Clinical Perspectives and Management of Edema in Chronic Venous Disease—What about Ruscus?
Abstract
:1. Introduction
2. Pathophysiology of Edema
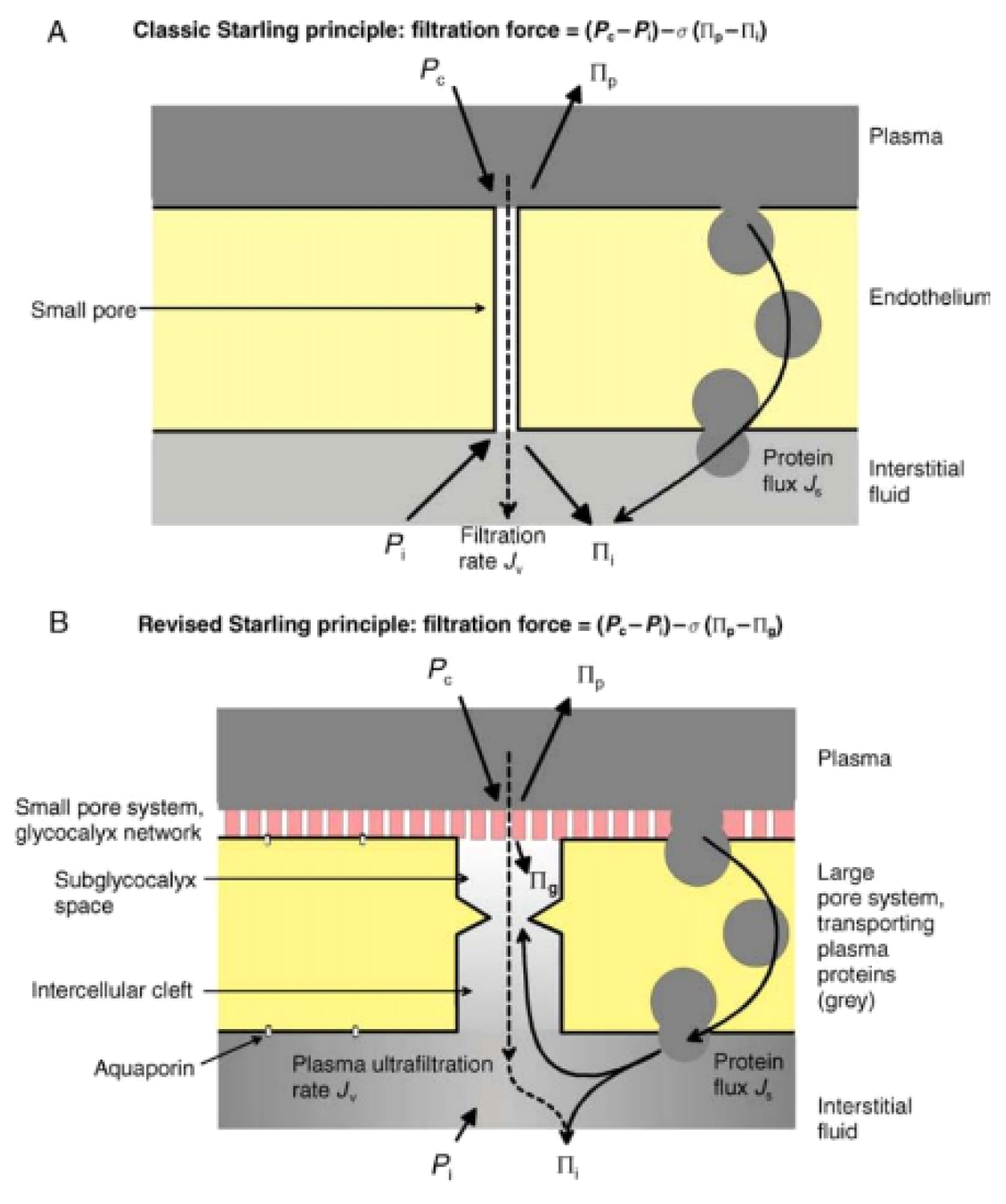
3. The Original Starling Principle
3.1. The Revised Starling Principle
3.2. Further Considerations on the Extended Starling Principle
3.3. The Importance of Colloid Osmotic Pressure
4. Edema from a Clinical Perspective
5. Signs and Symptoms of Edema
6. The Prevalence of Edema
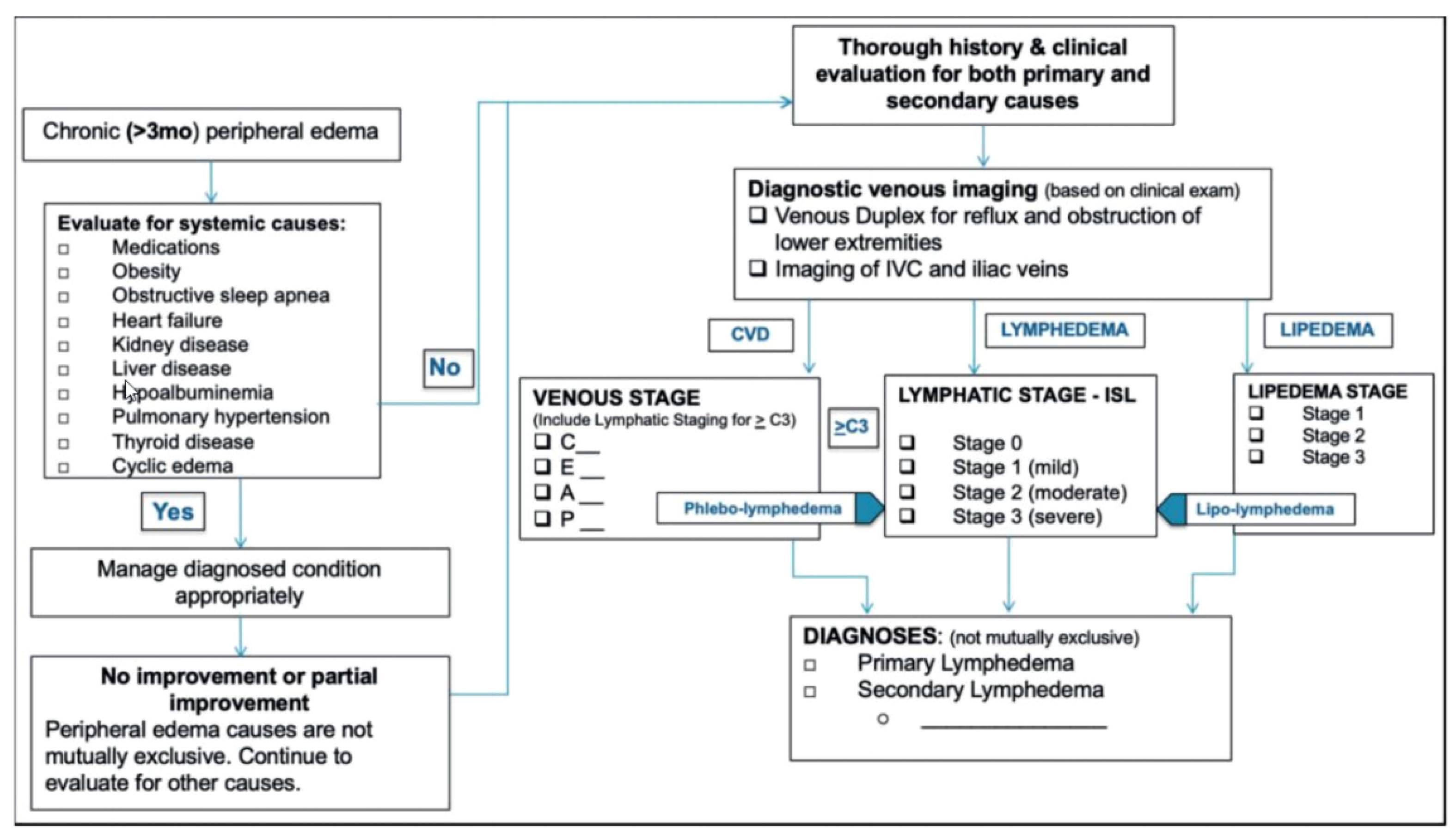
7. A Diagnostic Algorithm for Chronic Lower Extremity Swelling
8. CEAP Classification
9. The Benefits of Ruscus Extract on Edema
10. Clinical Studies with Ruscus/HMC/Vit C
11. Ruscus/HMC/Vit C in the Chronic Venous Disease Guidelines
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jawien, A.; Grzela, T.; Ochwat, A. Prevalence of chronic venous insufficiency in men and women in Poland: Multicentre cross-sectional study in 40,095 patients. Phlebology 2003, 18, 110–122. [Google Scholar] [CrossRef]
- Eklof, B.; Perrin, M.; Delis, K.T.; Rutherford, R.B.; Gloviczki, P.; American Venous; European Venous; International Union; American College; International Union. Updated terminology of chronic venous disorders: The VEIN-TERM transatlantic interdisciplinary consensus document. J. Vasc. Surg. 2009, 49, 498–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Medicines Agency. European Union Herbal Monograph on Ruscus aculeatus L., Rhizoma (EMA/HMPC/188804/2017). 20 November 2018. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/european-union-herbal-monograph-ruscus-aculeatus-l-rhizoma-revision-1_en.pdf (accessed on 20 June 2022).
