Effect of Methyl β-cyclodextrin on Radical Scavenging Kinetics of Olive Leaf Extracts and Interactions with Ascorbic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of the Deep Eutectic Solvent (DES)
2.3. Plant Material
2.4. Batch Extraction Procedure and Sample Handling
2.5. Total Polyphenol Determination
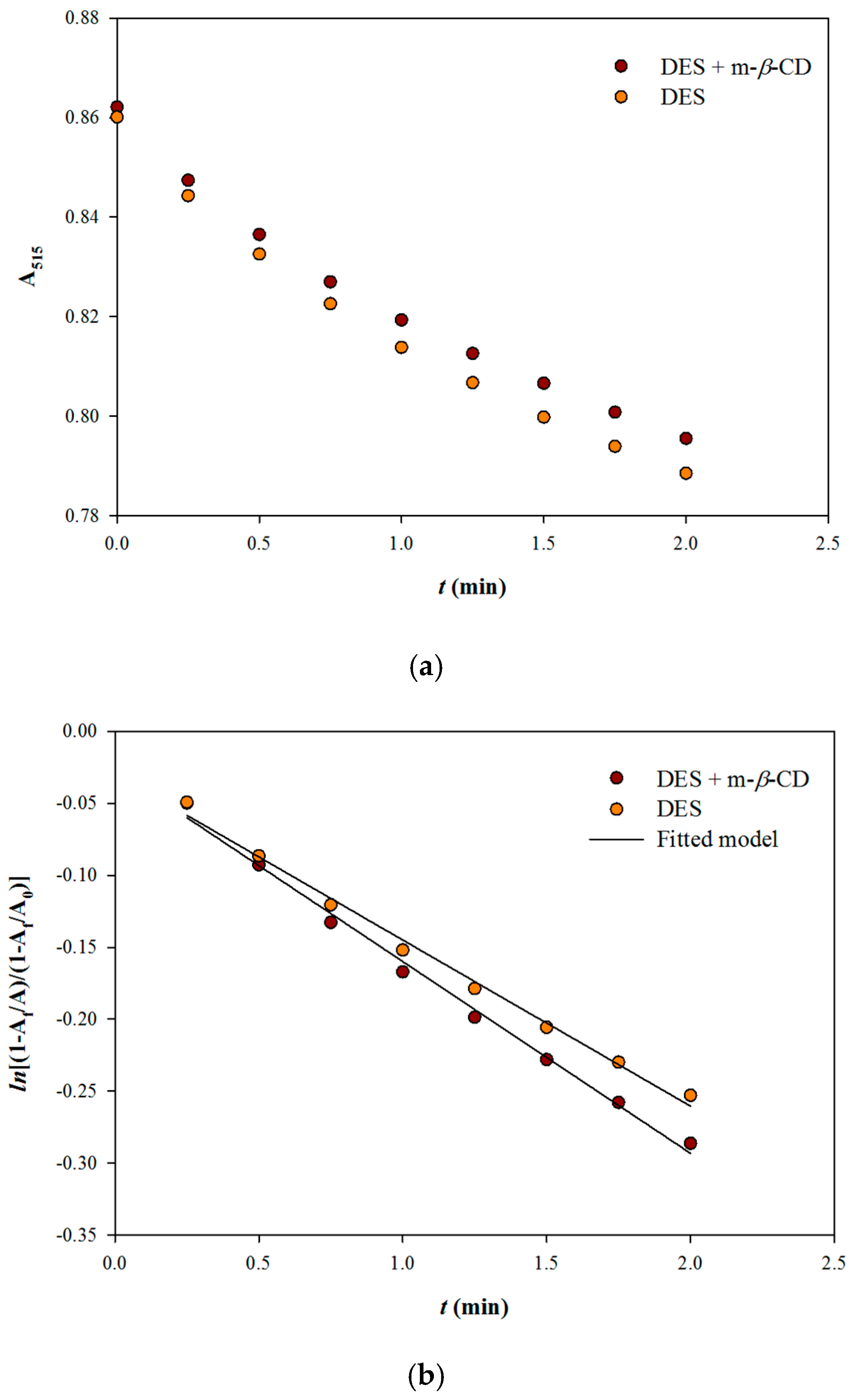
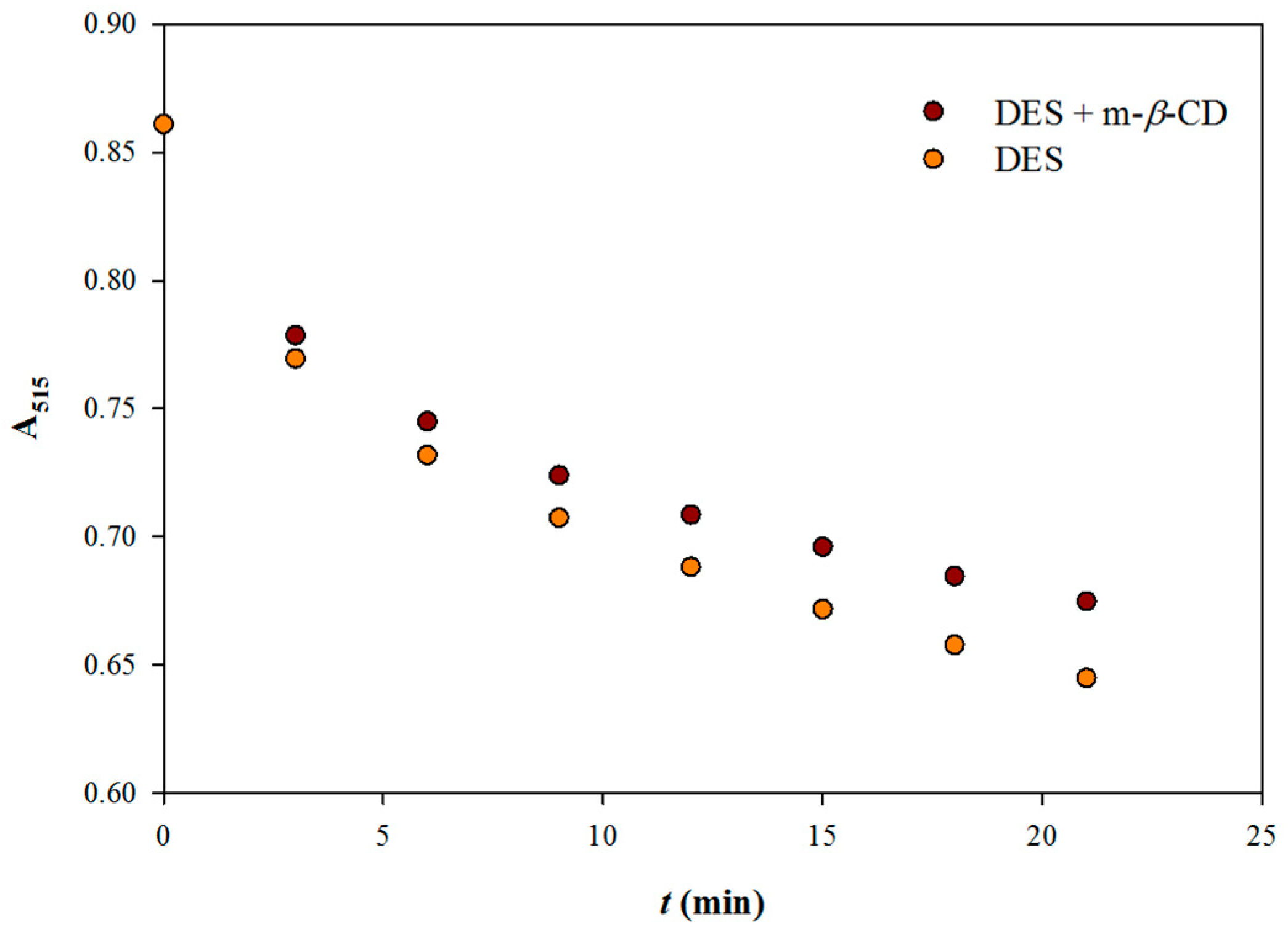
2.6. Kinetic Assay
2.7. Interaction with Ascorbic Acid
2.8. Statistics
3. Results and Discussion
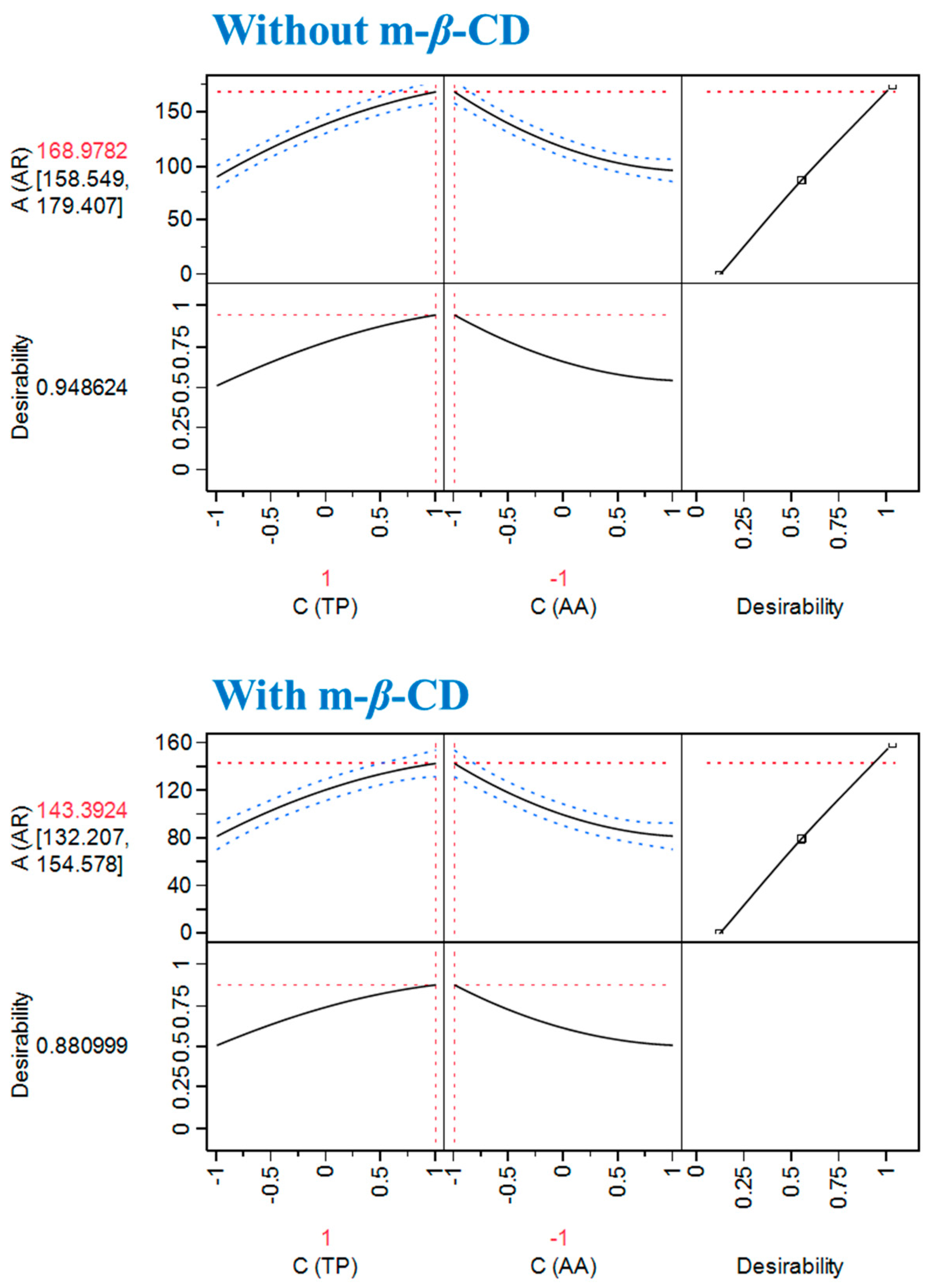
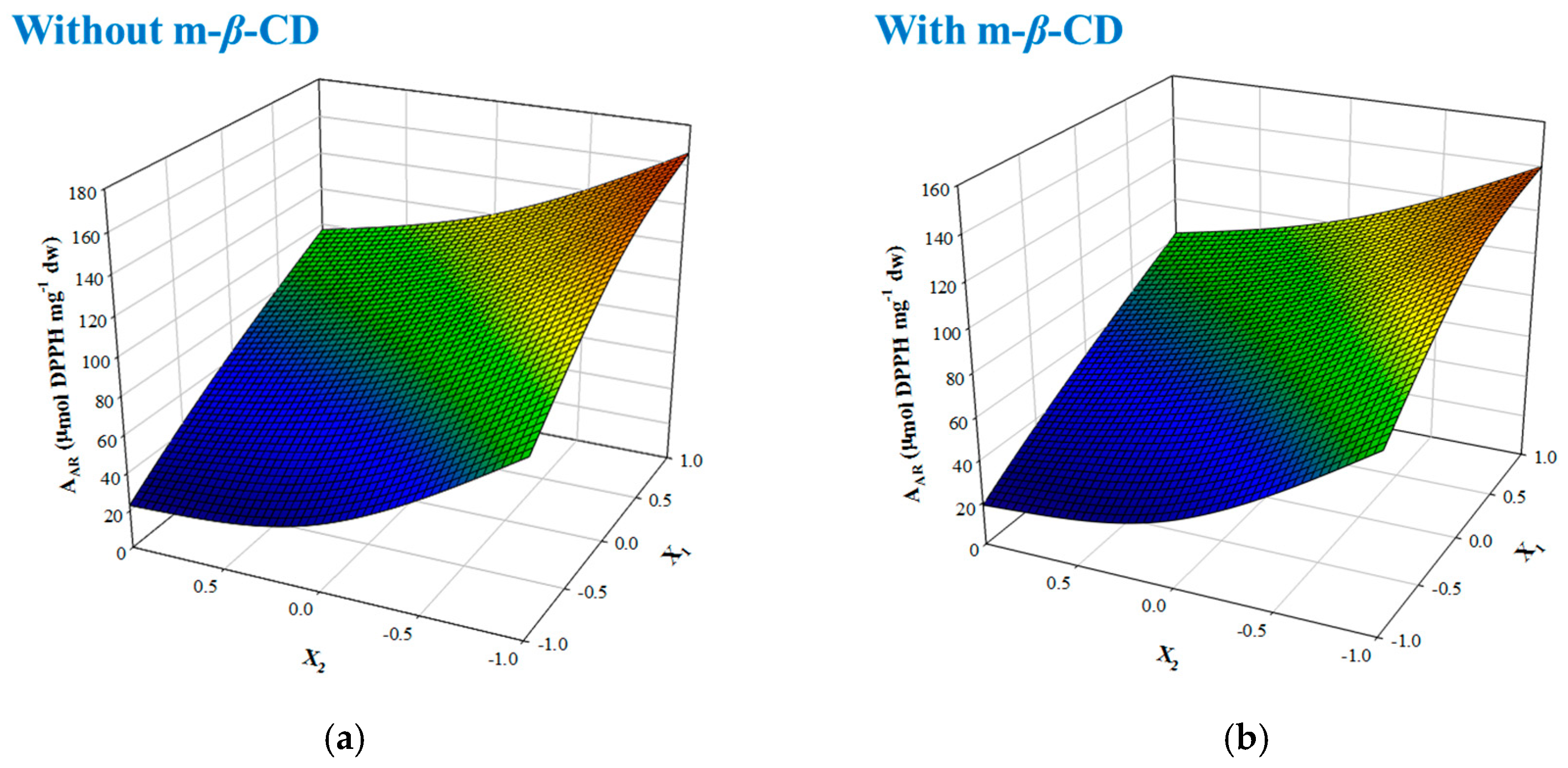
3.1 Reaction Stoichiometries and the Effect of m-β-Cyclodextrin
3.2 Interactions with Ascorbic Acid
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| A0 | initial absorbance at 515 nm |
| At | absorbance at 515 nm at any time t |
| AAR | antiradical activity (μmol DPPH g−1 dry weight) |
| c1 | initial antioxidant concentration (mol L−1) |
| c0 | initial DPPH concentration (mol L−1) |
| CAA | ascorbic acid concentration (mg L−1) |
| CTP | total polyphenol concentration (mg L−1) |
| ε | molar absorptivity (M−1 cm−1) |
| k | second-order rate constant (M−1 s−1) |
| k1 | second-order rate constant of the first abstracted H-atom (M−1 s−1) |
| nt | total stoichiometry (dimensionless) |
| R0 | initial reaction rate (M−1 s−1) |
| t | time (min) |
Abbreviations
| AA | ascorbic acid |
| DES | deep eutectic solvent |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical |
| HBA | hydrogen bond acceptor |
