1. Introduction
The analysis of soil mineral content in the vicinity of industrial plants is of fundamental importance for the assessment of ecological risks and efficient phytoremediation technology development [
1]. The Nikel settlement in the Murmansk region, situated in the vicinity of the Norwegian and Finnish border, is known for its Cu/Ni mining and processing, with intensive environmental pollution causing the formation of vast anthropogenically contaminated zones affected by soil degradation, with a related reduction in microbiological biodiversity [
2,
3], toxic element absorption by growing plants, and a negative effect on human health [
4,
5]. Native acidic soils promote the mobility and bioavailability of heavy metals [
6], while Arctic conditions slow down the activity of soil microorganisms. Until 2020, this region was among the most polluted regions in Russia, due to intensive sulfur oxide, nickel, and copper emissions starting from the 1930s [
7]. Multiple investigations of the anthropogenic risks [
3,
8,
9,
10,
11] and the proximity of the state border led to the decision to shut down the Ni/Cu smelting plant in 2020, which decreased sulfur dioxide emissions by 70% compared to that recorded in 2015. Nevertheless, the assessment of the long-term consequences of plant functioning and the development of a unified technological plan of phytoremediation remain an unsolved issue. In this respect, selenium, which is present in soil deposits along with S and Cu [
12], may be a contamination factor because of either possible Se toxicosis or its beneficial effect on biota due to the well-known antagonism with heavy metals [
13].
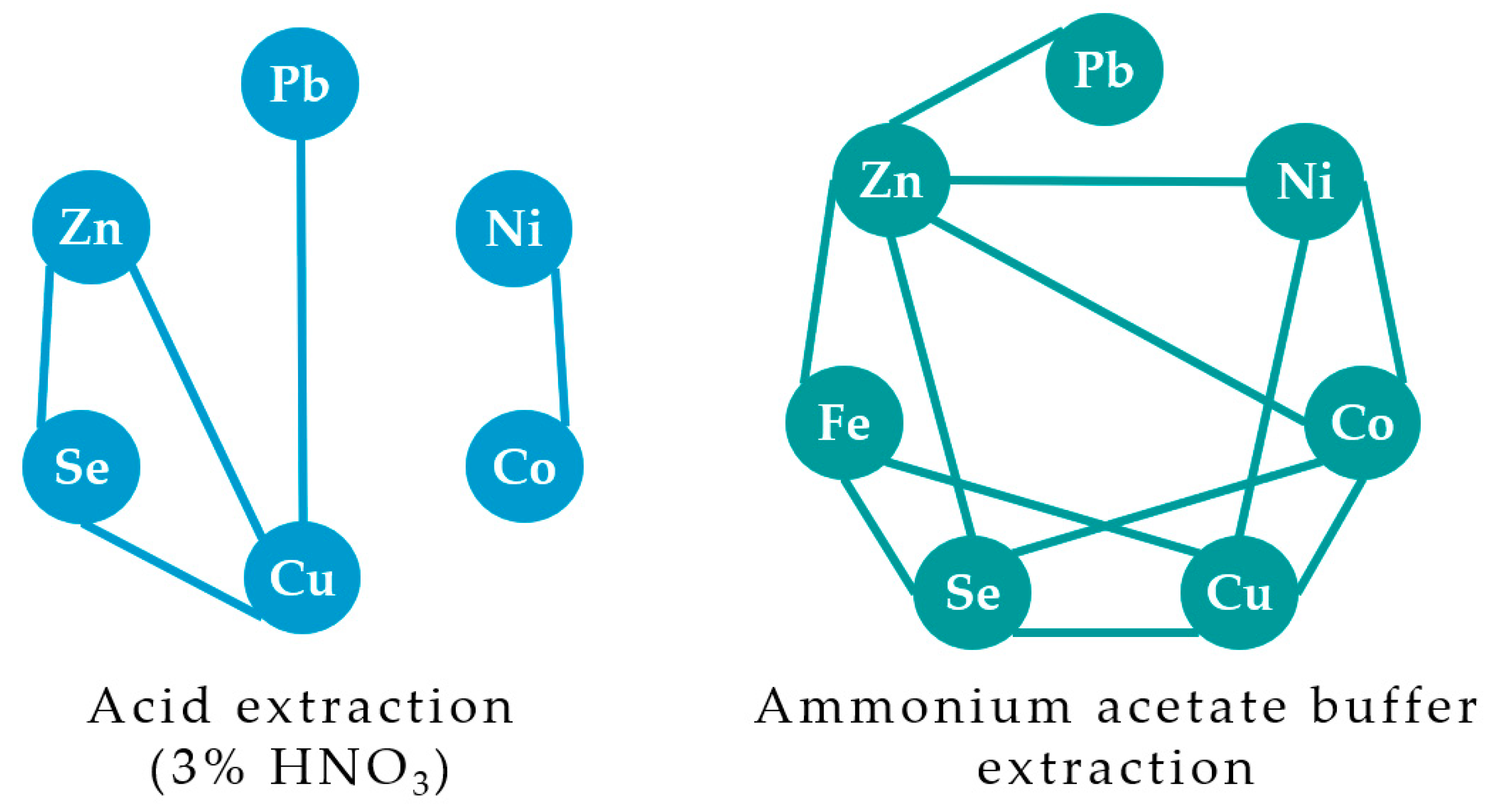
The amount of heavy metals that may be easily absorbed by plants, or the so-called metal bioavailability, is of utmost importance as a major qualitative indicator of the geochemical barrier efficiency, necessary for phytoremediation [
14]. In this respect, soil fractioning using different extracting agents provides important information on the peculiarities of soil–plant interactions. Indeed, extraction of soil heavy metals using water, EDTA [
15], CaCl
2 [
16], NaNO
3 [
17], NH
4NO
3 [
18,
19], ammonium acetate buffer [
20], and diluted nitric acid [
21,
22] is often used for the characterization of different forms of heavy metals in soils and their effect on plants. Brady et al. [
21] demonstrated that in marine sediments, 3% nitric acid solution provided deeper element extraction, compared to the ammonium acetate buffer, capable of extracting metal ions only from the weakest sorption centers and destroying some metal complexes due to the complexing ability of the acetate ion [
23]. So far, only the total content of heavy metals in soils from the vicinity of Pechenganikel plant has been determined [
3]. However, the levels of heavy metal accumulation in living organisms are preferable as an integral indicator of ecological risks. The latter may be applicable only to the south-western part of Pechenganikel plant’s vicinity, but not to the north-eastern part of the area, where the predominant north-easterly wind caused the formation of an anthropogenic desert lacking any vegetation except biocrusts. Thus, the determination of heavy metal content in mushrooms of Pechenga district was achieved only in unpolluted areas [
24], contrary to biocrusts, which lack any information about the peculiarities of their mineral composition in the above-mentioned conditions. Being the primary organisms under extreme environmental stresses, biocrusts are able to concentrate macro- and microelements, and can thus provide important information about the ecological situation of the polluted area [
25].
