date, no publication is available yet, being specifically addressed on ferrite formation in 0Crl7Ni4Cu4Nb stainless steel. The current study intends to reveal the mechanism attributable to the steel’s compelling advantages in mechanical stability and corrosion resistance by exploring ferrite formation in stainless steel.
4. Discussion
Ferrite is the origin of crack initiation and corrosion commencement of steels [
10]. Theoretically, minimizing ferrite formation and thickening ferrite-free surface zones has the potential measures to improve the mechanical stability and corrosion resistance of steel. Thus, it is necessary to understand the mechanism of ferrite formation and distribution in the process of steel forming.
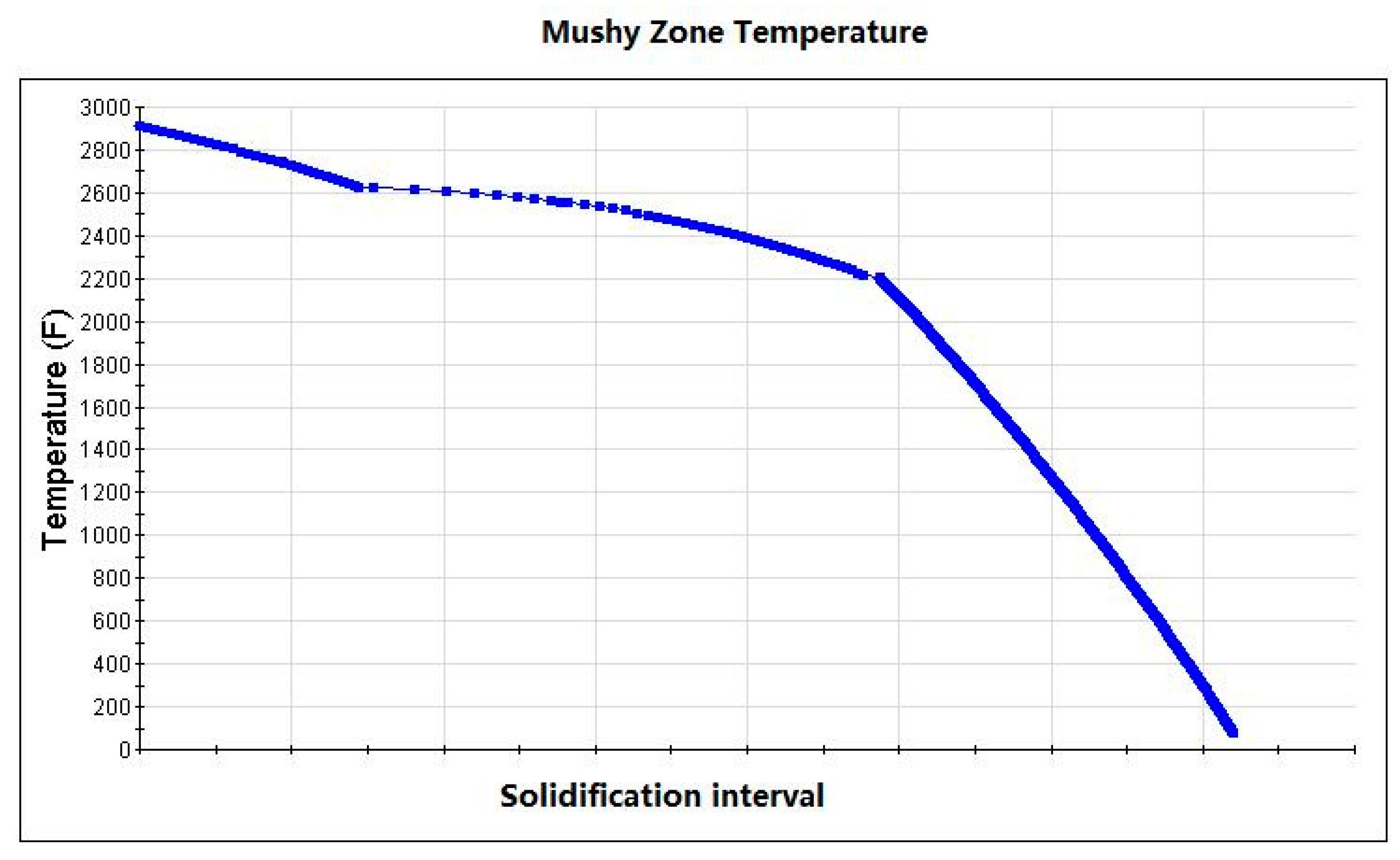
The current study investigated the correlation of ferrite formation to chemical composition and the mushy zone temperature (the difference between solidus and liquidus temperatures T
L−T
S). Ferrite is generally formed at high temperatures [
10]. At the beginning of ingot forming, the L to Delta transformation occurs first, then followed by the peritectic transformation to form austenite Gamma. Ferrite is often nucleated and grown up in the material when the processing temperature reaches Delta and Gamma phase or Delta single-phase. It could be retained in the tissue if the formed ferrite was not completely transformed into austenite gamma when cooling [