- Jawien, A.; Bouskela, E.; Allaert, F.A.; Nicolaides, A.N. The place of Ruscus extract, hesperidin methyl chalcone, and vitamin C in the management of chronic venous disease. Int. Angiol. 2017, 36, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, A.; Kakkos, S.; Baekgaard, N.; Comerota, A.; de Maeseneer, M.; Eklof, B.; Giannoukas, A.D.; Lugli, M.; Maleti, O.; Myers, K.; et al. Management of chronic venous disorders of the lower limbs. Guidelines According to Scientific Evidence. Int. Angiol. 2018, 37, 181–254. [Google Scholar] [CrossRef] [PubMed]
- Trayes, K.P.; Studdiford, J.S.; Pickle, S.; Tully, A.S. Edema: Diagnosis and management. Am. Fam. Physician 2013, 88, 102–110. [Google Scholar] [PubMed]
- Levick, J.R.; Michel, C.C. Microvascular fluid exchange and the revised Starling principle. Cardiovasc. Res. 2010, 87, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, T.E.; Michel, C.C. Advances in the Starling Principle and microvascular fluid exchange; consequences and implications for fluid therapy. Front. Vet. Sci. 2021, 8, 623671. [Google Scholar] [CrossRef]
- Darwish, A.; Lui, F. Physiology, Colloid Osmotic Pressure. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Wiig, H.; Swartz, M.A. Interstitial fluid and lymph formation and transport: Physiological regulation and roles in inflammation and cancer. Physiol. Rev. 2012, 92, 1005–1060. [Google Scholar] [CrossRef]
- Hahn, R.G.; Dull, R.O.; Zdolsek, J. The Extended Starling principle needs clinical validation. Acta Anaesthesiol. Scand. 2020, 64, 884–887. [Google Scholar] [CrossRef] [Green Version]
- Tornudd, M.; Hahn, R.G.; Zdolsek, J.H. Fluid distribution kinetics during cardiopulmonary bypass. Clinics 2014, 69, 535–541. [Google Scholar] [CrossRef]
- Guthe, H.J.; Indrebo, M.; Nedrebo, T.; Norgard, G.; Wiig, H.; Berg, A. Interstitial fluid colloid osmotic pressure in healthy children. PLoS ONE 2015, 10, e0122779. [Google Scholar] [CrossRef]
- Noddeland, H. Influence of body posture on transcapillary pressures in human subcutaneous tissue. Scand. J. Clin. Lab. Investig. 1982, 42, 131–138. [Google Scholar] [CrossRef]
- Wu, P.Y.; Udani, V.; Chan, L.; Miller, F.C.; Henneman, C.E. Colloid osmotic pressure: Variations in normal pregnancy. J. Perinat. Med. 1983, 11, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Fauchald, P.; Norseth, J.; Jervell, J. Transcapillary colloid osmotic gradient, plasma volume and interstitial fluid volume in long-term type 1 (insulin-dependent) diabetes. Diabetologia 1985, 28, 269–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dull, R.O.; Hahn, R.G. Transcapillary refill: The physiology underlying fluid reabsorption. J. Trauma Acute Care Surg. 2021, 90, e31–e39. [Google Scholar] [CrossRef]
- Ozpelit, E.; Ozpelit, M.E.; Albayrak, G.; Karabay, O.; Nesli Sahin, B.; Gonencer, J.Z.; Badak, O. Arterial stiffness and cardiac functions in patients with chronic venous disease. Int. Angiol. 2015, 34, 582–588. [Google Scholar]
- Gasparis, A.P.; Kim, P.S.; Dean, S.M.; Khilnani, N.M.; Labropoulos, N. Diagnostic approach to lower limb edema. Phlebology 2020, 35, 650–655. [Google Scholar] [CrossRef]
- Rabe, E.; Carpentier, P.; Maggioli, A. Understanding lower leg volume measurements used in clinical studies focused on venous leg edema. Int. Angiol. 2018, 37, 437–443. [Google Scholar] [CrossRef]
- Priollet, P. Venous edema of the lower limbs. Phlebolymphology 2006, 13, 183–187. [Google Scholar]
- Kazandjieva, J.; Christoff, G. Angioedema as a systemic disease. Clin. Dermatol. 2019, 37, 636–643. [Google Scholar] [CrossRef]