Indeed, biocrusts compose a complex interaction system of soil with bacteria (mostly cyanobacteria), algae, yeast, fungi along with lichens, and bryophytes [
26], increasing soil biological diversity, improving primary production, water and carbon storage, nitrogen fixation, soil aggregation, and providing a long-term promotion of vascular plant growth [
27,
28]. The biocrust matrix is formed from polymeric substances, predominantly polysaccharides distributed in the thin upper layer of the soil [
29,
30]. The species composition of biocrusts varies depending on the stage of their development, from cyanobacteria as the primary colonizer [
31] to a complex of algae, lichen, and bryophyte association, with a predominance of bryophytes in polar territories [
30,
32]. Nitrogen, phosphorus, and water deficiency, as well as high soil acidity, are the main factors affecting the development of biocrusts [
33,
34]. Investigation of bryophyte and lichen tolerance to heavy metals near Monchegorsk ‘Severonikel’ plant in subarctic conditions [
32] revealed three Marchantiales species that were highly tolerant to Ni/Cu contamination among the 18 tested:
Gymnocolea inflate,
Isopaches bicrenatus, and
Solenostoma confertissimum. Unfortunately, no data are available regarding the composition of biocrusts in the vicinity of Pechenganikel smelting plant.
To date, most of the investigations conducted, especially in the Arctic, have been devoted to biocrust diversity, carbon fixation, and water availability regulation [
26,
27,
29,
30,
31,
33,
34,
35,
36,
37,
38,
39], leaving aside the biochemical aspects of biocrust protection. This is particularly important in the zones affected by high anthropogenic pollution with specific loading in toxic elements. No literature information is available regarding the mineral composition of biocrusts grown at the extremely high anthropogenically polluted territory in the vicinity of the Pechenganikel smelting plant.
The present investigation aimed to (1) compare soil Ni, Cu, Co, Co, and Fe levels using two extracting solvents, i.e., 3% nitric acid and ammonium acetate buffer, pH 4.8, and determine the Se content in the vicinity of the Ni/Cu smelting plant and in areas far away from the plant; (2) characterize biocrust mineral composition and antioxidant status; and (3) study the relationships between biocrust and soil parameters.
2. Material and Methods
2.1. Climate Parameters and Sampling Places
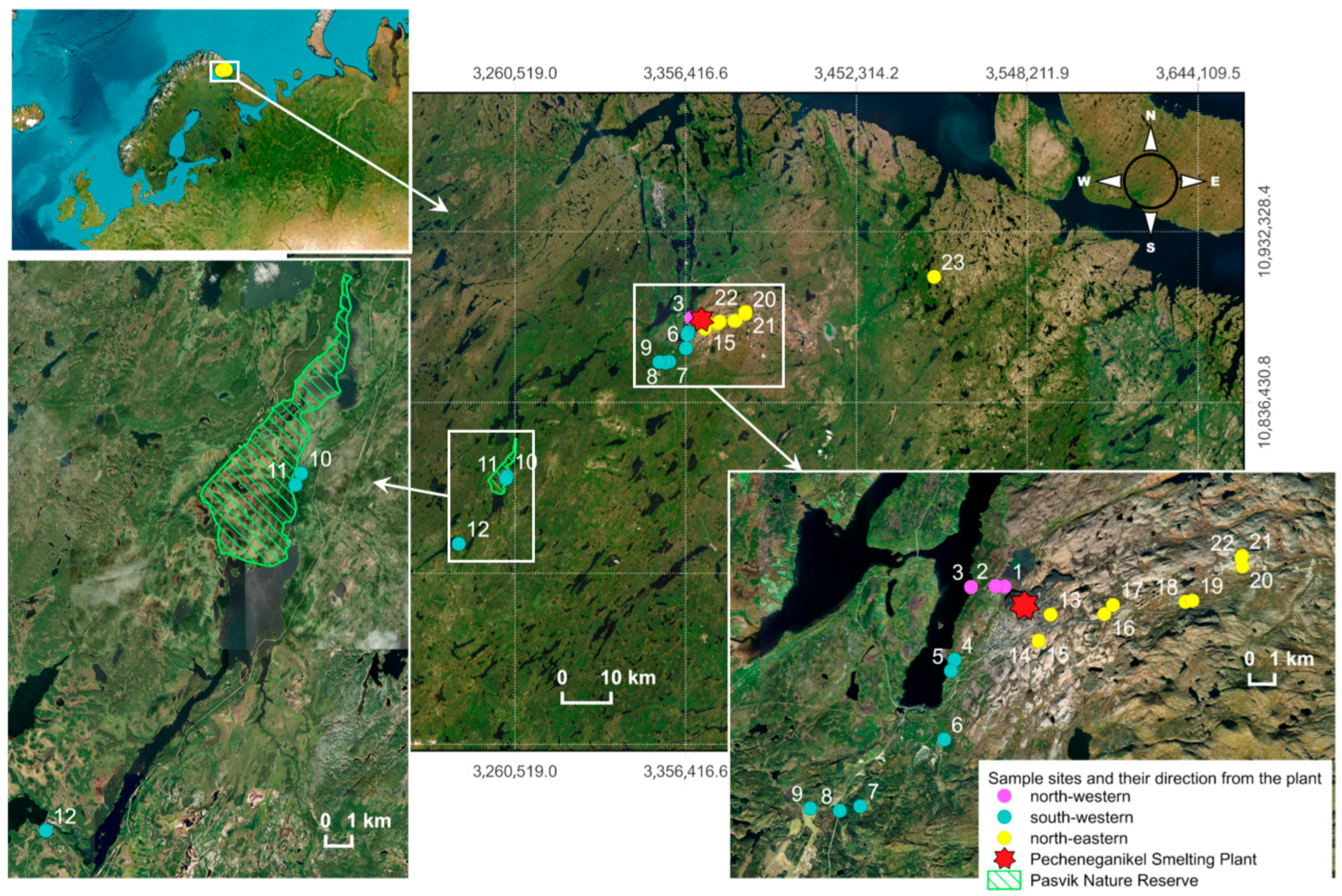
The investigated territory is situated in the subarctic region in the north-western part of European Russia near the Norwegian and Finnish border. Pechenga district is situated in the tundra and taiga soil and vegetation zone with the predominance of tundra, bog–podzolic, podzolic, bog, and sod soils. To evaluate the peculiarities of soil mineral content from 16 to 21 August 2024, 23 soil samples were taken at 8–12 cm depth at variable distance from 0.8 to 65.7 km from the Pechenganikel Ni/Cu smelting plant, Murmansk region, while biocrust samples were collected at the N-E locations 13–22 (
Supplementary Table S1;
Figure 1). Taking into account that imported soil is widely used in the Nikel settlement, soil was not sampled at the territory of Nikel. Soil sampling places were chosen according to the preferred wind directions: N-E (16.4%), S-W (6.9%), and N-W (10.2%). The geographical coordinates of sampling places, north-west, south-west, and north-east from the smelting plant, were determined by a GPS navigator.
