Leptomeningeal Carcinomatosis in a Patient with Pancreatic Cancer: A Rare Phenomenon?
Abstract
:1. Introduction
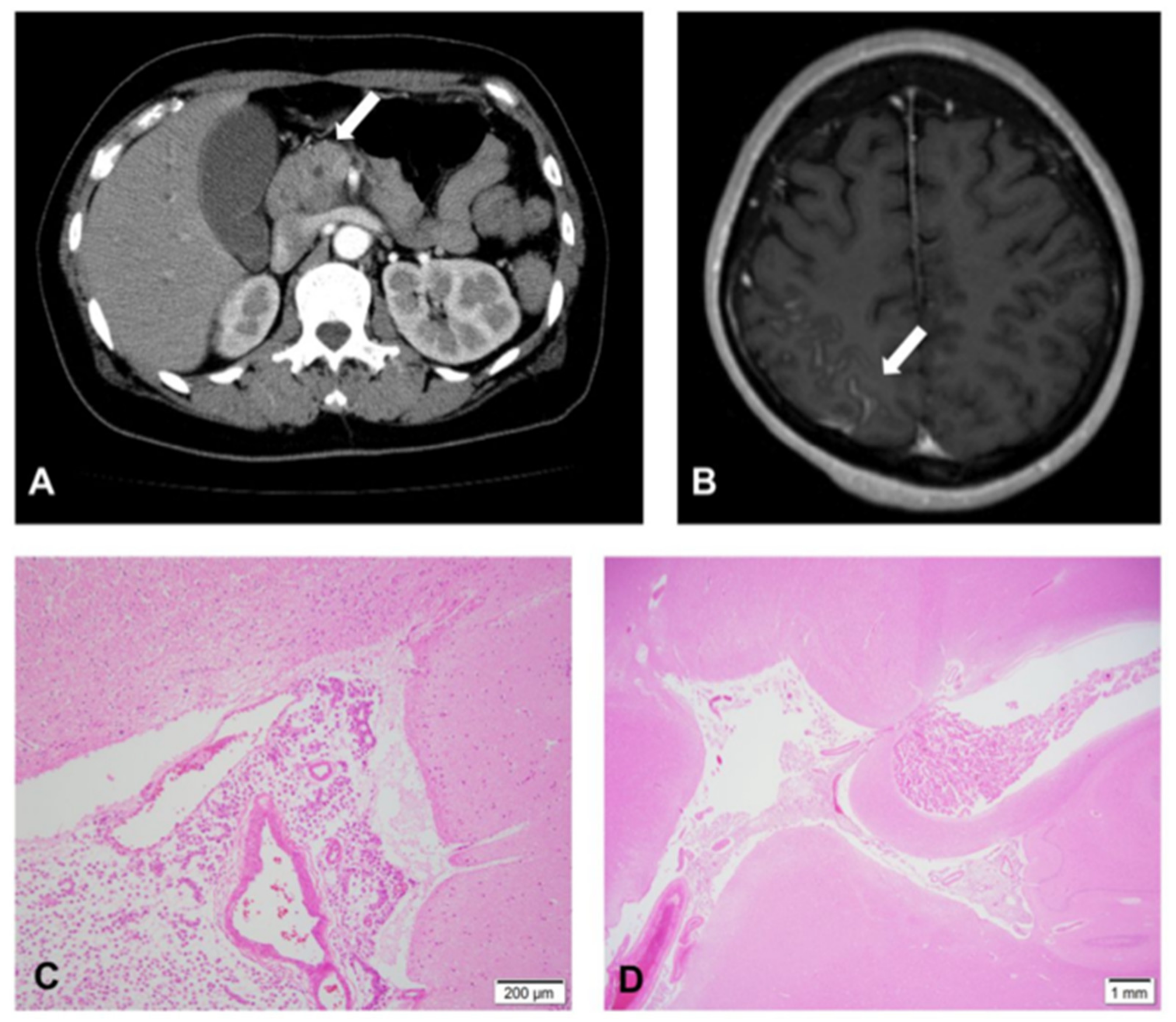
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; De La Fouchardière, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, J.G.; DeSouza, T.G.; Farkash, A.; Shafran, B.; Pack, D.; Rehman, F.; Fuks, J.; Portenoy, R. Leptomeningeal metastases: Comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemias. J. Neuro-Oncol. 1990, 9, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Anne, M.; Ahmad, N.; Lee, P.; Aziz, M.; Lebowicz, Y. An Unusual Presentation of Isolated Leptomeningeal Disease in Carcinoma of Unknown Primary with Pancreatic Features. J. Investig. Med. High Impact Case Rep. 2013, 18, 2324709613494830. [Google Scholar] [CrossRef]
- Brower, J.V.; Saha, S.; Rosenberg, S.A.; Hullett, C.R.; Robins, H.I. Management of leptomeningeal metastases: Prognostic factors and associated outcomes. J. Clin. Neurosci. 2016, 27, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Yoo, I.K.; Lee, H.S.; Kim, C.D.; Chun, H.J.; Jeen, Y.T.; Keum, B.; Kim, E.S.; Choi, H.S.; Lee, J.M.; Kim, S.H.; et al. Rare case of pancreatic cancer with leptomeningeal carcinomatosis. World J. Gastroenterol. 2015, 21, 1020–1023. [Google Scholar] [CrossRef]
- Trinh, V.T.; Medina-Flores, R.; Chohan, M.O. Leptomeningeal carcinomatosis as primary manifestation of pancreatic cancer. J. Clin. Neurosci. 2016, 30, 124–127. [Google Scholar] [CrossRef]
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014, 371, 1039–1049. [Google Scholar] [CrossRef]
- Hidalgo, M. Pancreatic cancer. N. Engl. J. Med. 2010, 362, 1605–1617. [Google Scholar] [CrossRef] [Green Version]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, K.; Okusaka, T.; Shimizu, K.; Furuse, J.; Ito, Y.; Hanada, K.; Shimosegawa, T.; Yamaguchi, K.; Okusaka, T.; Shimizu, K.; et al. EBM-based Clinical Guidelines for Pancreatic Cancer (2013) issued by the Japan Pancreas Society: A synopsis. Jpn. J. Clin. Oncol. 2014, 44, 883–888. [Google Scholar] [CrossRef] [Green Version]