- Goss, J.A.; Greene, A.K. Sensitivity and specificity of the Stemmer sign for lymphedema: A clinical lymphoscintigraphic study. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2295. [Google Scholar] [CrossRef] [PubMed]
- Raju, S.; Knepper, J.; May, C.; Knight, A.; Pace, N.; Jayaraj, A. Ambulatory venous pressure, air plethysmography, and the role of calf venous pump in chronic venous disease. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 428–440. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, T.F., Jr.; Rasmussen, J.C.; Sevick-Muraca, E.M. New diagnostic modalities in the evaluation of lymphedema. J. Vasc. Surg. Venous Lymphat. Disord. 2017, 5, 261–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Largeau, B.; Cracowski, J.L.; Lengellé, C.; Sautenet, B.; Jonville-Béra, A.P. Drug-induced peripheral oedema: An aetiology-based review. Br. J. Clin. Pharmacol. 2021, 87, 3043–3055. [Google Scholar] [CrossRef] [PubMed]
- Chrysant, S.G. Proactive compared with passive adverse event recognition: Calcium channel blocker–associated edema. J. Clin. Hypertens. 2008, 10, 716–722. [Google Scholar] [CrossRef] [Green Version]
- Kjeldsen, S.E.; Sica, D.; Haller, H.; Cha, G.; Gil-Extremera, B.; Harvey, P.; Heyvaert, F.; Lewin, A.J.; Villa, G.; Mancia, G. Nifedipine plus candesartan combination increases blood pressure control regardless of race and improves the side effect profile: DISTINCT randomized trial results. J. Hypertens. 2014, 32, 2488. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.G.; Nguyen, T.V.; Day, R.O. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann. Intern. Med. 1994, 121, 289–300. [Google Scholar] [CrossRef]
- Curtis, E.; Fuggle, N.; Shaw, S.; Spooner, L.; Ntani, G.; Parsons, C.; Corp, N.; Honvo, G.; Baird, J.; Maggi, S.; et al. Safety of Cyclooxygenase-2 Inhibitors in Osteoarthritis: Outcomes of a Systematic Review and Meta-Analysis. Drugs Aging 2019, 36, 25–44. [Google Scholar] [CrossRef] [Green Version]
- Afshari, R.; Maxwell, S.R.; Webb, D.J.; Bateman, D.N. Morphine is an arteriolar vasodilator in man. Br. J. Clin. Pharmacol. 2009, 67, 386–393. [Google Scholar] [CrossRef] [Green Version]
- Gardner-Nix, J. Opioids causing peripheral edema. J. Pain Symptom Manag. 2002, 23, 453–455. [Google Scholar] [CrossRef]
- Jeong, G.H.; Lee, K.H.; Lee, I.R.; Oh, J.H.; Kim, D.W.; Shin, J.W.; Kronbichler, A.; Eisenhut, M.; van der Vliet, H.J.; Abdel-Rahman, O. Incidence of capillary leak syndrome as an adverse effect of drugs in cancer patients: A systematic review and meta-analysis. J. Clin. Med. 2019, 8, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpentier, P.H.; Poulain, C.; Fabry, R.; Chleir, F.; Guias, B.; Bettarel-Binon, C.; Venous Working Group of the Societe Francaise; de Medecine, V. Ascribing leg symptoms to chronic venous disorders: The construction of a diagnostic score. J. Vasc. Surg. 2007, 46, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Pani, S.P.; Vanamail, P.; Yuvaraj, J. Limb circumference measurement for recording edema volume in patients with filarial lymphedema. Lymphology 1995, 28, 57–63. [Google Scholar] [PubMed]
- Rapprich, S.; Dingler, A.; Podda, M. Liposuction is an effective treatment for lipedema-results of a study with 25 patients. J. Dtsch. Dermatol. Ges. 2011, 9, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Rabe, E.; Pannier-Fischer, F.; Bromen, K.; Schuldt, K.; Stang, A.; Poncar, C.; Wittenhorst, M.; Bock, E.; Weber, S.; Jöckel, K.-H. Bonner venenstudie der deutschen gesellschaft für phlebologie. Phlebologie 2003, 32, 1–14. [Google Scholar]