11]. As shown in
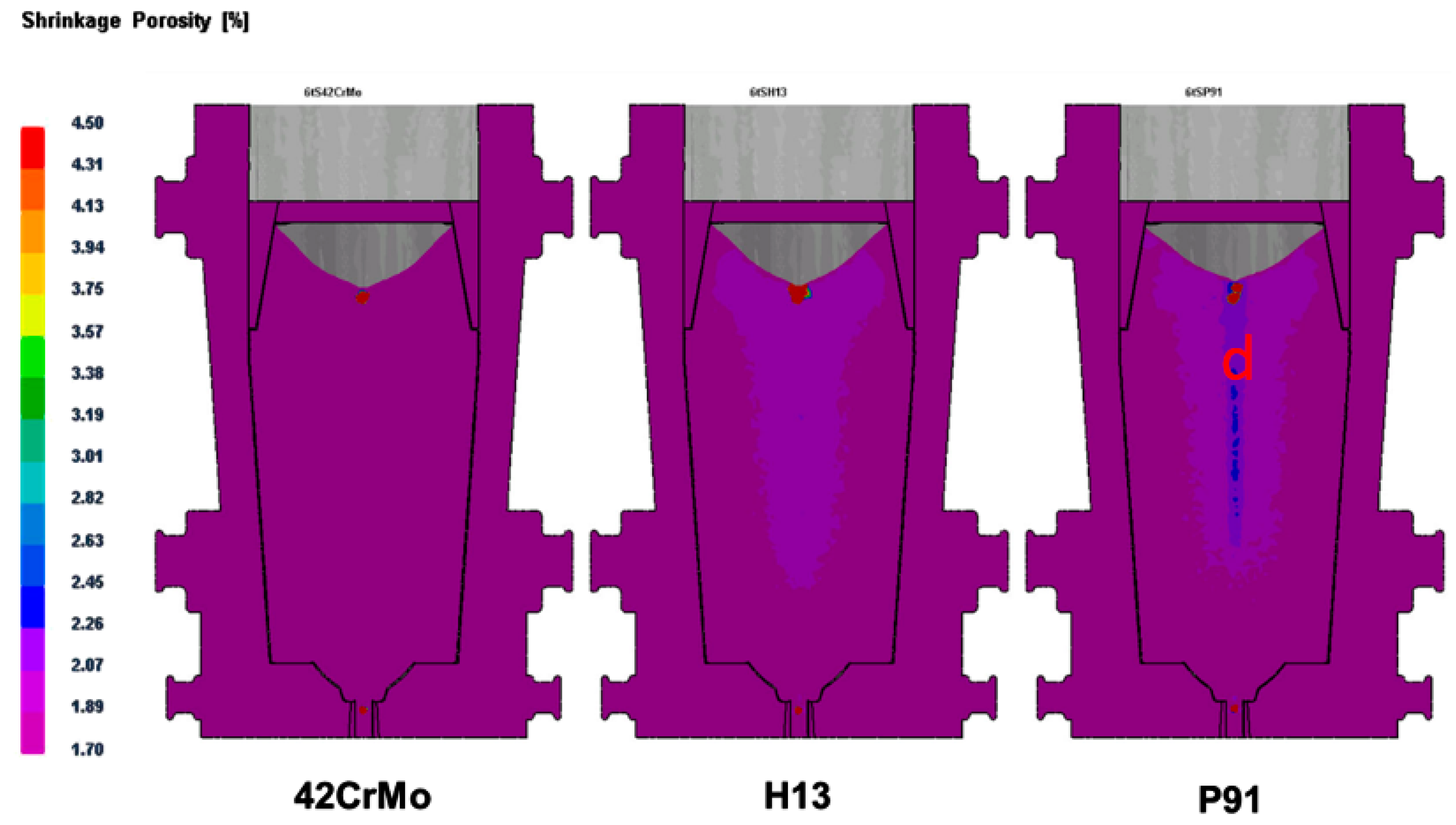
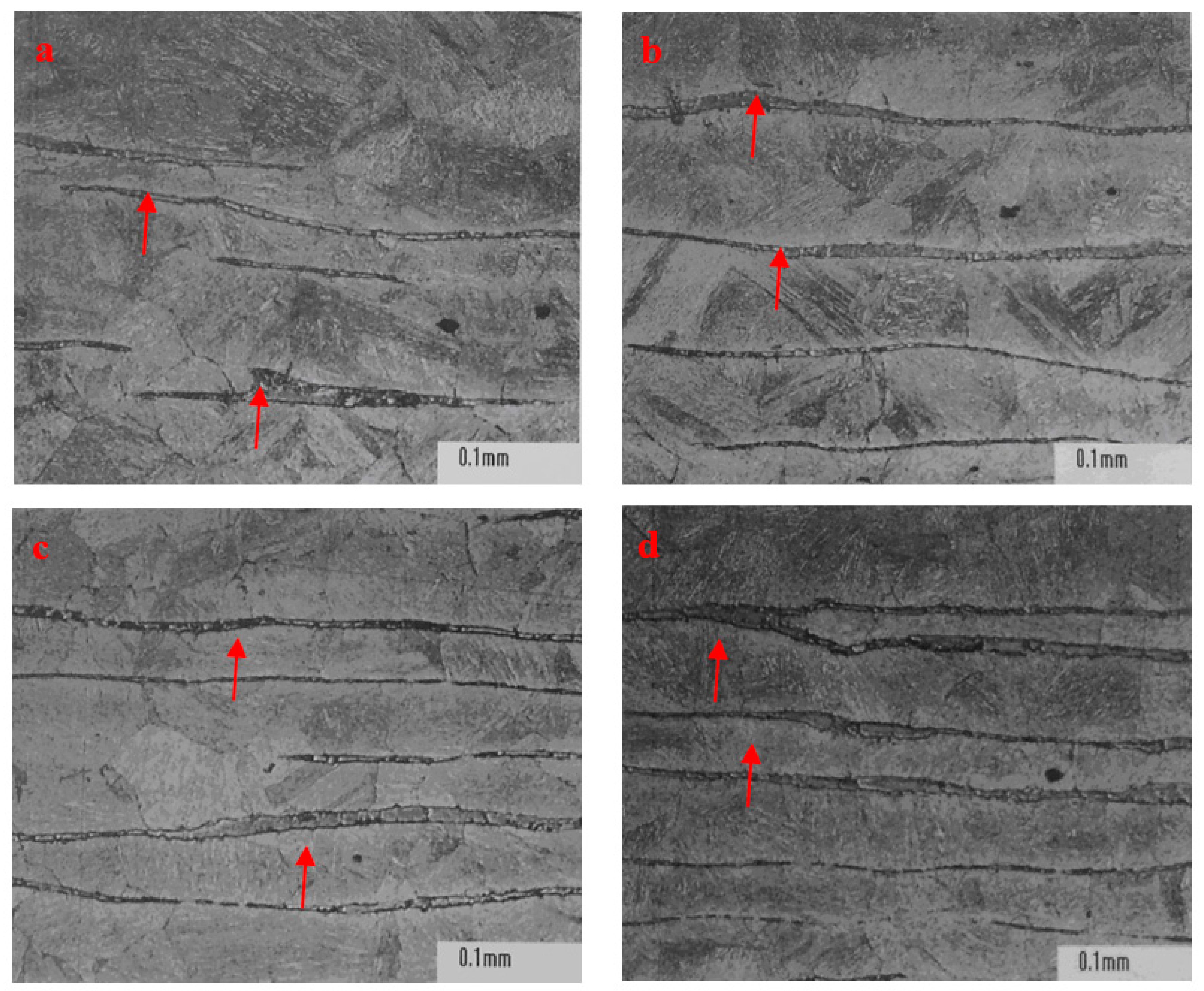
Figure 6, the morphology, density, and distribution pattern of ferrite in stainless steel P91 were different in the zones away from surface. More ferrite showing as continuous densified banded strips were observed in the core region than in the regions close to surface of ingot. This phenomenon could be attributable to the central segregation propensity of ferrite forming elements, in the solidification process, in addition to the temperature in the core region being higher due to a relatively slower heat exchange compared to the region close to surface.