| HBD | hydrogen bond donor |
| m-β-CD | methyl β-cyclodextrin |
| OLL | live leaves |
References
- Moon, J.-K.; Shibamoto, T. Antioxidant assays for plant and food components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Obied, H.K.; Prenzler, P.D.; Omar, S.H.; Ismael, R.; Servili, M.; Esposto, S.; Taticchi, A.; Selvaggini, R.; Urbani, S. Pharmacology of olive biophenols. Adv. Mol. Toxicol. 2012, 6, 195–242. [Google Scholar]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Goupy, P.; Dufour, C.; Loonis, M.; Dangles, O. Quantitative kinetic analysis of hydrogen transfer reactions from dietary polyphenols to the DPPH radical. J. Agric. Food Chem. 2003, 51, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadis, V.; Grigorakis, S.; Lalas, S.; Makris, D.P. Methyl β-cyclodextrin as a booster for the extraction of Olea europaea leaf polyphenols with a bio-based deep eutectic solvent. Biomass Convers. Bioref. 2017. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Grigorakis, S.; Lalas, S.; Makris, D.P. Highly efficient extraction of antioxidant polyphenols from Olea europaea leaves using an eco-friendly glycerol/glycine deep eutectic solvent. Waste Biomass Valorization 2017. [Google Scholar] [CrossRef]
- Makris, D.P.; Passalidi, V.; Kallithraka, S.; Mourtzinos, I. Optimization of polyphenol extraction from red grape pomace using aqueous glycerol/tartaric acid mixtures and response surface methodology. Prep. Biochem. Biotechnol. 