Abstract
Petrological knowledge on weathering processes controlling the mobility of chemical elements is still limited in the dry tropical zone of Cameroon. This study aims to investigate the mobility of major and trace elements during rhyolite weathering and soil formation in Mobono by understanding the mineralogical and elemental vertical variation. The studied soil was classified as Cambisols containing mainly quartz, K-feldspar, plagioclase, smectite, kaolinite, illite, calcite, lepidocrocite, goethite, sepiolite, and interstratified clay minerals. pH values ranging between 6.11 and 8.77 indicated that hydrolysis, superimposed on oxidation and carbonation, is the main process responsible for the formation of secondary minerals, leading to the formation of iron oxides and calcite. The bedrock was mainly constituted of SiO2, Al2O3, Na2O, Fe2O3, Ba, Zr, Sr, Y, Ga, and Rb. Ce and Eu anomalies, and chondrite-normalized La/Yb ratios were 0.98, 0.67, and 2.86, respectively. SiO2, Al2O3, Fe2O3, Na2O, and K2O were major elements in soil horizons. Trace elements revealed high levels of Ba (385 to 1320 mg kg−1), Zr (158 to 429 mg kg−1), Zn (61 to 151 mg kg−1), Sr (62 to 243 mg kg−1), Y (55 to 81 mg kg−1), Rb (1102 to 58 mg kg−1), and Ga (17.70 to 35 mg kg−1). LREEs were more abundant than HREEs, with LREE/HREE ratio ranging between 2.60 and 6.24. Ce and Eu anomalies ranged from 1.08 to 1.21 and 0.58 to 1.24 respectively. The rhyolite-normalized La/Yb ratios varied between 0.56 and 0.96. Mass balance revealed the depletion of Si, Ca, Na, Mn, Sr, Ta, W, U, La, Ce, Pr, Nd, Sm, Gd and Lu, and the accumulation of Al, Fe, K, Mg, P, Sc, V, Co, Ni, Cu, Zn, Ga, Ge, Rb, Y, Zr, Nb, Cs, Ba, Hf, Pb, Th, Eu, Tb, Dy, Ho, Er, Tm and Yb during weathering along the soil profile.
1. Introduction
Characterization of geological materials is a key in soil genesis studies, since parent material has long been recognized as a fundamental factor in soil formation [1]. Parent material has a strong influence on chemical weathering rates, profile depth and clay content [2,3]. Its variation may cause significant soil modifications [4,5,6]. The state of equilibrium in the lithosphere due to its contact with the atmosphere and the biosphere gives way to the establishment of a well-defined morphology with different physical, chemical, and mineralogical properties based on climatic zones [6,7,8,9,10].
Rhyolites have been described in many parts of the world, especially in the United States, Papua New Guinea, Chile, New Zealand, Brazil, Malaysia and Cameroon [11,12,13,14,15]. Different mineralogical pathways occurring during rhyolite weathering under humid tropical climate result in a variety of soils that are still poorly known [13,16]. In Brazil, these soil classes include Neosols, Cambisols and Argisols [14]. Weathering and neoformation of allophane, imogolite and halloysite in rhyolitic ashes were described in the humid tropical zone of Mexico [16]. Characterization of a weathering profile developed on rhyolite in humid tropical Malaysia Peninsular underlined the formation of kaolinite, illite and sericite [14]. Weathering and soil formation in rhyolitic tephra along a moisture gradient on Alcedo Volcano, Galápagos lead to the formation of halloysite, allophane, and ferrihydrite [17].
Strongly influenced by the climatic factors [17], soils preferentially develop in humid tropical zones under hot and humid tropical climates, as compared to dry tropical climates [18,19,20,21]. Humid tropic soils captivated the interest of scientists and engineers, especially in Central Africa [21,22,23,24,25]. Characterized by multimetric (≤30 m) weathering cover, the development of soils in humid tropical areas is due to the climate conditions favouring a strong weathering of primary minerals. These soils are characterized by ABC horizonations, with a mineralogy dominated by 1:1 clay type related to monosiallitization process characterized by a complete leaching of base cations but partial leaching of silica [26]. By contrast, the dry tropical zone is marked by slower weathering and contrasting soil mineralogy, dominated by smectite with some kaolinite [27,28,29]. Bisiallitization process is dominant in their geochemistry due to evaporation, low rainfall, very high temperatures and flat topography, leading to a partial leaching of both base cations and silica [30].
In the dry tropical zone of Cameroon, petrological knowledge on weathering processes controlling the mobility of chemical elements is still limited. Generally, major and trace elements distribution in soil profile reflects the different mineralogical transformations that occurred during weathering [31]. The accumulation of clay increases from shallow to intermediate horizons as rare earth elements (REE) contents increase during weathering processes [32]. The upper part of weathering profiles often contains fewer REE than the lower part, which may be enriched as a result of possible reprecipitation [33]. Their immobilization and fractionation depend on the nature of clay mineral [34], while their redistribution is linked to the properties of different REEs in soil solution and the nature of the primary phase [35]. REE contents and their mobility in soils are also linked to weathering intensity of parent materials, storage capacity of authigenic soil phases and fluctuations of redox conditions [36]. These knowledges are almost scarce in the study area. It is thus necessary to better understand the evolution of these formations in the study area and to identify factors that control these dynamic evolutions. Though much research works have been achieved on soils developed on rhyolites in the other parts of the world, to date, no work has been practically done on soils that developed on these formations in the dry tropical zone of Cameroon and even in Central Africa. To reduce gaps in knowledge regarding soils derived from acid volcanic rocks, the aim of this work is to investigate the mobility of major and trace elements during rhyolite weathering and soil formation in Mobono by understanding the mineralogical and elemental vertical variation.
2. Materials and Methods
2.1. Study Area
The study site, Mobono, is located in the Mindif subdivision, in the Far-North region of Cameroon (Figure 1). The climate is semi-arid, characterized by two seasons, including a long dry season from October to May (Aridity index < 20) and a short rainy season from June to September (Aridity index > 20) (Table 1). The landscape consists of a succession of hills, which emerge from the monotonous plain (average altitude 400 m a.s.l.) (Figure 2a). The studied relief unit is an asymmetric dome, rhyolitic in nature, culminating at about 450 m a.s.l. The vegetation is a dry savannah highly anthropogenized [37]. The substratum (Figure 2b) consists of metamorphic rocks (i.e., gneiss, micaschist, quartzite and cipolin) and magmatic rocks (i.e., granite, syenite, gabbro, basalt and rhyolite). They are associated to alluvial deposits of Quaternary age at the plain [15,38]. Main soil types found in the region are Vertisols, Regosols, Leptosols, Arenosols, Luvisols, Fluvisols, Gleysols and Cambisols [39]. Among these soils, Cambisols occurs on rhyolites in the high reliefs which emerge from the monotonous plain in the study area. They are associated to Leptosols which remained poorly represented.
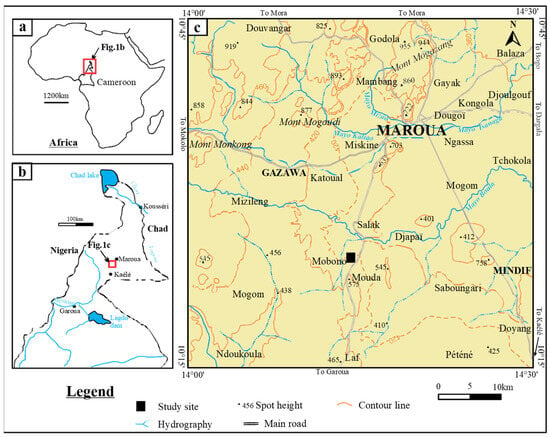
Figure 1.
(a) Location of Cameroon in Africa, (b) study area in North Cameroon, (c) study area detail topographic map.

Table 1.
Total annual rainfall, mean annual air temperature and aridity index of Maroua from 1980 to 2019.
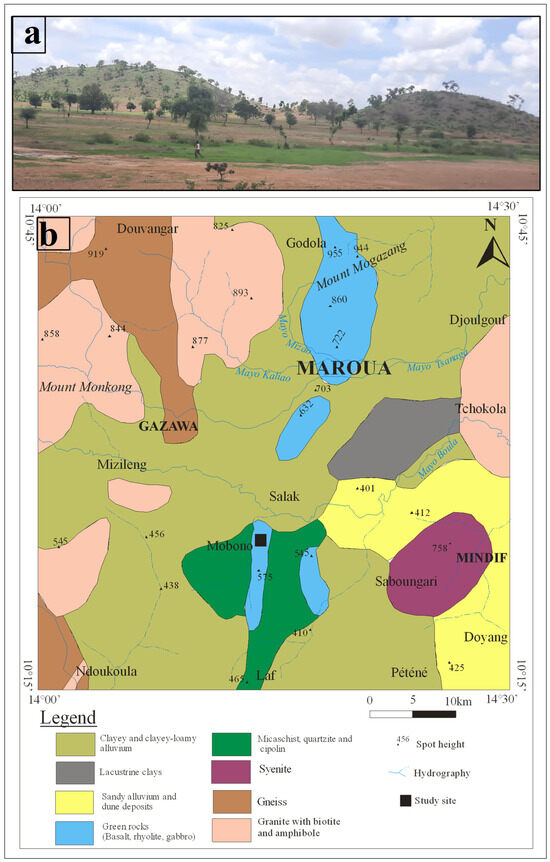
Figure 2.
(a) Landscape photograph showing hill units associated to plain in Mobono, North Cameroon; (b) Geological map of the studied area with the indication of Mobono (Extracted from the compilation of the Department of Mines and Geology of Cameroon in 1979 modified by Brabant and Gavaud [39]).
2.2. Soil Description and Sampling
From the bedrock (MBr) to the soil surface, horizons are merged into two sequences, i.e., an alteritic sequence and a solum (Figure 3). Soil description was made on trench walls opened for the exploitation of the quarry for aggregates production, and colours were determined in the field in their dry state using the Munsell colour chart.
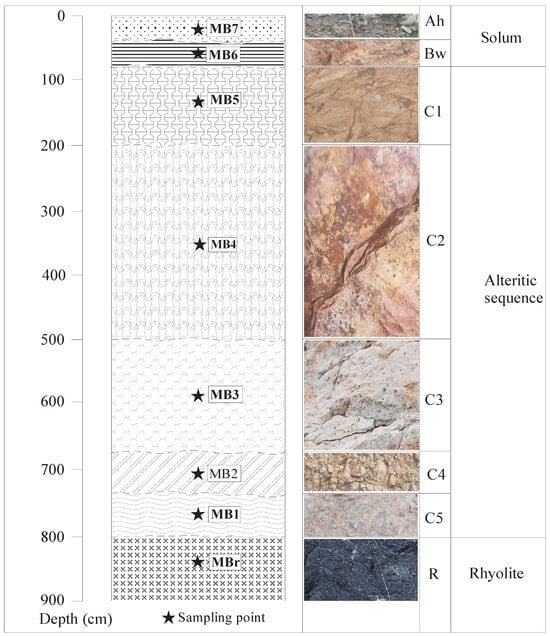
Figure 3.