Previous studies concluded that solidus and liquidus temperatures of a stainless steel were determined by its chemical composition [
10,
11]. Proper forging temperature relevant to solidus and liquidus temperatures can minimize the secondary ferrite formation and extend ferrite-free surface zone during forging process. Several computational models have been developed for the simulation of ferrite formation in correlation to temperature in stainless steel. Equation (3) is highly reliable in predicting ferrite formation tendency [
10,
11,
12,
13,
14]. The reliability of Equation (3) was validated with experimental data from several studies tested with various steels. Based on the formula, optimal temperatures for thermal treatment to various steels have been established. For example, the forging temperatures of stainless steel and heat-resistant steel are generally set up below 1180 °C [
12], the initial forging and final forging temperatures of 0Cr13, 1Cr13, 2Cr13, and 3Cr13, the GB standard martensite stainless steels being composed of 12–14% Cr, 82–84% Fe, and other trace alloying elements are 1150 °C and 850 °C, respectively [
15]; the initial forging and final forging temperatures of 1Cr18Ni9Ti are 1180 °C and 850 °C [
16]; the initial forging and final forging temperatures of 2Cr3WMoV are 1150 °C and 800 °C [
12,
16]. Equation (3) was further validated with C controlled steel, as shown in
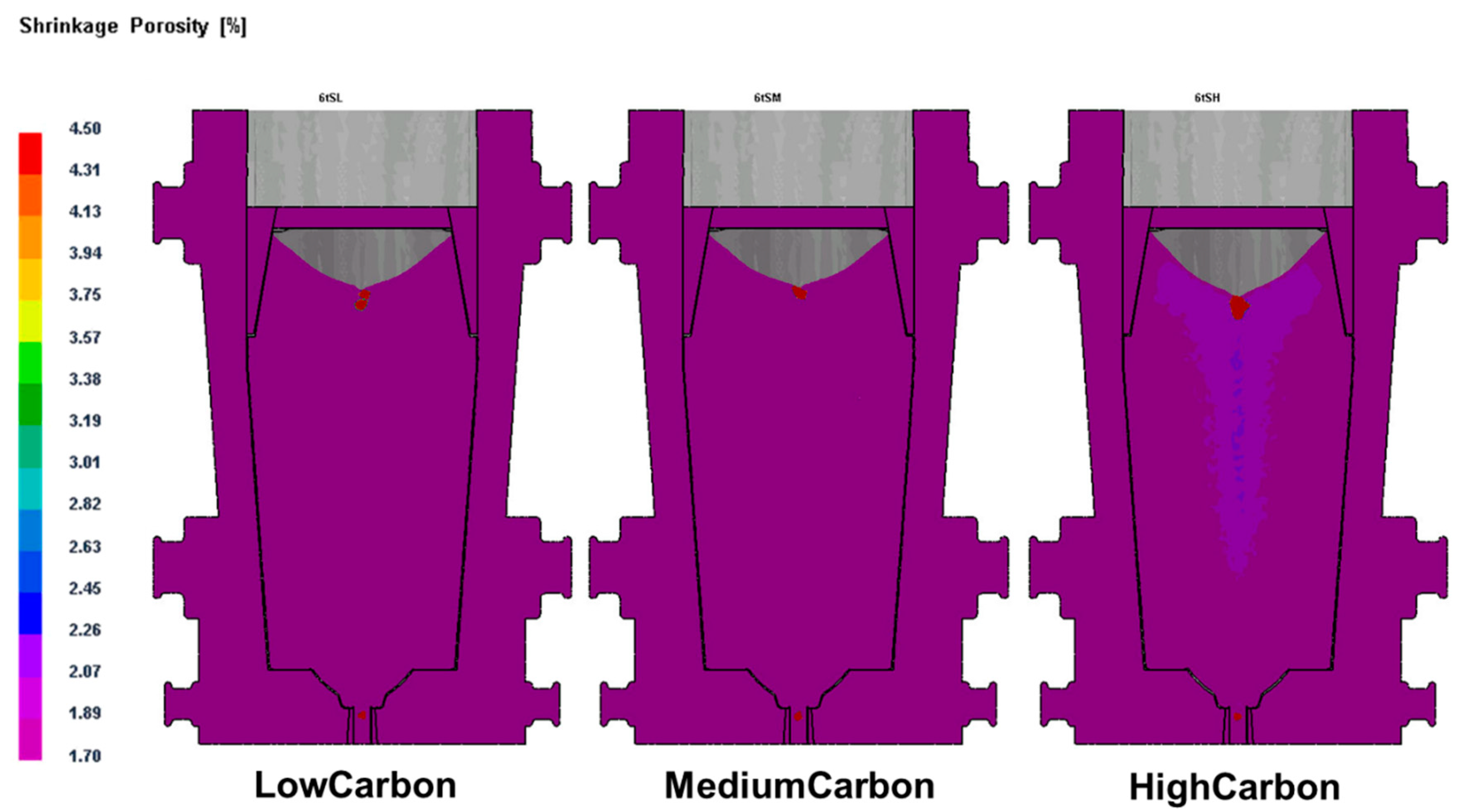
Figure 7. The simulation result was complied with the established conclusion that high Cr and temperature, and low C promote ferrite formation; in return, the result verified the reliability of Equations (1) and (3) in the prediction of ferrite formation.
Computational programs have been also used to predict ferrite formation in carbon steel precipitation hardening processes [
13,
14,
16]. Those programs took elements referring to chemical composition as simulating parameters, which can predict tendency, but do not quantitatively estimate ferrite formation. The chromium Equation (1) was up-graded to Equations (2)–(4) in considering the numerous ferrite formation affecting factors of chemical composition vs. the mushy zone and forging temperatures. The effect of each element of chemical composition on ferrite formation was expressed in chromium equivalence. The chemical composition elements (Cr, Mo, W, V, Nb, Al, and Si) were considered as factors favorable to ferrite formation, while the austenite forming elements (C, N, Mn, Ni, and Cu) were regarded as inhibitors to the appearance of ferrite. According to the chemical composition of 0Crl7Ni4Cu4Nb steel, Cr equivalent E