2016, 46, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Karvela, E.; Makris, D.P.; Karathanos, V.T. Implementation of response surface methodology to assess the antiradical behaviour in mixtures of ascorbic acid and α-tocopherol with grape (Vitis vinifera) stem extracts. Food Chem. 2012, 132, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction optimisation using water/glycerol for the efficient recovery of polyphenolic antioxidants from two Artemisia species. Sep. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Dangles, O.; Fargeix, G.; Dufour, C. One-electron oxidation of quercetin and quercetin derivatives in protic and non protic media. J. Chem. Soc. Perkin Trans. 2 1999, 1387–1396. [Google Scholar] [CrossRef]
- Alluis, B.; Dangles, O. Quercetin (= 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one) glycosides and sulfates: Chemical synthesis, complexation, and antioxidant properties. Helv. Chim. Acta 2001, 84, 1133–1156. [Google Scholar] [CrossRef]
- Villano, D.; Fernández-Pachón, M.; Moyá, M.; Troncoso, A.; García-Parrilla, M. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Mourtzinos, I.; Papadakis, S.E.; Igoumenidis, P.; Karathanos, V.T. Encapsulation of Melissa officinalis leaf's active compounds in β-cyclodextrin and modified starch. Procedia Food Sci. 2011, 1, 1679–1685. [Google Scholar] [CrossRef]
