Salubrious Effects of Green Tea Catechins on Fatty Liver Disease: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Literature Search and PRISMA Diagrams
2.2. Risk of Bias Assessment
3. Results
3.1. Findings from Rodent Studies
3.1.1. Clinicopathologic Effects
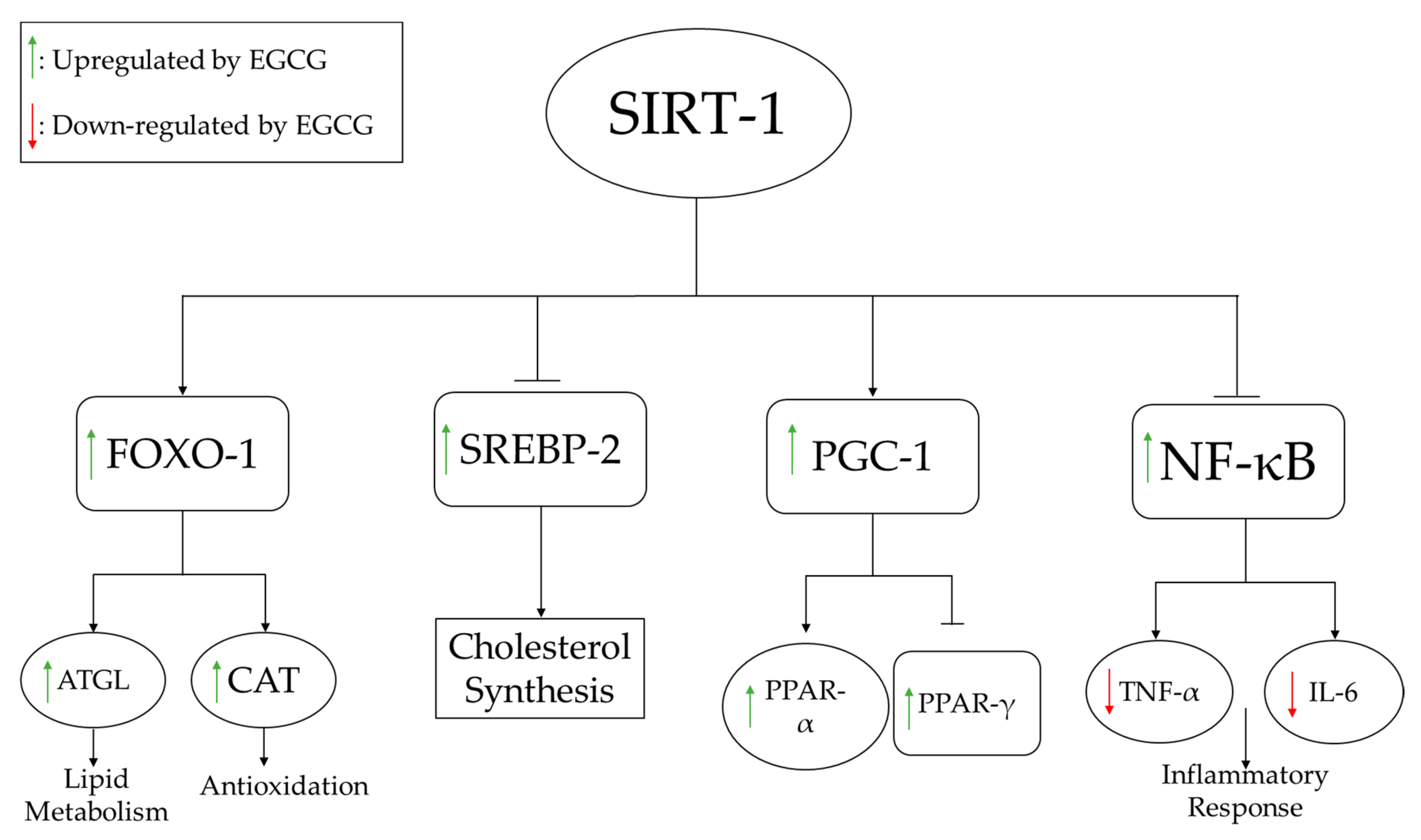
3.1.2. Lipid Metabolism
3.1.3. Carbohydrate Metabolism
3.1.4. Inflammatory Markers
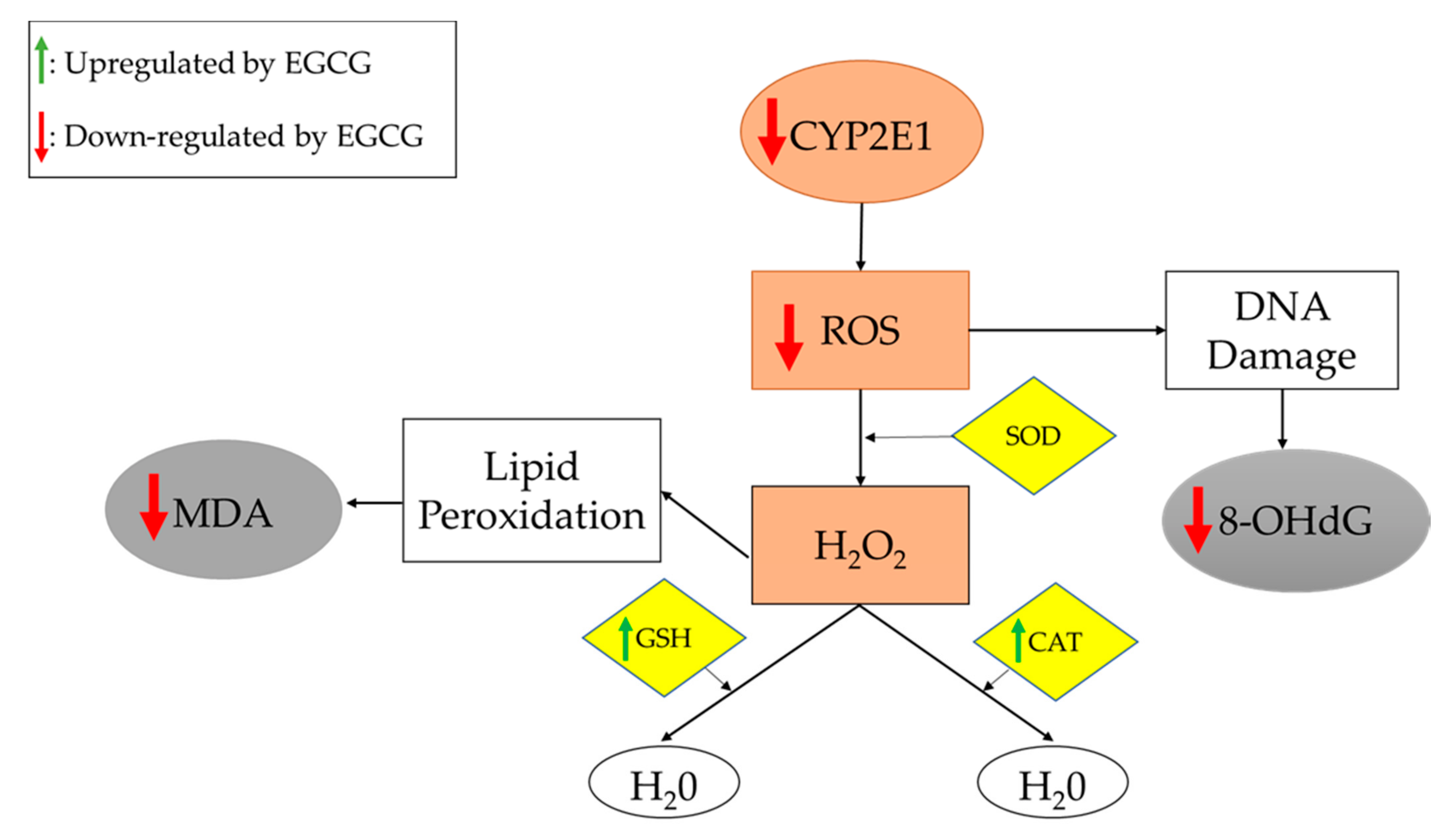
3.1.5. Oxidative Stress Markers
3.1.6. Biochemical Markers of Liver Damage
3.2. Findings from Human Studies
3.2.1. Clinicopathologic Effects
3.2.2. Lipid Metabolism
3.2.3. Carbohydrate Metabolism
3.2.4. Inflammatory Markers
3.2.5. Oxidative Stress Markers
3.2.6. Liver Enzymes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, B.W.; Adams, L.A. Non-alcoholic fatty liver disease. Crit. Rev. Clin. Lab. Sci. 2011, 48, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Kneeman, J.M.; Misdraji, J.; Corey, K.E. Secondary causes of nonalcoholic fatty liver disease. Ther. Adv. Gastroenterol. 2011, 5, 199–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.M.; Blissett, D.; Blissett, R.; Henry, L.; Stepanova, M.; Younossi, Y.; Racila, A.; Hunt, S.; Beckerman, R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016, 64, 1577–1586. [Google Scholar] [CrossRef]
- Beaton, M.D. Current treatment options for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Can. J. Gastroenterol. 2012, 26, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Sakata, R.; Nakamura, T.; Torimura, T.; Ueno, T.; Sata, M. Green tea with high-density catechins improves liver function and fat infiltration in non-alcoholic fatty liver disease (NAFLD) patients: A double-blind placebo-controlled study. Int. J. Mol. Med. 2013, 32, 989–994. [Google Scholar] [CrossRef] [Green Version]
- Wolfram, S. Effects of Green Tea and EGCG on Cardiovascular and Metabolic Health. J. Am. Coll. Nutr. 2007, 26, 373S–388S. [Google Scholar] [CrossRef]
- Hodges, J.K.; Sasaki, G.Y.; Bruno, R.S. Anti-inflammatory activities of green tea catechins along the gut–liver axis in nonalcoholic fatty liver disease: Lessons learned from preclinical and human studies. J. Nutr. Biochem. 2020, 85, 108478. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Hosseini, R.; Kazemi, A.; Ofori-Asenso, R.; Mazidi, M.; Mazloomi, S.M. Effects of green tea or green tea catechin on liver enzymes in healthy individuals and people with nonalcoholic fatty liver disease: A systematic review and meta-analysis of randomized clinical trials. Phytother. Res. 2020, 34, 1587–1598. [Google Scholar] [CrossRef]