Cr Equation (5), as shown below, was established corresponding to the effective index of each chemical element.
E
Cr in Equation (5) was regarded as the simulating index of chemical composition controlling ferrite formation in tapping steel. C, N, and V, as seen in the formula, have greater impacts on ferrite formation, followed by Si, Mo, Ni, and Mn. Cr is the key element attributable to the corrosion resistance of stainless steel, mainly due to its fundamental property against oxidizing medium, such as acidic chloride. The level of Cr content also affects the resistance to intergranular, electrolytic, and the crevice corrosions of steel. Carbon is a harmful element to stainless steel. C and Cr in the steel can form a high Cr carbide of Cr23C6 at 650–850 °C, resulting in Cr-depletion that leads to the decrease of corrosion resistance, particularly the intergranular corrosion resistance of stainless steel [
4,
5]. Thus, the C content is generally controlled to be at a low level for the optimization of corrosion resistance. However, the inappropriate low level of C content can reduce a single austenite stability and increase the cost of the smelting process. Moreover, carbon is a strong austenite forming element that helps expand and stabilize austenite forming. The potentiality of carbon to austenite forming is about 30 times that of nickel (Ni). Ni is a main austenitic stabilizing element in stainless steel forming. Ferrite content in the solidification structure of Fe alloy steel may be significantly changed by adjusting Ni content and Ni equivalent elements [
15]. Cu is the element that can reduce the tendency to the cold hardening phase in steel forming, which improves the hardness and strength, corrosion resistance, plasticity, and cold workability of austenitic stainless steel [
15,
16]. Theoretically, ferrite could be minimized, or even totally avoided in steel tissue by an attentive control of chemical components within appropriate ranges, based on Equations (2) and (5).