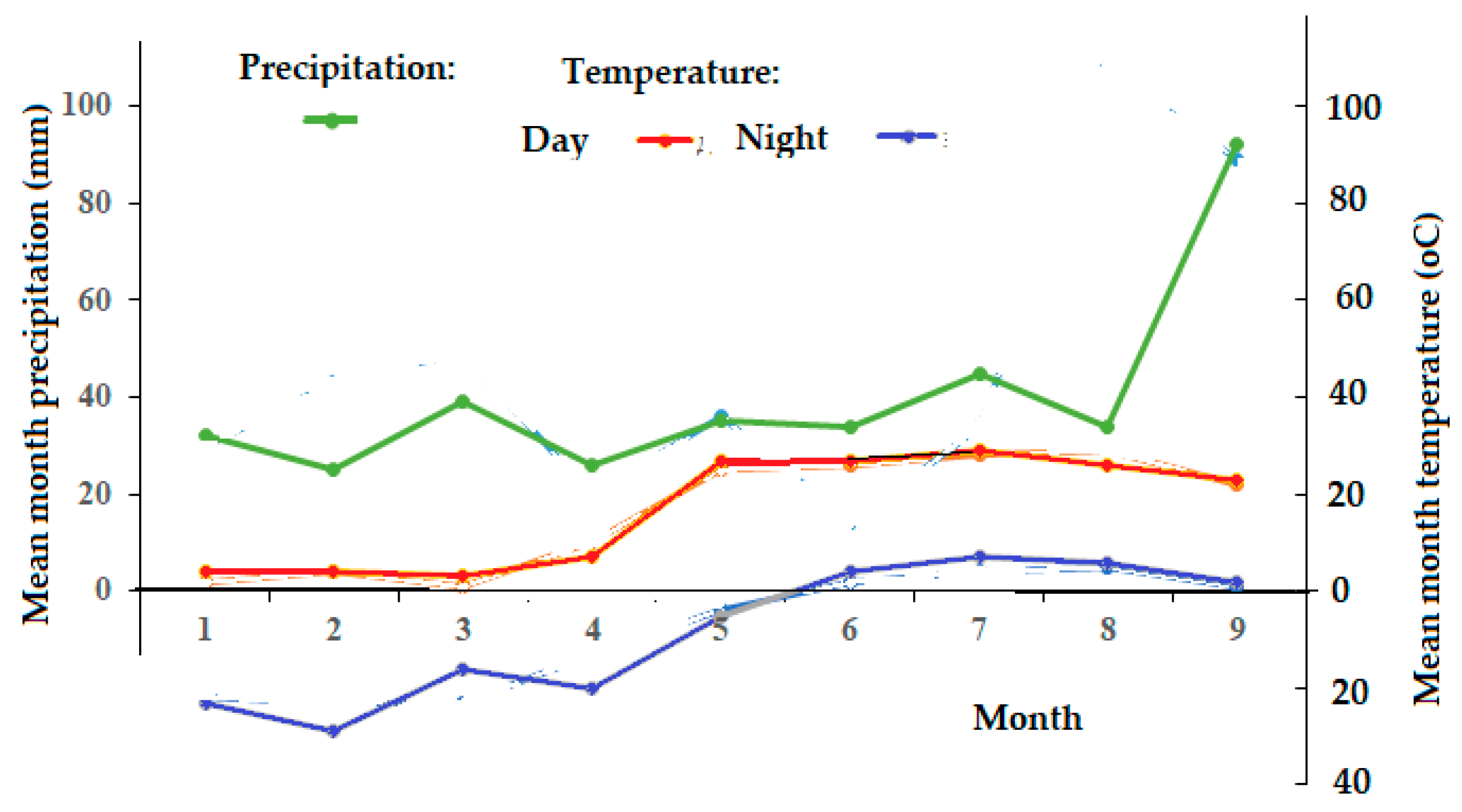
Mean annual temperature in the region studied reaches −0.5 °C due to the proximity of the Polar Circle and severe winters lasting about 6 months, bringing sudden cold snaps with temperatures dropping to −30 °C or even lower. Summer is short and cool, with mean temperatures from 10 to 15 °C. During the expedition in 2024, August was unusually dry and hot (
Figure 2).
2.2. Collection and Preparation of Soil and Biocrust Samples
Each soil sample of about 1 kg represents a mixture of soils collected at a depth of 8–12 cm at three different points. The samples were dried at room temperature, homogenized after removing stones and plant residues, sieved through a 1 mm nylon sieve, and used for the elemental analysis.