Morphological characteristics of the studied soil profile in Mobono, North Cameroon.
The alteritic sequence of ~7.20 m thick is made from bottom to top of five horizons (i.e., a C5 horizon at the base, median C4, C3, and C2 horizons and, an upper C1 horizon), described below.
- -
- The C5 horizon (MB1) (0.70 m) has a whitish colour (7.5YR 8/1), a massive structure, and a silty texture. It is compact and has few small spots which show effervescence with hydrochloric acid, characteristic of the presence of calcite. It is cracked into undifferentiated blocks, clogged with dark red materials, mostly iron oxides, and/or secondary, manganese precipitations. Broken blocks show yellow to reddish-yellow surfaces with hard and rounded dark red ferruginous domains (about 3%). The transition is gradual and irregular.
- -
- The C4 horizon (MB2) (10YR 7/8) is compact, characterized by massive structure and silty texture. About 0.50 m thick, it shows no internal differentiation. As in the lower horizon, there are cracks clogged by iron oxides and manganese precipitations. The transition is distinct and irregular.
- -
- The C3 horizon (MB3) (7.5YR 8/1) is compact and very dense, characterized by a massive structure and a silty texture. Its thickness is about 1.80 m. The transition is diffuse and irregular.
- -
- The C2 horizon (MB4) (10R4/4) is similar to the underlying horizon except that it is less dense and less compact, characterized by the presence of dark red ferruginous indurations and blackish spots. Its thickness is about 3 m. The transition is diffuse and irregular.
- -
- The C1 horizon (MB5) (10YR 7/8) in its upper part of ~1.2 thick. It is less compact. Ferruginous indurations and iron oxides and manganese precipitations are more important upwards. The transition is gradual and irregular.
The solum is approximately 0.80 m thick. It is subdivided in two horizons of different thickness and colour, i.e., the Bw horizon (MB6) and the Ah horizon (MB7). The Bw 0.50 m thick-horizon is characterized by the presence of numerous millimetric to centimetric roots and rootlets. There are many coarse elements (80%) embedded in a scarce fine matrix, with a clayey-silty texture and reddish brown (2.5YR 5/4) colour. The fresh fracture of coarse elements shows the preserved structure of the bedrock. The transition is gradual but irregular. The dark (7.5YR 5/2) 0.30 m thick Ah horizon (MB7) has a crumby structure and a silty texture. It is characterized by strong bioturbation, significant matrix porosity with many roots and rootlets.
The Bw horizon (MB6) is clayey-silty with fine blocky structure characterized by the presence of saprolith fragments. The thickness is 0.50 m. The colour is reddish brown (2.5YR 5/4). These characteristics are those of a cambic horizon, according to the IUSS Working Group WRB [40], leading to the classification of the studied soil as Cambisols.
Seven samples of soil and one rock sample were taken for further laboratory analyses.
2.3. Analytical Technics
The mineralogical and geochemical approach help to determine the mineralogical composition of soils and the concentration of the major, trace and rare earth elements which are used to reveal the weathering stages of the soils. Chemical indices, due to their quantitative nature, are efficient tools in determining the stage of soil development.
2.3.1. Mineralogical Analysis
Mineralogical analyses performed are X-ray diffraction (XRD), thermal analysis and infrared (IR) spectrometry. These analyses were carried out at the Clay Geochemistry and Sedimentary Environment Laboratory (AGEs) of the University of Liège. For XRD, data were recorded using a Brucker D8-Advance (Bruker, Siegsdorf, Germany) type diffractometer using copper Kα radiation (λ = 1.5418 Å). The measurement speed of the goniometer is 10 s/step, rotation speed 2°2θ/min, inducing an analysis time of several minutes to cover an angular range going from 2 to 45° 2θ for non-oriented powders (bulk sample < 250 µm) and 2 to 30° 2θ for oriented slats (clay fraction < 2 µm) (EG: ethylene glycol, N: normal, H: heat). Infrared spectra covering the 400–4000 cm−1 range were recorded on a Nicolet NEXUS (Thermo Fisher Scientific, Waltham, MA, USA) spectrometer, from 2 mg of sample diluted in a 180 mg KBr pellet. The resolution was 1 cm−1. Thermal analyses were achieved using differential thermal analysis (DTA) coupled with thermogravimetry analysis (TGA) on different soil samples. Samples (80 mg each) were heated from room temperature to 1000 °C under dry air at a rate of 5 °C/min using a TGA-2000 Analyzer (Precisa Gravimetrics AG, Dietikon, Switzerland) type.
2.3.2. Geochemical Analysis, Mass Balance and Chemical Indices
Pulverized samples (50–60 g) were used for geochemical analyses (details available at www.alsglobal.com, accessed on 28 May 2025) at ALS Minerals Global Group, Vancouver, Canada. Major and trace elements were done by ICP-AES (Inductively Coupled Plasma-Atomic Emission) and ICP-MS (Inductively Coupled Plasma Mass Spectrometry) from pulps respectively. The procedure consists of mixing 1.50 g of lithium metaborate (LiBO2) with 2 g of sample powder and fused at 1000 °C in a furnace. After, the melted sample was cooled and dissolved in 100 mm3 of 5% HNO3 acid solution. For REE contents, sample powder (0.25 g) was dissolved with four acid digestions while for the remaining trace elements, a split of sample pulp (0.50 g) was digested in aqua regia, and determined by ICP-MS. The weight difference after ignition (1000 °C) helped to determine Loss-on-ignition (LOI). For the major elements, detection limits were about 0.01 wt%, except for P2O5 (0.005 wt%) and Na2O (0.05 wt%). For trace elements, they were as follows in mg kg−1: Rb, Hf and Nb (0.20); Th (0.04); Y, Ba and Co (0.50); Zr (2); Cu, V, and Ni (0.5)0; Sr, Ta, and Ga (0.10); Cs (0.01); and U (0.05); Ge (0.05), Sc (1), Zn (0.50), Pb (0.05), W (0.05). REE values were as follows in mg kg−1: La, Ce (0.50); Nd and Lu (0.10); Tm, Tb, and Ho (0.01); Pr, Sm, Eu, Er, and Yb (0.03); Gd and Dy (0.05). For the analytical concentrations, two repeated samples were used to calculate the precision of analyses, which fall within 2% for major elements and 5% for REE. However, for standards used, the calculated accuracy of both (major oxides and REE) lie within typical uncertainty of the ICP-MS data.
Several indices, based on the ratio of the base cations (Ca, Mg, K and Na) to Al and Si, have been proposed to evaluate the degree of chemical weathering in soils. The chemical indices used in this study included: the Chemical Index of Alternation (CIA = (100) [Al2O3/(Al2O3 + CaO* + Na2O + K2O)]; [41]), the Ruxton Ratio (R = SiO2/Al2O3; [42]), the Mineralogical Index of Alteration (MIA = 2* (CIA-50); [43]), the Residual Index (V = (Al2O3 + K2O)/(MgO + CaO + Na2O); [44]), the Weathering potential index (WPI = (K2O + Na2O + CaO–H2O +). 100/(SiO2 + Al2O3 + Fe2O3 + TiO2 + CaO + MgO + Na2O + K2O); [42]), the Sesquioxide Content (SEC = Al2O3 + Fe2O3; [45]), and the Mobile index (Imob, mole ratio = [(K2O + Na2O + CaO)fresh–(K2O + Na2O + CaO)weathered]/[(K2O + Na2O + CaO)fresh]; [45]).
Other parameters as Loss of Ignition (LOI; [46]), pH (oxygen potential), Eh (redox potential) and rH2 (redox power) [47] were measured in addition to the geochemical content. The determination of pH and potential redox (Eh) was done in the same solution (soil/solution ratio 1/2.5) using a direct reading pH meter HQ11D. The redox power rH2 was calculated using the formula rH2 = Eh + 0.06 pH/0.03.
The behaviour of chemical elements, throughout the weathering mantle was approached by calculating elemental mass balance, according to the “mass balance method” [48,49] by considering titanium as an invariant element.
3. Results
3.1. Petrology and Geochemical Composition of the Rhyolite
Macroscopically, the rhyolite (MBr) is dark, dense (2.40 g/cm3), characterized by conchoidal fracture and whitish streaks, essentially made of calcite (Figure 4a). Microscopically, it shows a porphyritic microlithic texture with numerous phenocrysts of sanidine, quartz, calcite and opaque minerals, with a low amount of plagioclase, amphibole and micas (Figure 4b–d).

Figure 4.
Macroscopic (a) and analyzed polarized light microscopic view (b–d) of the Mobono rhyolite (MBr). Sa: sanidine; Qz: quartz; Ca: calcite.
Chemical analysis of the rhyolite revealed that SiO2 (70.59%) and Al2O3 (13.73%) are the main elements. These elements were followed by Na2O (5.89%), Fe2O3 (4.12%), CaO (1.74%) and K2O (1.55%). Other element contents were below 0.4% (Table 2). Main trace elements were Ba (611 mg kg−1), Zr (259.30 mg kg−1), Sr (147.60 mg kg−1), Y (48 mg kg−1), Ga (17 mg kg−1), Rb (14.90 mg kg−1). The remaining trace elements had contents below 10 mg kg−1 or were below the detection limit (Table 2). The sum of REE reached 143.06 mg kg−1 in the rhyolite, dominated by LREE (112.13 mg kg−1, LREE/HREE = 3.63). The (La/Yb)N ratio was low (2.86) (Table 2). The chondrite–normalized REE pattern (Figure 5) showed a negative Eu anomaly in the rock (Eu/Eu* = 0.67) but no Ce anomaly (Ce/Ce* = 0.98).

Table 2.
Major, trace and rare-earth elements concentrations in studied soils.
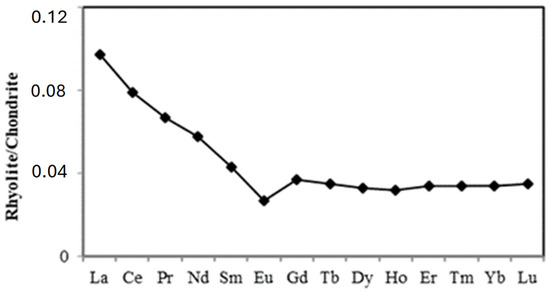
Figure 5.
Chondrite-normalized rare-earth element patterns for rhyolite in Mobono. Chondrite normalizing values after McDonough and Sun [50].