Ferrite could be also formed during the forging process for implementing an inappropriate high temperature in thermal treatment, or an overtime heat preservation procedure [
2,
3]. Ferrite formation, in correspondence to temperature, was estimated by Equation (4).
The observation of the current study is aligned with the previous reported results that ferrite formation in stainless steels is different among those with dissimilar compositions [
2,
3,
4,
5,
10,
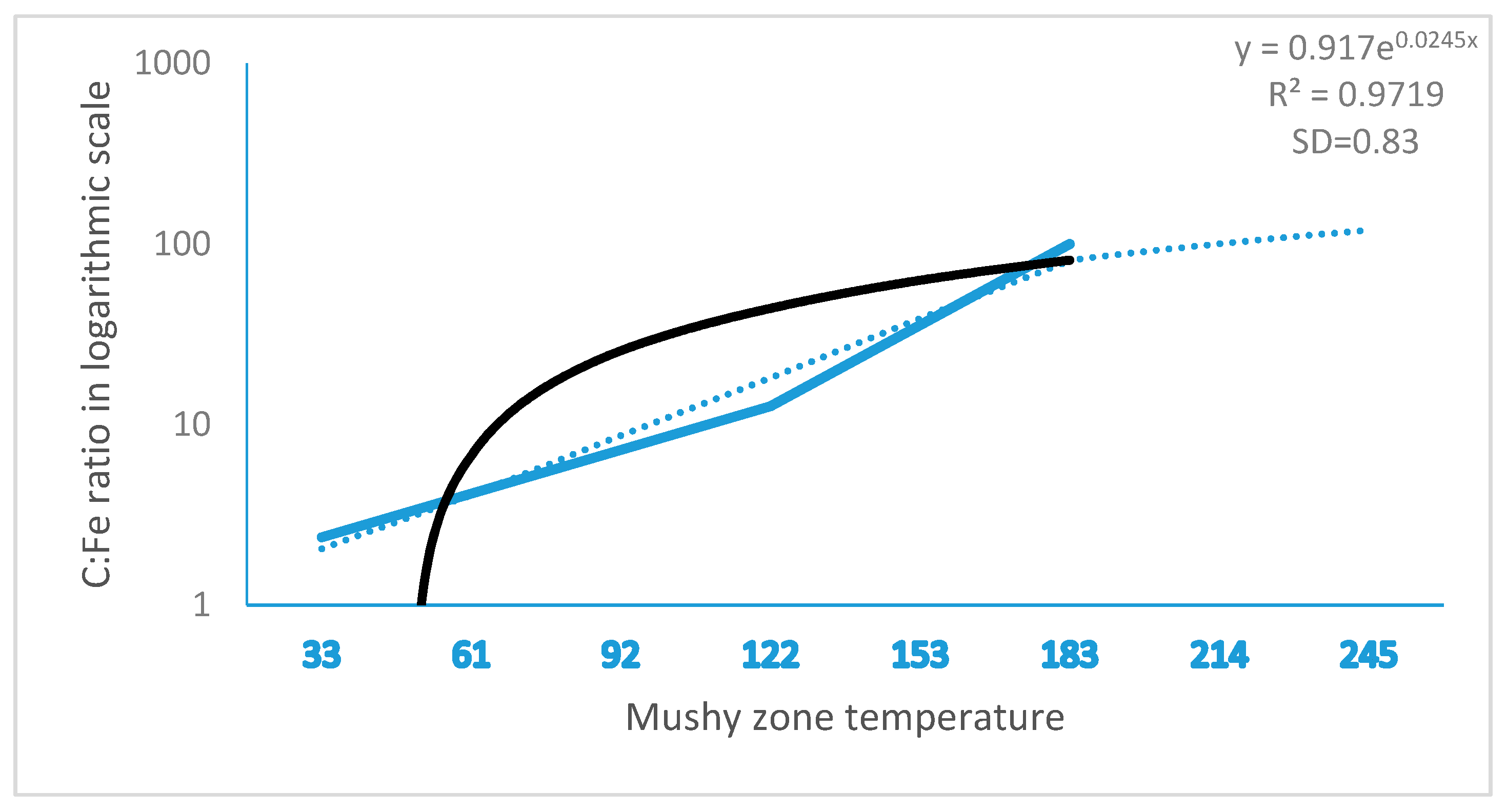
11]. In the study, we found that ferrite formation in steel ingots was highly relevant to the mushy zone temperature. The mushy zone temperature is a critical parameter for the proper adjustment of models (physical or numerical) or in the final stage of applied research of the real process. It indeed represents temperature gradients that significantly affect shrinkage porosity and ferrite formation, as shown in
Figure 4 and
Figure 5. We hypothesize that a wider mushy zone indicates a higher temperature gradient, which was susceptible to arouse crystalline rain in the supercooling process and provoked ferrite formation. More ferrites were observed in as-cast steels with high mushy zone temperatures, which supports our hypothesis. We found that ratios of Cr:Fe and C:Fe are more correlated to mushy zone temperature, which attracts our attention on the role of Fe, the major chemical content in ferrite formation. Based on our observation, we assume that ratios of Cr:Fe, C:Fe and Cr:C, rather arbitrary Cr and C contents, could be more relevant to ferrite formation in stainless steel, as shown in
Figure 2,
Figure 3,
Figure 4 and
Figure 5. Notably, as presented in
Table 4, both the distribution pattern and density of ferrite in 0Cr17Ni4Cu4Nb ingot risers and tails were deviated from the phenomenon of central segregation observed in all other examined steels in the study. Moreover, ferrite content was much lower than that in the standard reference steel GB/T8732, although Cr concentration was higher and the mushy zone temperature reached up to the higher level at 222 °C. Bizarrely, fewer ferrite presented in 0Cr17Ni4Cu4Nb tissue, though its mushy zone temperature and Cr content were the highest among the examined steels. The controversial observation on ferrite formation in 0Cr17Ni4Cu4Nb steel remains inexplicable and further studies will be required.