Biocrust samples were gathered in similar locations to the soil, along the N-E direction from the plant, dried at room temperature, and transferred in separate bags to the laboratory, where the 3 mm upper layer was carefully separated with a thin scalpel and homogenized.
2.3. Determination of Microelements and Heavy Metals
The contents of Cu, Fe, Zn, Co, Ni, and Pb in soils were determined using the flame Atomic Absorption Spectrometry method (AAS) with a Shimadzu GFA-7000 spectrophotometer (Shimadzu, Kioto, Japan), using soil extracts with (a) 3% nitric acid solution and (b) ammonium acetic buffer with pH 4.8. First, 5 g of each soil sample was mixed with the appropriate solvent according to the soil–solute ratio (1:10, w/v). The mixture was stirred vigorously at room temperature for one hour, and then filtered through filter paper, and the eluate was used for the AAS measurement of the analyzed elements. Certified Reference Material GBN07408 (Institute of Geophysical and Geochemical Exploration, Langfang, China) was used as an external standard. All determinations were made in triplicate, and the limits of detection (µg L−1) were as follows: Ni 2.1; Cu 0.8; Co 1.8; Zn 1.9; and Pb 8.0; the recovery levels were in the range of 98.6–98.9%.
The elemental composition of biocrusts was determined via sample digestion (1 g) at 20–425 °C and subsequent extraction of the obtained white powder with 20 mL of 3% nitric acid. The filtrate was used for the analysis.
2.4. Selenium Content
The microfluorimetric method of Se measurement [
40] adapted to the soil/biocrust analysis [
22] was used in the present work. First, 100 mg of dry homogenized soil/biocrust probe was mixed with 1.5 mL of nitric/perchloric acid (10:7,
v/
v) and digested according to the temperature regimes: 120 °C—1 h; 150 °C—1 h; and 180 °C—1 h. Traces of nitric acid were removed via heating the samples with 1–2 drops of 30% hydrogen peroxide for 10 min at 150 °C, while the reduction of the selenate to selenite was achieved by sample treatment with 1 mL of 6 N HCl at 120 °C for 10 min. The reaction was stopped by adding 1 mL of distilled water; the upper layer was decanted after full separation of phases, and the residue was extracted once more using 1 mL of 10% EDTA solution. The combined liquid extract was mixed with 1 mL of 10% EDTA solution, and the pH was adjusted to 1 with ammonia solution. A complex of selenite with 2,3-diaminonaphtalene (piasoselenol) was obtained via sample heating with 1 mL of freshly prepared 0.1% 2,3-diaminonaphtalene solution in 1% HCl at 53 °C for half an hour. The selenium concentration was determined using the fluorescence value of piasoselenol extracted with hexane using a λ excitation wavelength 376 nm and λ emission at 519 nm using a Fluorate 4M spectrophotometer (Lumex, St. Peterburg, Russia). Three replicates were used for the analysis. A soil sample with known Se content (240 µg kg
−1 d.w.; Federal Scientific Vegetable Center) was used as an external standard. The calibration curve was obtained using 5 different Se concentrations (as sodium selenate): 0, 2, 5, 10, and 15 ng. The sensitivity value of the method was 0.8 ng, and the recovery rate was 98.8%.
In general, only the total soil Se was analyzed due to the significant variability in the results at low concentrations [
41].
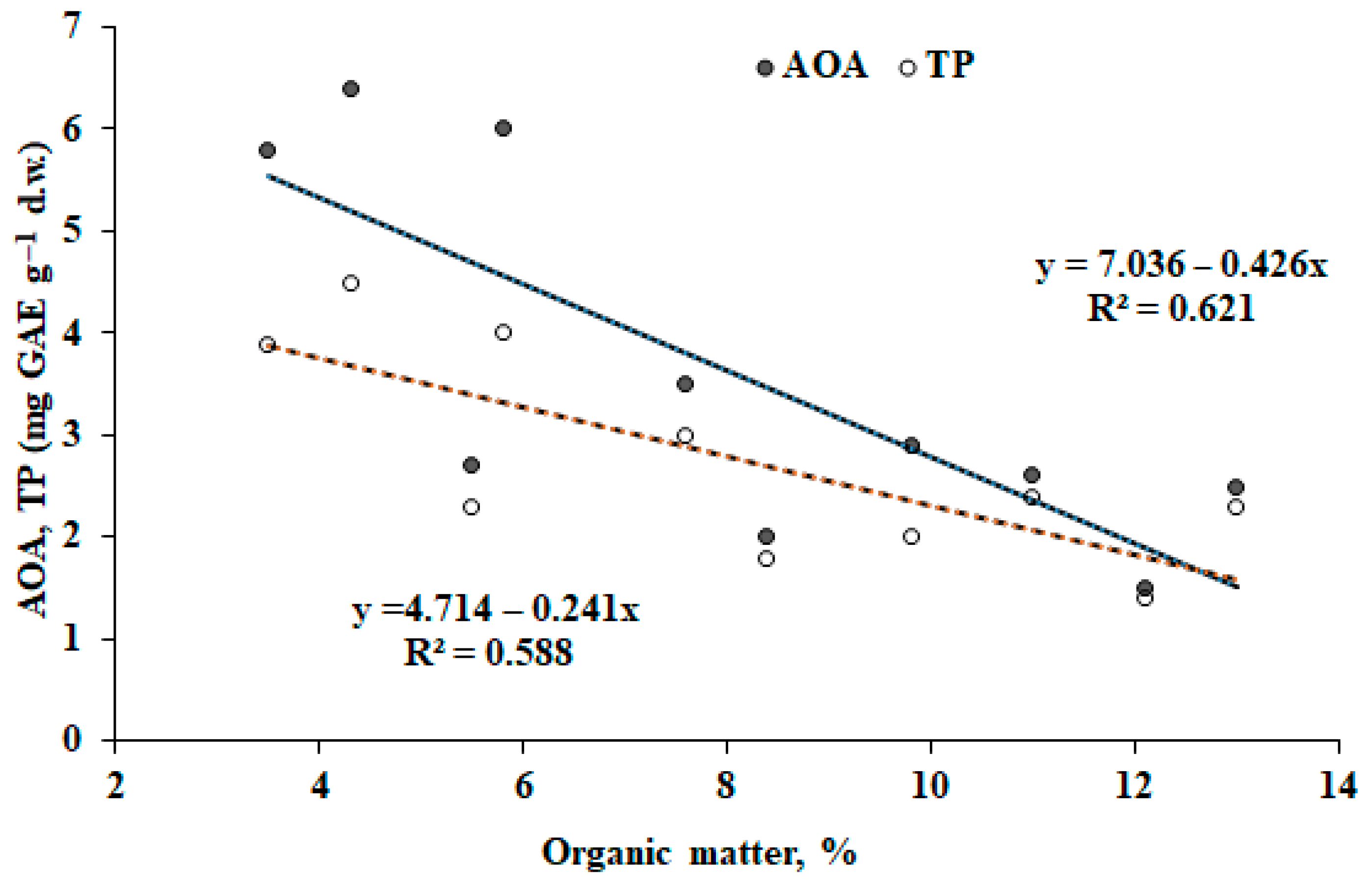
2.5. Organic Matter (OM)
The organic matter content (OM) was determined according to [
42].