3.2. Mineralogical Characteristics of Soils
In the alteritic sequence, diffractograms (Figure 6a,b) show that the soil mineralogy consists of primary minerals (quartz, K-feldspar, plagioclase) and secondary minerals (kaolinite, smectite, illite). Furthermore, a 7Å peak was seen in some diffractograms after heating at 500 °C, characteristic of interstratified minerals of 1:1/2:1 type [51]. Depending on the horizon, certain minerals appeared or disappeared. The C5 and C4 horizons were composed of quartz, K-feldspar, plagioclase, smectite, kaolinite and illite (Figure 6a). The C1, C2 and C3 horizons displayed the same mineralogical composition compared to the underlying horizons. In the C3 horizon the characteristic peak of smectite at 16 Å after ethylene glycol solvatation (EG) was not well developed (Figure 6b). In the C2 horizon, the smectite peak was expressed by a slight shoulder around 18Å on the EG spectra. On the bulk powder spectrum of C1 horizon sample, no clay minerals were identified; they were only observed after heating and saturation with ethylene glycol (Figure 6a,b). The mineralogical assemblage of the solum (i.e., Bw and Ah horizons) is similar to that observed in the alteritic sequence (Figure 6a,b).
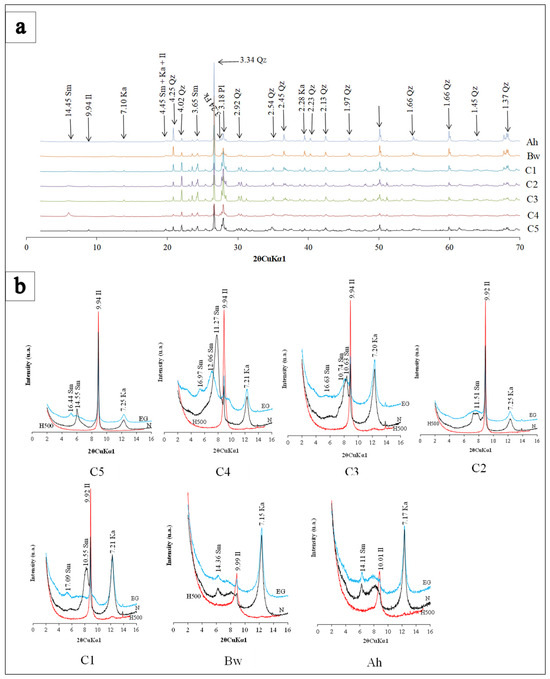
Figure 6.
(a) X-ray powder diffraction patterns of bulk material, Sm: smectite, Ka: kaolinite, Il: illite, Qz: Quartz; Fk: K-feldspar; Pl: plagioclase; (b) XRD patterns of the oriented <2 µm fraction of clay material, N: air-dried condition; EG: solvatation under ethylene-glycol for 24 h; H: heated at 500 °C for 4 h. MB1: C5; MB2: C4; MB3: C3; MB4: C2; MB5: C1; MB6: Bw; MB7: Ah.
The infrared spectrum confirms the presence of quartz and aluminium phyllosilicates shown by the Si-O vibration bands between 900–1200 cm−1 or 750–800 cm−1 and the Al-O band between 400–700 cm−1 (Figure 7). Quartz is identified by peaks at 999 cm−1 and 787 cm−1. Kaolinite is identified by the three peaks at 3698 cm−1, 3626 cm−1 and 910 cm−1. Smectite was also identified by the 1618 cm−1 peak. In addition to the minerals identified by X-ray diffraction, the presence of calcite (723 cm−1), lepidocrocite (743 cm−1) and goethite (640 cm−1) was noted. Except goethite that is observed in all samples, the infrared spectrum show some variations in the mineral content of the samples. In the C5 horizon, calcite (723 cm−1) and lepidocrocite (743 cm−1) are present. Calcite is absent in the C4 horizon. Lepidocrocite is only present in the C1 horizon. In the Bh horizon, sepiolite is present, a mineral characteristic of dry environments, identified here by the peak at 530 cm−1. The Ah horizon has an IR spectrum similar to that of the Bw horizon (Figure 7). However, the vibration band of the C-H bonds between 2700–2900 cm−1 characteristic of aliphatic compounds of organic matter is absent. This is in agreement with the very low abundance of organic matter in this semi-arid environment.
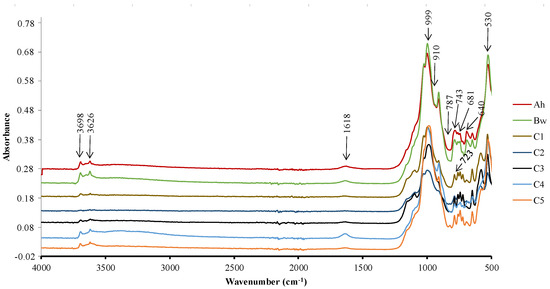
Figure 7.
IR curves of studied soils in Mobono, North Cameroon. MB1: C5; MB2: C4; MB3: C3; MB4: C2; MB5: C1; MB6: Bw; MB7: Ah.
The thermal behaviour of studied soil is similar from one horizon to the other but with variable weight loss (Figure 8). An endothermic peak between 50–125 °C or 40–150 °C indicates the disappearance of surface water or intra-layer water from clay minerals [52]. An exothermic peak is observed between 175 and 250 °C. The high peak intensity indicates the presence of a swelling (smectite) and a non-swelling phase (illite), characteristic of interstratified minerals [53]. An intense endothermic peak is also observed between 450 and 550 °C, accompanied by a weight loss ranging from 2 to 5%. This weight loss was associated with dehydroxylation according to the reaction 2 (OH)→H2O + Or (r, residual) [54,55]. The peak is characteristic of the loss of hydroxyl groups from clay structures indicating the presence of kaolinite [56]. The bulging in the range 300–450 °C marks the presence of a swelling phase (smectite) with a non-swelling phase (illite) characteristic of an interlayer mineral. For the Ah horizon, there is about 4% weight loss. An exothermic peak between 250–475 °C corresponds to the dehydroxylation or dehydration of illite-smectite interstratified minerals. The weight loss recorded between 600 °C and 900 °C reflects the presence of 2:1 clay minerals which lose their constitution water between this temperature interval. In all TGA curves, the weight loss observed between 275 °C and 300 °C could reflect the presence of iron oxyhydroxides (goethite) in the studied soil horizons.
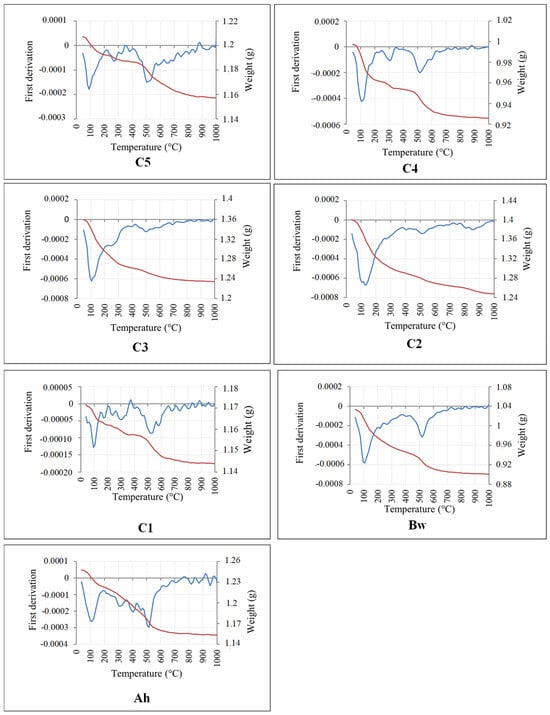
Figure 8.
Thermal analysis of studied soils in Mobono, North Cameroon. MB1: C5; MB2: C4; MB3: C3; MB4: C2; MB5: C1; MB6: Bw; MB7: Ah.
3.3. Geochemical Characteristics and Relative Soil Elements Mobility
3.3.1. Characteristics and Mobility of Major Elements
In the C5 horizon, SiO2 was the most abundant element (51.70%) followed by Al2O3 (25.30%), Fe2O3 (13.50%), K2O (6.80%) and Na2O (4.31%) (Table 2). Other elements were in negligible quantities (<2%) (Table 2). The C4 horizon had a similar geochemical composition with the underlying C5 horizon. However, there was an increase in Fe2O3 contents (24.96%) and a decrease in Al2O3 and SiO2 contents. The three horizons, C1, C2 and C3 had a close geochemical composition, similar to that of the underlying C4 horizon. Compared to the previously described horizons, SiO2 contents were higher in the three horizons C1, C2 and C3, while there is a decrease in the Al2O3, Fe2O3, Na2O and K2O contents. The Bw and the Ah horizons had a geochemical composition similar to that of the C3, C2 and C1 horizons. However, the Ah horizon was characterized by a higher SiO2 content (60.10%) and high LOI (6.66%) compared to other horizons.
SiO2 and Al2O3 display an opposite variation in concentrations along the depth profile (Figure 9). MgO concentrations also present an opposite evolution with respect to SiO2. The concentrations of Al2O3, Fe2O3, TiO2 and K2O have a similar variation trend with depth. CaO, Na2O and MnO concentrations significantly decrease with increasing depth except in the C1 horizon marked by an increase in MnO concentration (Figure 9).
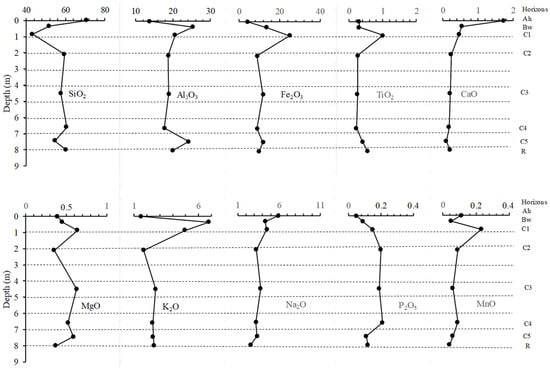
Figure 9.
Concentrations of major elements (wt %) with respect to profile depth in Mobono, North Cameroon. MB1: C5; MB2: C4; MB3: C3; MB4: C2; MB5: C1; MB6: Bw; MB7: Ah.
Among the major elements, Si, Ca, Na and Mn were depleted in all parts of the soil profile except for Si and Mn which are accumulated in the C1 horizon, as confirmed respectively by a mean mobilization rate of −27.41%, −86.58%, −46.18% and −37.18%. Al, Mg and K were depleted at the base in the C4 horizon and in the Ah horizon, and were accumulated instead in the other part of the soil profile. P is only depleted in the C4 horizon. Fe was the only major element accumulated in all parts of the soil profile during weathering (Table 3). Calcium and sodium are more mobile with mean mobilization rates of −87% and −46% respectively.

Table 3.
Mass balance evaluation (in %) of the major oxides, trace and rare-earth elements.