- Wrona, M.; Jockel, K.H.; Pannier, F.; Bock, E.; Hoffmann, B.; Rabe, E. Association of Venous Disorders with Leg Symptoms: Results from the Bonn Vein Study 1. Eur. J. Vasc. Endovasc. Surg. 2015, 50, 360–367. [Google Scholar] [CrossRef] [Green Version]
- Rabe, E.; Regnier, C.; Goron, F.; Salmat, G.; Pannier, F. The prevalence, disease characteristics and treatment of chronic venous disease: An international web-based survey. J. Comp. Eff. Res. 2020, 9, 1205–1218. [Google Scholar] [CrossRef]
- Lurie, F.; Passman, M.; Meisner, M.; Dalsing, M.; Masuda, E.; Welch, H.; Bush, R.L.; Blebea, J.; Carpentier, P.H.; De Maeseneer, M.; et al. The 2020 update of the CEAP classification system and reporting standards. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 342–352. [Google Scholar] [CrossRef]
- Pouget, G.; Ducros, L.; Marcelon, G. Effect of Ruscus extract on pheripheral lymphatic vessel pressure and flow. In Return Circulation and Norepinephrine: An Update; Vanhoutte, P.M., Ed.; John Lybbey Eurotext: Paris, France, 1991; pp. 89–95. [Google Scholar]
- Bouaziz, N.; Michiels, C.; Janssens, D.; Berna, N. Effect of Ruscus extract and hesperidin methylchalcone on hypoxia-induced activation of endothelial cells. Int. Angiol. 1999, 18, 306. [Google Scholar]
- Pinho-Ribeiro, F.A.; Hohmann, M.S.; Borghi, S.M.; Zarpelon, A.C.; Guazelli, C.F.; Manchope, M.F.; Casagrande, R.; Verri, W.A., Jr. Protective effects of the flavonoid hesperidin methyl chalcone in inflammation and pain in mice: Role of TRPV1, oxidative stress, cytokines and NF-kappaB. Chem. Biol. Interact. 2015, 228, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Grosso, G.; Bei, R.; Mistretta, A.; Marventano, S.; Calabrese, G.; Masuelli, L.; Giganti, M.G.; Modesti, A.; Galvano, F.; Gazzolo, D. Effects of vitamin C on health: A review of evidence. Front. Biosci. 2013, 18, 1017–1029. [Google Scholar] [CrossRef]
- Kakkos, S.K.; Allaert, F.A. Efficacy of Ruscus extract, HMC and vitamin C, constituents of Cyclo 3 fort(R), on improving individual venous symptoms and edema: A systematic review and meta-analysis of randomized double-blind placebo-controlled trials. Int. Angiol. 2017, 36, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Florea, I.; Stoica, L.E.; Ţolea, I. Chronic venous insufficiency–Clinical-Evolutional Aspects. Chart 2011, 4, 5. [Google Scholar]
- Allaert, F.; Hugue, C.; Cazaubon, M.; Renaudin, J.; Clavel, T.; Escourrou, P. Correlation between improvement in functional signs and plethysmographic parameters during. Int. Angiol. 2011, 30, 272–277. [Google Scholar]
- De Oca Narváez, J.M.; Torres, V.M.A.; Peñaloza, R.M.M. Eficacia del FABROVEN en la sintomatología funcional de la insuficiencia venosa crónica de miembros inferiores. Rev. Mex. Angiol. 2007, 35, 70–77. [Google Scholar]
- Guex, J.J.; Avril, L.; Enrici, E.; Enriquez, E.; Lis, C.; Taieb, C. Quality of life improvement in Latin American patients suffering from chronic venous disorder using a combination of Ruscus aculeatus and hesperidin methyl-chalcone and ascorbic acid (quality study). Int. Angiol. 2010, 29, 525–532. [Google Scholar]
- Guex, J.J.; Enrici, E.; Boussetta, S.; Avril, L.; Lis, C.; Taieb, C. Correlations between ankle circumference, symptoms, and quality of life demonstrate the clinical relevance of minimal leg swelling reduction: Results of a study in 1036 Argentinean patients. Dermatol. Surg. 2008, 34, 1666–1675. [Google Scholar] [CrossRef]
