JUUL™ing and Heating Lead to a Worsening of Arterial Stiffness
Abstract
1. Introduction
2. Materials and Methods
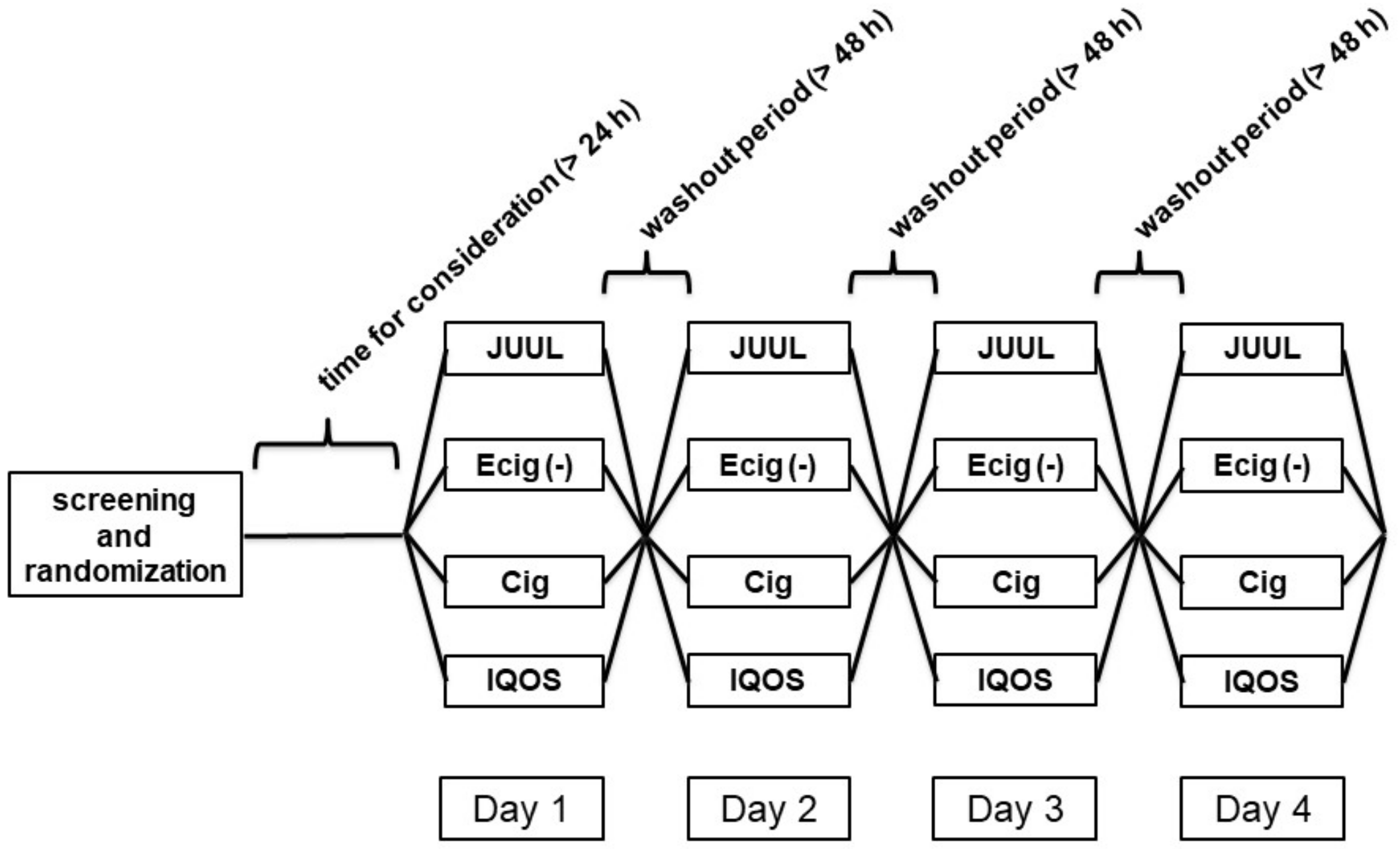
2.1. Study and Cohort Design
2.2. Measurement of Endothelial Vasodilator Function and Arterial Stiffness
2.3. Power Calculation and Statistical Analyses
3. Results
3.1. Study Design and Baseline Characteristics
3.2. Cigarette, HTP, and E-Cigarette Increased CO in Exhalation
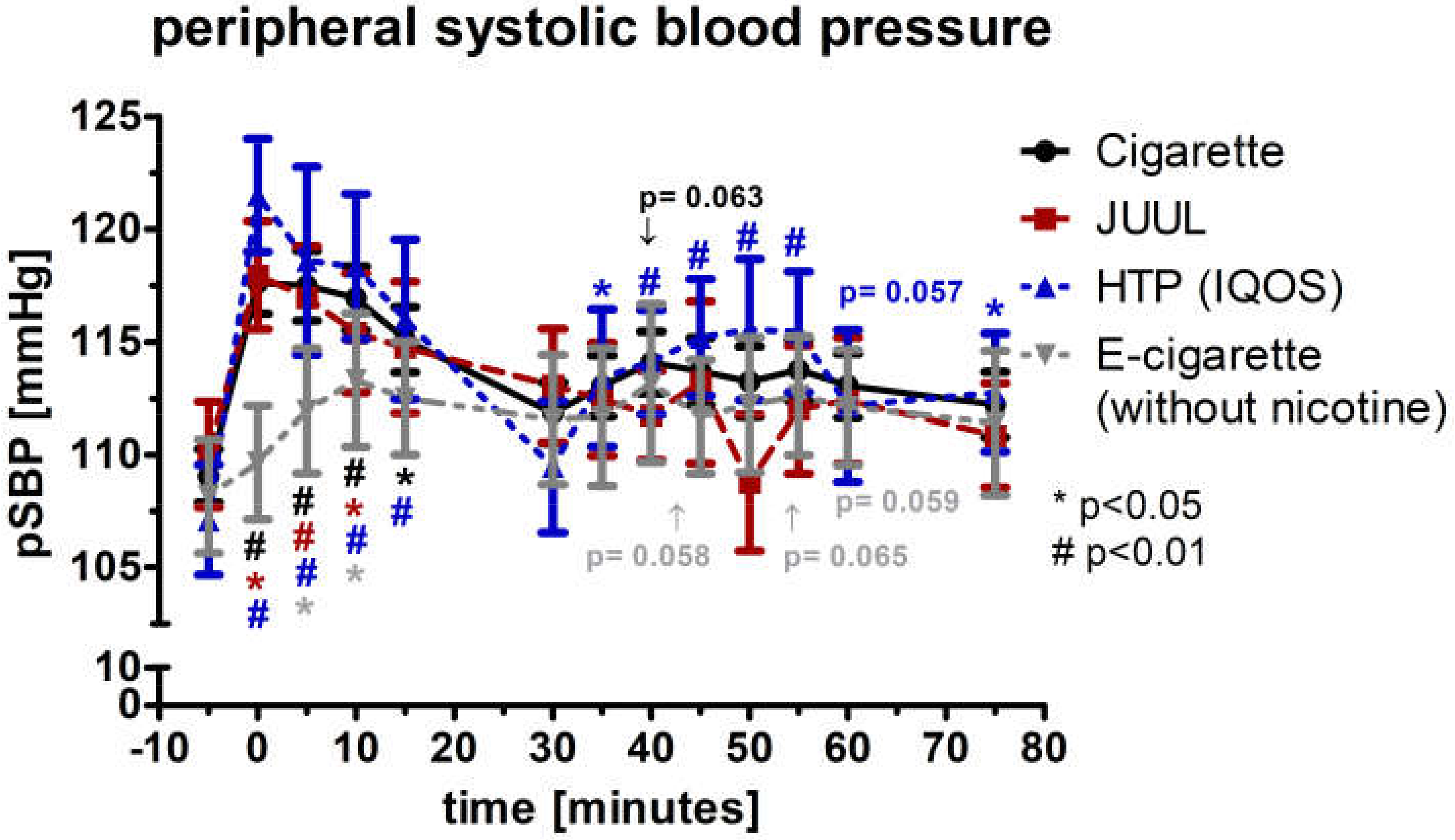
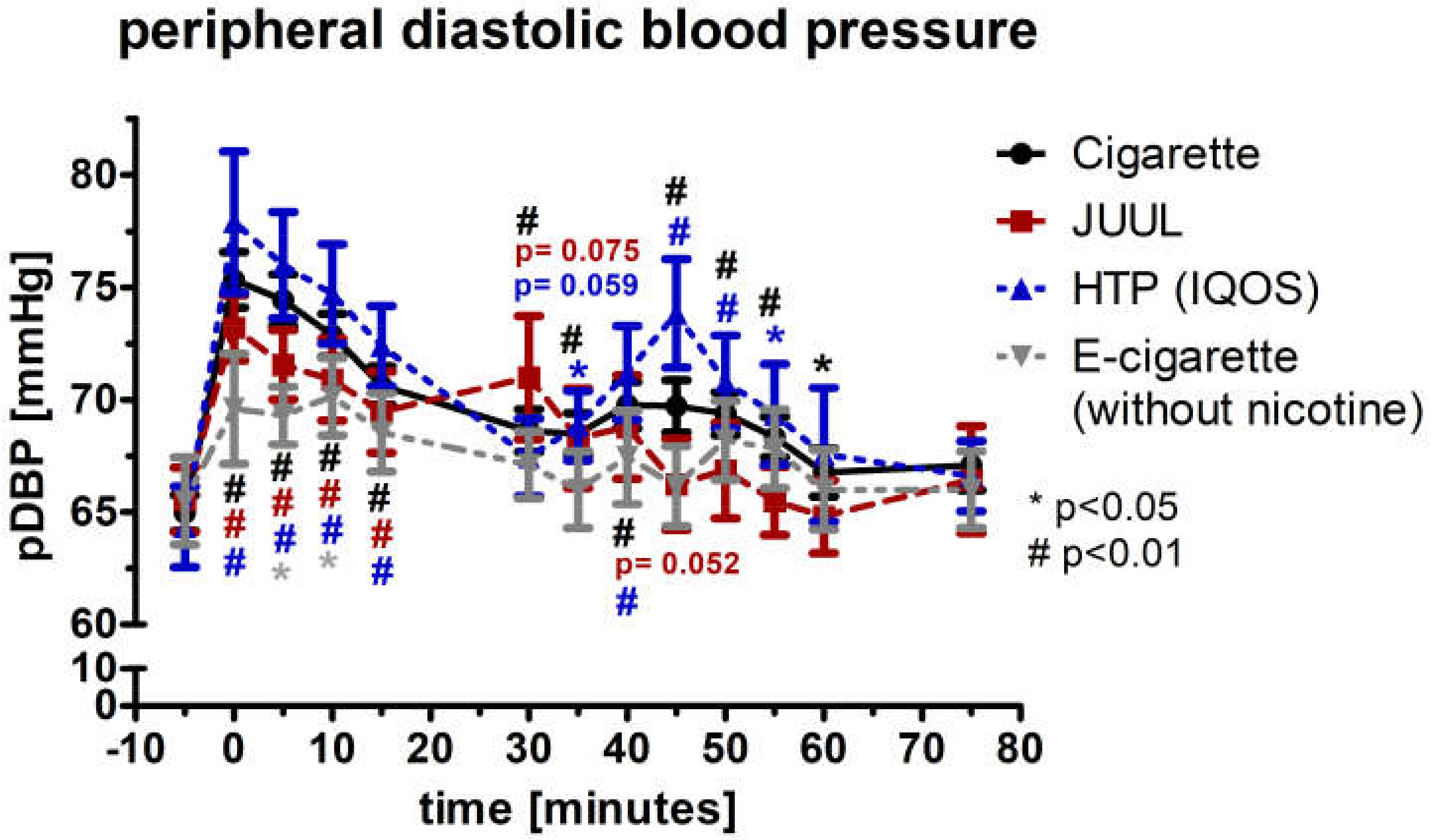
3.3. Peripheral Systolic Blood Pressure and Diastolic Blood Pressure
3.4. Increase in the Heart Rate in the Groups of Cigarette, JUUL™, and HTP
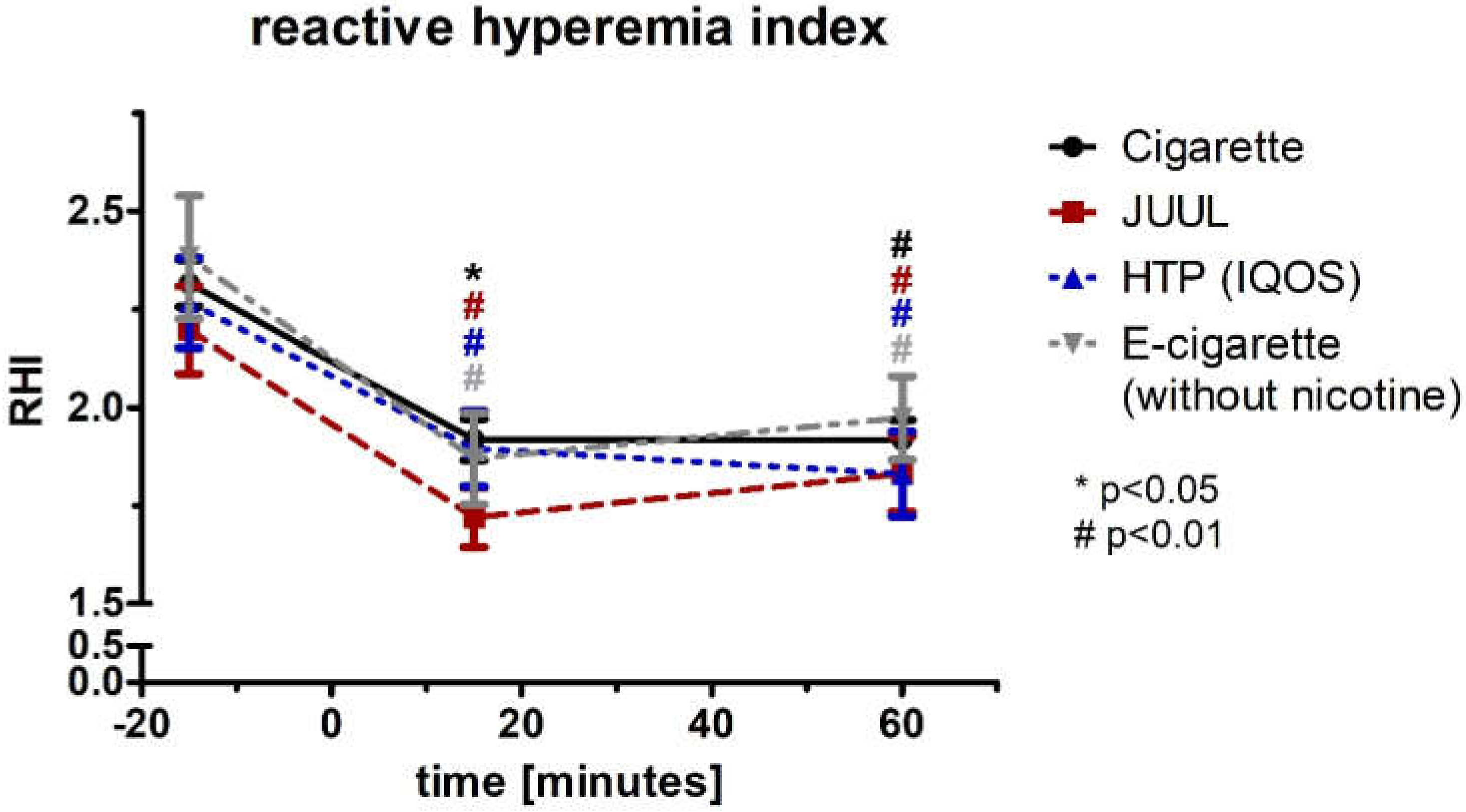
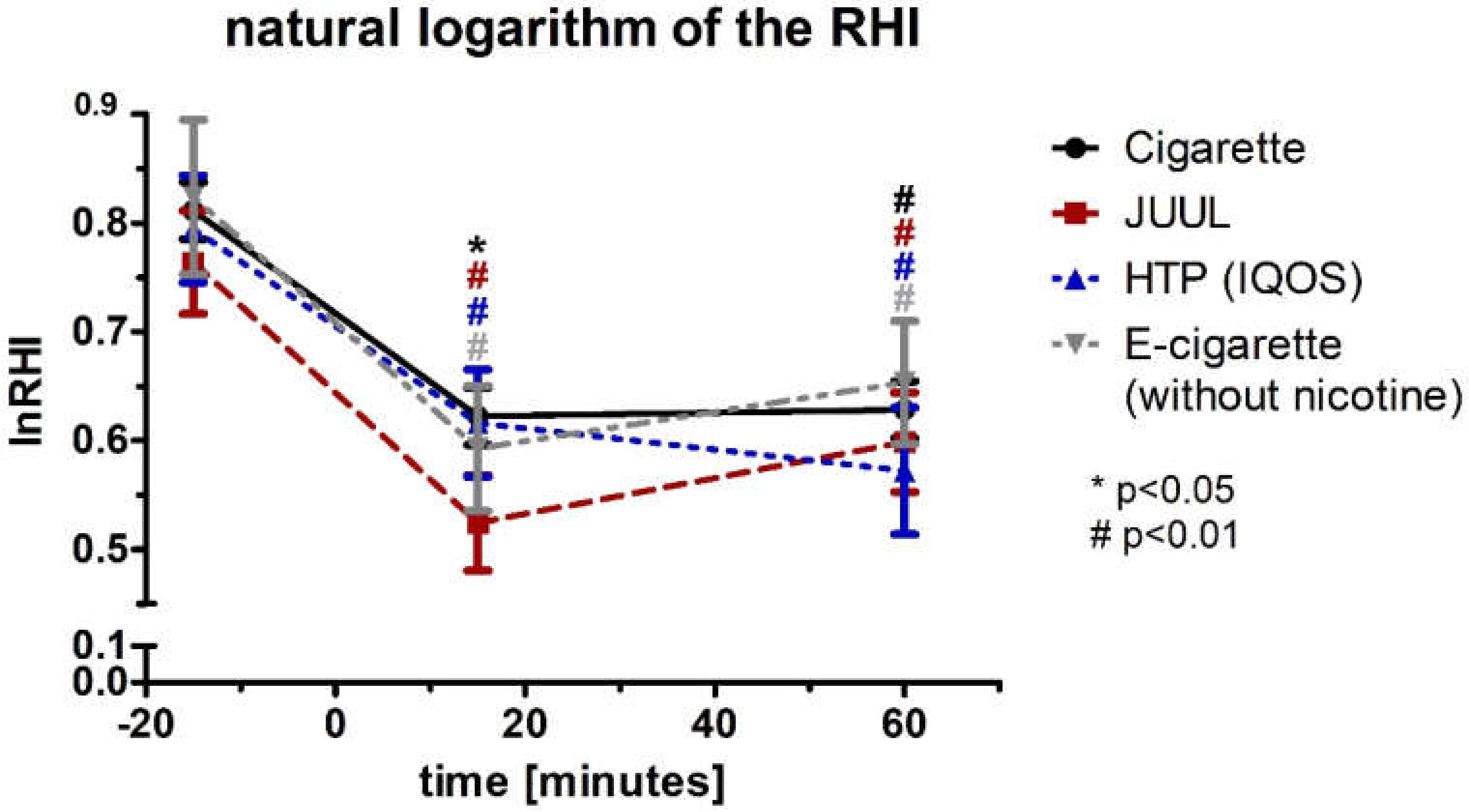
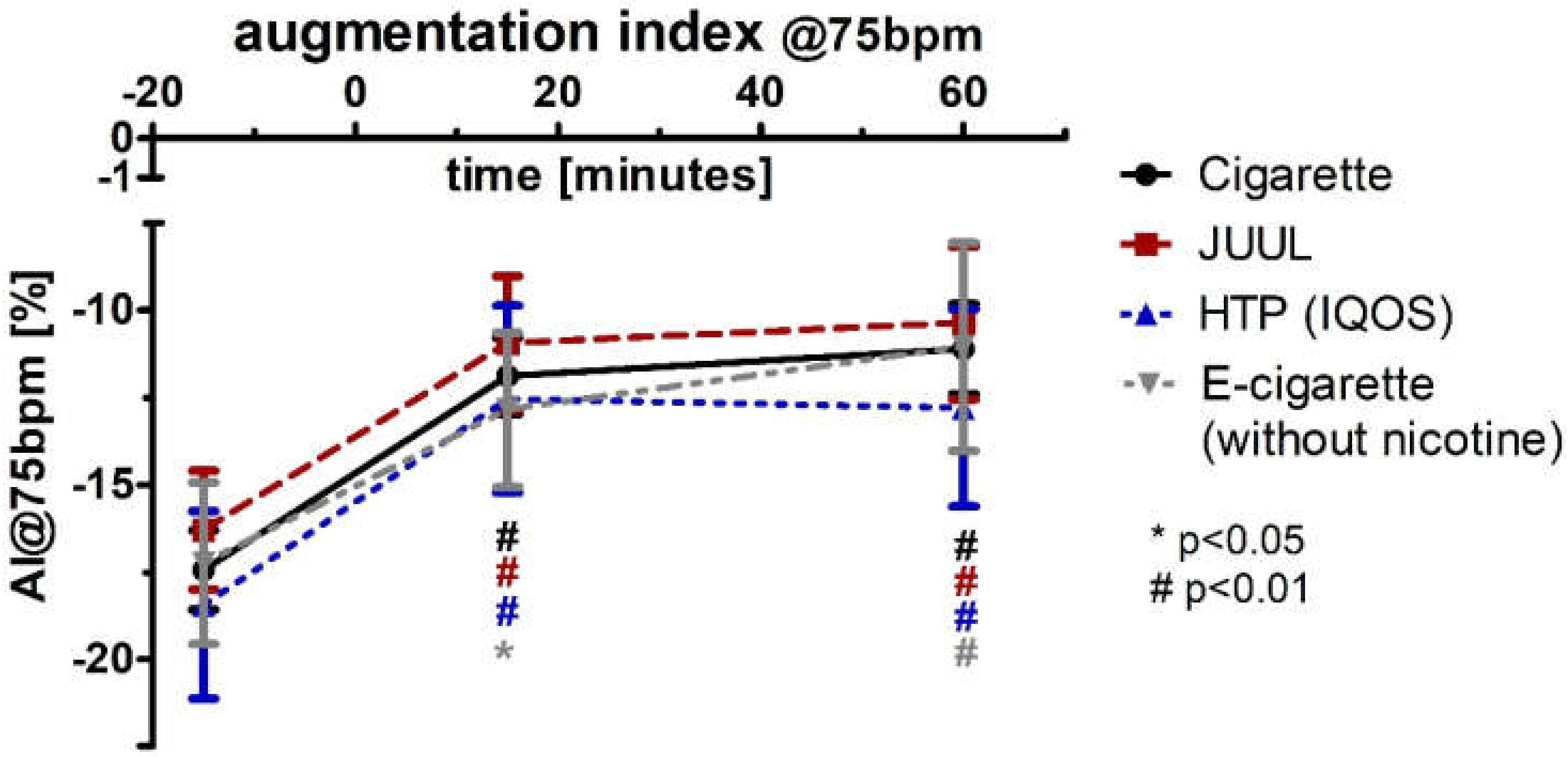
3.5. Aggravation of the Augmentation Index and Reactive Hyperemia Index after Using Any Type of Device
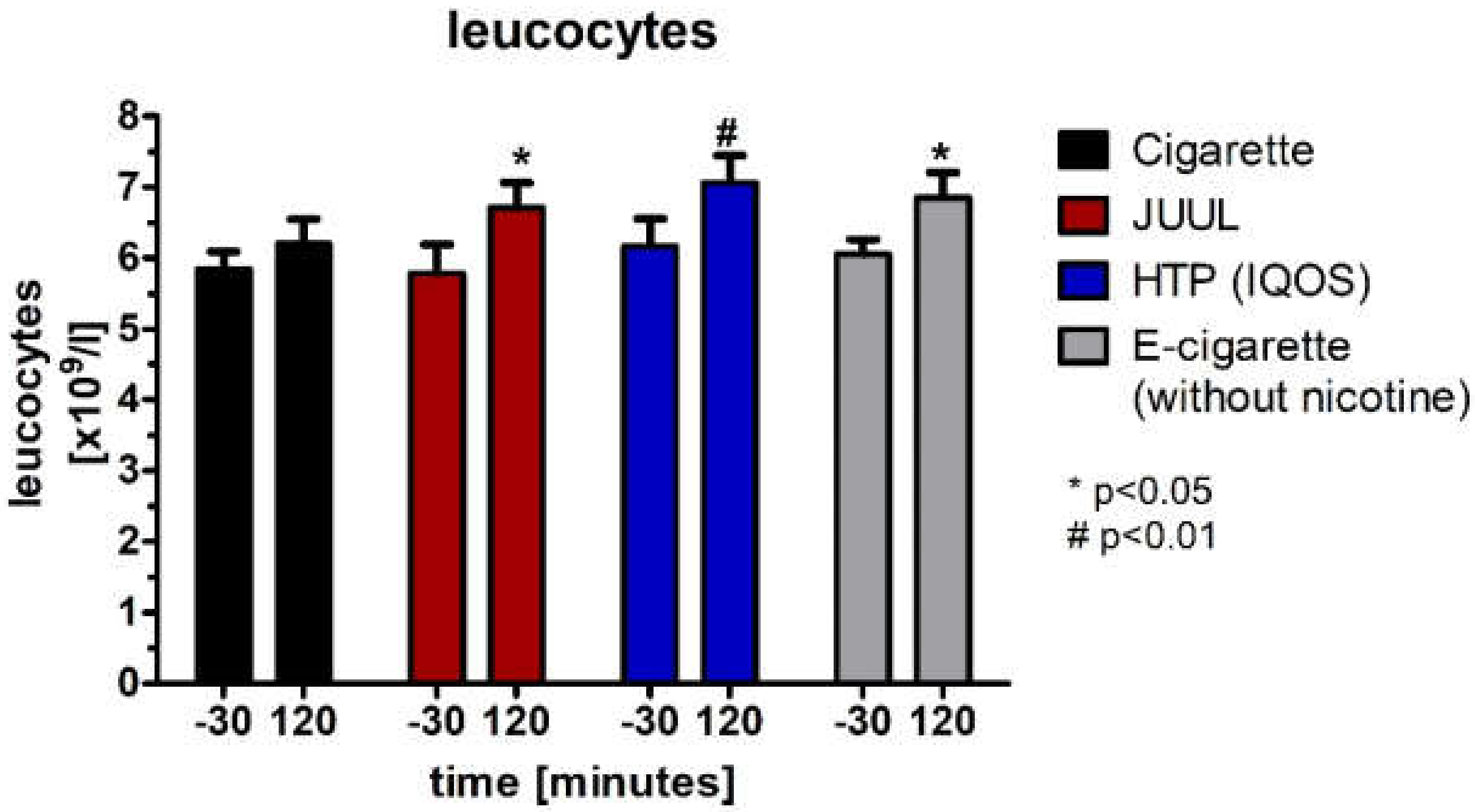
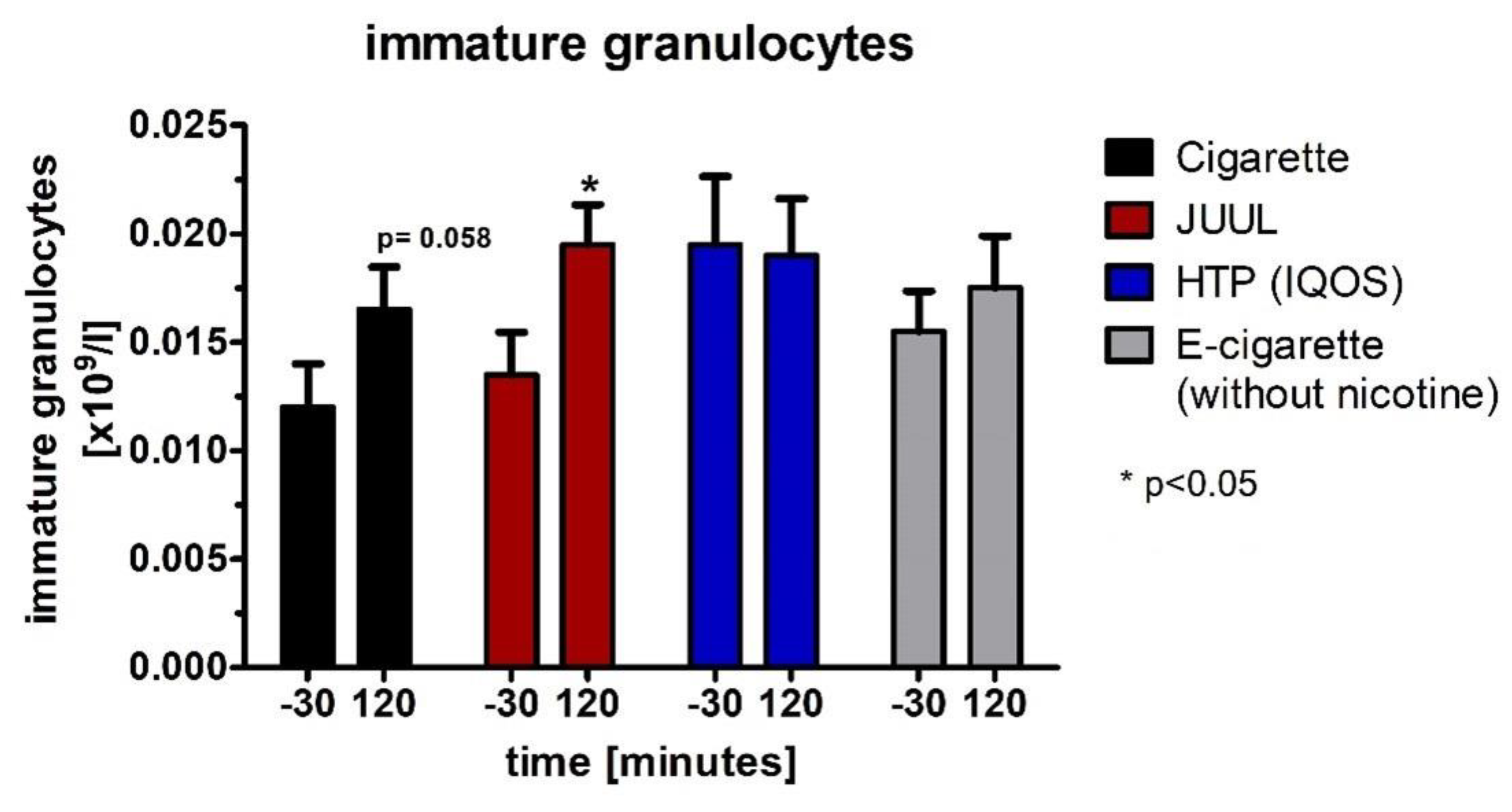