3.3.2. Characteristics and Mobility of Trace Elements
The distribution of trace elements varied from one horizon to another (Table 2). Trace elements show high level of Ba (385 to 1320 mg kg−1), Zr (158 to 429 mg kg−1), Zn (61 to 151 mg kg−1), Sr (62 to 243 mg kg−1), Y (55 to 81 mg kg−1), Rb (1102 to 58 mg kg−1) and Ga (17.70 to 35 mg kg−1). Some elements present high values only at the base of the soil profile, i.e., Sc (29 mg kg−1), V (24 to 194 mg kg−1), Co (21 mg kg−1), Cu (25 mg kg−1), Ni (10.2 mg kg−1) and Nb (10.4 to 22 mg kg−1), then decreased upwards (Table 2). On the contrary, Pb (17.4 mg kg−1) and Th (7 mg kg−1) displayed higher values in the upper part of the soil profile and decreased with depth. Except for Hf (4.1 to 10.6 mg kg−1), all other element contents were low, (<3 mg kg−1).
Sr is the only element depleted in all horizons of the soil profile, with a depletion rate ranging between −16.85 and −76.35% and corresponding to a mean mobilization rate of −44.24%. Other depleted elements are Ta, W and U, with −15.42%, −28.01% and −5.65% as mean mobilization rate respectively. U is depleted in C4 and Bw horizons. W is depleted along the soil profile except in the C1 horizon, while Ta is depleted in C4, Bw and Ah horizons. Cs, Zn and Pb are accumulated in all horizons of the soil profile. Their mobilization rates range respectively between 131.82 and 800.00%, 8409.09 and 24,172.73% and between 12,672.68 and 177,172.73%, corresponding respectively to a mean mobilization rate of 345.28%, 14,287.88% and 60,893.34%. Pb is the most highly imported trace elements, all the other trace elements being either depleted or accumulated in different horizons of the soil profile (Table 3).
3.3.3. Characteristics and Mobility of Rare Earth Elements
The sum of REE varied between 112.75 and 250.84 mg kg−1 (average value 160 mg kg−1). The highest REE value is observed in the C4 horizon while the lowest one is observed in the C1 horizon. There is a relative high abundance of LREE (La-Eu) than HREE (Gd-Lu). The LREE/HREE ratio varies between 2.6 to 6.24, with the highest value observed in the C4 horizon. Average concentrations of the LREE and HREE in studied soil are 124 and 35.6 mg kg−1 respectively. The rhyolite-normalized pattern showed a positive Eu anomaly (Figure 10a). Ce/Ce and Eu/Eu ratios values varied from 1.08 to 1.21 and 0.58 to 1.24 respectively. The C4 horizon shows the highest concentration values of LREE and HREE, with a strong negative anomaly in Ce after rhyolite/soil sample normalization (Figure 10a). The negative Ce anomaly is also observed in the C3 horizon while it is positive in the Bw horizon. The strong negative anomaly in Ce is also observed in the C4 horizon associated to a slightly negative Eu anomaly in all soil horizons after chondrite/soil sample normalization (Figure 10b).
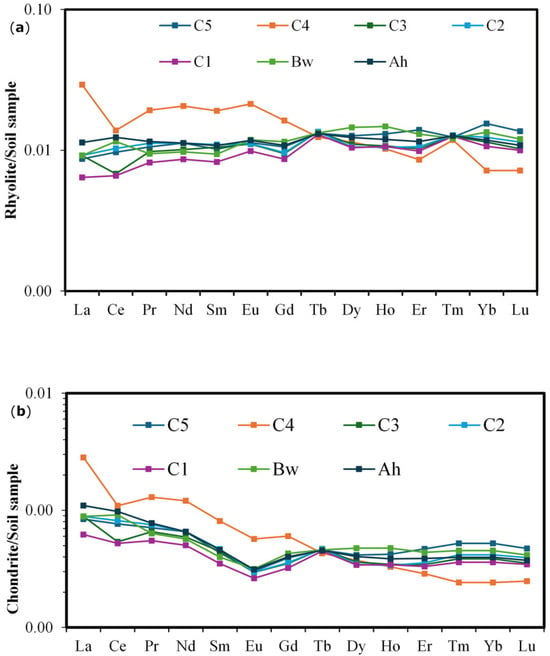
Figure 10.
(a) Rhyolite-normalized rare-earth element patterns for soil samples; (b) Chondrite-normalized Rare-Earth Element patterns for soil samples. Chondrite normalizing values after McDonough and Sun [51]. MB1: C5; MB2: C4; MB3: C3; MB4: C2; MB5: C1; MB6: Bw; MB7: Ah.
REE present different trends along the soil profile. Based on the mean mobilization rate, LREE are most depleted in the soil profile except Eu while HREE are most accumulated except Gd and Lu (Table 3). The most depleted element is Ce while the most accumulated element is Tb with a mean mobilization rate of −16% to 19%. All REE are depleted in the C4 horizon and the Ah horizon. They are also depleted in the Bw horizon except for Dy, Ho and Yb (Table 3).
3.4. Chemical Indices
The calculated chemical indices are listed in Table 4. The bedrock CIA is 59.93, indicating a slight weathering. CIA values for the C5 horizon (68.44) and C4 horizon (67.29) fit with slightly weathered horizon, as in the bedrock. The C1, C2, C3, Bw and Ah horizons are classified as moderately weathered (73.96 < CIA < 80.21), meaning that at least 50% of material was decomposed and/or disintegrated to soil, according to Nesbitt and Young [41]. The highest CIA value is observed in the Bw horizon. The MIA values ranged between 19.86 and 60.43, with a perfect correlation between MIA and CIA values (r = 1).

Table 4.
Chemical weathering indices of studied soils.
The Si/Al values of all soil horizons range from 2.95 to 6.05, the higher value being observed in C1 horizon. Due to their mobility during chemical weathering, alkaline and alkaline earth elements may undergo prominent changes, resulting in the lowest values on the Vogt index (V) for fresh samples. Weathering Potential Index (WPI) values ranged between 7.91 and −1.41, with increments over depth. In contrast with CIA, the highest value of WPI is observed in the bedrock. The sesquioxide content (SEC) reflects the insoluble Fe2O3 and Al2O3 oxide content in a sample. An increase of SEC indicates either a higher intensity of leaching or oxidation resulting from the enrichment of ferric iron. Its values range between 17.85 in the bedrock and 45.45 in the C4 horizon. LOI values vary between 1.40 and 6.66, with the lowest values in the bedrock and in the C1, C2 and C3 horizons while highest values are observed in the Bw and Ah horizons. The mobile index (Imob) serves as an index of the degree of decomposition of feldspar-bearing rocks [57]. It varies from −0.27 to 0.43 with the lowest values observed at the base of the soil profile (−0.27 to 0.00) (Table 4). Soil pH provides information on the pedogenetic process in place as well as on its trophic state. The pH and the Eh redox potential are the two main physicochemical parameters influencing the dynamics of major elements. Since these two parameters are isolated, their combined effects through a third parameter, which is the redox power denoted rH2. The pH of the studied soil is weakly acidic to acidic for the C5 horizon, the C4 horizon and the solum. It varies between 5.16 in the Ah horizon and 6.18 in the Bw horizon (Table 4). In contrast, the pH of C1, C2 and C3 horizons is basic, ranging between 7.73 and 8.77. The evolution of Eh is opposed to that of pH. The highest Eh value is observed in the Ah (0.11 volt) while the lowest value is noted in the C3 horizon (−0.08 volt). The rH2 redox power of different soil horizons varies between 14 and 14.42.
3.5. Soil Pedogenic Indices Based on Trace Elements and REE
Some elemental ratios (Table 5) enable to study the geochemical weathering trends of soil profiles. The Th/U ratios vary between 2.76 and 4.09. Except for C4 horizon, all the others display higher values compared to the fresh rhyolite.

Table 5.
Soil genetic indices based on trace and rare earth elements in studied soils.
The Ba/Nb ratios range between 32.08 in C3 and 126.92 in C4 horizons. Generally, C4, C2 and C1 horizons show high values compared to the fresh rhyolite while other horizons show lower values. Ti/Nb ratios are low, even in the fresh rhyolite, and almost constant along the soil profile, ranging from 0.01 to 0.10. The Zr/Rb ratio decreases from the bedrock until the C4 horizon, increases from C4 to C3 horizons, decreases from C3 to C2 horizons, increases again from C2 to C1 horizons, and decreases thereafter until the surface Ah horizon, ranging between 3.43 and 27.41. The La/Lu ratio on contrary, decreases from the bedrock to C5 horizon (26.94 to 17.16), increases from C5 to C4 horizons (17.16 to 109.84) and decreases thereafter from C4 to C5 horizons (109.84 to 17.29). It increases later from 17.29 in C1 horizon to 28.26 in the surface Ah horizon. The La/Yb ratios are 4.22 for the fresh rhyolite but range from 2.37 to 17.18 in the weathered materials. All the horizons (except C4 horizon) show ratio values lower than that of the bedrock. The La/Sm ratio fluctuates between 2.83 and 5.58 in the weathered materials but shows a value of 3.64 in the fresh rhyolite. The La/Sm ratios slightly vary along the soil profile, ranging from 2.83 to 3.82 (Table 5). As an exception, it reaches 5.58 in the C4 horizon. The (Rb + Sr)/Sr ratios are low with narrow variation along the soil profile, ranging from 1.10 to 1.76. The Y/Ho ratio varies between 28.57 and 31.15 in the weathered materials. These values are slightly higher than that of the bedrock which has a low value of 27.27. The higher value is observed in the Bw horizon (31.15). All the calculated soil pedogenic indices present an irregular zigzag evolution over depth.