- Raederstorff, D.G.; Schlachter, M.F.; Elste, V.; Weber, P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J. Nutr. Biochem. 2003, 14, 326–332. [Google Scholar] [CrossRef]
- Fiorini, R.N.; Donovan, J.L.; Rodwell, D.; Evans, Z.; Cheng, G.; May, H.D.; Milliken, C.E.; Markowitz, J.S.; Campbell, C.; Haines, J.K.; et al. Short-term administration of (-)-epigallocatechin gallate reduces hepatic steatosis and protects against warm hepatic ischemia/reperfusion injury in steatotic mice. Liver Transplant. 2005, 11, 298–308. [Google Scholar] [CrossRef]
- Kuzu, N.; Bahcecioglu, I.H.; Dagli, A.F.; Ozercan, I.H.; Ustündag, B.; Sahin, K. Epigallocatechin gallate attenuates experimental non-alcoholic steatohepatitis induced by high fat diet. J. Gastroenterol. Hepatol. 2008, 23, e465–e470. [Google Scholar] [CrossRef] [PubMed]
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.; Yang, C.S. The Major Green Tea Polyphenol, (-)-Epigallocatechin-3-Gallate, Inhibits Obesity, Metabolic Syndrome, and Fatty Liver Disease in High-Fat–Fed Mice. J. Nutr. 2008, 138, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Kim, C.-T.; Kim, Y. Green Tea (–)-Epigallocatechin-3-Gallate Reduces Body Weight with Regulation of Multiple Genes Expression in Adipose Tissue of Diet-Induced Obese Mice. Ann. Nutr. Metab. 2009, 54, 151–157. [Google Scholar] [CrossRef]
- Ueno, T.; Torimura, T.; Nakamura, T.; Sivakumar, R.; Nakayama, H.; Otabe, S.; Yuan, X.; Yamada, K.; Hashimoto, O.; Inoue, K.; et al. Epigallocatechin-3-gallate improves nonalcoholic steatohepatitis model mice expressing nuclear sterol regulatory element binding protein-1c in adipose tissue. Int. J. Mol. Med. 2009, 24, 17–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, N.; Bezzina, R.; Hinch, E.; Lewandowski, P.A.; Cameron-Smith, D.; Mathai, M.L.; Jois, M.; Sinclair, A.J.; Begg, D.P.; Wark, J.D.; et al. Green tea, black tea, and epigallocatechin modify body composition, improve glucose tolerance, and differentially alter metabolic gene expression in rats fed a high-fat diet. Nutr. Res. 2009, 29, 784–793. [Google Scholar] [CrossRef]
- Chen, Y.-K.; Cheung, C.; Reuhl, K.R.; Liu, A.B.; Lee, M.-J.; Lu, Y.-P.; Yang, C.S. Effects of Green Tea Polyphenol (−)-Epigallocatechin-3-gallate on Newly Developed High-Fat/Western-Style Diet-Induced Obesity and Metabolic Syndrome in Mice. J. Agric. Food Chem. 2011, 59, 11862–11871. [Google Scholar] [CrossRef] [Green Version]
- Sae-Tan, S.; Grove, K.A.; Kennett, M.J.; Lambert, J.D. (−)-Epigallocatechin-3-gallate increases the expression of genes related to fat oxidation in the skeletal muscle of high fat-fed mice. Food Funct. 2011, 2, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, C.; Nishimatsu, S.; Moriyama, T.; Ozasa, S.; Kawada, T.; Sayama, K. Catechins and Caffeine Inhibit Fat Accumulation in Mice through the Improvement of Hepatic Lipid Metabolism. J. Obes. 2012, 2012, 520510. [Google Scholar] [CrossRef] [Green Version]