- Diamanti, A.C.; Igoumenidis, P.E.; Mourtzinos, I.; Yannakopoulou, K.; Karathanos, V.T. Green extraction of polyphenols from whole pomegranate fruit using cyclodextrins. Food Chem. 2017, 214, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Medronho, B.; Valente, A.J.; Costa, P.; Romano, A. Inclusion complexes of rosmarinic acid and cyclodextrins: Stoichiometry, association constants, and antioxidant potential. Colloid Polym. Sci. 2014, 292, 885–894. [Google Scholar] [CrossRef]
- Shao, P.; Zhang, J.; Fang, Z.; Sun, P. Complexing of chlorogenic acid with β-cyclodextrins: Inclusion effects, antioxidative properties and potential application in grape juice. Food Hydrocol. 2014, 41, 132–139. [Google Scholar] [CrossRef]
- Lu, Z.; Cheng, B.; Hu, Y.; Zhang, Y.; Zou, G. Complexation of resveratrol with cyclodextrins: Solubility and antioxidant activity. Food Chem. 2009, 113, 17–20. [Google Scholar] [CrossRef]
- Çelik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. Antioxidant capacity of quercetin and its glycosides in the presence of β-cyclodextrins: Influence of glycosylation on inclusion complexation. J. Inclus. Phenom. Macrocycl. Chem. 2015, 83, 309–319. [Google Scholar] [CrossRef]
- Folch-Cano, C.; Jullian, C.; Speisky, H.; Olea-Azar, C. Antioxidant activity of inclusion complexes of tea catechins with β-cyclodextrins by ORAC assays. Food Res. Int. 2010, 43, 2039–2044. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Salta, F.; Yannakopoulou, K.; Chiou, A.; Karathanos, V.T. Encapsulation of olive leaf extract in β-cyclodextrin. J. Agric. Food Chem. 2007, 55, 8088–8094. [Google Scholar] [CrossRef] [PubMed]