4. Discussion
4.1. Mineralogical Characteristics of Weathering Products
The isalteritic sequence, constituted of C5, C4, C3, C2 and C1 horizons, is made of smectite, kaolinite, illite, calcite, lepidocrocite, goethite, and interstratified clay minerals of 1:1/2:1 type, associated to primary minerals such as quartz and plagioclase. This paragenesis is similar to that commonly observed in the dry tropical zone [27,29,58]. It differs from the paragenesis described in the humid tropical part of Cameroon [59] by the presence of interstratified clay minerals, calcite, illite and smectite. The development of these minerals is characteristic of confined and consequently less acidic environments, as confirmed by the measured pH values (6.11 to 8.77) [60]. These pH values indicate that the main process which presides over the formation of secondary minerals at the base of the soil profile in the study area is hydrolysis [27,28], which is superimposed on the oxidation responsible for the observed iron oxides and the carbonation responsible for calcite precipitation. This hydrolysis process of primary minerals leads to profound change in bedrock mineralogy due to the fact that the rock-forming minerals exhibit different degrees of stability. According to mineral-stability series of Goldish [61], mineral weathering begin by calcite, follows plagioclase, sanidine, amphibole, mica and quartz (most resistant mineral), leading to progressive release of chemical elements (Si, Al, Fe, Ca, Mg, K, Na, Mn, …) in the study environment. The accumulation of calcite here is accelerated by the presence of this mineral in the bedrock. The predominance of 2:1 clay minerals indicates that the geochemical process underlying the soil profile in the study area is bisiallitization. The latter is favoured by the low rainfall and the long dry season which generate silica and basic cations—rich soil solutions, leading to the formation of 2:1 clays minerals, illite and smectite [27]. The presence of kaolinite along with illite and smectite at the base of the profile indicates that monosiallitization also occurs alongside bisiallitization. Kaolinite neoformation is favoured by the morphoclimatic and hydrological conditions which prevail in the study area, even bisiallitization remains dominant. Coexistence of kaolinite and smectite have been reported in the literature [27,62,63]. Similar results were obtained by Tsozué et al. [64] on gabbro in Maroua, also in the Far-North region of Cameroon. In subtropical climates, lepidocrocite (γ-FeOOH) with its concretion and crust’s aspect is generally found around roots and voids in soils, with patchy distribution, corresponding to reductomorphic soils’ characteristic [65,66]. It is formed during the initial stages of pedogenesis in young soils [67]. This initial stage is attested in the studied soil by the morphological organization of the soil greatly dominated by the weathering sequence and the presence of feldspar and quartz in all soil horizons. The mineralogical composition of the solum in the upper part of the soil profile is similar to that observed at the base of soil, with the presence of sepiolite. Sepiolite present in the Bw horizon is a fibrous mineral, which is usually neoformed. It is not stable in humid climates and its presence is favoured in soils in dry or semi-dry climates, where the environment rich in alkaline elements, silica and magnesium favours their formation [68]. As a mineral of the palygorskite group, sepiolite could also form from the transformation of smectite by a dissolution process already described by Jones and Galán [69]. The presence of quartz and feldspars reflects an incomplete alteration of primary minerals, in agreement with the presence of coarse lithorelitual elements noted in the field and the climatic characteristics of the study area. Calcite is absent in this sequence, in agreement with the acidic pH values (5.2–6.2).
4.2. Weathering Indices and Pedogenic Processes
To evaluate changes over a soil profile, weathering indices are used. They are calculated in molecular proportions from elemental oxide concentrations. These indices systematically change with depth along soil profiles developed on homogeneous parent rocks [70]. The Ruxton indices (3.61 and 6.06) are typical of the predominance 2:1 minerals represented by smectite, illite and sepiolite in the clay fraction, with however an important quantity of quartz mineral already identified in the bedrock. CIA, MIA and V present similar evolution from the bedrock to the Ah horizon. The CIA values reflect both the gradual removal of labile cations from minerals during chemical weathering and the proportion of primary and secondary minerals in the bulk sample [41]. Its values range from ~ 50 for fresh igneous rocks to ~100 for soils originating from intensely weathering rocks containing minerals such as kaolinite [71,72,73]. The increase of CIA values from the bedrock (59.93) to Bw horizon (80.21) and the Ah horizon (78.90), with a simultaneous decrease in soluble cations quantities marked the progressive development of the soil profile, with the bedrock classified as very slightly weathered material (50 to 60), the C5 and the C4 horizons as slightly weathered material (60 to 70), and the C1, C2 and C3 horizons and the solum as moderately weathered material (70 to 80) [70]. High CIA values reflect the removal of labile cations relative to stable residual constituents during weathering [41]. These different weathering stages are confirmed by MIA values which respectively correspond to incipient, weak and moderate weathering [43,74]. The sesquioxide content (SEC) reflects the Fe2O3 and Al2O3 content in the studied soil which are insoluble oxides. Its values vary from 17.85 in the bedrock to 45.45 in the C4 horizon and it is higher in all part of the soil profile than in the bedrock. An increase of SEC indicates either a higher intensity of leaching or oxidation resulting from the enrichment of ferric iron from the oxidation of ferrous iron [75], producing lepidocrocite and goethite. The weathering potential index (WPI) provides a relative measure of the removal of elements, which includes most of the mobile cations, during weathering. Its values increase with depth, ranging from −1.41 in the Ah horizon to 17.91 in the bedrock. According to Ng et al. [75], the smaller the measured WPI value, the greater the intensity of weathering and leaching is. The mobile index (Imob) generally reflects the degree of decomposition of feldspar-bearing rocks [57]. The index measures the difference between the concentration of mobile cations (K2O, Na2O and CaO) and combines data from both the weathering products and their fresh parent materials. It indicates the compositional changes occurring in the bedrocks during weathering. High values are observed in the C1, C2 and C3 horizons and the solum (0.28 to 0.45) while the lower ones are observed in the C4 horizon (−0.08) and in the bedrock (0.00). The higher the index, the bigger the difference in the abundance of mobile cations present in the weathered and fresh rocks and the more intense weathering is [75], confirming the increase in intensity of weathering from the base of the studied soil profile to the upper surface horizons as observed through the other chemical indices. These results indicate that soil formation on rhyolite at Mobono in the Far-North region of Cameroon is due to moderate progressive weathering of the original parent material and its transformation to more stable minerals, clays and oxides, as consequence of the removal of mobile elements leading thus to the differentiation and the progressive evolution of the study soil profile. Independent of weathering rates or climatic conditions, the influence of rhyolite’s mineralogy or the rate of change in chemical properties appears to accelerate with weathering time resulting in the observed soil type. The evolution of LOI with depth is modelled on that of CIA, MIA and WPI and according to Sueoka et al. [46], it is a good indicator for the degree of chemical weathering. All soil pedogenic indices calculated, present a zigzag evolution with depth. This marks the differentiated behaviour of various elements depending on the nature of different parameters responsible for the mobilization of each of them along the profile, indicating an irregular change in the study soil profile with depth [70].
There are two limitations on the use of iron in WPI and SEC formula. First, any increase in ferric iron is dependent on the relative concentrations of ferrous and ferric iron in the parent rock. Second, the abundance of iron reflects redox conditions which may not be constant throughout a weathering profile.
4.3. Behaviour of Chemical Elements and Weathering Trends
4.3.1. Major Elements
Phosphorus, iron, potassium, aluminium, and magnesium have positive mean mobilization rates, which indicate their accumulation in the soil profile. High Al2O3 content and its accumulation in the soil horizons is consistent with the presence of aluminum-rich clay minerals, kaolinite, smectite, and illite. Iron is the only element accumulated in all part of the soil profile, in line with the presence of iron oxides identified in all horizons. The high Fe2O3 content in C4 horizon (24.96%) may indicate the presence of other iron-bearing minerals or amorphous iron phases. The others are depleted in the C4 horizon and the Ah horizon. Silicon is the least depleted major element although its accumulation is noted in the C1 horizon (17%), as the consequence of poor drainage, leading to the formation of 2:1 clay minerals [27]. High SiO2 content in the soil horizons corresponds to the presence of quartz. The significant variations in SiO2 content characterized by depletion and accumulation along the soil profile, may suggest the presence of other silica phases or dissolution/precipitation processes affecting silica. Like silicon, other depleted major elements are calcium, sodium and manganese. The CaO content is higher in the C5 horizon (0.50%) where it is less depleted (−69%), consistent with the presence of calcite. The presence of CaO in other horizons (e.g., 0.46% in C4) without evidence of calcite, could indicate other calcium-bearing phases not identified by XRD analysis. The dynamic of Mn and Fe is influenced by the redox condition of the environment, characterized by a rH2 of 14. This value, included in the interval 13–15, corresponds to the transition zone between aerobic and anaerobic environments favourable to the mobilization of Mn [47]. The manganese precipitation in the cracks marks the return of conditions favourable to Mn immobilization. Manganese, unlike Fe2+, diffused more easily out of the pedological volumes because of the slow oxidation kinetics of the former, especially at pH < 8.50 [76]. In the alteritic sequence, a rise in iron percentage is observed in the C5 and the C1, C2, and C3 horizons, leading to its recrystallization in the lower part of the soil profile in the form of goethite and lepidocrocite, while a decrease is observed in the surface horizons under the action of organic acids. The Ruxtons indices in the studied soil remain too high (3.61 to 6.05). This is in agreement with the geochemical trend, characterized by the partial depletion of silica and bases, characteristic of bisiallitization process which act together with monosiallitization throughout the soil profile.
4.3.2. Trace Elements
The distribution of trace elements varies significantly from the parent rock to the pedological volumes. This distribution of trace elements depends on the weathering intensity. Sr is the only element depleted in all part of the soil profile. Sr cannot be incorporated into structures of secondary minerals without producing a charge imbalance given that it is divalent cation. It tends to be excluded from these stable minerals leading to its depletion [77]. Other depleted elements are Ta, W and U, with −15.42, −28.01 and −5.65% as mean mobilization rate respectively along the soil profile. The valence of Ta is +5 while that of W and U range respectively from +2 to +6 and +3 to +6. These three elements could be included in structures of secondary minerals and/or can be strongly sorbed by these minerals. Their depletion means that inclusion and sorption are not major factors in the long-term persistence of these elements in the studied soil [77]. It might be linked to the rH2 redox powers of the different soil horizons. Ranging between 14 and 14.42, there are favourable conditions to the mobilization of chemical elements [47]. This process might be enhanced by the form of the studied rhyolitic dome, which culminate at about 450 m a.s.l.
On the other hand, Cs, Zn and Pb are accumulated in all parts of the soil profile. Their mobilization rates range respectively between 131 and 177,172%. These trace elements are depleted along the soil profile under acid pH [77]. Their accumulation in studied soil might be due to the weakly acidic to neutral pH. The accumulation of Zn might be due to its incorporation into iron minerals. The sorption is pH-dependent, weak at pH < 6 but stronger at the pH of the studied soil (5.16 to 8.77) [77]. Pb is the most highly imported trace element. The adsorption of Pb on mineral surfaces is strong at weakly acidic to neutral pH values, as in the studied soil [78]. The accumulation of Pb in soils would also depend on ion exchange and complexation phenomena [79]. Fe oxides and 2:1 clay minerals would be particularly favourable substrates for the complexation and immobilization of Pb in soil [79]. All the other trace elements are depleted or imported in different parts of the soil profile, but present a positive mobilization rate meaning that they are also imported in the soil profile. They include Sc, V, Co, Ni, Cu, Ga, Ge, Rb, Y, Zr, Nb, Cs, Ba, Hf and Th. Their accumulation in the soil profile, might thus be the result of exclusion/inclusion, sorption and complexation processes which occur alternatively along the soil profile according to the soil pH [77,78,79]. However, even after weathering of primary minerals, trace element can still, partially or totally, be incorporated again in secondary minerals and remain immobilized [80].
