Abstract
Phytoremediation has emerged as a sustainable and cost-effective strategy for mitigating contamination in soil and water systems, utilizing plants and their associated microbial consortia to uptake, degrade, or immobilize pollutants. This review synthesizes findings from over 100 peer-reviewed publications and case studies to identify key parameters influencing phytoremediation efficiency, including contaminant bioavailability, chemical speciation, concentration levels (ranging from trace to >100 mg/L), plant species suitability, hydraulic retention time, and temperature ranges (10–35 °C). Despite its proven potential, the absence of standardized design frameworks limits consistent implementation and cross-site performance comparability. To address this, the study proposes a conceptual system design framework supported by measurable performance metrics—such as pollutant removal efficiency (often >70% for heavy metals) and biomass uptake capacity. The review further examines regulatory and policy gaps that hinder the technology’s integration into national remediation strategies, particularly in low- and middle-income countries. It underscores the need for technical guidelines, regulatory benchmarks, and protocols for post-treatment biomass management to enable safe, effective, and scalable deployment. By advocating a multi-stakeholder, evidence-based approach, the study aims to bridge the gap between scientific innovation and environmental governance, positioning phytoremediation as a viable tool for pollution control, ecosystem restoration, and alignment with global sustainability targets.
1. Introduction
Environmental contamination of water sources by both organic and inorganic pollutants has become a pressing global issue due to industrial expansion, agricultural intensification, and urbanization [1,2,3,4]. Heavy metals, pesticides, hydrocarbons, and pharmaceuticals are increasingly detected in aquatic ecosystems, posing threats to human health, ecosystem stability, and long-term sustainability [5,6,7,8]. Traditional remediation techniques, while effective, are often energy-intensive, cost-prohibitive, and ecologically disruptive, particularly in low-income regions [9,10,11,12,13]. In this context, phytoremediation, a plant-based, eco-compatible approach, has garnered global attention as a sustainable, low-cost, and visually unobtrusive alternative to conventional treatment technologies [1,14,15]. Unlike mechanical or chemical methods that may compromise soil or water integrity, phytoremediation operates in harmony with natural biogeochemical cycles and relies on plant metabolism and microbial interactions to detoxify or sequester pollutants [12,16].
Over the past three decades, numerous studies have demonstrated the effectiveness of phytoremediation for removing a wide range of contaminants, including heavy metals (Pb, Cd, Zn, As), organic compounds such as polycyclic aromatic hydrocarbons (PAHs), pesticides, and emerging contaminants like pharmaceuticals and personal care products [17,18,19,20,21,22,23,24,25,26,27,28,29,30]. Plant species such as Pteris vittata, Eichhornia crassipes, Lemna minor, Brassica juncea, and Typha spp. have been evaluated for their capacity to absorb, accumulate, and transform pollutants in situ [6,9,10]. Several system configurations—constructed wetlands, hydroponic systems, and rhizofiltration beds—have been developed to facilitate this process [11,12]. However, despite a growing body of empirical and field-based evidence, there remains a lack of harmonization in system design, operational parameters, and performance metrics across studies [13,14,15,16]. For instance, variation in plant selection, hydraulic retention time, nutrient load, and contaminant concentration hampers the ability to replicate or scale up successful models across regions.
Today, as environmental pollutants are increasingly detected in various environmental compartments [31,32,33,34,35], phytoremediation has become a suitable solution because it avoids the need for multiple costly treatment steps. An overview of representative plant species and their associated contaminants is provided in Table 1. Phytoremediation utilizes various plant species to remove, stabilize, or degrade environmental pollutants, with the selection of specific plants depending on the type of contaminant and the site conditions. Different types of contaminants that cause harmful effects on human health and other biological systems can be removed by appropriate plant species. These plants absorb pollutants from the environment and detoxify their toxic effects.

Table 1.
Overview of selected plant species used for the removal of specific pollutants in water.
A critical review of the current literature reveals substantial heterogeneity in phytoremediation system design and performance reporting. While numerous case studies provide promising results under controlled or small-scale conditions, they often lack the systematization necessary to inform real-world application or policymaking. Moreover, only a limited number of countries have developed regulatory frameworks that explicitly incorporate phytoremediation into environmental management or land-use planning [60,61,62,63,64,65,66]. The disconnect between research outcomes and policy implementation is further exacerbated by the absence of standard operational guidelines or universally accepted evaluation metrics. Developing countries, despite being most in need of low-cost solutions, face barriers such as limited technical expertise, insufficient regulatory support, and fragmented pilot initiatives that hinder scale-up [63,64,65,66,67]. The current state of the literature points toward the need for a standardized design framework that could bridge scientific understanding with actionable policy and technical application.
The objective of this review is to consolidate and critically evaluate existing knowledge on phytoremediation of water contaminants, with a focus on identifying key environmental and biological variables that affect performance. Specifically, this paper reviews (i) common contaminant classes addressed through phytoremediation, (ii) effective plant species and their physiological mechanisms, (iii) system design parameters including influent concentration, temperature, pH, and the hydraulic loading rate, and (iv) real-world examples of phytoremediation implementation. Furthermore, the study evaluates how policy frameworks in different countries support or limit the adoption of phytoremediation technologies. Special attention is given to identifying gaps in design standardization, regulatory harmonization, and performance benchmarking that limit practical application.
To achieve these goals, we adopted a structured review methodology, incorporating both quantitative and qualitative data sources. Peer-reviewed articles, technical reports, and policy documents were retrieved from databases such as Scopus, Web of Science, and Google Scholar using targeted keywords including “phytoremediation,” “water pollution,” “standardized framework,” and “environmental policy.” Selection criteria focused on publications that provided explicit information on contaminant removal efficiency, operational parameters, and contextual factors (e.g., climate, economic setting). Table 1 compiles representative plant species used in phytoremediation, categorized by contaminant class and plant type. Further tables and figures synthesize system-level variables and identify performance ranges critical for optimizing phytoremediation under diverse environmental conditions. Figure 1 illustrates the conceptual layout of a standardized phytoremediation system, linking policy, research, and application dimensions within the proposed framework.
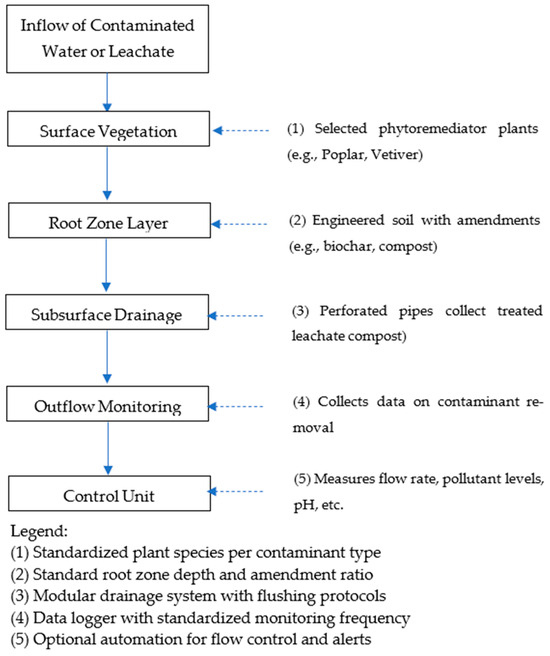
Figure 1.
Schema of standard phytoremediation system.
The significance of this review lies in its potential to inform the development of a coherent, cross-sectoral approach to phytoremediation. By synthesizing the dispersed knowledge into a unified framework, this work aims to support the design of context-appropriate phytoremediation systems that are both effective and implementable. The alignment of scientific data with policy considerations is especially vital in advancing phytoremediation beyond pilot scales, particularly in low-resource settings. Moreover, by proposing clear operational criteria and performance thresholds, the review provides actionable guidance for environmental engineers, land planners, and policymakers. Finally, this work contributes to global efforts toward sustainable development and circular economy principles by demonstrating how nature-based solutions such as phytoremediation can reconcile ecological health with economic feasibility.