Taking into account that soil composes only a small part of biocrust, the Folin–Ciocâlteu colorimetric method for the determination of polyphenol content and the titrimetric method for the determination of the total antioxidant activity in plants [
43] were adapted for biocrust analysis. Only dried homogenized samples of biocrust were used in the determination, which provides representativeness of the determination under the low sampling mass applied.
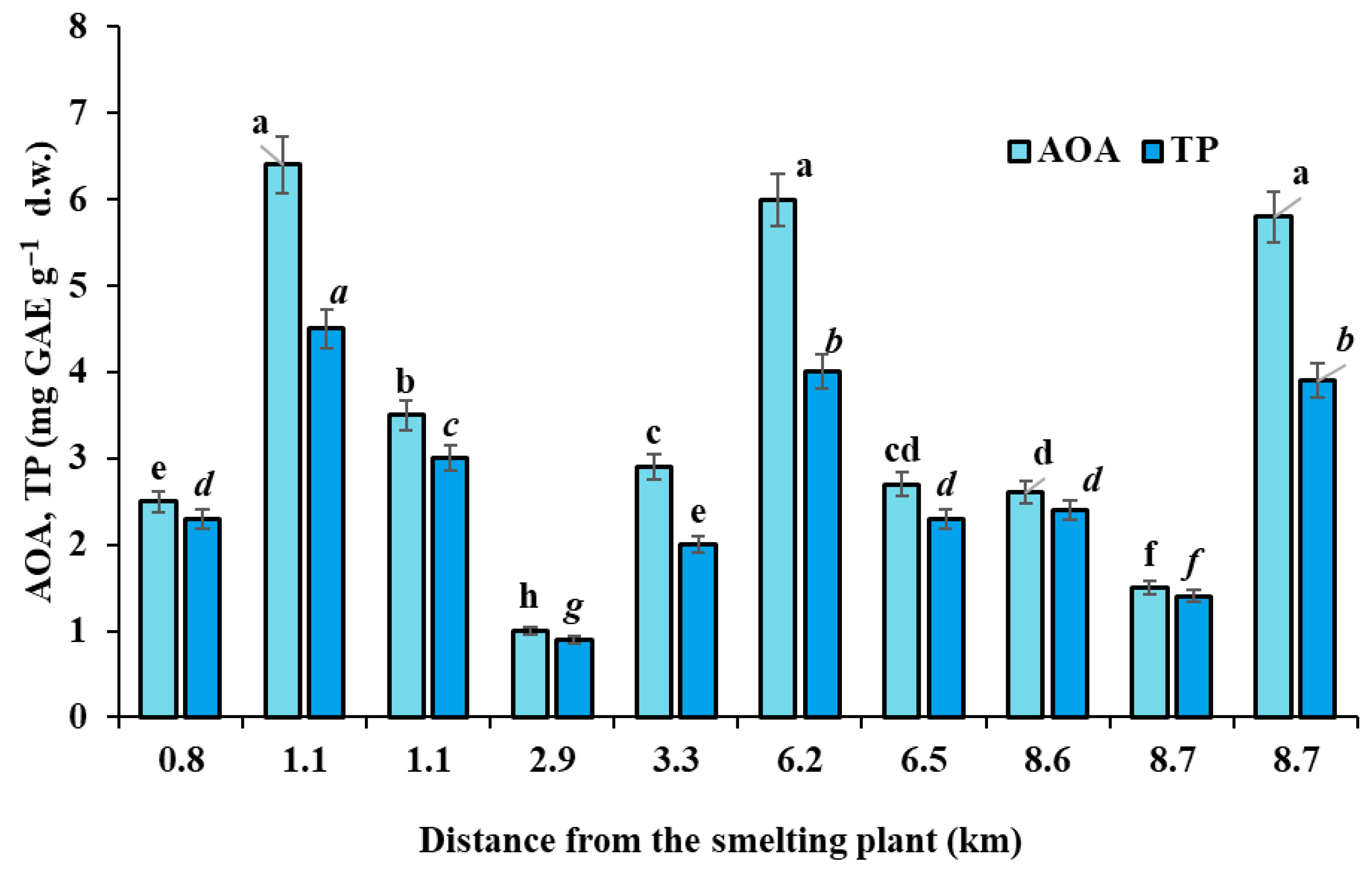
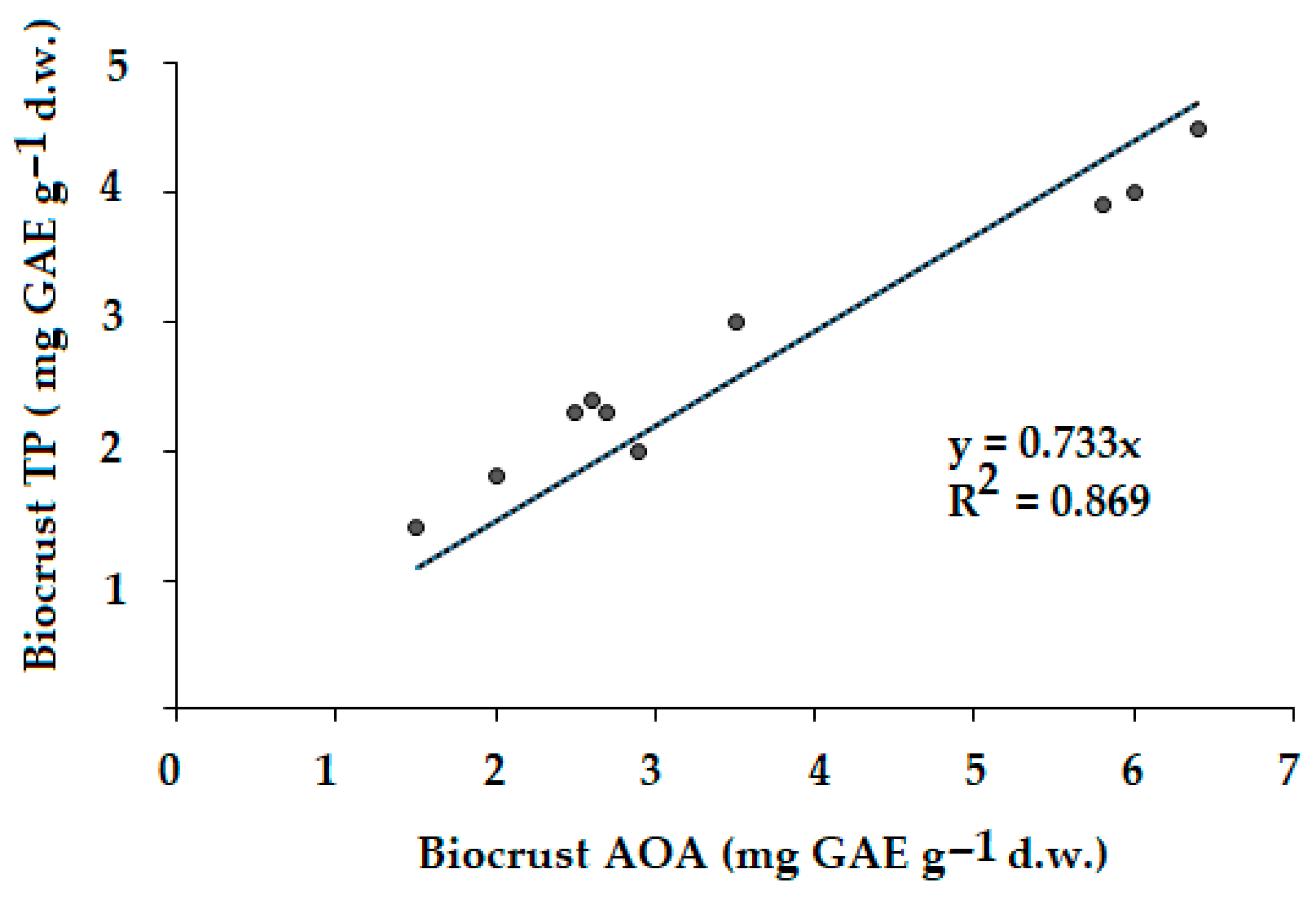
2.6. Antioxidant Activity (AOA)
The total antioxidant activity of dried biocrust powder was assessed in ethanolic extracts after heating the samples in 70% ethanol at 80 °C for 1 h using a redox titration method developed previously for plant samples with gallic acid as an external standard [
43]. A calibration curve was constructed using different concentrations of gallic acid (10 to 70 mg L
−1); the recovery level was 98.5%, and the analysis was made in triplicate.
2.7. Total Polyphenols (TPs)
Biocrust polyphenol content was assessed using the Folin–Ciocâlteu colorimetric method, according to [
43] with a small modification. After the extraction of one gram of dry biocrust with 20 mL of 70% ethanol at 80 °C for 1 h, the mixture was cooled down and quantitatively transferred to a volumetric flask, and the volume was adjusted to 25 mL. After thorough separation of the solid fraction, 1 mL of the resulting solution was used for the reaction with Folin–Ciocâlteu reagent in the presence of saturated Na
2CO