4.3.3. Rare Earth Elements
The sum of REE in studied soils varies between 112.75 and 250.84 mg kg−1, with an average value of 160 mg kg−1, and a high abundance of LREE than HREE. This mean value is about half of the mean value currently obtained in Cameroonian soils [81]. It is higher than that obtained in Cuba (74.10 mg kg−1), Brazil (108 mg kg−1), Japan (98.40 mg kg−1), China (155 mg kg−1), Swedish (89.30 mg kg−1) and Europe (125 mg kg−1) soils [82]. It is also higher than that of the continental crust which vary from 0.30 to 43 mg kg−1 [83]. Hu et al. [84] reported that the concentration of an individual REE depends on the parent material and soil types. Soils derived from alkaline igneous rocks usually show high REE contents than soils from acid igneous rocks [82]. The higher average ΣLREE in the study soils (124 mg kg−1) compared with soils of other countries (Cuba, Brazil, Japan, Swedish, Europe) is in line with the felsic nature of the parent rock. Light REEs are considered to be incompatible elements that tend to be higher in felsic rocks and lower in mafic rocks [82].
Eu anomalies observed in the plotted normalized patterns in rhyolite/soil and chondrite/soil are respectively positive and slightly negative. The partial substitution of Ca2+ and Sr2+ by europium during dissolution of feldspar associated to some supergene mechanisms might have led to positive anomaly [35,85]. The negative Ce anomaly confirmed its trivalent form as other REE [86]. C3 horizon shows negative Ce anomaly while in Bw horizon it is positive. Under different oxido-redox conditions in the natural environment, the depletion or enrichment of Ce and Eu is generally related to their oxidation state and mobility. In an environment characterized by a higher oxygen fugacity where Ce3+ is less mobile, there is an easy transformation of Ce3+ into Ce4+, leading thus to a positive Ce anomaly [87]. Similar results were obtained by Alsalam et al. [88] in Triassic sandstone, claystone and conglomerates and the Triassic limestones and dolomites in the mountainous environments in Turkey, were cerium monitors cases of oxidation due to the potential oxidation bond between Ce3+ and Ce4+ during pedogenesis processes in soil profiles. This implies that in the weathering of the studied soil profile, Ce and Eu will decompose on a smaller scale.
The REE contents and their mobility in soils are linked to weathering intensity of parent materials, the storage capacity of authigenic soil phases and fluctuations of redox conditions [34,81]. Basically, their contents in the soils are mainly controlled by the geological background of the area, but the sources of REEs are also related to weathering and external disturbance. Based on their mean mobilization rate, LREE are most depleted in the soil profile except Eu while HREE are most imported except Gd and Lu. The HREEs enrichment in the soil profile is likely associated with their more limited soil mobility [24]. A high correlation between REEs and Fe was observed in tropical soils [82]. Weathering and crystallization of Fe oxides is often accompanied by REE release [89]. The depletion of LREE in the studied soil might be due to the accumulation of Fe. Generally, REE are released from primary minerals and sorbed at the surface of secondary minerals [90], represented in the study area by clay minerals and iron oxides. Studied soil genesis consisting of migration and accumulation of clay minerals, Al, Fe, and Mn controls the vertical distribution of REEs, because processes like sorption, complexation, and precipitation modify the REE contents in the soil profile [91]. pH is the dominant parameter controlling REE behaviour. Under acidic conditions, REE are easily removed from weathering products, but they are fixed by major scavengers under neutral to alkaline conditions [92].
In addition to the contribution of rock mineral weathering as the main source of elements in soils and accumulated elements in particular, it is important to note input from precipitation or surface water. This is in line with Bouba et al. [93] which mention a significant dust storm aerosol concentration in the region in dry season.
The weathering of the parent rhyolite rock constituted of sanidine, quartz, calcite, opaque minerals, plagioclase, amphibole and micas, leads to the mobility and transport of elements in soil solution, both vertically and horizontally. The mobility and transport of elements are influenced by a complex interplay of factors [94]. These factors include soil properties (clay type, organic matter, soil structure and texture), water movement (rainfall and drainage, capillary action, speed of water movement), and the chemical characteristics of elements themselves (element charge and solubility). Water, acting as a solvent, might play a decisive role in carrying these elements through the entire soil profile, leading thus to the differentiation of the study soil profile, with some elements depleted and others accumulated, under the monosiallitization and bisiallitization processes.
5. Conclusions
The aim of this study was to understand the mineralogical and elemental vertical variation in soil formation developed on rhyolites thoroughly the mobility of major and trace elements. The following conclusions can be drawn:
- -
- In situ weathering of the rhyolite parent rock in the dry tropical zone of Cameroon led to the differentiation of seven horizons which are C5, C4, C3, C2, C1, Bw and Ah from bottom to top.
- -
- This differentiation leads to chemical weathering of primary minerals into smectite, kaolinite, illite, calcite, lepidocrocite, goethite, sepiolite and interstratified clay minerals. The presence of both 2:1 (smectite, illite, sepiolite, and interstratified clay minerals) and 1:1 clay minerals (kaolinite) underlines the contribution of both bisiallitization and monosiallitization, together with oxidation and carbonation processes.
- -
- This result in a high mobilization of major elements with strong leaching of Si (−27%), Ca (−87%), Na (−46%) and Mn (−37%), and the accumulation of Al (34%), Fe (148%), K (86%), Mg (15%) and P (194%) along the soil profile.
- -
- Concerning trace elements, Sr is the most depleted element (−44%), followed by W (−28%%), Ta (−15%) and U (−6%). All the other elements (i.e., Sc, V, Co, Ni, Cu, Zn, Ga, Ge, Rb, Y, Zr, Nb, Cs, Ba, Hf, Pb and Th) accumulated in the soil profile result from exclusion/inclusion, sorption and complexation processes which occur alternatively along the soil profile depending on the soil pH.
- -
- The REE contents and their mobility in soils are linked to weathering intensity of parent materials, the storage capacity of authigenic soil phases and fluctuations of redox conditions. Average concentrations of the LREE and HREE in the studied soil are 124 and 35 mg kg−1 respectively. LREE are most depleted (except Eu) while HREE are most imported (except Gd and Lu), along the soil profile. Ce/Ce and Eu/Eu ratios values vary from 1.08 to 1.21 and 0.58 to 1.24 respectively.
- -
- The understanding of the vertical variation of the mineralogical composition and elemental distribution might play an important role in shaping soil organic matter composition during soil weathering across large timescales through its stabilization and protection. Further studies are recommended to assess this link in order to gain a better understanding of how mineral matrix and geochemical composition affect soil organic matter composition across study area. This will lead to the development of strategies for soil management and conservation in the Sudano-Sahelian zone.
Author Contributions
All the authors substantially contributed to this article. The conceptualization of the study was done by D.T. and A.N.N., with input from M.G.D., E.L.T.M. and N.F. The data acquisition, investigation, methodology, and visualization for the paper were performed by D.T., A.N.N. and N.F., with substantial input from M.G.D. and E.L.T.M., D.T. and A.N.N. wrote the initial draft, and E.L.T.M., M.G.D. and N.F. were involved in the reviewing, editing, and the validation of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The research data will be freely available on request from the corresponding author.
Acknowledgments
Authors thank Joël Otten for XRD and TGA analyses (University of Liège) and Hervé Ngatanko (University of Maroua) for English editing. The manuscript was improved by the comments and suggestions of two anonymous reviewers.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Regassa, A.; Van Daele, K.; De Paepe, P.; Dumon, M.; Deckers, J.; Asrat, A.; Van Ranst, E. Characterizing weathering intensity and trends of geological materials in the Gilgel Gibe catchment, southwestern Ethiopia. J. Afr. Earth Sci. 2014, 99, 568–582. [Google Scholar] [CrossRef]
- Palumbo, B.; Angelone, M.; Bellanca, A.; Dazzo, C.; Hauser, S.; Neri, R.; Wilson, J. Influence of inheritance and pedogenesis on heavy metal distribution in soils of Sicily, Italy. Geoderma 2000, 95, 247–266. [Google Scholar] [CrossRef]
- Driese, S.G.; Jacobs, J.R.; Nordt, L.C. Comparison of modern and ancient Vertisols developed on limestone in terms of their geochemistry and parent material. Sediment. Geol. 2003, 157, 49–69. [Google Scholar] [CrossRef]
- Schaetzl, R.J.; Anderson, S. Soil Genesis and Geomorphology; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Tunçay, T.; Dengiz, O. Chemical weathering rates and geochemical-mineralogical characteristics of soils developed on heterogenous parent material and toposequence. Carpathian J. Earth Environ. Sci. 2016, 11, 583–598. [Google Scholar]