- Sumi, T.; Shirakami, Y.; Shimizu, M.; Kochi, T.; Ohno, T.; Kubota, M.; Shiraki, M.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. (−)-Epigallocatechin-3-gallate suppresses hepatic preneoplastic lesions developed in a novel rat model of non-alcoholic steatohepatitis. SpringerPlus 2013, 2, 690. [Google Scholar] [CrossRef] [Green Version]
- Kochi, T.; Shimizu, M.; Terakura, D.; Baba, A.; Ohno, T.; Kubota, M.; Shirakami, Y.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. Non-alcoholic steatohepatitis and preneoplastic lesions develop in the liver of obese and hypertensive rats: Suppressing effects of EGCG on the development of liver lesions. Cancer Lett. 2014, 342, 60–69. [Google Scholar] [CrossRef]
- Xiao, J.; Ho, C.T.; Liong, E.C.; Nanji, A.A.; Leung, T.M.; Lau, T.Y.H.; Fung, M.L.; Tipoe, G.L. Epigallocatechin gallate attenuates fibrosis, oxidative stress, and inflammation in non-alcoholic fatty liver disease rat model through TGF/SMAD, PI3 K/Akt/FoxO1, and NF-kappa B pathways. Eur. J. Nutr. 2014, 53, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, T.R.; Velusamy, P.; Srinivasan, A.; Ganesan, T.; Mangaiah, S.; Narasimhan, K.; Chakrapani, L.N.; Thanka, J.; Walter, C.E.J.; Durairajan, S.; et al. EGCG mediated downregulation of NF-AT and macrophage infiltration in experimental hepatic steatosis. Exp. Gerontol. 2014, 57, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Meng, Z.-J.; Xiong, R.-B.; Guo, J.-Q.; Lu, X.-C.; Zheng, Z.-W.; Deng, Y.-P.; Luo, B.-D.; Zou, F.; Li, H. Green tea polyphenol epigallocatechin-3-gallate ameliorates insulin resistance in non-alcoholic fatty liver disease mice. Acta Pharmacol. Sin. 2015, 36, 597–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Sun, X.; Chen, Y.; Deng, Y.; Qian, K. Epigallocatechin gallate attenuated non-alcoholic steatohepatitis induced by methionine- and choline-deficient diet. Eur. J. Pharmacol. 2015, 761, 405–412. [Google Scholar] [CrossRef]
- Santamarina, A.B.; Carvalho-Silva, M.; Gomes, L.M.; Okuda, M.H.; Santana, A.A.; Streck, E.L.; Seelaender, M.; Nascimento, C.M.O.D.; Ribeiro, E.B.; Lira, F.S.; et al. Decaffeinated green tea extract rich in epigallocatechin-3-gallate prevents fatty liver disease by increased activities of mitochondrial respiratory chain complexes in diet-induced obesity mice. J. Nutr. Biochem. 2015, 26, 1348–1356. [Google Scholar] [CrossRef] [Green Version]