2. Application Status of Phytoremediation
Over the past two decades, phytoremediation has emerged as a promising and sustainable approach for remediating contaminated environments, particularly in areas affected by industrialization, mining, and agricultural runoff [68,69]. Despite its scientific maturity and ecological advantages, the application of phytoremediation varies significantly across regions due to differences in environmental policies, technological readiness, and socio-economic priorities [70,71,72,73,74]. In North America and Europe, countries such as the United States, Canada, Germany, and the Netherlands have advanced phytoremediation beyond laboratory research into pilot-scale and full-scale remediation projects. For instance, the U.S. Environmental Protection Agency (EPA) officially recognizes phytoremediation as a viable method for treating heavy metals and organic pollutants, especially at Superfund and brownfield sites [75]. In Germany, successful use of species like Salix (willows) and Populus (poplars) for remediation of cadmium- and zinc-contaminated soils has influenced broader land-use rehabilitation strategies, integrating phytoremediation into environmental management policies [76,77].
In Asia, countries such as China and India face severe environmental contamination from mining and industrial activities. Both nations are actively researching and gradually adopting phytoremediation. China has invested heavily in identifying hyperaccumulator plants like Pteris vittata for arsenic removal [78,79], while India has demonstrated the cost-effectiveness of species such as Vetiveria zizanioides and Brassica juncea for remediation of lead- and chromium-contaminated soils through field trials [80,81]. Southeast Asian countries, including Thailand and Vietnam, have initiated phytoremediation projects in response to heavy metal pollution from craft villages and agricultural runoff, although these remain limited in scale and are hindered by the absence of standardized frameworks for design and implementation [82,83]. In Latin America and Africa, phytoremediation is an area of growing interest but remains largely exploratory. Countries like Brazil and Chile focus on native plants adapted to local contamination, especially in mining-affected zones [84,85,86,87,88,89]. Similarly, Nigeria, Ghana, and South Africa have launched research programs on phytoremediation; however, practical application is restricted by limited financial resources, low policy support, and insufficient technical expertise [90,91,92,93].
Despite these regional advances, global challenges persist, including the lack of protocols for system design, plant selection, and performance evaluation; variability in climatic, soil, and hydrological conditions that complicate replicability; and limited integration of phytoremediation into national remediation policies, which results in inconsistent funding and oversight [94,95]. Recognizing these challenges, international organizations such as the United Nations Environment Programme (UNEP) and the Food and Agriculture Organization (FAO) emphasize the role of green remediation technologies, including phytoremediation, in meeting Sustainable Development Goals (SDGs) [96,97]. The creation of transnational networks and knowledge-sharing platforms is critical to accelerating the global adoption of phytoremediation. Bridging scientific advances with coherent policy frameworks is essential for realizing the full potential of phytoremediation as a sustainable environmental management tool worldwide.
3. Current Design Approaches in Phytoremediation Systems
Phytoremediation has evolved into a widely recognized green remediation strategy, relying on plant-based systems to remove, stabilize, or degrade environmental pollutants. As its applications move from experimental studies toward operational-scale deployment, the demand for standardized design methodologies has become increasingly critical [98]. The absence of consistent technical frameworks limits the comparability of project outcomes, hinders regulatory acceptance, and slows the broader adoption of phytoremediation technologies. Among the core components of system design is plant species selection, which must be grounded in pollutant specificity, site-specific environmental conditions, and ecological compatibility [56,99]. At present, such choices are often guided by empirical experience or region-specific practices. The development of standardized selection protocols, incorporating critical plant traits such as tolerance thresholds, accumulation efficiency, growth rates, and seasonal adaptability, would enhance performance predictability and project scalability [100]. Establishing curated databases of approved phytoremediation species for defined contaminant classes would be a significant step toward harmonizing practices across projects and regions.
In addition to plant selection, the spatial and structural design of phytoremediation systems plays a pivotal role in optimizing remediation efficiency [101,102,103]. Parameters such as planting density, spatial configuration (e.g., contour planting, wetland modules), and root depth must be matched to site typology and contaminant characteristics [104,105]. Standardizing these variables according to application type, whether landfill leachate management or heavy metal-laden soils, would support scalable and repeatable designs [106,107,108]. The integration of engineered features, such as modular drainage systems, hydraulic management units, and flow control infrastructure, further enhances system stability. Likewise, soil and substrate amendments are essential for optimizing nutrient conditions and contaminant bioavailability [109,110]. However, the inconsistent use of enhancers such as biochar, compost, and chelating agents results in performance variability and potential environmental risk. Defining best-practice protocols for amendment composition, application rates, and environmental risk assessment would contribute significantly to safe and effective system operation [104,111,112,113]. Hydrological design, particularly in groundwater or leachate treatment systems, also demands standardization in terms of flow regime, retention time, and subsurface drainage layout, to ensure consistent exposure of pollutants to root zones and rhizosphere microbial activity.
To support long-term system functionality and regulatory transparency, operational monitoring and maintenance must also follow uniform protocols. Standardized procedures for contaminant sampling, plant health evaluation, biomass harvesting, and frequency of system performance checks are essential. Advanced tools such as remote sensing, real-time sensors, and GIS-based analytics offer scalable solutions for large-area deployments and should be integrated into national monitoring standards [74]. Figure 1 presents a conceptual framework for a standardized phytoremediation system, featuring five key components: (1) species selected from a pollutant-specific catalog, (2) prescribed root zone depths and amendment ratios, (3) engineered modular drainage systems with back-flush options, (4) data logging stations with defined monitoring frequencies, and (5) optional automation units for real-time flow and parameter control. Together, these design elements represent a move toward codified frameworks that facilitate regulatory approval, ensure technical robustness, and improve stakeholder confidence. Standardized design criteria, when coupled with adaptive site-based customization, will be instrumental in advancing phytoremediation from niche application to mainstream environmental management strategy.
4. Critical Environmental Factors Affecting Phytoremediation Efficiency
Water plays a central role in phytoremediation systems, particularly in designs aimed at treating contaminated wastewater, surface runoff, or groundwater. The quality, quantity, and dynamics of water within these systems significantly influence contaminant mobility, plant health, and microbial activity. To enhance the reliability and scalability of phytoremediation, there is an increasing need to standardize key water-related environmental parameters across various applications and site conditions.
The chemical composition of the water, such as pH, electrical conductivity (EC), dissolved oxygen (DO), nutrient concentrations (e.g., nitrogen, phosphorus), and presence of heavy metals or organic pollutants, directly affects contaminant bioavailability and plant uptake mechanisms. Standardizing baseline water quality assessments (e.g., pH 6.0–8.0, DO ≥ 5 mg/L) and defining threshold values for phytotoxicity would ensure consistent treatment performance across systems. Table 2 summarizes the standardized values for key water quality parameters relevant to phytoremediation system design.

Table 2.
Water Chemical Parameters and Proposed Standardized Values for Phytoremediation.
The rate at which water is introduced into the system influences retention time, contaminant contact with the rhizosphere, and oxygen levels. Overloading can reduce treatment efficiency and cause plant stress. A standardized HLR range (e.g., 2–10 cm/day for constructed wetlands) could guide the dimensioning of phytoremediation units and ensure optimal hydraulic conditions for different plant species and contaminant types. Recommended Hydraulic Loading Rates (HLRs) for various phytoremediation systems are listed in Table 3.

Table 3.
Proposed Hydraulic Loading Rates (HLR) for Phytoremediation Systems.
When applying the Hydraulic Loading Rate (HLR), it is essential to adjust the rate according to the specific type of phytoremediation system and treatment objectives to ensure optimal performance. An excessively high HLR can lead to insufficient water retention time, reducing contaminant removal efficiency and causing stress to plants. Conversely, a very low HLR may result in the accumulation of pollutants and decreased treatment effectiveness due to oxygen deficiency and limited microbial activity. Additionally, climatic conditions and the plant species used significantly influence the optimal HLR, and these factors should be carefully considered during system design.
Whether water is applied continuously, intermittently, or in pulses affects root oxygenation, microbial activity, and system resilience. Standardizing operational modes and recommending flow regimes tailored to system type (e.g., horizontal vs. vertical flow wetlands) would help stabilize treatment performance under variable loading. The effects of different flow regimes on phytoremediation performance are summarized in Table 4.

Table 4.
Effects of Flow Regimes on Phytoremediation System Performance.
The selection of an appropriate flow regime depends on the type of system, the nature of the contaminants to be treated, and the specific environmental conditions. Vertical subsurface flow systems (VSSF) commonly employ intermittent or pulsed flow modes to enhance oxygenation and microbial activity, whereas horizontal subsurface flow systems (HSSF) typically operate under continuous flow to maintain stable conditions. Standardizing flow regimes and ensuring proper system design are crucial steps to optimize treatment efficiency and support long-term system sustainability.