- Snelgrove, D.W.; Lusztyk, J.; Banks, J.; Mulder, P.; Ingold, K. Kinetic solvent effects on hydrogen-atom abstractions: Reliable, quantitative predictions via a single empirical equation. J. Am. Chem. Soc. 2001, 123, 469–477. [Google Scholar] [CrossRef]
- García-Padial, M.; Martínez-Ohárriz, M.C.; Navarro-Blasco, I.; Zornoza, A. The role of cyclodextrins in ORAC-fluorescence assays. Antioxidant capacity of tyrosol and caffeic acid with hydroxypropyl-β-cyclodextrin. J. Agric. Food Chem. 2013, 61, 12260–12264. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Parrilla, E.; Palos, R.; Rosa, L.A.; Frontana-Uribe, B.A.; González-Aguilar, G.A.; Machi, L.; Ayala-Zavala, J.F. Formation of two 1:1 chlorogenic acid: β-cyclodextrin complexes at pH 5: Spectroscopic, thermodynamic and voltammetric study. J. Mex. Chem. Soc. 2010, 54, 103–110. [Google Scholar]
- Choueiri, L.; Chedea, V.S.; Calokerinos, A.; Kefalas, P. Antioxidant/pro-oxidant properties of model phenolic compounds. Part II: Studies on mixtures of polyphenols at different molar ratios by chemiluminescence and LC-MS. Food Chem. 2012, 133, 1039–1044. [Google Scholar] [CrossRef]
- Karvela, E.; Makris, D.; Karathanos, V. Evaluation of mixture effects in binary solutions of ascorbic acid with grape (Vitis vinifera) seed extracts using response surface methodology. Int. Food Res. J. 2013, 20, 2193–2198. [Google Scholar]