- Ceccon, G.; Wollring, M.; Brunn, A.; Deckert, M.; Waldschmidt, D.; Fink, G.R.; Galldiks, N. Leptomeningeal Carcinomatosis in a Patient with Pancreatic Cancer Responding to Nab-Paclitaxel plus Gemcitabine. Case Rep. Oncol. 2020, 13, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.S.; Kurt, H.; Elder, J.B. Asynchronous leptomeningeal carcinomatosis from pancreatic cancer: A case report and review of the literature. Clin. J. Gastroenterol. 2014, 7, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Filho, A.F.; Cardoso, F.; Di Leo, A.; Awada, A.; da Silva, V.D.; Tovar, R.B.; Schwartsmann, G. Carcinomatous meningitis as a clinical manifestation of pancreatic carcinoma. Ann. Oncol. 2001, 12, 1757–1759. [Google Scholar] [CrossRef] [PubMed]
- Kurzaj, E.; Kopczynski, S.; Barowska-Lehman, J.; Ludwiczak, R. Subdural haematoma associated with dural carcinomatosis in a patient with primary carcinoma of pancreas. Neurochirurgia 1980, 23, 13–17. [Google Scholar] [CrossRef]
- Mack, F.; Baumert, B.; Schäfer, N.; Hattingen, E.; Scheffler, B.; Herrlinger, U.; Glas, M. Therapy of leptomeningeal metastasis in solid tumors. Cancer Treat. Rev. 2016, 43, 83–91. [Google Scholar] [CrossRef]
- Sandberg, D.I.; Bilsky, M.H.; Souweidane, M.M.; Bzdil, J.; Gutin, P.H. Ommaya reservoirs for the treatment of leptomeningeal metastases. Neurosurgery 2000, 47, 49–54, discussion 54–55. [Google Scholar]
- Yagi, Y.; Nishimura, Y.; Nakatsugawa, S.; Fukuoka, T.; Hirota, M.; Okamoto, K.; Sato, T.; Ichihara, T. A case of meningeal carcinomatosis from pancreatic cancer during chemotherapy using gemcitabine. Jpn. J. Gastoenterol. Surg. 2006, 39, 1683–1688. [Google Scholar] [CrossRef]
- Hirota, M.; Yagi, Y.; Yamashita, K.; Okamoto, K.; Sato, T.; Ichihara, T. A long survival case of unresectable pancreatic cancer by chemoradiotherapy with gemcitabine as key drug. Cancer Chemother. 2008, 35, 2413–2416. [Google Scholar]
- Minchom, A.; Chan, S.; Melia, W.; Shah, R. An unusual case of pancreatic cancer with leptomeningeal infiltration. J. Gastrointest. Cancer 2010, 41, 107–109. [Google Scholar] [CrossRef]
- Blows, S.J.; Morgan, R.; Dhariwal, U.; Petts, G.; Roncaroli, F. Pancreatic adenocarcinoma presenting with sudden onset bilateral deafness secondary to metastatic leptomeningeal infiltration. Age Ageing 2012, 41, 818–819. [Google Scholar] [CrossRef] [Green Version]
- Rao, R.; Sadashiv, S.K.; Goday, S.; Monga, D. An extremely rare case of pancreatic cancer presenting with leptomeningeal carcinomatosis and synchronous intraparenchymal brain metastasis. Gastrointest. Cancer Res. 2013, 6, 90–92. [Google Scholar] [PubMed]