- da Silva, R.J.A.B.; da Silva, Y.J.A.B.; Straaten, V.P.; do Nascimento, C.W.A.; Biondi, C.M.; da Silva, Y.J.A.B.; Filho, J.C.D.A. Influence of parent material on soil chemical characteristics in a semi-arid tropical region of Northeast Brazil. Environ. Monit. Assess. 2022, 194, 331. [Google Scholar] [CrossRef]
- Costa, E.U.C.; Santos, J.C.B.; Azevedo, A.C.; Araujo Filho, J.C.; Correa, M.M.; Neves, L.V.M.W.; Vidal-Torrado, P.; Souza Junior, V.S. Mineral alteration and genesis of Al-rich soils derived from conglomerate deposits in Cabo Basin, NE Brazil. Catena 2018, 167, 198–211. [Google Scholar] [CrossRef]
- Niu, S.; Gao, L.; Wang, X. Characterization of contamination levels of heavy metals in agricultural soils using geochemical baseline concentrations. J. Soils Sediments 2019, 19, 1697–1707. [Google Scholar] [CrossRef]
- Thiombane, M.; Di Bonito, M.; Albanese, S.; Zuzolo, D.; Lima, A.; De Vivo, B. Geogenic versus anthropogenic behaviour and geochemical footprint of Al, Na, K and P in the Campania region (Southern Italy) soils through compositional data analysis and enrichment factor. Geoderma 2019, 335, 12–26. [Google Scholar] [CrossRef]
- Zinn, Y.L.; Faria, J.A.; Araujo, M.A.; Skorupa, A.L.A. Soil parent material is the main control on heavy metal concentrations in tropical highlands of Brazil. Catena 2020, 185, 104319. [Google Scholar] [CrossRef]
- Heckman, K.; Welty, B.A.; Rasmussen, C.; Schwartz, E. Geologic controls of soil carbon cycling and microbial dynamics in temperate conifer forests. Chem. Geol. 2009, 267, 12–23. [Google Scholar] [CrossRef]
- Heckman, K.; Rasmussen, C. Lithologic controls on regolith weathering and mass flux in forested ecosystems of the southwestern USA. Geoderma 2011, 164, 99–111. [Google Scholar] [CrossRef]
- Brilhante, S.A.; Santos, J.C.B.; Souza Júnior, V.S.; Araújo, J.K.S.; Ribeiro Filho, M.R.; Corrêa, M.M. Weathering of rhyolites and soil formation in an Atlantic Forest fragment in northeastern Brazil. Rev. Bras. Ciência Solo 2017, 41, e0160558. [Google Scholar] [CrossRef]
- Raj, J.K. Physical characterization of a deep weathering profile over rhyolite in humid tropical peninsular Malaysia. Geotech. Geol. Eng. 2018, 36, 3793–3809. [Google Scholar] [CrossRef]
- Gountié, D.M.; Asobo, N.E.; Fozing, E.M.; Tchamabé, B.C.; Zangmo, T.G.; Nguihdama, D.; Tchokona, S.D.; Kamgang, P.; Aka, T.F.; Ohbak, T. Petrology and geochemistry of lavas from Gawar, Minawao and Zamay volcanoes of the northern segment of the Cameroon volcanic line (Central Africa): Constraints on mantle source and geochemical evolution. J. Afr. Earth Sci. 2019, 153, 3–41. [Google Scholar] [CrossRef]
- Dubroeucq, D.; Geissert, D.; Quantin, P. Weathering and soil forming processes under semi-arid conditions in two Mexican volcanic ash soils. Geoderma 1998, 86, 99–122. [Google Scholar] [CrossRef]
- Candra, I.N.; Gerzabek, M.H.; Ottner, F.; Tintner, J.; Wriessnig, K.; Zehetner, F. Weathering and soil formation in rhyolitic tephra along a moisture gradient on Alcedo Volcano, Galapagos. Geoderma 2019, 343, 215–225. [Google Scholar] [CrossRef]
- Tardy, Y. Petrology of Laterites and Tropical Soils; Balkema: Leiden, Amsterdam, 1997. [Google Scholar]
- Bitom, D.; Volkoff, B.; Abossolo-Angue, M. Evolution and alteration in situ of a massive iron duricrust in Central Africa. J. Afr. Earth Sci. 2003, 37, 89–101. [Google Scholar] [CrossRef]
- Bitom, D.; Volkoff, B.; Beauvais, A.; Seyler, F.; Ndjigui, P.D. Rôle des héritages latéritiques et du niveau des nappes dans l’évolution des modelés et des sols en zone intertropicale forestière humide. Comptes Rendus Géoscience 2004, 336, 1161–1170. [Google Scholar] [CrossRef]
- Beauvais, A. Ferricrete biochemical degradation on the rainforest-savannas boundary of central african republic. Geoderma 2009, 150, 379–388. [Google Scholar] [CrossRef]
- Oyelami, C.A.; Van Rooy, J.L. A review of the use of lateritic soils in the construction/development of sustainable housing in Africa: A geological perspective. J. Afr. Earth Sci. 2016, 119, 226–237. [Google Scholar] [CrossRef]
- Nouazi, M.M.; Beauvais, A.; Tematio, P.; Ambrosi, J.-P.; Yemefack, M.; Palmer, K.Y.B.; Yongue-Fouateu, R. Lateritic weathering of trachyte, and bauxite formation in West Cameroon: Morphological and geochemical evolution. J. Geochem. Explor. 2019, 205, 106324. [Google Scholar] [CrossRef]
- Azinwi, T.P.; Kouankap, N.D.G.; Wotchokoa, P.; Tchagnian, M.B.; Tene, D.J.F.; Kamgang, K.V.; Bitom, D. Geochemistry of a lateritic mantle developed on basalt in the Cameroon Western Highlands (Cameroon Volcanic Line). Geoderma 2020, 376, 114569. [Google Scholar] [CrossRef]
- Onana, L.V.; Ndome, E.E.; Noa Tang, D.S.; Kamgang, K.V.; Ekodeck, E.G. Chemical weathering intensity and rare earth elements release from a chlorite schist profile in a humid tropical area, Bengbis, Southern Cameroon. J. Cameroon Acad. Sci. 2020, 16, 123–145. [Google Scholar] [CrossRef]
- Kessoum, A.J.-M.; Noa Tang, S.D.; Sababa, E.; Onana, V.L. Weathering profiles developed on gneisses from Batchenga and Doua areas, central Cameroon: Climate and topography controls. J. Afr. Earth Sci. 2021, 184, 104367. [Google Scholar] [CrossRef]
- Nguetnkam, J.P.; Kamga, R.; Villiéras, F.; Ekodeck, G.E.; Yvon, J. Variable weathering response of granite in tropical zones. Example of two sequences studied in Cameroon (Central Africa). Comptes Rendus Geosci. 2008, 340, 451–461. [Google Scholar] [CrossRef]
- Nguetnkam, J.-P.; Villieras, F.; Kamga, R.; Ekodeck, G.E.; Yvon, J. Mineralogy and geochemical behaviour during weathering of greenstone belt under tropical dry conditions in the extreme North Cameroon (Central Africa). Chem. Erde 2014, 74, 185–193. [Google Scholar] [CrossRef]
- Temga, J.P.; Maché, J.R.; Balo, M.A.; Nguetnkam, J.P.; Bitom, D.L. Ceramics applications of clay in Lake Chad Basin, Central Africa. Appl. Clay Sci. 2019, 171, 118–132. [Google Scholar] [CrossRef]
- Ngounou Ngatcha, B.; Mudry, J.; Sigha Nkamdjou, L.; Njitchoua, R.; Naah, E. Climate variability and impacts on an alluvial aquifer in a semi-arid climate, the Logone-Chari plain (South of Lake Chad). Int. Assoc. Hydrol. Sci. 2005, 295, 94–100. [Google Scholar]
- Eze, P.N.; Molwalefhe, L.N.; Kebonye, N.M. Geochemistry of soils of a deep pedon in the Okavango Delta, NW Botswana: Implications for pedogenesis in semi-arid regions. Geoderma Reg. 2021, 24, e00352. [Google Scholar] [CrossRef]
- Fu, W.; Li, X.; Feng, Y.; Feng, M.; Peng, Z.; Yu, H.; Lin, H. Chemical weathering of S-type granite and formation of Rare Earth Element (REE)-rich regolith in South China: Critical control of lithology. Chem. Geol. 2019, 520, 33–51. [Google Scholar] [CrossRef]
- Ma, Y.; Huo, R.; Liu, C. Speciation and fractionation of rare earth elements in a lateritic profile from southern China; identification of the carriers of Ce anomalies. Geochim. Cosmochim. Acta 2002, 66, 471. [Google Scholar]
- Laveuf, C.; Cornu, S. A review on the potentiality of rare earth elements to trace pedogenetic processes. Geoderma 2009, 154, 1–12. [Google Scholar] [CrossRef]
- Panahi, A.; Young, G.M.; Rainbird, R.H. Behaviour of major and trace elements (including REE) during Paleoproterozoic pedogenesis and diagenetic alteration of an archaean granite near Ville Marie, Québec, Canada. Geochim. Cosmochim. Acta 2000, 64, 2199–2220. [Google Scholar] [CrossRef]
- Cao, Y.-W.; Liu, X.-M.; Wang, C.; Bai, E.; Wu, N. Rare earth element geochemistry in soils along arid and semiarid grasslands in northern China. Ecol. Process. 2022, 11, 29. [Google Scholar] [CrossRef]
- Van der zon APM. Graminées du Cameroun Volume I, Phytogéographie et Pâturages; Wageningen Agricultural University Papers; Wageningen University and Research: Wageningen, The Netherlands, 1992. [Google Scholar]
- Tamen, J.; Nkoumbou, C.; Reusser, E.; Tchoua, F. Petrology and geochemistry of mantle xenoliths from the Kapsiki plateau (Cameroon volcanic line): Implications for lithospheric upwelling. J. Afr. Earth Sci. 2015, 101, 119–134. [Google Scholar] [CrossRef]
- Brabant, P.; Gavaud, M. Les Sols et Les Ressources en Terres du Nord Cameroun; Collection Notice Explicative N° 103; Editions de l’ORSTOM: Marseille, France, 1985. [Google Scholar]
- IUSS Working Group. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Nesbitt, Y.W.; Young, G.M. Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 1982, 299, 715–717. [Google Scholar] [CrossRef]
- Ruxton, pp. Measures of the degree of chemical weathering of rock. J. Geol. 1968, 76, 518–527. [Google Scholar] [CrossRef]
- Voicu, G.; Bardoux, M.; Jebrak, M.; Voicu, D. Normative mineralogical calculations for tropical weathering profiles. Geol. Assoc. Can. Miner. Assoc. Can. Program Abstr. 1996, 21, A-69. [Google Scholar]
- Vogt, T. Sulitjelmafeltets geologiog petrografi. Nor. Geol. Unders. 1927, 121, 1–560. [Google Scholar]
- Irfan, T.Y. Mineralogy, fabric properties and classification of weathered granite in Hong Kong. Q. J. Eng. Geol. 1996, 29, 5–35. [Google Scholar] [CrossRef]
- Sueoka, T.; Lee, I.K.; Muramatsu, M.; Imamura, S. Geomechanical properties and engineering classification for decomposed granite soils in Kaduna district, Nigeria. In Proceedings of the First International Conference Geomechanical in Tropical Lateritic and Saprolitic Soils, Brasilia, Brazil, 11–14 February 1985; Volume 1, pp. 175–186. [Google Scholar]
- Rodier, J. L’analyse de L’eau: Eaux Naturelles, Eaux Résiduaires et Eaux de mer: Chimie, Physico-Chimie, Microbiologie, Biologie, Interprétation des Résultats, 8th ed.; Dunod: Malakoff, France, 1996. [Google Scholar]
- Brimhall, G.H.; Alpers, C.N.; Cunningham, A.B. Analysis of supergene ore–forming processes and grand-Water solute transport using mass balance principles. Econ. Geol. 1985, 80, 1227–1256. [Google Scholar] [CrossRef]