3 solution. The concentration of polyphenols was determined one hour later using the absorption value of the resulting solution at 730 nm on a Unico 2804 UV spectrophotometer (Suite E, Dayton, NJ, USA) based on the appropriate values of the external standard 0.02% gallic acid solution. The recovery level was 98.5%, and the analysis was performed in triplicate.
2.8. Statistical Analysis
All statistical analyses were performed using the R 4.3.2 and Python 3.11 environments with the statsmodels and scipy packages. Metal concentrations in soil and biocrust samples were log10-transformed prior to analysis to improve normality and homoscedasticity of residuals. To account for the nested structure of the data (multiple observations within individual sampling sites), linear mixed-effects models (MixedLM) were applied with the sampling site included as a random intercept.
For soil data, models of the form (1)
were fitted separately for each element, where
Distance is the distance from the emission source (km),
Direction indicates the sector relative to the smelter (N–E vs. S–W),
Soil pool refers to the chemical fraction (NH
4OAc or 3% HNO
3), and
SOM is the soil organic matter content (%).
For biocrusts, the response variable was the log10-transformed metal concentration, and alternative models were fitted with soil predictors from NH4OAc or HNO3 pools. Models included Distance, Direction, and SOM as covariates, with Site as a random effect. The best model for each element was selected using the Akaike Information Criterion (AIC).
To explore potential transfer processes, transfer factors (TFs) were calculated as the ratio between metal concentrations in biocrusts and soils for both pools (TF_NH
4OAc and TF_HNO
3). Mixed-effects models with the structure
were used to test the effects of spatial gradient and soil properties on TF values.