3.6. Decrease in Plasma Cortisol Concentration and Changes within the Hemogram after Using the Devices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hammond, D.; Wackowski, O.A.; Reid, J.L.; O’Connor, R.J. Use of JUUL E-cigarettes Among Youth in the United States. Nicotine Tob. Res. 2020, 22, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Nielsen. Tobacco “All Channel” Data. Equity Research; Wells Fargo Securities: San Francisco, CA, USA, 2018. [Google Scholar]
- Cullen, K.A.; Ambrose, B.K.; Gentzke, A.S.; Apelberg, B.J.; Jamal, A.; King, B.A. Notes from the Field: Use of Electronic Cigarettes and Any Tobacco Product Among Middle and High School Students—United States, 2011–2018. MMWR Morb. Mortal Wkly. Rep. 2018, 67, 1276–1277. [Google Scholar] [CrossRef] [PubMed]
- Fadus, M.C.; Smith, T.T.; Squeglia, L.M. The rise of e-cigarettes, pod mod devices, and JUUL among youth: Factors influencing use, health implications, and downstream effects. Drug Alcohol Depend. 2019, 201, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Willett, J.G.; Bennett, M.; Hair, E.C.; Xiao, H.; Greenberg, M.S.; Harvey, E.; Cantrell, J.; Vallone, D. Recognition, use and perceptions of JUUL among youth and young adults. Tob. Control 2019, 28, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Duan, Z.; Kwok, J.; Binns, S.; Vera, L.E.; Kim, Y.; Szczypka, G.; Emery, S.L. Vaping versus JUULing: How the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob. Control 2019, 28, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Parliament, E. Directive 2014/40/EU of the European Parliament and of the Council. Available online: https://ec.europa.eu/health/tobacco/docs/dir_201440_en.pdf (accessed on 1 February 2022).
- Duell, A.K.; Pankow, J.F.; Peyton, D.H. Free-Base Nicotine Determination in Electronic Cigarette Liquids by (1)H NMR Spectroscopy. Chem. Res. Toxicol. 2018, 31, 431–434. [Google Scholar] [CrossRef]