- Mi, Y.; Qi, G.; Fan, R.; Ji, X.; Liu, Z.; Liu, X. EGCG ameliorates diet-induced metabolic syndrome associating with the circadian clock. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1575–1589. [Google Scholar] [CrossRef]
- Huang, J.; Feng, S.; Liu, A.; Dai, Z.; Wang, H.; Reuhl, K.; Lu, W.; Yang, C.S. Green Tea Polyphenol EGCG Alleviates Metabolic Abnormality and Fatty Liver by Decreasing Bile Acid and Lipid Absorption in Mice. Mol. Nutr. Food Res. 2018, 62, 1700696. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, M.-Z.; Zhang, Y.-B.; Wen, B.-B.; An, H.-M.; Ou, X.-C.; Xiong, Y.-F.; Lin, H.-Y.; Liu, Z.-H.; Huang, J.-A. Coadministration of epigallocatechin-3-gallate (EGCG) and caffeine in low dose ameliorates obesity and nonalcoholic fatty liver disease in obese rats. Phytother. Res. 2019, 33, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, S. Epigallocatechin gallate suppresses hepatic cholesterol synthesis by targeting SREBP-2 through SIRT1/FOXO1 signaling pathway. Mol. Cell. Biochem. 2018, 448, 175–185. [Google Scholar] [CrossRef]
- Sheng, L.; Jena, P.K.; Liu, H.; Hu, Y.; Nagar, N.; Bronner, D.N.; Settles, M.L.; Baümler, A.J.; Wan, Y.Y. Obesity treatment by epigallocatechin-3-gallate−regulated bile acid signaling and its enriched Akkermansia muciniphila. FASEB J. 2018, 32, 6371–6384. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Gao, C.; Yan, P.; Zhang, M.; Wang, Y.; Hu, Y.; Wu, X.; Wang, X.; Sheng, J.; Li, F.; et al. EGCG Reduces Obesity and White Adipose Tissue Gain Partly Through AMPK Activation in Mice. Front. Pharmacol. 2018, 9, 1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ushiroda, C.; Naito, Y.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Higashimura, Y.; Yasukawa, Z.; Okubo, T.; Inoue, R.; Honda, A.; et al. Green tea polyphenol (epigallocatechin-3-gallate) improves gut dysbiosis and serum bile acids dysregulation in high-fat diet-fed mice. J. Clin. Biochem. Nutr. 2019, 65, 34–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, H.; Yang, W.; Bao, S.; Cao, Y. Epigallocatechin Gallate Suppresses Inflammatory Responses by Inhibiting Toll-like Receptor 4 Signaling and Alleviates Insulin Resistance in the Livers of High-fat-diet Rats. J. Oleo Sci. 2020, 69, 479–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, P.; Olmstead, B.D.; Sasaki, G.Y.; Vodovotz, Y.; Yu, Z.; Bruno, R.S. Epigallocatechin gallate but not catechin prevents nonalcoholic steatohepatitis in mice similar to green tea extract while differentially affecting the gut microbiota. J. Nutr. Biochem. 2020, 84, 108455. [Google Scholar] [CrossRef]