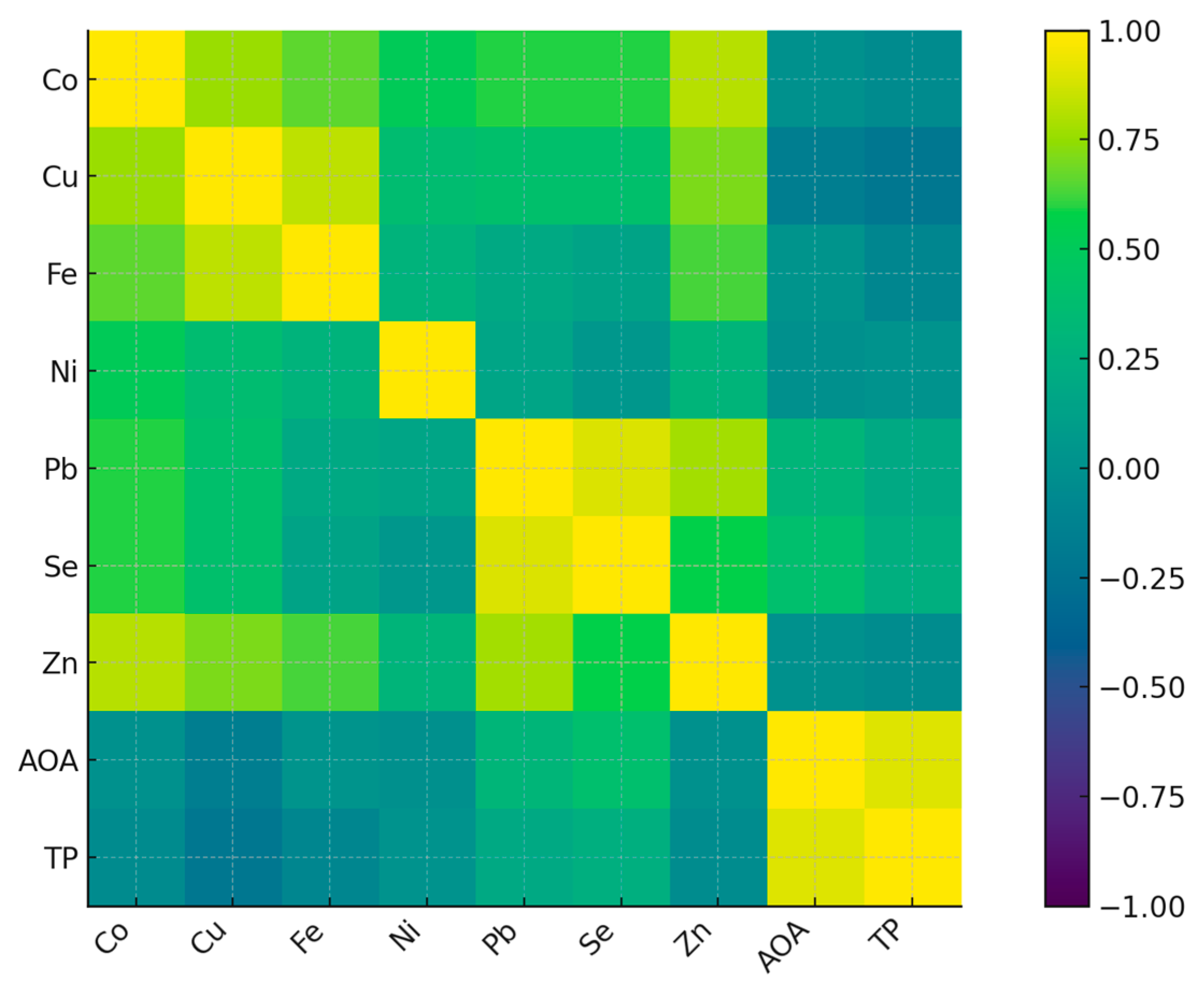
Pairwise correlations between metals in biocrusts and biocrust functional indicators (AOA and TP) were assessed using Spearman’s rank correlation coefficients. Partial correlations controlling for distance were also calculated. For all families of tests (model coefficients or correlation matrices), p-values were adjusted for multiple comparisons using the Benjamini–Hochberg false discovery rate (FDR) procedure. Results were reported as adjusted q-values, with q < 0.05 considered statistically significant.
Spatial autocorrelation of model residuals was evaluated using Moran’s I with k-nearest neighbors (k = 4) and permutation tests (499 randomizations). In addition, sensitivity analyses were carried out by excluding imported soil samples to test the robustness of the results.
All descriptive statistics, model outputs, correlation matrices, and TF calculations are provided in the
Supplementary Information.
4. Conclusions
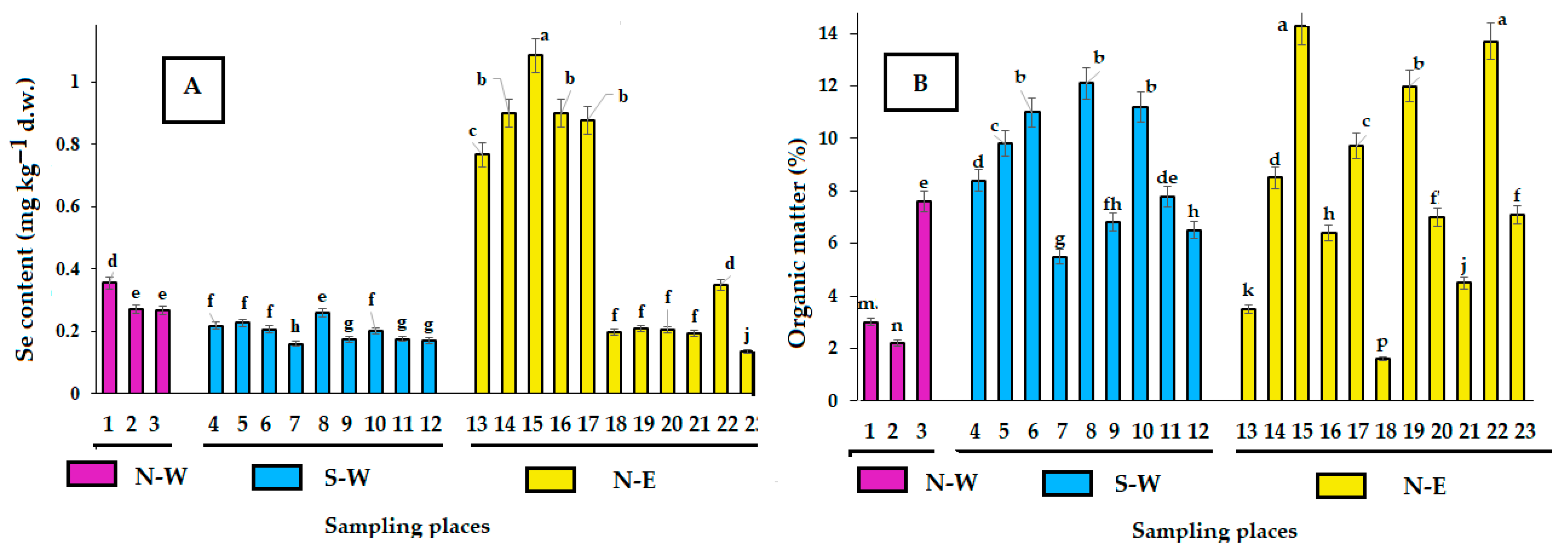
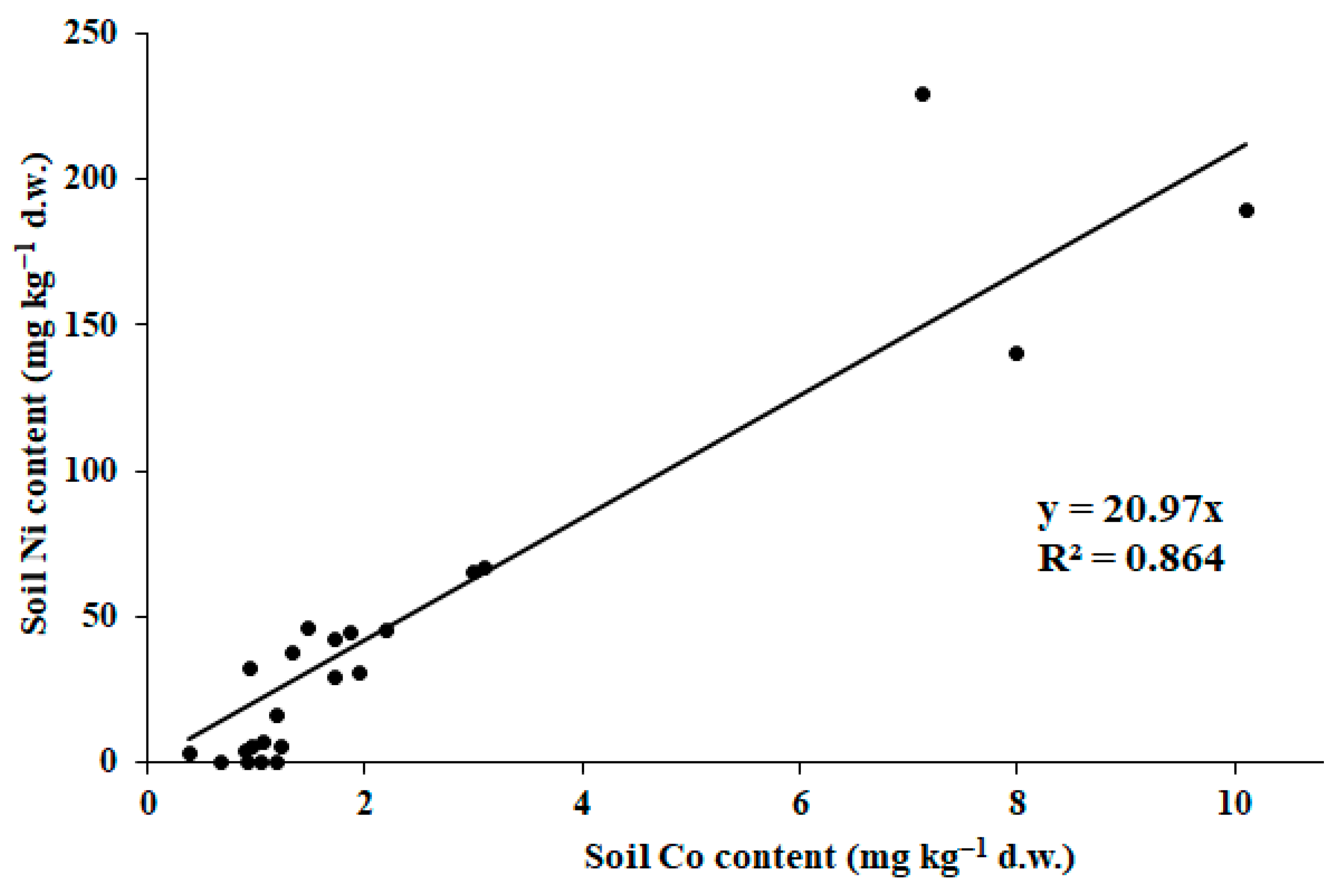
The consequences of Pechenganikel operation over decades revealed complex Ni, Cu, Co, Pb, Se, and Zn distribution both in soils and biocrusts, with correlations between the elements greatly affected by the soil extraction method. Local Se contamination in the vicinity of Pechenganikel plant and a positive correlation between Ni and Co, and Cu and Se upon the ammonium acetate buffer application are important environmental characteristics of the territory in 2024. Biocrust formation in conditions of intensive anthropogenic influence is directly related to the organic matter content in soil, while its accumulation abilities in Ni, Co, Se, and Pb are directly connected with soil mineral content, which demonstrates a complex relationship with soil Fe and Cu (buffer extract), Se, and Co and Zn (nitric acid extract). Further investigations are expected to deepen the study of the biocrust–soil interaction mechanisms in conditions of intensive environmental pollution.
The application of linear mixed-effects modelling and transfer factor analysis revealed significant spatial gradients in transfer factors for Ni, Cu, and Zn, with the strongest associations observed for NH4OAc-based fractions. This emphasizes the sensitivity of mobile pools in explaining biocrust–soil interactions; therefore, biocrusts serve as effective bioindicators of both absolute metal contamination and its bioavailable fractions.
Future studies are expected to address temporal changes in soil–biocrust metal dynamics, explore the role of microbial community composition in accumulation patterns, and integrate biocrust-based monitoring into regional environmental management frameworks.