- Ghosh, A.; Beyazcicek, O.; Davis, E.S.; Onyenwoke, R.U.; Tarran, R. Cellular effects of nicotine salt-containing e-liquids. J. Appl. Toxicol. 2021, 41, 493–505. [Google Scholar] [CrossRef]
- NIDA. References. National Institute on Drug Abuse Website. 3 August 2021. Available online: https://nida.nih.gov/publications/research-reports/tobacco-nicotine-e-cigarettes/references (accessed on 5 April 2022).
- Initiative, T. Behind the Explosive Growth of JUUL. Available online: https://truthinitiative.org/research-resources/emerging-tobacco-products/behind-explosive-growth-juul (accessed on 1 February 2022).
- Walley, S.C.; Wilson, K.M.; Winickoff, J.P.; Groner, J. A Public Health Crisis: Electronic Cigarettes, Vape, and JUUL. Pediatrics 2019, 143, e20182741. [Google Scholar] [CrossRef]
- Blount, B.C.; Karwowski, M.P.; Shields, P.G.; Morel-Espinosa, M.; Valentin-Blasini, L.; Gardner, M.; Braselton, M.; Brosius, C.R.; Caron, K.T.; Chambers, D.; et al. Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N. Engl. J. Med. 2019, 382, 697–705. [Google Scholar] [CrossRef]
- Schier, J.G.; Meiman, J.G.; Layden, J.; Mikosz, C.A.; VanFrank, B.; King, B.A.; Salvatore, P.P.; Weissman, D.N.; Thomas, J.; Melstrom, P.C.; et al. CDC 2019 Lung Injury Response Group. Severe Pulmonary Disease Associated with Electronic-Cigarette-Product Use—Interim Guidance. MMWR Morb. Mortal Wkly. Rep. 2019, 68, 787–790. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Newly Signed Legislation Raises Federal Minimum Age of Sale of Tobacco Products to 21. Available online: https://www.fda.gov/tobacco-products/ctp-newsroom/newly-signed-legislation-raises-federal-minimum-age-sale-tobacco-products-21 (accessed on 11 February 2022).
- Administration, Center of Disease Control (CDC). Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products. Available online: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html (accessed on 8 February 2022).