In responding to the inevitable ferrite formation in steels, strategies to improve steel quality should include minimizing ferrite formation and avoiding ferrite accumulation in the steel forming process. The current main-stream study has been focusing on the control of ferrite forming. As an alternative approach to extend the service life of steel, more attention has been attracted to exploit the ferrites existing in steel tissue. A biological metal coating technology developed in Canada is a typical example to take advantage of existing ferrite to improve steel’s corrosion resistance (publication in process).
5. Conclusions
The current study verified previous studies that concluded Cr and high temperature treatment promoted ferrite formation, and ferrites generally gathered in the core region of steel ingot. We further found, in the study, that ferrite formation was highly correlated to the mushy zone temperature, while the mushy zone temperature was closely associated with ratios of Cr:Fe and C:Fe in steel composition. This is the first report to draw attention to the effect of Fe—the key component of steel on ferrite formation in steel. However, the ferrite formation in 0Cr17Ni4Cu4Nb stainless steel was deviated from the verified concept—steel with a high mushy zone temperature would have a greater propensity to ferrite formation. Fewer ferrites in random distributions were observed in the zones of 0Cr17Ni4Cu4Nb steel tissue, though the steel had a high Cr content and mushy zone temperature. We assume that disseminated ferrites had less probability to gather together and trigger local premature cracking, i.e., less crack formation in steel microstructure, thus improving the steel’s mechanical stability. While, the observation remains inexplicable, a few inferences could be drawn from the study: (1) Fe, the major component of steel could impact on ferrite formation through its proportion to other chemical components, such as Cr, Cu, Ni, and C in stainless steels; (2) mushy zone temperature was an important intrinsic factor affecting ferrite formation; and (3) the advantages of 0Crl7Ni4Cu4Nb in corrosion resistance and mechanical stability could arise from the fact that fewer ferrite and no central segregation in ferrite distribution in the steel. Contributions of Ni and Cu to the advantages of 0Crl7Ni4Cu4Nb need to be further studied.