- Ning, K.; Lu, K.; Chen, Q.; Guo, Z.; Du, X.; Riaz, F.; Feng, L.; Fu, Y.; Yin, C.; Zhang, F.; et al. Epigallocatechin Gallate Protects Mice against Methionine–Choline-Deficient-Diet-Induced Nonalcoholic Steatohepatitis by Improving Gut Microbiota to Attenuate Hepatic Injury and Regulate Metabolism. ACS Omega 2020, 5, 20800–20809. [Google Scholar] [CrossRef]
- Yuan, H.; Li, Y.; Ling, F.; Guan, Y.; Zhang, D.; Zhu, Q.; Liu, J.; Wu, Y.; Niu, Y. The phytochemical epigallocatechin gallate prolongs the lifespan by improving lipid metabolism, reducing inflammation and oxidative stress in high-fat diet-fed obese rats. Aging Cell 2020, 19, e13199. [Google Scholar] [CrossRef]
- Huang, J.; Li, W.; Liao, W.; Hao, Q.; Tang, D.; Wang, D.; Wang, Y.; Ge, G. Green tea polyphenol epigallocatechin-3-gallate alleviates nonalcoholic fatty liver disease and ameliorates intestinal immunity in mice fed a high-fat diet. Food Funct. 2020, 11, 9924–9935. [Google Scholar] [CrossRef]
- Du, Y.; Paglicawan, L.; Soomro, S.; Abunofal, O.; Baig, S.; Vanarsa, K.; Hicks, J.; Mohan, C. Epigallocatechin-3-Gallate Dampens Non-Alcoholic Fatty Liver by Modulating Liver Function, Lipid Profile and Macrophage Polarization. Nutrients 2021, 13, 599. [Google Scholar] [CrossRef]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.G.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative Stress in Obesity: A Critical Component in Human Diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Chantre, P.; Lairon, D. Recent findings of green tea extract AR25 (Exolise) and its activity for the treatment of obesity. Phytomedicine 2002, 9, 3–8. [Google Scholar] [CrossRef]
- Kovacs, E.M.R.; Lejeune, M.P.G.M.; Nijs, I.; Westerterp-Plantenga, M.S. Effects of green tea on weight maintenance after body-weight loss. Br. J. Nutr. 2004, 91, 431–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagao, T.; Komine, Y.; Soga, S.; Meguro, S.; Hase, T.; Tanaka, Y.; Tokimitsu, I. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am. J. Clin. Nutr. 2005, 81, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Nagao, T.; Hase, T.; Tokimitsu, I. A Green Tea Extract High in Catechins Reduces Body Fat and Cardiovascular Risks in Humans. Obesity 2007, 15, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Auvichayapat, P.; Prapochanung, M.; Tunkamnerdthai, O.; Sripanidkulchai, B.-O.; Auvichayapat, N.; Thinkhamrop, B.; Kunhasura, S.; Wongpratoom, S.; Sinawat, S.; Hongprapas, P. Effectiveness of green tea on weight reduction in obese Thais: A randomized, controlled trial. Physiol. Behav. 2008, 93, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Coates, A.; Buckley, J.; Ross, R.; Thielecke, F.; Howe, P.R. Can EGCG Reduce Abdominal Fat in Obese Subjects? J. Am. Coll. Nutr. 2007, 26, 396S–402S. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Tsai, T.-H.; Kao, Y.-H.; Hwang, K.-C.; Tseng, T.-Y.; Chou, P. Effect of green tea extract on obese women: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2008, 27, 363–370. [Google Scholar] [CrossRef]