- Brimhall, G.H.; Dietrich, W.E. Constitutive mass balance relations between chemical composition, volume, density, porosity and strain in metasomatic hydrochemical systems: Results on weathering and pedogenesis. Geochim. Cosmochim. Acta 1987, 51, 567–587. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.S. The composition of the earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Thorez, J. Pratical Identification of Clay Minerals; Lelotte, G., Ed.; Dison: Singapore, 1976. [Google Scholar]
- Baran, B.; Ertürk, T.; Sarikaya, Y.; Alemdaroglu, T. Workability test method for metals applied to examine a workability measure (plastic limit) for clays. Appl. Clay Sci. 2001, 20, 53–63. [Google Scholar] [CrossRef]
- Srasra, E. Caractérisation Minéralogiques, Propriétés Physico-Chimiques et Application des Argiles du Gisement Haidoudi. Ph.D. Thesis, Université de Tunis: Tunis, Tunisia, 1987. [Google Scholar]
- Wolters, F.; Emmerich, K. Thermal reactions of smectites-relation of dehydroxylation temperature to octahedral structure. Thermochim. Acta 2007, 462, 80–88. [Google Scholar] [CrossRef]
- Ayari, F.; Srasra, E.; Trabelsi-Ayadi, M. Characterization of bentonitic clays and their use as adsorbent. Desalination 2005, 185, 391–397. [Google Scholar] [CrossRef]
- Nzeukou, N.A. Minéralogie, Géochimie et Propriétés Céramiques des Argiles Alluviales de la Sanaga Entre Nanga-Eboko et Ebebda (Région du Centre-Cameroun). Ph.D. Thesis, University of Yaoundé I, Yaoundé, Cameroon, 2014; 154p. [Google Scholar]
- Aristizabal, E.; Roser, B.; Yokota, S. Tropical chemical weathering of hillslope deposits and bedrock source in the Aburra Valley, northern Colombian Andes. Eng. Geol. 2005, 81, 389–406. [Google Scholar] [CrossRef]
- Basga, S.D.; Temga, J.P.; Tsozué, D.; Danbé, D.; Nguetnkam, J.P. Morphological, mineralogical and geochemical features of topomorphic vertisols used for sorghum production in North Cameroon. Eurasian J. Soil Sci. 2018, 7, 346–354. [Google Scholar] [CrossRef]
- Tsozué, D.; Bitom, D.; Yongue-Fouateu, R. morphology, mineralogy and geochemistry of a lateritic soil sequence developed on micaschist in the abong-mbang region, southeast Cameroon. S. Afr. J. Geol. 2012, 115, 103–116. [Google Scholar] [CrossRef]
- Francis, M.L.; Fey, M.V.; Ellis, F.; Poch, R.M. Petroduric and petrosepiolitic horizons in soils of Namaqual and, South Africa. Span. J. Soil Sci. 2012, 2, 8–25. [Google Scholar]
- Goldich, S.S. A study in rock-weathering. J. Geol. 1938, 46, 17–58. [Google Scholar] [CrossRef]
- Amouric, M.; Olives, J. Transformation mechanisms and interstratifications in conversion of smectite to kaolinite: An HRTEM study. Clays Clay Miner. 1998, 46, 521–527. [Google Scholar] [CrossRef]
- Tsozué, D.; Basga, S.D.; Nzeukou, N.A. Spatial variation of soil weathering processes in the tropical high reliefs of Cameroon (Central Africa). Eurasian J. Soil Sci. 2020, 9, 92–104. [Google Scholar] [CrossRef][Green Version]
- Tsozué, D.; Nzeukou, N.A.; Azinwi, T.P. Genesis and classification of soils developed on gabbro in the high reliefs of Maroua region, North Cameroon. Eurasian J. Soil Sci. 2017, 6, 168–177. [Google Scholar] [CrossRef]
- Van Breemen, N. Long-term chemical, mineralogical and morphological effects of iron-redox processes in periodically flooded soils. In Iron in Soils and Clay Minerals; Stucki, J., Goodman, B., Schwertmann, U., Eds.; NATO ASI Series; D. Reidel Publishing: Dordrecht, The Netherlands, 1988. [Google Scholar]
- Jordanova, N. Magnetism of materials occurring in the environment—Basic overview. In Soil Magnetism: Applications in Pedology, Environmental Science and Agriculture, 1st ed.; Kindle Edition; Academic Press: San Diego, CA, USA, 2016; pp. 1–28. [Google Scholar]
- Cornell, R.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrence and Uses; Wiley: Weinheim, Germany; New York, NY, USA, 2003. [Google Scholar]
- Singer, A. Pedogenic palygorskite in the arid environment. In Palygorskite-Sepiolite. Occurrences, Genesis and Uses; Singer, A., Galán, E., Eds.; Developments in Sedimentology 37; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Jones, B.F.; Galán, E. Sepiolite and palygorskite. In Hydrous Phyllosilicates (Exclusive of Micas); Bailey, S.W., Ed.; Reviews in Mineralogy 19; Mineralogical Society of America: Washington, DC, USA, 1988. [Google Scholar]
- Tunçay, T.; Dengiz, O.; Bayramin, I.; Kilic, S.; Baskan, O. Chemical weathering indices applied to soils developed on old lake sediments in a semi-arid region of Turkey. Eurasian J. Soil Sci. 2019, 8, 60–72. [Google Scholar] [CrossRef]
- Fedo, C.M.; Nesbitt, H.W.; Young, G.M. Unraveling the effects of potassium metasomatism in sedimentary rocks and paleosols with implications for paleoweathering conditions and provenance. Geology 1995, 23, 921–924. [Google Scholar] [CrossRef]
- Ao, H.; Deng, C.; Dekkers, M.J.; Sun, Y.; Liu, Q.; Zhu, R. Pleistocene environmental evolution in the Nihewan Basin and implication for early human colonization of North China. Quat. Int. 2010, 223–224, 472–478. [Google Scholar] [CrossRef]
- Özaytekin, H.H.; Mutlu, H.H.; Dedeoglu, M. Soil formation on a calcic chronosequence of ancient Lake Konya in Central Anatolia, Turkey. J. Afr. Earth Sci. 2012, 76, 66–74. [Google Scholar] [CrossRef]
- Voicu, G.; Bardoux, M. Geochemical behaviour under tropical weathering of the Barama-Mazaruni greenstone belt at Omai gold mine. Guiana Shield. Appl. Geochem. 2002, 17, 321–336. [Google Scholar] [CrossRef]
- Ng, C.W.W.; Guan, P.; Shang, Y.J. Weathering mechanisms and indices of the igneous rocks of Hong Kong. Q. J. Eng. Geol. Hydrogeol. 2001, 34, 133–151. [Google Scholar] [CrossRef]
- Thamdrup, B.; Fossing, H.; Jørgensen, B.B. Manganese, iron, and sulfur cycle in a coastal marine sediment, Aahus Bay, Denmark. Geochim. Cosmochim. Acta 1994, 23, 5115–5129. [Google Scholar] [CrossRef]
- Marques, J.J.; Schulze, D.G.; Curi, N.; Mertzman, S.A. Trace element geochemistry in Brazilian Cerrado soils. Geoderma 2004, 121, 31–43. [Google Scholar] [CrossRef]
- Blanc, P.; Burnol, A.; Guyonnet, D. Atténuation des Métaux de la Liste de Substances Prioritaires Dans la Zone Non Saturée. Rapport BRGM/53096-FR. 2004. Available online: http://infoterre.brgm.fr/rapports/RP-53096-FR.pdf (accessed on 5 May 2025).
- Morin, G.; Juillot, F.; Ildefonse, P.; Calas, G.; Samama, J.C.; Chevallier, P.; Brown, G.E. Mineralogy of lead in a soil developed on a Pb-mineralized sandstone (Largentière, France). Am. Mineral. 2001, 86, 92–104. [Google Scholar] [CrossRef]
- Gnandi, K.; Tobschall, H.J. Distribution patterns of rare-earth elements and uranium in tertiary sedimentary phosphorites of Hahotoé–Kpogamé, Togo. J. Afr. Earth Sci. 2003, 37, 1–10. [Google Scholar] [CrossRef]
- Temga, P.J.; Sababa, E.; Mamdem, L.E.; Ngo Bijeck, M.L.; Azinwi, T.P.; Tehna, N.; Zo’o Zame, P.; Onana, V.L.; Nguetnkam, J.P.; Bitom, L.D.; et al. Rare earth elements in tropical soils, Cameroon soils (Central Africa). Geoderma Reg. 2021, 25, e00369. [Google Scholar] [CrossRef]
- Alfaro, M.R.; Do Nascimento, C.W.A.; Biondi, C.M.; da Silva, Y.J.A.B.; de Aguiar Accioly, A.M.; Montero, A.; Muñiz Ugarte, O.; Estevez, J. Rare-earth-element geochemistry in soils developed in different geological settings of Cuba. Catena 2018, 162, 317–324. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust. In The Crust; Rudnick, R.L., Ed.; Elsevier-Pergamon: Oxford, UK, 2003; pp. 1–64. [Google Scholar] [CrossRef]
- Hu, Z.; Haneklaus, S.; Sparovek, G.; Schnug, E. Rare earth elements in soils. Commun. Soil Sci. Plant Anal. 2006, 37, 1381–1420. [Google Scholar] [CrossRef]
- Ndjigui, P.-D.; Badinane, M.F.B.; Nyeck, B.; Nandjip, H.P.K.; Bilong, P. Mineralogical and geochemical features of the coarse saprolite developed on orthogneiss in the SW of Yaoundé, South Cameroon. J. Afr. Earth Sci. 2013, 79, 125–142. [Google Scholar] [CrossRef]
- Marsh, J.S. REE fractionation and Ce anomalies in weathered Karoo dolerite. Chem. Geol. 1990, 90, 189–194. [Google Scholar] [CrossRef]
- Wang, L.; Liang, T. Geochemical fractions of rare earth elements in soil around a mine tailing in Baotou, China. Sci. Rep. 2015, 5, 12483. [Google Scholar] [CrossRef]
- Alsalam, O.; Şeker, C.; Dedeoğlu, M. Quantifying the role of chemical weathering rates on soil developed along an altitudinal transect in the mountainous environments, Turkey. Eurasian J. Soil Sci. 2020, 9, 140–150. [Google Scholar] [CrossRef][Green Version]
- Laveuf, C.; Cornu, S.; Guilerme, L.R.G.; Guerin, A.; Juillot, F. The impact of redox conditions on the rare earth element signature of redoximorphic features in a soil sequence developed from limestone. Geoderma 2012, 170, 25–38. [Google Scholar] [CrossRef]
- Karadaǧ, M.M.; Küpeli, S.; Arýk, F.; Ayhan, A.; Zedef, V.; Döyen, A. Rare earth element (REE) geochemistry and genetic implications of the Morta¸s-bauxite deposit (Seydi¸sehir/Konya-Southern Turkey). Chem. Erde Geochem. 2009, 69, 143–159. [Google Scholar] [CrossRef]
- Mihajlovic, J.; Bauriegel, A.; Stärkc, H.-J.; Roßkopf, N.; Zeitz, J.; Milbert, G.; Rinklebe, J. Rare earth elements in soil profiles of various ecosystems across Germany. Appl. Geochem. 2019, 102, 197–217. [Google Scholar] [CrossRef]
- Fleet, A.J. Aqueous and sedimentary geochemistry of the rare earth elements. In Rare Earth Element Geochemistry; Hendersonm, P., Ed.; Elsevier: Amsterdam, The Netherlands, 1984; pp. 343–373. [Google Scholar]
- Bouba, L.; Kémo, W.; Samba Assomo, p. Lithometeors and public health issue in the city of Maroua inthe Far North region of Cameroon (Central Africa). Nat. Hazards 2025, 121, 5901–5920. [Google Scholar] [CrossRef]
- Kodešová, R.; Vignozzi, N.; Rohošková, M.; Hájková, T.; Kočárek, M.; Pagliai, M.; Kozák, J.; Šimůnek, J. Impact of varying soil structure on transport processes in different diagnostic horizons of three soil types. J. Contam. Hydrol. 2009, 104, 107–125. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).