- Johnson, W.R.; Theeler, B.J.; Van Echo, D.; Young, P.; Kwok, M. Treatment of Leptomeningeal Carcinomatosis in a Patient with Metastatic Pancreatic Cancer: A Case Report and Review of the Literature. Case Rep. Oncol. 2018, 11, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Grossman, S.A.; Krabak, M.J. Leptomeningeal carcinomatosis. Cancer Treat. Rev. 1999, 25, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.; Le Rhun, E.; Taillibert, S. Carcinomatous meningitis: Leptomeningeal metastases in solid tumors. Surg. Neurol. Int. 2013, 4 (Suppl. 4), S265–S288. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, N. The diagnostic and therapeutic management of leptomeningeal carcinomatosis. Ann. Oncol. 2004, 15 (Suppl. 4), iv285–iv291. [Google Scholar] [CrossRef] [PubMed]
- Bendell, J.C.; Domchek, S.M.; Burstein, H.J.; Harris, L.; Younger, J.; Kuter, I.; Bunnel, C.; Rue, M.; Gelman, R.; Winer, E. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 2003, 97, 2972–2977. [Google Scholar] [CrossRef]
- Groves, M.D. Leptomeningeal disease. Neurosurg. Clin. 2011, 22, 67–78. [Google Scholar] [CrossRef]
 | |||||
|---|---|---|---|---|---|
| Time from Diagnosis | 0 Months | 15 Months | 19 Months | 21 Months | 24 Months |
| Events | Diagnosis | 1st recurrence | 2nd recurrence | 3rd recurrence | Before Death |
| T-Bil (mg/dL) | 4.3 | 1.0 | 0.60 | 0.40 | 0.20 |
| AST (U/L) | 394 | 36 | 30 | 19 | 54 |
| ALT (U/L) | 630 | 37 | 33 | 22 | 62 |
| CEA (ng/mL) | 3.0 | 9.6 | 12.4 | 135.2 | 20.4 |
| CA19-9 (kU/mL) | 269 | 460 | 13,240 | 50,000 | 16,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayanagi, T.; Ohishi, Y.; Katayama, M.; Tamura, R. Leptomeningeal Carcinomatosis in a Patient with Pancreatic Cancer: A Rare Phenomenon? Medicines 2022, 9, 39. https://doi.org/10.3390/medicines9070039
Sayanagi T, Ohishi Y, Katayama M, Tamura R. Leptomeningeal Carcinomatosis in a Patient with Pancreatic Cancer: A Rare Phenomenon? Medicines. 2022; 9(7):39. https://doi.org/10.3390/medicines9070039
Chicago/Turabian StyleSayanagi, Taichi, Yumiko Ohishi, Makoto Katayama, and Ryota Tamura. 2022. "Leptomeningeal Carcinomatosis in a Patient with Pancreatic Cancer: A Rare Phenomenon?" Medicines 9, no. 7: 39. https://doi.org/10.3390/medicines9070039
APA StyleSayanagi, T., Ohishi, Y., Katayama, M., & Tamura, R. (2022). Leptomeningeal Carcinomatosis in a Patient with Pancreatic Cancer: A Rare Phenomenon? Medicines, 9(7), 39. https://doi.org/10.3390/medicines9070039