Temperature plays a key role in the effectiveness of phytoremediation systems, influencing plant metabolism, microbial activity, and the solubility of contaminants. In cooler climates, treatment efficiency is often lower due to slower biological processes. Including recommended operational temperature ranges (e.g., 15–35 °C) in standardized guidelines helps define seasonal expectations and guide the selection of appropriate plant species. Below is a summary table showing how different water temperature ranges affect treatment performance and which plant species are best suited to each range. Table 5 summarizes the influence of temperature on treatment efficiency and corresponding suitable plant species.

Table 5.
Summary of Temperature Effects on Treatment Efficiency and Suitable Plant Species.
The optimal temperature range for phytoremediation is considered to be 20–30 °C, with peak heavy metal uptake observed at 25–30 °C. When temperatures exceed 30 °C, many species such as Eichhornia crassipes may experience heat stress, resulting in reduced treatment performance. Therefore, choosing plant species suitable for the local temperature conditions is critical to maintaining the system’s effectiveness.
To ensure the effective and comparable application of phytoremediation technologies across different sites, the establishment of standardized water conditions is crucial. This involves implementing baseline water quality testing protocols to support informed site selection and ongoing system monitoring. Additionally, it is necessary to define acceptable ranges for critical water parameters, including pH, dissolved oxygen (DO), electrical conductivity (EC), and nutrient concentrations. Developing comprehensive guidance documents that align hydraulic and flow design with water characteristics and treatment objectives will further enhance system performance. Moreover, promoting the creation of reference databases that document contaminant removal efficiencies by various plant species under specific water conditions will provide valuable insights for future applications. By integrating these standardized water-related design criteria into phytoremediation planning, the field can achieve more predictable outcomes and facilitate wider adoption as a viable environmental remediation strategy.
5. Contaminant Characteristics
The bioavailability of contaminants is a key factor determining the effectiveness of phytoremediation, as it reflects the extent to which pollutants can be absorbed and metabolized by plants in water or soil environments [56]. To work toward standardization and future policy development for phytoremediation applications, it is necessary to establish specific regulations for assessing and controlling the bioavailability of contaminants at treatment sites [52,86]. Environmental factors such as pH, temperature, soil composition, and the physicochemical properties of the medium must be measured and standardized to ensure bioavailability levels are suitable for maximizing plant uptake and degradation of pollutants [89]. Additionally, the role of chelating agents and microorganisms in modifying and enhancing bioavailability should be further studied and incorporated into technical guidelines, aiming to optimize treatment conditions and improve the efficiency of phytoremediation [82]. Establishing these standards will not only ensure consistency in technology implementation but also provide a solid legal foundation for deploying biological pollution treatment projects at national and international levels [42,100,113].
The chemical speciation of contaminants plays an important role in determining the effectiveness of phytoremediation, as different forms, such as ions, organic compounds, or metal complexes, exhibit varying levels of toxicity and plant uptake potential [96]. To standardize the application and management of this technology, it is essential to develop procedures for identifying and analyzing the chemical forms of pollutants at treatment sites to accurately assess pollution risk and the treatment capacity of plant systems. Furthermore, research on the transformation of contaminant species under different environmental conditions, as well as during the phytoremediation process, must be standardized to ensure proper control and optimization of operational conditions [7,56]. Establishing technical standards related to chemical speciation will enhance accuracy in evaluating treatment effectiveness and support the development of environmental management policies, enabling the long-term, safe, and efficient application of phytoremediation in the future.
The concentration of contaminants in the environment, such as in water, soil, or sediment, is one of the most direct factors influencing the effectiveness of phytoremediation. Determining and standardizing the presence levels of pollutants at treatment locations is essential to ensure that plant systems can operate optimally. Excessive concentrations can cause toxicity or stress in plants, reducing their ability to absorb and degrade contaminants, while concentrations that are too low may fail to adequately trigger effective treatment responses [89,96]. Therefore, establishing standards for optimal concentration thresholds for each contaminant type and plant species used in phytoremediation will help guide system selection and design, ensuring sustainability and long-term efficiency of environmental remediation projects. Policies and technical guidelines should be built on scientific data to manage and control contaminant concentrations, thereby facilitating the broad application of phytoremediation technologies in the future.
The interaction among properties such as chemical speciation, bioavailability, and concentration of contaminants plays a decisive role in the performance of plant-based treatment systems in phytoremediation [7,114]. The combination of contaminant speciation and bioavailability directly affects the absorption and transformation capacity of plants, while contaminant concentration determines the level of exposure and impact on organisms. Additionally, environmental factors such as pH, temperature, humidity, and the characteristics of the soil or aquatic medium also influence these properties, thereby affecting treatment efficiency and system stability. For practical and environmentally viable phytoremediation applications, the development of standards and management policies must emphasize integrated evaluation of these interacting factors to optimize operating conditions and ensure the effectiveness of pollution treatment in environmental projects.
6. Post-Harvest Management of Contaminant-Loaded Biomass
Once phytoremediation plants have accumulated pollutants, the harvested biomass becomes a secondary waste stream that must be managed safely and economically. Best practice begins with controlled harvesting schedules that minimize mechanical disturbance and prevent re-release of contaminants to water bodies or adjacent soil [80,114]. Immediately after harvest, biomass should be characterized for total contaminant load, moisture content, and calorific value to determine an appropriate end-of-life pathway [87].
Post-harvest treatment of phytoremediator plants is critical to prevent secondary contamination. In most field and pilot-scale applications, harvested biomass is subjected to one or more of the following decontamination or stabilization steps: air drying, incineration at controlled temperatures (typically 500–700 °C), composting with microbial amendments to accelerate degradation, or encapsulation in inert matrices. For instance, Eichhornia crassipes collected from metal-contaminated waters is often dried and incinerated, while species like Brassica juncea are composted under monitored aerobic conditions to degrade residual organics and reduce bioavailability of metals
- -
- Secure Containment and Disposal—For biomass containing high levels of persistent or highly toxic elements (e.g., Hg, Cd, Pb), secure landfill disposal or hazardous-waste incineration remains the most widely accepted option. Ashes from high-temperature incineration must be stabilized or vitrified before landfilling to avoid leaching.
- -
- Thermochemical Conversion—Where metals are present at moderate concentrations, pyrolysis, gasification, or controlled combustion can recover energy and leave a reduced-volume ash that can be further treated. Recent studies show that co-firing Typha latifolia and Eichhornia crassipes with conventional biomass can yield renewable heat while concentrating metals into an ash phase for recycling or secure disposal [3].
- -
- Phytomining and Metal Recovery—Hyperaccumulator biomass rich in Ni, Zn, or Au can be processed to recover valuable metals through smelting or bio-hydrometallurgical leaching. Pilot projects using Pteris vittata for As and Brassica juncea for Pb recovery demonstrate technical feasibility, although economic viability depends on metal market prices and biomass logistics [13,87].
- -
- Composting and Biochar Production—For biomass primarily laden with nutrients or low-toxicity organics, composting can recycle organic matter, provided periodic leachate monitoring confirms contaminant levels remain below agronomic thresholds. Alternatively, converting biomass to biochar via low-temperature pyrolysis immobilizes many metals and generates a sorptive material useful for further water treatment [104].
- -
- Carbon Sequestration and Ecosystem Services—Fast-growing species such as willow and poplar can be harvested in short rotations; their incorporation into bioenergy-carbon-capture chains contributes to negative-emission strategies while safely removing pollutants from aquatic systems [85].
- -
- Regulatory Alignment—National waste regulations should classify phytoremediation biomass based on contaminant thresholds, specify transport and storage protocols, and set emission limits for thermochemical processing. Harmonized criteria will streamline permitting and assure public safety [53,100].
- -
- Life-Cycle Assessment (LCA)—Incorporating LCA into design guidelines helps compare disposal routes on the basis of greenhouse gas emissions, resource recovery, and long-term liability, guiding stakeholders toward the most resilient option [108].
Implementing a decision matrix that matches contaminant profile, biomass quantity, and local infrastructure with the best end-use pathway will close the loop between phytoremediation and waste management, ensuring that environmental gains are not offset by downstream risks.