- Xie, W.; Tackett, A.P.; Berlowitz, J.B.; Harlow, A.F.; Kathuria, H.; Galiatsatos, P.; Fetterman, J.L.; Cho, J.; Blaha, M.J.; Hamburg, N.M.; et al. Association of Electronic Cigarette Use with Respiratory Symptom Development among US Young Adults. Am. J. Respir. Crit. Care Med. 2020, 58, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Bals, R.; Boyd, J.; Esposito, S.; Foronjy, R.; Hiemstra, P.S.; Jimenez-Ruiz, C.A.; Katsaounou, P.; Lindberg, A.; Metz, C.; Schober, W.; et al. Electronic cigarettes: A task force report from the European Respiratory Society. Eur. Respir. J. 2019, 53, 1801151. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, R.; Kaldewai, D.; Lindemann, F.; Merfert-Diete, C. (Eds.) Jahrbuch Sucht 2009; Neuland Verlagsgesellschaft: Hamm, Germany, 2009. [Google Scholar]
- Hutzler, C.; Paschke, M.; Kruschinski, S.; Henkler, F.; Hahn, J.; Luch, A. Chemical hazards present in liquids and vapors of electronic cigarettes. Arch. Toxicol. 2014, 88, 1295–1308. [Google Scholar] [CrossRef] [PubMed]
- Goniewicz, M.L.; Smith, D.M.; Edwards, K.C.; Blount, B.C.; Caldwell, K.L.; Feng, J.; Wang, L.; Christensen, C.; Ambrose, B.; Borek, N.; et al. Comparison of Nicotine and Toxicant Exposure in Users of Electronic Cigarettes and Combustible Cigarettes. JAMA Netw. Open 2018, 1, e185937. [Google Scholar] [CrossRef]
- Kalkhoran, S.; Glantz, S.A. E-cigarettes and smoking cessation in real-world and clinical settings: A systematic review and meta-analysis. Lancet Respir. Med. 2016, 4, 116–128. [Google Scholar] [CrossRef]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Bohm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood Press 2014, 23, 3–16. [Google Scholar] [CrossRef]
- Linton, M.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; Vickers, K.C. The Role of Lipids and Lipoproteins in Atherosclerosis; Feingold, K.R., Anawalt, B., Boyce, A., et al., Eds.; Endotext [Internet]: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Sassalos, K.; Vlachopoulos, C.; Alexopoulos, N.; Gialernios, T.; Aznaouridis, K.; Stefanadis, C. The acute and chronic effect of cigarette smoking on the elastic properties of the ascending aorta in healthy male subjects. Hellenic. J. Cardiol. 2006, 47, 263–268. [Google Scholar]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.M.; Kjeldsen, S.E.; Laurent, S.; et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2007, 25, 1105–1187. [Google Scholar] [CrossRef]
- Moerland, M.; Kales, A.J.; Schrier, L.; van Dongen, M.G.; Bradnock, D.; Burggraaf, J. Evaluation of the EndoPAT as a Tool to Assess Endothelial Function. Int. J. Vasc. Med. 2012, 2012, 904141. [Google Scholar] [CrossRef]
- Franzen, K.F.; Willig, J.; Cayo Talavera, S.; Meusel, M.; Sayk, F.; Reppel, M.; Dalhoff, K.; Mortensen, K.; Droemann, D. E-cigarettes and cigarettes worsen peripheral and central hemodynamics as well as arterial stiffness: A randomized, double-blinded pilot study. Vasc. Med. 2018, 23, 419–425. [Google Scholar] [CrossRef]
- Antoniewicz, L.; Brynedal, A.; Hedman, L.; Lundbäck, M.; Bosson, J.A. Acute Effects of Electronic Cigarette Inhalation on the Vasculature and the Conducting Airways. Cardiovasc. Toxicol. 2019, 19, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, T.; Pena, I.; Temesgen, N.; Glantz, S.A. Association Between Electronic Cigarette Use and Myocardial Infarction. Am. J. Prev. Med. 2018, 55, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Osei, A.D.; Mirbolouk, M.; Orimoloye, O.A.; Dzaye, O.; Uddin, S.M.I.; Dardari, Z.A.; DeFilippis, A.P.; Bhatnagar, A.; Blaha, M.J. The association between e-cigarette use and asthma among never combustible cigarette smokers: Behavioral risk factor surveillance system (BRFSS) 2016 & 2017. BMC Pulm. Med. 2019, 19, 180. [Google Scholar] [CrossRef]
- Mallock, N.; Trieu, H.L.; Macziol, M.; Malke, S.; Katz, A.; Laux, P.; Henkler-Stephani, F.; Hahn, J.; Hutzler, C.; Luch, A. Trendy e-cigarettes enter Europe: Chemical characterization of JUUL pods and its aerosols. Arch. Toxicol. 2020, 94, 1985–1994. [Google Scholar] [CrossRef]
- Franzen, K.F.; Belkin, S.; Goldmann, T.; Reppel, M.; Watz, H.; Mortensen, K.; Droemann, D. The impact of heated tobacco products on arterial stiffness. Vasc. Med. 2020, 25, 572–574. [Google Scholar] [CrossRef]
- D’Amario, D.; Migliaro, S.; Borovac, J.A.; Vergallo, R.; Galli, M.; Restivo, A.; Bonini, M.; Romagnoli, E.; Leone, A.M.; Crea, F. Electronic Cigarettes and Cardiovascular Risk: Caution Waiting for Evidence. Eur. Cardiol. 2019, 14, 151–158. [Google Scholar] [CrossRef]
- Lee, W.H.; Ong, S.G.; Zhou, Y.; Tian, L.; Bae, H.R.; Baker, N.; Whitlatch, A.; Mohammadi, L.; Guo, H.; Nadeau, K.C.; et al. Modeling Cardiovascular Risks of E-Cigarettes With Human-Induced Pluripotent Stem Cell-Derived Endothelial Cells. J. Am. Coll. Cardiol. 2019, 73, 2722–2737. [Google Scholar] [CrossRef]
- Van Bortel, L.M.; Duprez, D.; Starmans-Kool, M.J.; Safar, M.E.; Giannattasio, C.; Cockcroft, J.; Kaiser, D.R.; Thuillez, C. Clinical applications of arterial stiffness, Task Force III: Recommendations for user procedures. Am. J. Hypertens. 2002, 15, 445–452. [Google Scholar] [CrossRef]
- Agabiti-Rosei, E.; Mancia, G.; O’Rourke, M.F.; Roman, M.J.; Safar, M.E.; Smulyan, H.; Wang, J.G.; Wilkinson, I.B.; Williams, B.; Vlachopoulos, C. Central blood pressure measurements and antihypertensive therapy: A consensus document. Hypertension 2007, 50, 154–160. [Google Scholar] [CrossRef]
- Muthumalage, T.; Lamb, T.; Friedman, M.R.; Rahman, I. E-cigarette flavored pods induce inflammation, epithelial barrier dysfunction, and DNA damage in lung epithelial cells and monocytes. Sci. Rep. 2019, 9, 19035. [Google Scholar] [CrossRef]
- Winther, K.; Knudsen, J.B.; Gormsen, J.; Jensen, J. Effect of metoprolol and propranolol on platelet aggregation and cAMP level in hypertensive patients. Eur. J. Clin. Pharmacol. 1986, 29, 561–564. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Heated Tobacco Products (HTPs) Information Sheet—2nd Edition. Available online: https://www.who.int/publications/i/item/WHO-HEP-HPR-2020.2 (accessed on 8 February 2022).