- Matsuyama, T.; Tanaka, Y.; Kamimaki, I.; Nagao, T.; Tokimitsu, I. Catechin Safely Improved Higher Levels of Fatness, Blood Pressure, and Cholesterol in Children. Obesity 2008, 16, 1338–1348. [Google Scholar] [CrossRef]
- Maki, K.C.; Reeves, M.S.; Farmer, M.; Yasunaga, K.; Matsuo, N.; Katsuragi, Y.; Komikado, M.; Tokimitsu, I.; Wilder, D.; Jones, F.; et al. Green Tea Catechin Consumption Enhances Exercise-Induced Abdominal Fat Loss in Overweight and Obese Adults. J. Nutr. 2009, 139, 264–270. [Google Scholar] [CrossRef]
- Brown, A.L.; Lane, J.; Coverly, J.; Stocks, J.; Jackson, S.; Stephen, A.; Bluck, L.; Coward, A.; Hendrickx, H. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: Randomized controlled trial. Br. J. Nutr. 2009, 101, 886–894. [Google Scholar] [CrossRef]
- Di Pierro, F.; Menghi, A.B.; Barreca, A.; Lucarelli, M.; Calandrelli, A. Greenselect Phytosome as an adjunct to a low-calorie diet for treatment of obesity: A clinical trial. Altern. Med. Rev. 2009, 14, 154–160. [Google Scholar]
- Basu, A.; Du, M.; Sanchez, K.; Leyva, M.J.; Betts, N.M.; Blevins, S.; Wu, M.; Aston, C.E.; Lyons, T.J. Green tea minimally affects biomarkers of inflammation in obese subjects with metabolic syndrome. Nutrition 2011, 27, 206–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, A.; Sanchez, K.; Leyva, M.J.; Wu, M.; Betts, N.M.; E Aston, C.; Lyons, T.J. Green Tea Supplementation Affects Body Weight, Lipids, and Lipid Peroxidation in Obese Subjects with Metabolic Syndrome. J. Am. Coll. Nutr. 2010, 29, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Thielecke, F.; Rahn, G.; Böhnke, J.; Adams, F.; Birkenfeld, A.L.; Jordan, J.; Boschmann, M. Epigallocatechin-3-gallate and postprandial fat oxidation in overweight/obese male volunteers: A pilot study. Eur. J. Clin. Nutr. 2010, 64, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Lane, J.; Holyoak, C.; Nicol, B.; Mayes, A.E.; Dadd, T. Health effects of green tea catechins in overweight and obese men: A randomised controlled cross-over trial. Br. J. Nutr. 2011, 106, 1880–1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogdanski, P.; Suliburska, J.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jabłecka, A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr. Res. 2012, 32, 421–427. [Google Scholar] [CrossRef]