7. Implications for Policy and Large-Scale Adoption
7.1. Opportunities for National Environmental Remediation Programs
The establishment of standardized design frameworks for phytoremediation offers transformative potential for national environmental remediation programs, especially in the context of complex and diverse aquatic systems. Given the variability in physicochemical conditions and contaminant profiles across rivers, lakes, wetlands, and industrial effluents, a harmonized yet flexible system is essential to ensure predictable treatment performance. A central tenet of this standardization lies in the definition of key engineering and biological parameters, including plant species selection, planting density, root zone configuration, hydraulic retention time, and substrate composition. By formalizing such parameters, practitioners can tailor site-specific applications while adhering to overarching guidelines that facilitate replication, regulatory acceptance, and scalability. In addition, developing uniform protocols for the characterization of contaminant bioavailability and speciation, particularly for metals and persistent organic pollutants, will enable accurate estimation of phytoremediation potential and cross-site comparability.
Table 6 illustrates common challenges encountered in phytoremediation practices and the corresponding mitigation strategies drawn from empirical studies. For example, low biomass productivity, such as Lemna minor yielding approximately 30 g/m2/day, restricts contaminant uptake and may necessitate interventions like genetic modification or nutrient supplementation. Similarly, metal contaminants bound tightly in alkaline soils show bioavailability reductions exceeding 40%, requiring the use of chelating agents such as EDTA or citric acid. Phytotoxic concentrations, seasonal performance declines, and extended remediation timeframes also underscore the necessity of robust design that integrates supportive measures such as gradual exposure regimes, controlled environments, and combined treatment modalities. Addressing these challenges through standardized intervention protocols ensures more resilient and efficient phytoremediation systems, especially when implemented at the policy or national program level.

Table 6.
Common Challenges in Phytoremediation and Corresponding Mitigation Strategies.
Beyond system design, standardization offers critical advantages in monitoring, performance evaluation, and technology transfer. Establishing a suite of universal indicators, such as contaminant removal efficiency, plant physiological status, and changes in effluent water quality, will allow for objective assessments and ongoing system optimization. Integrating environmental variables (e.g., temperature, flow rate, pH) into monitoring frameworks enhances predictive accuracy and operational resilience. Moreover, the delineation of best-practice applications for enhancing agents like microbial consortia and substrate amendments will promote greater treatment efficacy. Importantly, clearly defined operational and safety thresholds provide a foundation for regulatory compliance, stakeholder confidence, and cost–benefit optimization. By embedding phytoremediation within national remediation strategies through standardized protocols, policymakers can drive widespread adoption of this ecologically sustainable and economically viable water treatment technology.
7.2. Integration into Regulatory Guidelines and Sustainability Plans
The formal integration of phytoremediation into national environmental regulatory systems represents a pivotal advancement in mainstreaming green technologies for water and soil decontamination. Jurisdictions such as the United States, through the Environmental Protection Agency’s Superfund program, have officially recognized phytoremediation as a viable remediation technique, particularly for heavy metal and organic contaminant cleanup. Likewise, the European Union’s Water Framework Directive promotes eco-efficient technologies, including phytoremediation, to meet water quality and ecological restoration objectives. India’s Central Pollution Control Board has similarly incorporated phytoremediation into guidelines for rejuvenating polluted aquatic ecosystems. These precedents underscore an increasing policy shift toward embracing nature-based solutions as integral to resilient environmental governance strategies
Nevertheless, in many national and regional contexts, the regulatory scaffolding necessary to support the widespread and safe deployment of phytoremediation remains underdeveloped. Existing environmental policies often lack specific, enforceable provisions addressing phytoremediation’s design, implementation, and monitoring, limiting its eligibility for public funding and formal project endorsement. Establishing clear regulatory guidelines is therefore essential to legitimize phytoremediation within statutory frameworks. These should include standardized protocols for site characterization, contaminant threshold values, species selection, and long-term performance monitoring. Moreover, attention must be paid to potential risks, such as contaminant accumulation in biomass, the possibility of species invasiveness, and unintended trophic transfer, to ensure ecological integrity is maintained. Addressing these regulatory gaps will not only foster responsible deployment but also bolster public and institutional confidence in the technology.
Beyond statutory mandates, embedding phytoremediation into national sustainability strategies offers multidimensional benefits. As an inherently low-input, ecologically restorative technology, phytoremediation aligns closely with green remediation principles by minimizing energy consumption, avoiding hazardous chemicals, and enhancing biodiversity. Figure 2 illustrates its relative efficiency compared to conventional remediation techniques, reinforcing its suitability for sustainable water resource management. Incorporating phytoremediation into integrated watershed management, wetland rehabilitation, and climate adaptation policies facilitates cross-sectoral cooperation among scientific institutions, government bodies, industry, and civil society. Such inclusion fosters innovation ecosystems, enables the replication of best practices, and supports community engagement in environmental stewardship. Ultimately, the convergence of regulatory clarity and sustainability planning creates the institutional infrastructure necessary for scaling up phytoremediation as a credible, standardized, and socially accepted solution for pollution mitigation and ecosystem restoration.
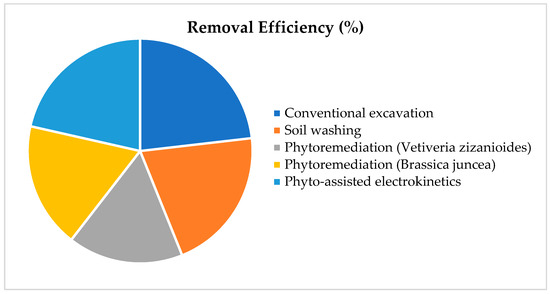
Figure 2.
Relative Efficiency of Phytoremediation vs. Other Remediation Techniques.
7.3. Recommendations for Policymakers and Stakeholders
To fully realize the potential of phytoremediation as an effective and environmentally viable water treatment technology, policymakers and stakeholders must take proactive and coordinated actions. Policymakers should prioritize funding for multidisciplinary research focused on improving phytoremediation techniques, including plant selection, contaminant bioavailability enhancement, and system optimization under diverse environmental conditions. Investment in developing standardized methodologies for site assessment, monitoring, and performance evaluation is critical to build a robust evidence base supporting regulatory approval and practical implementation. Stakeholders, including regulatory agencies, scientific communities, and industry experts, should collaborate to create comprehensive technical standards and guidelines that define best practices for phytoremediation design, operation, and monitoring. These standards will help ensure consistency, reliability, and safety across projects, facilitating regulatory acceptance and investor confidence. Raising awareness and building technical expertise among environmental managers, practitioners, and local communities are essential for the successful deployment of phytoremediation. Training programs and knowledge-sharing platforms should be established to equip stakeholders with the necessary skills to design, operate, and maintain phytoremediation systems effectively. Implementing pilot-scale phytoremediation projects in diverse settings can demonstrate the technology’s feasibility, identify operational challenges, and generate site-specific data. Governments and stakeholders should support these initiatives through grants, public–private partnerships, or incentives, enabling adaptive management and refinement of technical approaches. Effective phytoremediation deployment requires coordinated efforts among policymakers, researchers, industry players, non-governmental organizations, and affected communities. Establishing forums and networks to facilitate dialogue, share experiences, and align goals will strengthen cooperation and enhance the technology’s social acceptance and sustainability. Finally, policymakers should explicitly incorporate phytoremediation within national and regional environmental management strategies, pollution control plans, and sustainability agendas. Clear policy frameworks that recognize phytoremediation as a complementary or alternative treatment option will create a supportive environment for innovation and investment. By following these recommendations, policymakers and stakeholders can accelerate the responsible development and scaling of phytoremediation technologies, contributing to improved water quality, ecosystem health, and community well-being.
8. Conclusions
Phytoremediation has demonstrated considerable promise as an ecologically sound and economically viable strategy for remediating polluted water and soil systems. This review highlights the multifactorial dependencies influencing phytoremediation efficiency, such as contaminant speciation, bioavailability, hydrological regimes, temperature conditions, and the ecological adaptability of selected plant species. It emphasizes the necessity of establishing standardized design criteria and regulatory frameworks that integrate these critical variables to ensure safety, consistency, and performance reliability. The method’s cost-effectiveness and minimal infrastructure requirements render it particularly advantageous for low-income and developing nations, where conventional remediation technologies remain prohibitively expensive. By formulating technical standards and adopting phytoremediation into national environmental programs, such regions can address environmental degradation while advancing sustainable development priorities.