- Farsalinos, K.E.; Romagna, G.; Tsiapras, D.; Kyrzopoulos, S.; Voudris, V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: Implications for research protocol standards definition and for public health authorities’ regulation. Int. J. Environ. Res. Public Health 2013, 10, 2500–2514. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, J.H.; Goletiani, N.; Sholar, M.B.; Siegel, A.J.; Mello, N.K. Effects of smoking successive low- and high-nicotine cigarettes on hypothalamic-pituitary-adrenal axis hormones and mood in men. Neuropsychopharmacology 2008, 33, 749–760. [Google Scholar] [CrossRef]
- Kuvin, J.T.; Patel, A.R.; Sliney, K.A.; Pandian, N.G.; Sheffy, J.; Schnall, R.P.; Karas, R.H.; Udelson, J.E. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am. Heart J. 2003, 146, 168–174. [Google Scholar] [CrossRef]
- Adamopoulos, D.; Argacha, J.F.; Gujic, M.; Preumont, N.; Degaute, J.P.; van de Borne, P. Acute effects of nicotine on arterial stiffness and wave reflection in healthy young non-smokers. Clin. Exp. Pharmacol. Physiol. 2009, 36, 784–789. [Google Scholar] [CrossRef]
- Hong, T.; Wu, J.; Wijaya, D.; Xuan, Z.; Fetterman, J.L. JUUL the heartbreaker: Twitter analysis of cardiovascular health perceptions of vaping. Tob. Induc. Dis. 2021, 19, 1–6. [Google Scholar] [CrossRef]
- Rhee, M.Y.; Na, S.H.; Kim, Y.K.; Lee, M.M.; Kim, H.Y. Acute effects of cigarette smoking on arterial stiffness and blood pressure in male smokers with hypertension. Am. J. Hypertens. 2007, 20, 637–641. [Google Scholar] [CrossRef]
- Szoltysek-Boldys, I.; Sobczak, A.; Zielinska-Danch, W.; Barton, A.; Koszowski, B.; Kosmider, L. Influence of inhaled nicotine source on arterial stiffness. Przegl. Lek. 2014, 71, 572–575. [Google Scholar]
- Vlachopoulos, C.; Ioakeimidis, N.; Abdelrasoul, M.; Terentes-Printzios, D.; Georgakopoulos, C.; Pietri, P.; Stefanadis, C.; Tousoulis, D. Electronic Cigarette Smoking Increases Aortic Stiffness and Blood Pressure in Young Smokers. J. Am. Coll Cardiol. 2016, 67, 2802–2803. [Google Scholar] [CrossRef]
- Stefanadis, C.; Tsiamis, E.; Vlachopoulos, C.; Stratos, C.; Toutouzas, K.; Pitsavos, C.; Marakas, S.; Boudoulas, H.; Toutouzas, P. Unfavorable effect of smoking on the elastic properties of the human aorta. Circulation 1997, 95, 31–38. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Kosmopoulou, F.; Panagiotakos, D.; Ioakeimidis, N.; Alexopoulos, N.; Pitsavos, C.; Stefanadis, C. Smoking and caffeine have a synergistic detrimental effect on aortic stiffness and wave reflections. J. Am. Coll. Cardiol. 2004, 44, 1911–1917. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ikonomidis, I.; Vlastos, D.; Kourea, K.; Kostelli, G.; Varoudi, M.; Pavlidis, G.; Efentakis, P.; Triantafyllidi, H.; Parissis, J.; Andreadou, I.; et al. Electronic Cigarette Smoking Increases Arterial Stiffness and Oxidative Stress to a Lesser Extent Than a Single Conventional Cigarette: An Acute and Chronic Study. Circulation 2018, 137, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, A.; Feely, J. Effect of smoking on arterial stiffness and pulse pressure amplification. Hypertension 2003, 41, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.T. Vascular damage from smoking: Disease mechanisms at the arterial wall. Vasc. Med. 1998, 3, 21–28. [Google Scholar] [CrossRef]
- Kool, M.J.; Hoeks, A.P.; Struijker Boudier, H.A.; Reneman, R.S.; Van Bortel, L.M. Short- and long-term effects of smoking on arterial wall properties in habitual smokers. J. Am. Coll Cardiol. 1993, 22, 1881–1886. [Google Scholar] [CrossRef]
- Smith, C.J.; Kluck, L.A.; Ruan, G.J.; Ashrani, A.A.; Marshall, A.L.; Pruthi, R.K.; Shah, M.V.; Wolanskyj-Spinner, A.; Gangat, N.; Litzow, M.R.; et al. Leukocytosis and Tobacco Use: An Observational Study of Asymptomatic Leukocytosis. Am. J. Med. 2021, 134, e31–e35. [Google Scholar] [CrossRef]
- Kawada, T. Leukocytosis and Tobacco Use: A Risk Assessment. Am. J. Med. 2021, 134, e228. [Google Scholar] [CrossRef]
- Kawada, T. Smoking-induced leukocytosis can persist after cessation of smoking. Arch. Med. Res. 2004, 35, 246–250. [Google Scholar] [CrossRef]
- Chaaban, T. Acute eosinophilic pneumonia associated with non-cigarette smoking products: A systematic review. Adv. Respir. Med. 2020, 88, 142–146. [Google Scholar] [CrossRef]
- Adriaens, K.; Gucht, D.V.; Baeyens, F. IQOSTM vs. e-Cigarette vs. Tobacco Cigarette: A Direct Comparison of Short-Term Effects after Overnight-Abstinence. Int. J. Environ. Res. Public Health 2018, 15, 2902. [Google Scholar] [CrossRef]
- Nabavizadeh, P.; Liu, J.; Havel, C.M.; Ibrahim, S.; Derakhshandeh, R.; Jacob Iii, P.; Springer, M.L. Vascular endothelial function is impaired by aerosol from a single IQOS HeatStick to the same extent as by cigarette smoke. Tob. Control 2018, 27, s13–s19. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Auer, J.; Eber, B. Arterial pulse wave velocity but not augmentation index is associated with coronary artery disease extent and severity: Implications for arterial transfer function applicability. J. Hypertens. 2008, 26, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.J.; Devereux, R.B.; Kizer, J.R.; Lee, E.T.; Galloway, J.M.; Ali, T.; Umans, J.G.; Howard, B.V. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: The Strong Heart Study. Hypertension 2007, 50, 197–203. [Google Scholar] [CrossRef]