- Aoun, M.; Makris, D. Binary mixtures of natural polyphenolic antioxidants with ascorbic acid: Impact of interactions on the antiradical activity. Int. Food Res. J. 2012, 19, 603–606. [Google Scholar]
- Aoun, M.; Makris, D.P. Use of response surface methodology to evaluate the reducing power in binary solutions of ascorbic acid with natural polyphenolic antioxidants. Int. J. Food Stud. 2013, 2, 238–251. [Google Scholar] [CrossRef]
- Sahin, S.; Samli, R.; Birteksöz Tan, A.S.; Barba, F.J.; Chemat, F.; Cravotto, G.; Lorenzo, J.M. Solvent-free microwave-assisted extraction of polyphenols from olive tree leaves: Antioxidant and antimicrobial properties. Molecules 2017, 22, 1056. [Google Scholar] [CrossRef] [PubMed]
| Independent Variables | Code Units | Coded Variable Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| CTP (mg·L−1) | X1 | 10 | 40 | 70 |
| CAA (mg·L−1) | X2 | 10 | 40 | 70 |
| Design Point | Independent Variables | Response (AAR, μmol DPPH g−1 dw) | ||||
|---|---|---|---|---|---|---|
| Without m-β-CD | m-β-CD | |||||
| X1 | X2 | Measured | Predicted | Measured | Predicted | |
| 1 | −1 | −1 | 91.04 | 90.58 | 82.13 | 82.06 |
| 2 | −1 | 1 | 22.85 | 19.14 | 18.87 | 15.04 |
| 3 | 1 | −1 | 166.02 | 168.98 | 140.08 | 143.39 |
| 4 | 1 | 1 | 96.88 | 96.59 | 82.68 | 82.23 |
| 5 | −1 | 0 | 35.5 | 39.68 | 31.81 | 35.71 |
| 6 | 1 | 0 | 120.27 | 117.60 | 102.84 | 99.98 |
| 7 | 0 | −1 | 141.99 | 139.50 | 124.61 | 121.37 |
| 8 | 0 | 1 | 63.58 | 67.58 | 53.00 | 57.28 |
| 9 | 0 | 0 | 89.11 | 88.36 | 78.18 | 76.49 |
| 10 | 0 | 0 | 89.11 | 88.36 | 75.83 | 76.49 |