The findings of this review underscore the urgent need for a globally harmonized phytoremediation framework, one that aligns scientific innovation with coherent policy mechanisms. Integrating phytoremediation into environmental governance and sustainability plans will not only expand its application but also strengthen its contribution to ecosystem restoration and pollution control. Regulatory guidance should define site suitability parameters, target pollutant classes, and safe biomass disposal protocols to mitigate environmental and health risks. Furthermore, future research should prioritize pilot-scale studies, development of bioindicator systems, and public outreach to enhance community participation. Interdisciplinary collaboration across scientific, policy, and community stakeholders will be pivotal in transforming phytoremediation from a promising innovation into a mainstream solution for environmental resilience and pollution mitigation.
Author Contributions
Conceptualization, T.Q.N.; project administration, T.Q.N.; supervision, T.Q.N. and M.N.T.; writing—original draft, T.M.H., T.Q.N. and M.N.T.; writing—review and editing, T.Q.N., M.N.T. and T.N.N.; data curation, D.H.P., T.N.N. and H.M.T.; formal analysis, D.H.P., T.N.N. and H.M.T.; investigation, T.M.H., H.M.T. and M.Q.B.; validation, T.M.H., M.Q.B. and H.X.N.; resources, M.Q.B. and H.X.N.; software, D.H.P. and H.X.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Center for High Technology Research and Development is appreciated for partly supporting to this research. We thank Tran Ngoc Minh for technical and analytical assistance.
Conflicts of Interest
Author Duc Hung Pham was employed by the Hera Biopharmaceuticals Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BOD | Biological Oxygen Demand |
| COD | Chemical Oxygen Demand |
| EC | Electrical Conductivity |
| TDS | Total Dissolved Solids |
| WHO | World Health Organization |
| EPA | Environmental Protection Agency |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| BTEX | Benzene, Toluene, Ethylbenzene, Xylenes |
| TPH | Total Petroleum Hydrocarbons |
| PTEs | Potentially Toxic Elements |
| TSS | Total Suspended Solids |
| pH | Potential of Hydrogen |
References
- Ali, S.; Abbas, Z.; Rizwan, M.; Zaheer, I.; Yavaş, İ.; Ünay, A.; Abdel-Daim, M.; Bin-Jumah, M.; Hasanuzzaman, M.; Kalderis, D. Application of Floating Aquatic Plants in Phytoremediation of Heavy Metals Polluted Water: A Review. Sustainability 2020, 12, 1927. [Google Scholar] [CrossRef]
- Pivetz, B.E. Phytoremediation of Contaminated Soil and Ground Water at Hazardous Waste Sites; EPA/540/S-01/500; U.S. Environmental Protection Agency: Washington, DC, USA, 2001.
- Gallagher, J.L. Halophytic crops for cultivation at seawater salinity. Plant Soil 1985, 89, 323–336. [Google Scholar] [CrossRef]
- Sekomo, C.B.; Rousseau, D.P.L.; Saleh, S.A.; Lens, P.N.L. Heavy Metal Removal in Duckweed and Algae Ponds as a Polishing Step for Textile Wastewater Treatment. Ecol. Eng. 2012, 44, 102–110. [Google Scholar] [CrossRef]
- Guidi Nissim, W.; Castiglione, S.; Guarino, F.; Pastore, M.C.; Labra, M. Beyond Cleansing: Ecosystem Services Related to Phytoremediation. Plants 2023, 12, 1031. [Google Scholar] [CrossRef]
- Haris, H.; Fai, C.M.; Bahruddin, A.S.; Dinesh, A.A.A. Effect of Temperature on Nutrient Removal Efficiency of Water Hyacinth for Phytoremediation Treatment. Int. J. Eng. Technol. 2018, 7, 81–84. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture 2019: Moving Forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019. [Google Scholar]
- Nguyen, H.M.N.; Khieu, H.T.; Ta, N.A.; Le, H.Q.; Nguyen, T.Q.; Do, T.Q.; Hoang, A.Q.; Kannan, K.; Tran, T.M. Distribution of Cyclic Volatile Methylsiloxanes in Drinking Water, Tap Water, Surface Water, and Wastewater in Hanoi, Vietnam. Environ. Pollut. 2021, 285, 117260. [Google Scholar] [CrossRef]
- Kudo, H.; Qian, Z.; Inoue, C.; Chien, M.-F. Temperature Dependence of Metals Accumulation and Removal Kinetics by Arabidopsis halleri ssp. gemmifera. Plants 2023, 12, 877. [Google Scholar] [CrossRef] [PubMed]
- Cristaldi, A.; Oliveri Conti, G.; Cosentino, S.L.; Mauromicale, G.; Copat, C.; Grasso, A.; Zuccarello, P.; Fiore, M.; Restuccia, C.; Ferrante, M. Phytoremediation Potential of Arundo donax (Giant Reed) in Contaminated Soil by Heavy Metals. Environ. Res. 2020, 185, 109427. [Google Scholar] [CrossRef] [PubMed]
- Green, C.; Hoffnagle, A. Phytoremediation Field Studies Database for Chlorinated Solvents, Pesticides, Explosives, and Metals; EPA Report; U.S. Environmental Protection Agency: Washington, DC, USA, 2004.
- Al-Baldawi, I.A.; Abdullah, S.R.S.; Anuar, N.; Hasan, H.A. Phytotransformation of Methylene Blue from Water Using Aquatic Plant (Azolla pinnata). Environ. Technol. Innov. 2018, 11, 15–22. [Google Scholar] [CrossRef]
- Crum, S.J.H.; van Kammen-Polman, A.M.M.; Leistra, M. Sorption of Nine Pesticides to Three Aquatic Macrophytes. Arch. Environ. Contam. Toxicol. 1999, 37, 310–316. [Google Scholar] [CrossRef]
- Durjava, M.; Kolar, B. Bioavailability-Based Environmental Quality Standards for Metals under the Water Framework Directive. Acta Hydrotech. 2023, 36, 17–29. [Google Scholar] [CrossRef]
- Dincau, B.; Tang, C.; Dressaire, E.; Sauret, A. Clog Mitigation in a Microfluidic Array via Pulsatile Flows. Soft Matter 2022, 18, 1767–1778. [Google Scholar] [CrossRef] [PubMed]
- Calderon, J.L.; Kaunda, R.B.; Sinkala, T.; Workman, C.F.; Bazilian, M.D.; Clough, G. Phytoremediation and Phytoextraction in Sub-Saharan Africa: Addressing Economic and Social Challenges. Ecotoxicol. Environ. Saf. 2021, 226, 112864. [Google Scholar] [CrossRef]
- Cai, Y.; Cao, X.; Liu, B.; Lin, H.; Luo, H.; Liu, F.; Su, D.; Lv, S.; Lin, Z.; Lin, D. Saline-Alkali Tolerance Evaluation of Giant Reed (Arundo donax) Genotypes under Saline-Alkali Stress at Seedling Stage. Agronomy 2025, 15, 463. [Google Scholar] [CrossRef]
- Rizvi, Z.F.; Jamal, M.; Parveen, H.; Sarfraz, W.; Nasreen, S.; Khalid, N.; Muzammil, K. Phytoremediation Potential of Pistia stratiotes, Eichhornia crassipes, and Typha latifolia for Chromium with Stimulation of Secondary Metabolites. Heliyon 2024, 10, e29078. [Google Scholar] [CrossRef] [PubMed]
- Urucu, O.A.; Garosi, B.; Musah, R.A. Efficient Phytoremediation of Methyl Red and Methylene Blue Dyes from Aqueous Solutions by Juncus effusus. ACS Omega 2025, 10, 1943–1953. [Google Scholar] [CrossRef]
- Buta, E.; Borșan, I.L.; Omotă, M.; Trif, E.B.; Bunea, C.I.; Mocan, A.; Bora, F.D.; Rózsa, S.; Nicolescu, A. Comparative Phytoremediation Potential of Eichhornia crassipes, Lemna minor, and Pistia stratiotes in Two Treatment Facilities in Cluj County, Romania. Horticulturae 2023, 9, 503. [Google Scholar] [CrossRef]
- Paes, É.C.; Veloso, G.V.; de Castro Filho, M.N.; Barroso, S.H.; Fernandes-Filho, E.I.; Fontes, M.P.F.; Soares, E.M.B. Potential of Plant Species Adapted to Semi-Arid Conditions for Phytoremediation of Contaminated Soils. J. Hazard. Mater. 2023, 449, 131034. [Google Scholar] [CrossRef]
- Kola, E.; Munyai, C.; Dalu, T. A Review of Macrophyte Phytoremediation in Africa: Current Research and Challenges. Chem. Ecol. 2025, 41, 710–728. [Google Scholar] [CrossRef]
- Brown, D.S.; Kreissl, J.S.; Gearhart, R.A.; Kruzic, A.P.; Boyle, W.C.; Otis, R.J. Manual—Constructed Wetlands Treatment of Municipal Wastewaters; EPA/625/R-99/010 (NTIS PB2001-101833); U.S. Environmental Protection Agency: Washington, DC, USA, 2000.