- Suliburska, J.; Bogdanski, P.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jabłecka, A. Effects of Green Tea Supplementation on Elements, Total Antioxidants, Lipids, and Glucose Values in the Serum of Obese Patients. Biol. Trace Elem. Res. 2012, 149, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Mielgo-Ayuso, J.; Barrenechea, M.L.; Alcorta, P.; Larrarte, E.; Margareto, J.; Labayen, I. Effects of dietary supplementation with epigallocatechin-3-gallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: Randomised, double-blind, placebo-controlled clinical trial. Br. J. Nutr. 2014, 111, 1263–1271. [Google Scholar] [CrossRef] [Green Version]
- Askari, G.; Pezeshki, A.; Safi, S.; Feizi, A.; Karami, F. The effect of green tea extract supplementation on liver enzymes in patients with nonalcoholic fatty liver disease. Int. J. Prev. Med. 2016, 7, 28. [Google Scholar] [CrossRef]
- Hussain, M.; Rehman, H.U.; Akhtar, L. Therapeutic benefits of green tea extract on various parameters in non-alcoholic fatty liver disease patients. Pak. J. Med Sci. 2017, 33, 931–936. [Google Scholar] [CrossRef]
- Roberts, J.; Willmott, A.; Beasley, L.; Boal, M.; Davies, R.; Martin, L.; Chichger, H.; Gautam, L.; Del Coso, J. The Impact of Decaffeinated Green Tea Extract on Fat Oxidation, Body Composition and Cardio-Metabolic Health in Overweight, Recreationally Active Individuals. Nutrients 2021, 13, 764. [Google Scholar] [CrossRef]
- Khoubnasabjafari, M.; Ansarin, K.; Jouyban, A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. BioImpacts 2015, 5, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Feng, J.; Yang, Y.; Dai, C.; Lu, A.; Li, J.; Liao, Y.; Xiang, M.; Huang, Q.; Wang, N.; et al. Significance of Serum Total Oxidant/Antioxidant Status in Patients with Colorectal Cancer. PLoS ONE 2017, 12, e0170003. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, G.E., Jr.; Pruitt, W.M.; Pruitt, K. Diverse Roles of SIRT1 in Cancer Biology and Lipid Metabolism. Int. J. Mol. Sci. 2015, 16, 950–965. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Yoneda, M. Current and future pharmacological therapies for NAFLD/NASH. J. Gastroenterol. 2017, 53, 362–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, V.W.-S.; Singal, A.K. Emerging medical therapies for non-alcoholic fatty liver disease and for alcoholic hepatitis. Transl. Gastroenterol. Hepatol. 2019, 4, 53. [Google Scholar] [CrossRef] [PubMed]
- Van der Windt, D.J.; Sud, V.; Zhang, H.; Tsung, A.; Huang, H. The Effects of Physical Exercise on Fatty Liver Disease. Gene Expr. 2018, 18, 89–101. [Google Scholar] [CrossRef] [Green Version]
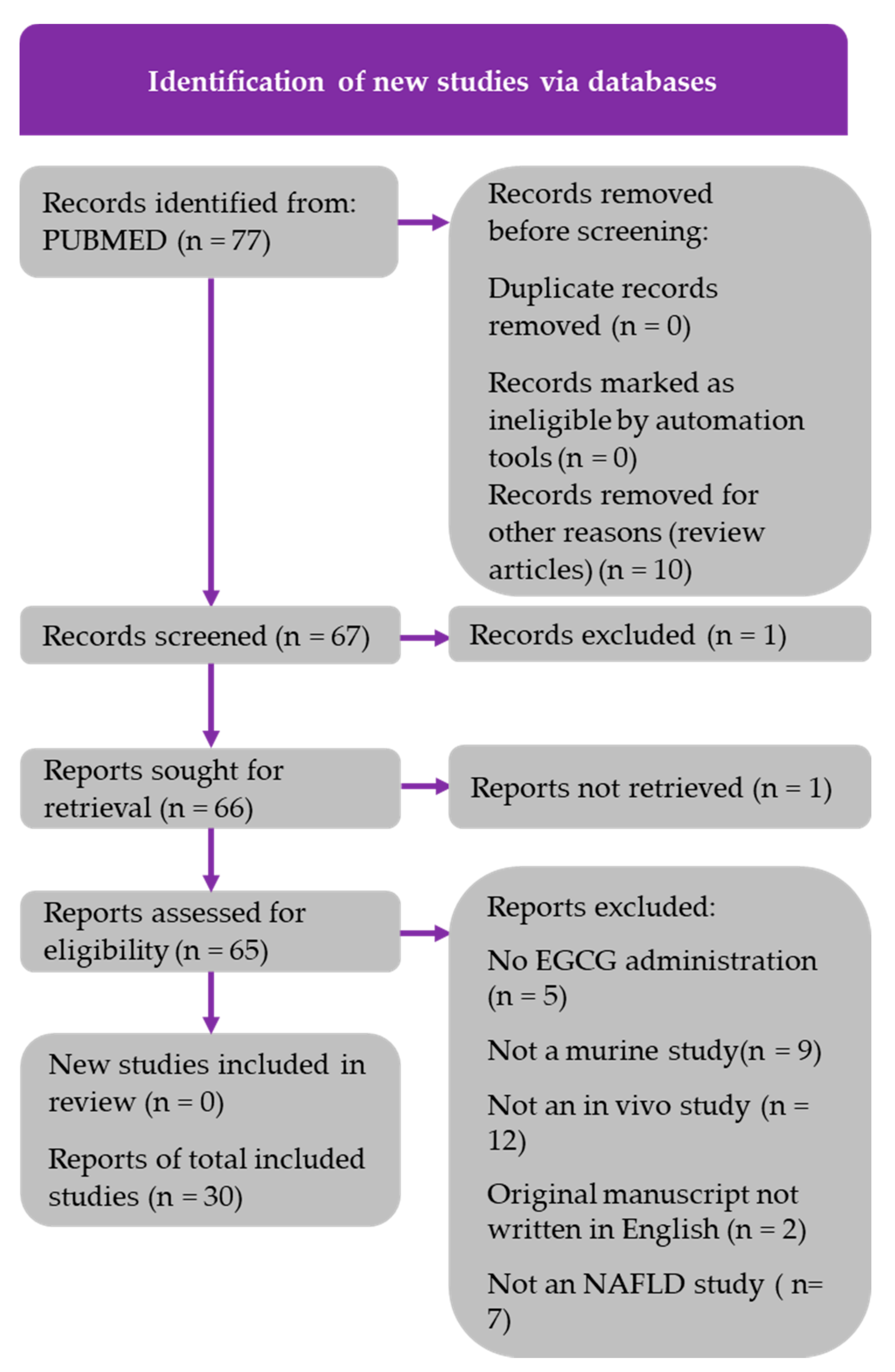
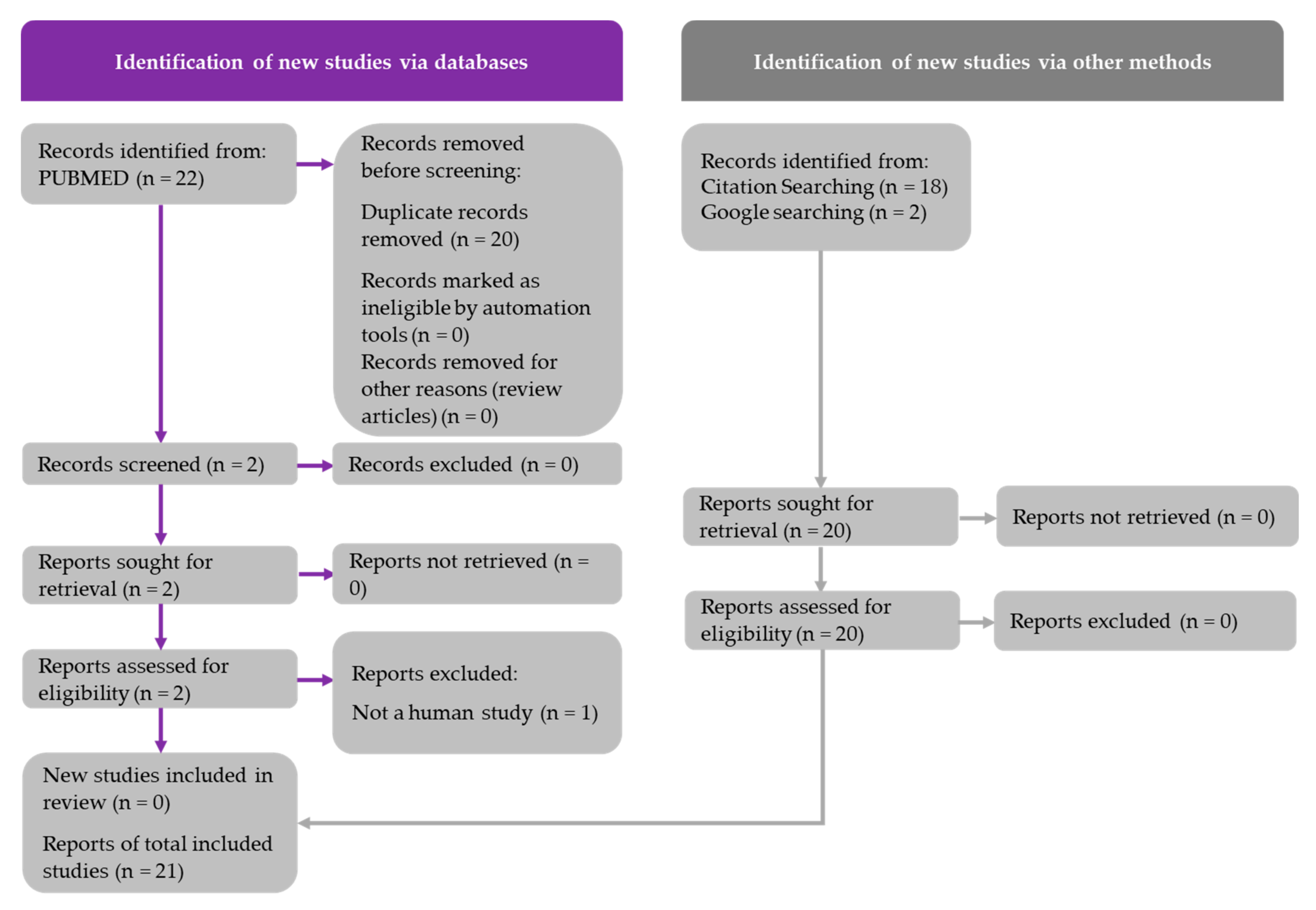
| Study [Ref] | Model | EGCG Intake | Duration | Clinical/Pathological Outcome | Lipid Metabolism | Carbohydrate Metabolism | Inflammatory Markers | Oxidative Stress Markers | Liver Injury Enzymes |