| Antioxidant Test | 2nd Order Polynomial Equations | R2 | p |
|---|---|---|---|
| Without m-β-CD | 88.36 + 38.96 − 35.96 − 9.72 + 15.18 | 1.00 | <0.0001 |
| With m-β-CD | 76.49 + 32.13 − 32.04 − 8.64 + 12.84 | 0.99 | 0.0002 |
| Antioxidant Test | Maximum Predicted Response | Optimal Ratio |
|---|---|---|
| Without m-β-CD | 168.98 ± 10.43 | 70/10 |
| With m-β-CD | 143.39 ± 11.18 | 70/10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athanasiadis, V.; Lalas, S.; Makris, D.P. Effect of Methyl β-cyclodextrin on Radical Scavenging Kinetics of Olive Leaf Extracts and Interactions with Ascorbic Acid. ChemEngineering 2017, 1, 6. https://doi.org/10.3390/chemengineering1010006
Athanasiadis V, Lalas S, Makris DP. Effect of Methyl β-cyclodextrin on Radical Scavenging Kinetics of Olive Leaf Extracts and Interactions with Ascorbic Acid. ChemEngineering. 2017; 1(1):6. https://doi.org/10.3390/chemengineering1010006
Chicago/Turabian StyleAthanasiadis, Vassilis, Stavros Lalas, and Dimitris P. Makris. 2017. "Effect of Methyl β-cyclodextrin on Radical Scavenging Kinetics of Olive Leaf Extracts and Interactions with Ascorbic Acid" ChemEngineering 1, no. 1: 6. https://doi.org/10.3390/chemengineering1010006
APA StyleAthanasiadis, V., Lalas, S., & Makris, D. P. (2017). Effect of Methyl β-cyclodextrin on Radical Scavenging Kinetics of Olive Leaf Extracts and Interactions with Ascorbic Acid. ChemEngineering, 1(1), 6. https://doi.org/10.3390/chemengineering1010006