- Ghosh, M.; Singh, S.P. A Review on Phytoremediation of Heavy Metals and Utilization of Its Byproducts. Appl. Ecol. Environ. Res. 2005, 3, 214–231. [Google Scholar] [CrossRef]
- Trinh, H.T.; Marcussen, H.; Hansen, H.C.B.; Le, G.T.; Duong, H.T.; Ta, N.T.; Nguyen, T.Q.; Hansen, S.; Strobel, B.W. Screening of Inorganic and Organic Contaminants in Floodwater in Paddy Fields of Hue and Thanh Hoa in Vietnam. Environ. Sci. Pollut. Res. 2017, 24, 7348–7358. [Google Scholar] [CrossRef]
- Parra, L.-M.M.; Torres, G.; Arenas, A.D.; Sánchez, E.; Rodríguez, K. Phytoremediation of Low Levels of Heavy Metals Using Duckweed (Lemna minor). In Abiotic Stress Responses in Plants; Springer: New York, NY, USA, 2012; pp. 451–463. [Google Scholar] [CrossRef]
- Sharma, R.; Saini, H.; Paul, D.R.; Chaudhary, S.; Nehra, S.P. Removal of Organic Dyes from Wastewater Using Eichhornia crassipes: A Potential Phytoremediation Option. Environ. Sci. Pollut. Res. 2021, 28, 7116–7122. [Google Scholar] [CrossRef]
- Pulford, I.; Watson, C. Phytoremediation of Heavy Metal-Contaminated Land by Trees: A Review. Environ. Int. 2003, 29, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Sawarkar, R.; Shakeel, A.; Kumar, T.; Ansari, S.A.; Agashe, A.; Singh, L. Evaluation of Plant Species for Air Pollution Tolerance and Phytoremediation Potential in Proximity to a Coal Thermal Power Station: Implications for Smart Green Cities. Environ. Geochem. Health 2023, 45, 7303–7322. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M. Phytoremediation Strategies for Mitigating Environmental Toxicants. Heliyon 2024, 10, e38683. [Google Scholar] [CrossRef] [PubMed]
- Chander, P.D.; Fai, C.M.; Kin, C.M. Removal of Pesticides Using Aquatic Plants in Water Resources: A Review. IOP Conf. Ser. Earth Environ. Sci. 2018, 164, 012027. [Google Scholar] [CrossRef]
- Truong, D.A.; Trinh, H.T.; Le, G.T.; Phan, T.Q.; Duong, H.T.; Tran, T.T.L.; Nguyen, T.Q.; Hoang, M.T.T.; Nguyen, T.V. Occurrence and Ecological Risk Assessment of Organophosphate Esters in Surface Water from Rivers and Lakes in Urban Hanoi, Vietnam. Chemosphere 2023, 331, 138805. [Google Scholar] [CrossRef]
- UN Environment. Global Environment Outlook—GEO-6: Healthy Planet, Healthy People; UN Environment: Nairobi, Kenya, 2019. [Google Scholar] [CrossRef]
- Malunguja, G.K.; Paschal, M. Evaluating Potential Phytoremediators to Combat Detrimental Impacts of Mining on Biodiversity: A Review Focused in Africa. Discov. Environ. 2024, 2, 94. [Google Scholar] [CrossRef]
- O’Donnell, M.S.; Whipple, A.L.; Inman, R.D.; Tarbox, B.C.; Monroe, A.P.; Robb, B.S.; Aldridge, C.L. Remote Sensing for Monitoring Mine Lands and Recovery Efforts; Circular 1525; U.S. Geological Survey: Reston, VA, USA, 2024. [CrossRef]
- Hudson, J.; Hogye, S.; Frederick, R.; Goo, R.; Kelly, S. US EPA Program Strategy for Decentralized Wastewater Systems. Proc. Water Environ. Fed. 2005, 2005, 5795–5801. [Google Scholar] [CrossRef]
- da Silva, J.; Rosa, G.B.; Sganzerla, W.G.; Ferrareze, J.P.; Simioni, F.J.; Campos, M.L. Strategies and Prospects in the Recovery of Contaminated Soils by Phytoremediation: An Updated Overview. Commun. Plant Sci. 2023, 13, 1–12. [Google Scholar] [CrossRef]
- Ahila, K.G.; Ravindran, B.; Muthunarayanan, V.; Nguyen, D.D.; Nguyen, X.C.; Chang, S.W.; Nguyen, V.K.; Thamaraiselvi, C. Phytoremediation Potential of Freshwater Macrophytes for Treating Dye-Containing Wastewater. Sustainability 2020, 13, 329. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Jayamuthunagai, J.; Praveenkumar, R.; Iyyappan, J. Phytoremediation Techniques for the Removal of Dye in Wastewater. In Bioremediation and Biotechnology: Sustainable Approaches to Pollution Degradation; Springer: Singapore, 2018; pp. 243–252. [Google Scholar] [CrossRef]
- McIntyre, T.C. Databases and Protocol for Plant and Microorganism Selection: Hydrocarbons and Metals. In Phytoremediation; Wiley: Hoboken, NJ, USA, 2003; pp. 887–904. [Google Scholar] [CrossRef]
- Olette, R.; Couderchet, M.; Biagianti, S.; Eullaffroy, P. Toxicity and Removal of Pesticides by Selected Aquatic Plants. Chemosphere 2008, 70, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Prasertsup, P.; Ariyakanon, N. Removal of Chlorpyrifos by Water Lettuce (Pistia stratiotes L.) and Duckweed (Lemna minor L.). Int. J. Phytoremediat. 2011, 13, 383–395. [Google Scholar] [CrossRef]
- Salido, A.L.; Hasty, K.L.; Lim, J.-M.; Butcher, D.J. Phytoremediation of Arsenic and Lead in Contaminated Soil Using Chinese Brake Ferns (Pteris vittata) and Indian Mustard (Brassica juncea). Int. J. Phytoremediat. 2003, 5, 89–103. [Google Scholar] [CrossRef]
- Singh, V.; Thakur, L.; Mondal, P. Removal of Lead and Chromium from Synthetic Wastewater Using Vetiveria zizanioides. Clean Soil Air Water 2015, 43, 538–543. [Google Scholar] [CrossRef]
- Alsghayer, R.; Salmiaton, A.; Mohammad, T.; Idris, A.; Ishak, C.F. Removal Efficiencies of Constructed Wetland Planted with Phragmites and Vetiver in Treating Synthetic Wastewater Contaminated with High Concentration of PAHs. Sustainability 2020, 12, 3357. [Google Scholar] [CrossRef]
- Anh, B.T.K.; Ha, N.T.H.; Danh, L.T.; Minh, V.V.; Kim, D.D. Phytoremediation Applications for Metal-Contaminated Soils Using Terrestrial Plants in Vietnam. In Phytoremediation; Springer: Cham, Switzerland, 2017; pp. 157–181. [Google Scholar] [CrossRef]
- Cheng, X.; Jiang, L.; Liu, W.; Song, X.; Kumpiene, J.; Luo, C. Phytoremediation of Trichloroethylene in the Soil/Groundwater Environment: Progress, Problems, and Potential. Sci. Total Environ. 2024, 954, 176566. [Google Scholar] [CrossRef]
- Boonsaner, M.; Borrirukwisitsak, S.; Boonsaner, A. Phytoremediation of BTEX Contaminated Soil by Canna×generalis. Ecotoxicol. Environ. Saf. 2011, 74, 1700–1707. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Le, T.G.; Nguyen, T.T.; Le, V.N.; Hoang, M.T.; Truong, N.M.; Nguyen, N.T.; Bui, Q.M. The Deadlock of Application Research Using Halophytes for Seawater Desalination Due to Rapid Evaporation of Water. SSRN Electron. J. 2023. [Google Scholar] [CrossRef]
- Virú-Vasquez, P.; Pilco-Nuñez, A.; Tineo-Cordova, F.; Madueño-Sulca, C.T.; Quispe-Ojeda, T.C.; Arroyo-Paz, A.; Alvarez-Arteaga, R.; Velasquez-Zuñiga, Y.; Oscanoa-Gamarra, L.L.; Saldivar-Villarroel, J.; et al. Integrated Biochar-Compost Amendment for Zea mays L. Phytoremediation in Soils Contaminated with Mining Tailings of Quiulacocha, Peru. Plants 2025, 14, 1448. [Google Scholar] [CrossRef]