- Guex, J.J.; Enriquez Vega, D.M.; Avril, L.; Boussetta, S.; Taieb, C. Assessment of quality of life in Mexican patients suffering from chronic venous disorder—Impact of oral Ruscus aculeatus-hesperidin-methyl-chalcone-ascorbic acid treatment—‘Quality Study’. Phlebology 2009, 24, 157–165. [Google Scholar] [CrossRef]
- Peralta, G.A.; Gardoqui, J.A.; Macías, F.L.; Ceja, V.N.; Cisneros, S.M.; Macías, C.M. Clinical and capillaroscopic evaluation in the treatment of chronic venous insufficiency with Ruscus aculeatus, hesperidin methylchalcone and ascorbic acid in venous insufficiency treatment of ambulatory patients. Int. Angiol. 2007, 26, 378. [Google Scholar]
- Parrado, F.; Buzzi, A. A study of the efficacy and tolerability of a preparation containing Ruscus aculeatus in the treatment of chronic venous insufficiency of the lower limbs. Clin. Drug Investig. 1999, 18, 255–261. [Google Scholar] [CrossRef]
| Description | |
|---|---|
| C0 | No visible or palpable signs of venous disease |
| C1 | Telangiectasias or reticular veins |
| C2 | Varicose veins |
| C2r | Recurrent varicose veins |
| C3 | Edema |
| C4 | Changes in skin and subcutaneous tissue secondary to CVD |
| C4a | Pigmentation or eczema |
| C4b | Lipodermatosclerosis or atrophie blanche |
| C4c | Corona phlebectatica |
| C5 | Healed venous ulcer |
| C6 | Active venous ulcer |
| C6r | Recurrent active venous ulcer |
| Reference | Country | Patients | N | Ruscus/HMC/Vit C Dose (Capsules/Day) | Duration | Effect on Edema |
|---|---|---|---|---|---|---|
| de Oca Narváes, et al. 2007 [48] | Mexico | CVI | 170 | 2 | 6 months | Proportion of patients with edema ↓ from 84% at baseline to 23% at study end |
| Peralta et al. 2007 [52] | Mexico | CVI | 124 | 2 | 12 weeks | Proportion of patients with edema ↓ from 82% at baseline to 0% at study end |
| Guex et al. 2008 [50] | Argentina | CVD (CEAP class C0 to C3) | 1036 | 3 | 12 weeks | Mean ankle circumference ↓ by 21 mm from baseline (p < 0.001) |
| Guex et al. 2009 [51] | Mexico | CVD (CEAP class C0 to C3) | 917 | 2 | 12 weeks | Mean ankle circumference ↓ from 247.8 mm at baseline to 234.64 mm at week 12 (p < 0.001) |
| Guex et al. 2010 [49] | Mexico and Argentina | CVD (CEAP class C0s to C3) | 1953 | 2 | 12 weeks | Sum of left and right mean ankle circumference ↓ from 509.4 mm at baseline to 488.1 at week 12 (p < 0.001) |
| Allaert et al. 2011 [47] | France | CVD (CEAP class C2s to C3s) | 65 | 3 | 28 days | Overall frequency of edema ↓ from 88% at baseline to 60% on day 28, and evening edema ↓ from 72% at baseline to 52% on day 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bihari, I.; Guex, J.-J.; Jawien, A.; Szolnoky, G. Clinical Perspectives and Management of Edema in Chronic Venous Disease—What about Ruscus? Medicines 2022, 9, 41. https://doi.org/10.3390/medicines9080041
Bihari I, Guex J-J, Jawien A, Szolnoky G. Clinical Perspectives and Management of Edema in Chronic Venous Disease—What about Ruscus? Medicines. 2022; 9(8):41. https://doi.org/10.3390/medicines9080041
Chicago/Turabian StyleBihari, Imre, Jean-Jérôme Guex, Arkadiusz Jawien, and Gyozo Szolnoky. 2022. "Clinical Perspectives and Management of Edema in Chronic Venous Disease—What about Ruscus?" Medicines 9, no. 8: 41. https://doi.org/10.3390/medicines9080041
APA StyleBihari, I., Guex, J.-J., Jawien, A., & Szolnoky, G. (2022). Clinical Perspectives and Management of Edema in Chronic Venous Disease—What about Ruscus? Medicines, 9(8), 41. https://doi.org/10.3390/medicines9080041