- Roman, M.J.; Devereux, R.B.; Kizer, J.R.; Okin, P.M.; Lee, E.T.; Wang, W.; Umans, J.G.; Calhoun, D.; Howard, B.V. High central pulse pressure is independently associated with adverse cardiovascular outcome the strong heart study. J. Am. Coll. Cardiol. 2009, 54, 1730–1734. [Google Scholar] [CrossRef]
- Goniewicz, M.L.; Knysak, J.; Gawron, M.; Kosmider, L.; Sobczak, A.; Kurek, J.; Prokopowicz, A.; Jablonska-Czapla, M.; Rosik-Dulewska, C.; Havel, C.; et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control 2014, 23, 133–139. [Google Scholar] [CrossRef]
- Stephens, W.E. Comparing the cancer potencies of emissions from vapourised nicotine products including e-cigarettes with those of tobacco smoke. Tob. Control 2017, 27, 10–17. [Google Scholar] [CrossRef]





| Sex | All subjects (n = 20) | Male (n = 10) | Female (n = 10) | p-Value |
|---|---|---|---|---|
| Mean ± SED | Mean ± SED | Mean ± SED | ||
| Age [years] | 25.2 ± 0.9 | 25.6 ± 0.4 | 24.7 ± 0.3 | 0.6063 |
| Weight [kg] | 78.2 ± 3.5 | 85.9 ± 1.9 | 68.8 ± 0.8 | 0.0104 |
| Height [m] | 1.76 ± 0.0 | 1.8 ± 0.0 | 1.7 ± 0.0 | 0.0040 |
| BMI [kg/m²] | 25.0 ± 0.8 | 25.9 ± 0.4 | 24.0 ± 0.2 | 0.2180 |
| Waist [cm] | 82.1 ± 2.0 | 86.1 ± 0.7 | 77.1 ± 0.7 | 0.0181 |
| Hip [cm] | 92.9 ± 2.0 | 93.5 ± 1.0 | 92.0 ± 0.7 | 0.7155 |
| Cigarettes per day | 2.1 ± 0.7 | 2.7 ± 0.1 | 1.2 ± 0.2 | 0.2832 |
| Fagerström-Test [points] | 0.5 ± 0.2 | 0.8 ± 0.1 | 0.1 ± 0.0 | 0.1180 |
| Combustible Cigarette | JUULTM | Heated Tobacco Product | E-Cigarette | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time [min] | Mean ± SED | Paired t-Test | Mean ± SED | Paired t-Test | Mean ± SED | Paired t-Test | Mean ±SED | Paired t-Test | ANOVA | |
| RHI | Baseline | 2.3 ± 0.06 | 2.2 ± 0.11 | 2.3 ± 0.11 | 2.4 ± 0.16 | 0.540 | ||||
| 15 | 1.9 ± 0.05 | 0.032 | 1.7 ± 0.08 | 0.001 | 1.9 ± 0.1 | 0.001 | 1.9 ± 0.12 | 0.002 | 0.016 | |
| 60 | 1.9 ± 0.05 | 0.001 | 10.8 ± 0.10 | 0.001 | 10.8 ± 0.11 | 0.004 | 20.0 ± 0.11 | 0.000 | 0.368 | |
| LnRHI | Baseline | 0.8 ± 0.03 | 0.8 ± 0.048 | 0.8 ± 0.05 | 0.8 ± 0.07 | 0.587 | ||||
| 15 | 0.6 ± 0.03 | 0.022 | 0.5 ± 0.04 | 0.000 | 0.6 ± 0.05 | 0.002 | 0.6 ± 0.06 | 0.002 | 0.017 | |
| 0 | 0.6 ± 0.03 | 0.001 | 0.6 ± 0.05 | 0.001 | 0.6 ± 0.06 | 0.003 | 0.7 ± 0.06 | 0.000 | 0.363 | |
| AI@75bpm [%] | Baseline | −17.4 ± 10.1 | −16.3 ± 1.7 | −18.5 ± 2.7 | −17.3 ± 2.31 | 0.928 | ||||
| 15 | −11.9 ± 10.1 | 0.000 | −110.0 ± 1.9 | 0.000 | −120.6 ± 2.7 | 0.000 | −12.9 ± 2.21 | 0.013 | 0.907 | |
| 60 | −110.1 ± 1.3 | 0.001 | −10.4 ± 2.2 | 0.002 | −120.8 ± 20.8 | 0.000 | −110.1 ± 2.99 | 0.008 | 0.888 | |
| Plasma Cortisol [mmol/L] | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Combustible Cigarette | JUULTM | Heated Tobacco Product | E-Cigarette | ||||||
| Time [min] | Mean ± SED | paired t-test | Mean ± SED | paired t-test | Mean ± SED | paired t-test | Mean ± SED | paired t-test | ANOVA |
| Baseline | 353.5 ± 21.7 | 358.5 ± 44.8 | 355.6 ± 47.1 | 375.5 ± 47.4 | 0.873 | ||||
| 12 | 332.5 ± 17.1 | 0.571 | 303.9 ± 33.2 | 0.049 | 347.6 ± 38.7 | 0.792 | 366.8 ± 38.2 | 0.786 | 0.527 |
| 120 | 196.6 ± 11.6 | 0.000 | 183.0 ± 20.9 | 0.000 | 207.4 ± 24.0 | 0.000 | 205.1 ± 24.9 | 0.000 | 0.865 |
| Hemogram | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Combustible Cigarette | JUULTM | Heated Tobacco Product | E-Cigarette | |||||||
| Type of cell | Time [min] | Mean ± SED | paired t-test | Mean ± SED | paired t-test | Mean ± SED | paired t-test | Mean ± SED | paired t-test | ANOVA |
| Leucocytes [G/l] | Baseline | 5.8 ± 0.3 | 5.8 ± 0.4 | 6.2 ± 0.4 | 6.1 ± 0.2 | 0.816 | ||||
| 120 | 6.2 ± 0.3 | 0.090 | 6.7 ± 0.4 | 0.047 | 7.1 ± 0.4 | 0.003 | 6.9 ± 0.4 | 0.024 | 0.374 | |
| Immature granulocytes [G/l] | Baseline | 0.012 ± 0.0 | 0.014 ± 0.0 | 0.02 ± 0.0 | 0.016 ± 0.0 | 0.119 | ||||
| 120 | 0.017 ± 0.0 | 0.058 | 0.02 ± 0.0 | 0.014 | 0.019 ± 0.0 | 0.847 | 0.018 ± 0.0 | 0.447 | 0.765 | |
| Neutrophil granulocytes [G/l] | Baseline | 3.2 ± 0.2 | 3.4 ± 0.3 | 3.6 ± 0.3 | 3.4 ± 0.2 | 0.743 | ||||
| 120 | 3.6 ± 0.3 | 0.051 | 4.0 ± 0.3 | 0.057 | 4.4 ± 0.4 | 0.006 | 4.3 ± 0.4 | 0.034 | 0.237 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benthien, J.; Meusel, M.; Cayo Talavera, S.; Eitel, I.; Drömann, D.; Franzen, K.F. JUUL™ing and Heating Lead to a Worsening of Arterial Stiffness. Medicines 2022, 9, 28. https://doi.org/10.3390/medicines9040028
Benthien J, Meusel M, Cayo Talavera S, Eitel I, Drömann D, Franzen KF. JUUL™ing and Heating Lead to a Worsening of Arterial Stiffness. Medicines. 2022; 9(4):28. https://doi.org/10.3390/medicines9040028
Chicago/Turabian StyleBenthien, Julia, Moritz Meusel, Silja Cayo Talavera, Ingo Eitel, Daniel Drömann, and Klaas F. Franzen. 2022. "JUUL™ing and Heating Lead to a Worsening of Arterial Stiffness" Medicines 9, no. 4: 28. https://doi.org/10.3390/medicines9040028
APA StyleBenthien, J., Meusel, M., Cayo Talavera, S., Eitel, I., Drömann, D., & Franzen, K. F. (2022). JUUL™ing and Heating Lead to a Worsening of Arterial Stiffness. Medicines, 9(4), 28. https://doi.org/10.3390/medicines9040028