- Tarla, D.N.; Erickson, L.E.; Hettiarachchi, G.M.; Amadi, S.I.; Galkaduwa, M.; Davis, L.C.; Nurzhanova, A.; Pidlisnyuk, V. Phytoremediation and Bioremediation of Pesticide-Contaminated Soil. Appl. Sci. 2020, 10, 1217. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Raskin, I.; Smith, R.D.; Salt, D.E. Phytoremediation of Metals: Using Plants to Remove Pollutants from the Environment. Curr. Opin. Biotechnol. 1997, 8, 221–226. [Google Scholar] [CrossRef]
- Vymazal, J. Constructed Wetlands for Wastewater Treatment. Water 2010, 2, 530–549. [Google Scholar] [CrossRef]
- Kaur, H.; Kumar, A.; Bindra, S.; Sharma, A. Phytoremediation: An Emerging Green Technology for Dissipation of PAHs from Soil. J. Geochem. Explor. 2024, 259, 107426. [Google Scholar] [CrossRef]
- Tan, K.A.; Morad, N.; Ooi, J.Q. Phytoremediation of Methylene Blue and Methyl Orange Using Eichhornia crassipes. Int. J. Environ. Sci. Dev. 2016, 7, 724–728. [Google Scholar] [CrossRef]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- Dordio, A.; Carvalho, A.J.P.; Teixeira, D.M.; Dias, C.B.; Pinto, A.P. Removal of Pharmaceuticals in Microcosm Constructed Wetlands Using Typha spp. and LECA. Bioresour. Technol. 2010, 101, 886–892. [Google Scholar] [CrossRef]
- Uysal, Y.; Taner, F. Bioremoval of Cadmium by Lemna minor in Different Aquatic Conditions. Clean Soil Air Water 2010, 38, 370–377. [Google Scholar] [CrossRef]
- Cunningham, S.D.; Ow, D.W. Promises and Prospects of Phytoremediation. Plant Physiol. 1996, 110, 715–719. [Google Scholar] [CrossRef]
- Rock, S.; Pivetz, B.; Madalinski, K.; Adams, N.; Wilson, T. Introduction to Phytoremediation; EPA/600/R-99/107 (NTIS PB2000-106690); U.S. Environmental Protection Agency: Washington, DC, USA, 2000.
- Córdoba-Tovar, L.; Marrugo-Madrid, S.; Castro, L.P.; Tapia-Contreras, E.E.; Marrugo-Negrete, J.; Díez, S. Exploring the Phytoremediation Potential of Plant Species in Soils Impacted by Gold Mining in Northern Colombia. Environ. Sci. Pollut. Res. 2025, 32, 3795–3808. [Google Scholar] [CrossRef] [PubMed]
- Bernardino, C.A.R.; Mahler, C.F.; Preussler, K.H.; Novo, L.A.B. State of the Art of Phytoremediation in Brazil, Review and Perspectives. Water Air Soil Pollut. 2016, 227, 272. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, W.; Tong, W. Clogging Processes Caused by Biofilm Growth and Organic Particle Accumulation in Lab-Scale Vertical Flow Constructed Wetlands. J. Environ. Sci. 2009, 21, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, R.V.; Boralkar, D.B.; Chavhan, R.D. Remediation Capabilities of Pilot-Scale Wetlands Planted with Typha angustifolia and Acorus calamus to Treat Landfill Leachate. J. Ecol. Environ. 2018, 42, 23. [Google Scholar] [CrossRef]
- Dennis, G.; Shin, P.E. Brownfields Technology Primer: Selecting and Using Phytoremediation for Site Cleanup; EPA 542-R-01-006; U.S. Environmental Protection Agency: Washington, DC, USA, 2001.
- Riccioli, F.; Guidi Nissim, W.; Masi, M.; Palm, E.; Mancuso, S.; Azzarello, E. Modeling the Ecosystem Services Related to Phytoextraction: Carbon Sequestration Potential Using Willow and Poplar. Appl. Sci. 2020, 10, 8011. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, T.; Carvalho, P.N.; Arias, C.A.; Chen, Z.; Brix, H. Removal of the Pharmaceuticals Ibuprofen and Iohexol by Four Wetland Plant Species in Hydroponic Culture: Plant Uptake and Microbial Degradation. Environ. Sci. Pollut. Res. 2016, 23, 2890–2898. [Google Scholar] [CrossRef] [PubMed]
- Siyar, R.; Doulati Ardejani, F.; Farahbakhsh, M.; Norouzi, P.; Yavarzadeh, M.; Maghsoudy, S. Potential of Vetiver Grass for the Phytoremediation of a Real Multi-Contaminated Soil, Assisted by Electrokinetic. Chemosphere 2020, 246, 125802. [Google Scholar] [CrossRef]
- Xia, H.; Ma, X. Phytoremediation of Ethion by Water Hyacinth (Eichhornia crassipes) from Water. Bioresour. Technol. 2006, 97, 1050–1054. [Google Scholar] [CrossRef]
- Brix, H. Do Macrophytes Play a Role in Constructed Treatment Wetlands? Water Sci. Technol. 1997, 35, 11–17. [Google Scholar] [CrossRef]
- Abdallah, M.A.M. Phytoremediation of Heavy Metals from Aqueous Solutions by Two Aquatic Macrophytes, Ceratophyllum demersum and Lemna gibba L. Environ. Technol. 2012, 33, 1609–1614. [Google Scholar] [CrossRef]
- Ahmadi, F.; Mohammadkhani, N.; Servati, M. Halophytes Play Important Role in Phytoremediation of Salt-Affected Soils in the Bed of Urmia Lake, Iran. Sci. Rep. 2022, 12, 12223. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.A.; Bashir, O.; Ul Haq, S.A.; Amin, T.; Rafiq, A.; Ali, M.; Américo-Pinheiro, J.H.P.; Sher, F. Phytoremediation of Heavy Metals in Soil and Water: An Eco-Friendly, Sustainable and Multidisciplinary Approach. Chemosphere 2022, 303, 134788. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wan, X.; Lei, M.; Wang, L.; Chen, T. Research Advances in Mechanisms of Arsenic Hyperaccumulation of Pteris vittata: Perspectives from Plant Physiology, Molecular Biology, and Phylogeny. J. Hazard. Mater. 2023, 460, 132463. [Google Scholar] [CrossRef]
- Akinpelu, E.A.; Nchu, F. Advancements in Phytoremediation Research in South Africa (1997–2022). Appl. Sci. 2024, 14, 7660. [Google Scholar] [CrossRef]
- Muthusaravanan, S.; Sivarajasekar, N.; Vivek, J.S.; Paramasivan, T.; Naushad, M.; Prakashmaran, J.; Gayathri, V.; Al-Duaij, O.K. Phytoremediation of Heavy Metals: Mechanisms, Methods and Enhancements. Environ. Chem. Lett. 2018, 16, 1339–1359. [Google Scholar] [CrossRef]
- Baker, A.J.M.; McGrath, S.P.; Reeves, R.D.; Smith, J.A.C. Metal Hyperaccumulator Plants: A Review of the Ecology and Physiology of a Biological Resource for Phytoremediation of Metal-Polluted Soils. In Phytoremediation of Contaminated Soils; Terry, N., Vangronsveld, J., Banuelos, G., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 85–107. [Google Scholar]
- Danh, L.T.; Truong, P.; Mammucari, R.; Foster, N. Economic Incentive for Applying Vetiver Grass to Remediate Lead, Copper and Zinc Contaminated Soils. Int. J. Phytoremediat. 2010, 13, 47–60. [Google Scholar] [CrossRef]
- de Lima Barizão, A.C.; Silva, M.F.; Andrade, M.; Brito, F.C.; Gomes, R.G.; Bergamasco, R. Green Synthesis of Iron Oxide Nanoparticles for Tartrazine and Bordeaux Red Dye Removal. J. Environ. Chem. Eng. 2020, 8, 103618. [Google Scholar] [CrossRef]
- Zhao, F.; Han, Y.; Shi, H.; Wang, G.; Zhou, M.; Chen, Y. Arsenic in the Hyperaccumulator Pteris vittata: A Review of Benefits, Toxicity, and Metabolism. Sci. Total Environ. 2023, 896, 165232. [Google Scholar] [CrossRef]
- Le, T.M.; Pham, P.T.; Nguyen, T.Q.; Nguyen, T.Q.; Bui, M.Q.; Nguyen, H.Q.; Vu, N.D.; Kannan, K.; Tran, T.M. A Survey of Parabens in Aquatic Environments in Hanoi, Vietnam and Its Implications for Human Exposure and Ecological Risk. Environ. Sci. Pollut. Res. 2022, 29, 46767–46777. [Google Scholar] [CrossRef]
- Lee, S.-H.; Park, H.; Kim, J.-G. Current Status of and Challenges for Phytoremediation as a Sustainable Environmental Management Plan for Abandoned Mine Areas in Korea. Sustainability 2023, 15, 2761. [Google Scholar] [CrossRef]
- Fooladi, M.; Moogouei, R.; Jozi, S.A.; Golbabaei, F.; Tajadod, G. Phytoremediation of BTEX from Indoor Air by Hyrcanian Plants. Environ. Health Eng. Manag. 2019, 6, 233–240. [Google Scholar] [CrossRef]
- Dubey, N.K.; Kumar, V.; Chandra, R. Phytoremediation of Environmental Pollutants; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- UN-HABITAT. Constructed Wetlands Manual; UN-HABITAT Water for Asian Cities Programme: Nairobi, Nepal, 2008. [Google Scholar]
- Odoh, C.K.; Zabbey, N.; Sam, K.; Eze, C.N. Status, Progress and Challenges of Phytoremediation, An African Scenario. J. Environ. Manag. 2019, 237, 365–378. [Google Scholar] [CrossRef]
- Cruz, F.V.S.; Venne, P.; Segura, P.; Juneau, P. Effect of Temperature on the Physiology and Phytoremediation Capacity of Spirodela polyrhiza Exposed to Atrazine and S-metolachlor. Aquat. Toxicol. 2025, 282, 107304. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, A.N.; Waris, A.; Ilyas, M.; Zamel, D. Phytoremediation of Pollutants from Wastewater: A Concise Review. Open Life Sci. 2022, 17, 488–496. [Google Scholar] [CrossRef]
- Nazir, M.; Idrees, I.; Idrees, P.; Ahmad, S.; Ali, Q.; Malik, A. Potential of Water Hyacinth (Eichhornia crassipes L.) for Phytoremediation of Heavy Metals from Waste Water. Biol. Clin. Sci. Res. J. 2020, 2020, 1–6. [Google Scholar] [CrossRef]
- Gomes, M.P. Climate Change and Aquatic Phytoremediation of Contaminants: Exploring the Future of Contaminant Removal. Phyton 2024, 93, 2127–2147. [Google Scholar] [CrossRef]
- Anh, B.T.K.; Minh, N.N.; Ha, N.T.H.; Kim, D.D.; Kien, N.T.; Trung, N.Q.; Cuong, T.T.; Danh, L.T. Field Survey and Comparative Study of Pteris vittata and Pityrogramma calomelanos Grown on Arsenic Contaminated Lands with Different Soil pH. Bull. Environ. Contam. Toxicol. 2018, 100, 720–726. [Google Scholar] [CrossRef]
- Ahmad, A. Phytoremediation of Heavy Metals and Total Petroleum Hydrocarbon and Nutrients Enhancement of Typha latifolia in Petroleum Secondary Effluent for Biomass Growth. Environ. Sci. Pollut. Res. 2022, 29, 5777–5786. [Google Scholar] [CrossRef] [PubMed]
- Lazo, A.; Lazo, P.; Urtubia, A.; Lobos, M.G.; Hansen, H.K.; Gutiérrez, C. An Assessment of the Metal Removal Capability of Endemic Chilean Species. Int. J. Environ. Res. Public Health 2022, 19, 3583. [Google Scholar] [CrossRef] [PubMed]
- Kristanti, R.A.; Tirtalistyani, R.; Tang, Y.Y.; Thao, N.T.T.; Kasongo, J.; Wijayanti, Y. Phytoremediation Mechanism for Emerging Pollutants: A Review. Trop. Aquat. Soil Pollut. 2023, 3, 88–108. [Google Scholar] [CrossRef]
- Bui, T.K.A.; Dang, D.K.; Nguyen, T.K.; Nguyen, N.M.; Nguyen, Q.T.; Nguyen, H.C. Phytoremediation of Heavy Metal Polluted Soil and Water in Vietnam. J. Viet. Environ. 2014, 6, 47–51. [Google Scholar] [CrossRef]
- Khandare, R.V.; Govindwar, S.P. Phytoremediation of Textile Dyes and Effluents: Current Scenario and Future Prospects. Biotechnol. Adv. 2015, 33, 1697–1714. [Google Scholar] [CrossRef]
- Hanh, D.T.; Kadokami, K.; Matsuura, N.; Trung, N.Q. Screening Analysis of a Thousand Micro-Pollutants in Vietnamese Rivers. Southeast Asian Water Environ. 2013, 5, 195–202. [Google Scholar]
- Salt, D.E.; Smith, R.D.; Raskin, I. Phytoremediation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 643–668. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, Plant Selection and Enhancement by Natural and Synthetic Agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Vymazal, J.; Kröpfelová, L. Wastewater Treatment in Constructed Wetlands with Horizontal Sub-Surface Flow; Springer: Dordrecht, The Netherlands, 2008; Volume 14. [Google Scholar] [CrossRef]
- Wang, H.; Sheng, L.; Xu, J. Clogging Mechanisms of Constructed Wetlands: A Critical Review. J. Clean. Prod. 2021, 295, 126455. [Google Scholar] [CrossRef]
- Wang, J.; Aghajani Delavar, M. Techno-Economic Analysis of Phytoremediation: A Strategic Rethinking. Sci. Total Environ. 2023, 902, 165949. [Google Scholar] [CrossRef] [PubMed]
- Zulkernain, N.H.; Uvarajan, T.; Ng, C.C. Roles and Significance of Chelating Agents for Potentially Toxic Elements (PTEs) Phytoremediation in Soil: A Review. J. Environ. Manag. 2023, 341, 117926. [Google Scholar] [CrossRef]
- El-Sadaawy, M.M.; Agib, N.S. Removal of Textile Dyes by Ecofriendly Aquatic Plants from Wastewater: A Review on Plant Species, Mechanisms, and Perspectives. Blue Econ. 2024, 2, 1. [Google Scholar] [CrossRef]
- Lazo, P.; Lazo, A. Assessment of Native and Endemic Chilean Plants for Removal of Cu, Mo and Pb from Mine Tailings. Minerals 2020, 10, 1020. [Google Scholar] [CrossRef]
- Reed, S.C.; Crites, R.W.; Middlebrooks, E.J. Natural Systems for Waste Management and Treatment, 2nd ed.; McGraw Hill: New York, NY, USA, 1995. [Google Scholar]
- Interstate Technology & Regulatory Council (ITRC). Phytotechnology Technical and Regulatory Guidance and Decision Trees, Revised; PHYTO-3; ITRC: Washington, DC, USA, 2009. [Google Scholar]
- Kaewtubtim, P. Heavy Metal Phytoremediation Potential of Plant Species in a Mangrove Ecosystem in Pattani Bay, Thailand. Appl. Ecol. Environ. Res. 2016, 14, 367–382. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S. Treatment Wetlands, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar] [CrossRef]
- Gerhardt, K.E.; Huang, X.-D.; Glick, B.R.; Greenberg, B.M. Phytoremediation and Rhizoremediation of Organic Soil Contaminants: Potential and Challenges. Plant Sci. 2009, 176, 20–30. [Google Scholar] [CrossRef]
- O’Brien, R.M.; Phelan, T.J.; Smith, N.M.; Smits, K.M. Remediation in Developing Countries: A Review of Previously Implemented Projects and Analysis of Stakeholder Participation Efforts. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1259–1280. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of Heavy Metals: Concepts and Applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).