|---|---|---|---|---|---|---|---|---|---|
| Raederstorff 2003 [9] | HFD (R) | 0.25–1% (CD) | 4 weeks | ↑ Fecal fat/cholesterol/lipid excretion; ↔ Body weight, liver weight, food intake | ↓ TC, LDL, HDL, TG | ||||
| Fiorini 2005 [10] | I/R (M) | 85 mg/kg (DW/IP) | 5 days | ↓ Body weight, steatosis; ↔ Food intake | ↓ FAS | ↑ GSH; ↔ UCP | ↓ ALT | ||
| Kuzu 2007 [11] | HFD (R) | 1 g/L (DW) | 6 weeks | ↓ Body weight, liver weight, steatosis, inflammation; ↔ Degeneration, necrosis | ↓ TG; ↔ TC | ↓ Insulin, IR | ↓ MDA, CYP2E1; ↑ GSH | ↓ ALT; ↔ ALP, AST | |
| Bose 2008 [12] | HFD (M) | 3.2 g/kg (CD) | 16 weeks | ↓ Body weight, liver weight, MAT, VAT, EAT, RAT | ↓ TG; ↑ Fecal lipids | ↓ Glucose, insulin, IR | ↓ ALT | ||
| Lee 2008 [13] | HFD (M) | 0.2–0.5% (CD) | 8 weeks | ↓ Body weight, EAT, VAT, RAT; ↔ Liver weight, energy intake | ↓ TC, LDL, PPAR-γ, FAS, LPL; ↑ CPT-I, HSL, ATGL | ↑ UCP-II | ↔ ALT, AST | ||
| Ueno 2009 [14] | NASH (M) | 0.05–0.1% (DW) | 42 weeks | ↓ Steatosis, intralobular fibrosis, ballooning; ↔ Body weight | ↓ TC, TG; ↔ FFA | ↓ Glucose | ↓ pAkt, pIKKß, pNF-κB | ↓ 8-OhdG | ↓ ALT; ↔ AST |
| Chen 2009 [15] | HFD (R) | 1 mg/kg (DW) | 23 weeks | ↓ WAT;↑ Body weight; ↔ Food intake | ↑ PPAR-γ; ↔ TC, LDL, HDL, TG, SREBP-1C, PPAR-α, CPT-II, FAS, ACC | ↓ Glucose | ↑ UCP-II; ↔ ACO, MCD | ||
| Chen 2011 [16] | HFD (M) | 0.32% (CD) | 17 weeks | ↓ Body weight, BAT, steatosis; ↔ Food intake | ↓ TG; ↑ Fecal lipids | ↓ Glucose, insulin, IR | ↓ ALT, HSL | ||
| Sae-tan 2011 [17] | HFD (M) | 0.32% (CD) | 15 weeks | ↓ Body weight, liver weight; ↔ Food intake | ↓ TG | ↓ Glucose, insulin | ↓ ALT | ||
| Sugiura 2012 [18] | HFD (M) | 0.1% (DW) | 4 weeks | ↔ Body weight, liver weight, food intake, IPAT | ↔ TC, TG, FAS, CPT-II | ↔ ACO | |||
| Sumi 2013 [19] | HFD (R) | 0.01–0.1% (DW) | 7 weeks | ↓ Liver fibrosis, steatosis; ↔ Body weight, liver weight | ↓ TG | ↑ TNF-α, IL-6 | ↓ GPx-1, GST-P+, 8-OHdG, d-ROM; ↑ CAT | ↓ ALT | |
| Kochi 2013 [20] | HFD (R) | 0.1% (DW) | 9 weeks | ↓ Steatosis; ↑ Body weight | ↓ MDA, 8-OHdG, GST-P+, d-ROM, CYP2E1; ↑ GPx, CAT | ||||
| Xiao 2013 [21] | HFD (R) | 50 mg/kg (IP) | 8 weeks | ↓ Body weight, food intake, steatosis, fibrosis | ↓ TNF-α, COX-2 | ↑ GPx, CAT; ↔ SOD | |||
| Krishnan 2014 [22] | HFD (R) | 100 mg/kg (OG) | 30 days | ↓ Steatosis, inflammation | ↓ NF-κB, TNF-α | ||||
| Gan 2015 [23] | HFD (M) | 10–40 mg/kg (IP) | 24 weeks | ↓ Energy intake, body weight, liver weight, steatosis, VAT; ↑ Hepatic cells | ↓ TC, TG, LDL; ↑ HDL | ↓ Glucose, insulin, IR, glucose intolerance | |||
| Ding 2015 [24] | MCDD (M) | 25–100 mg/kg (IP) | 4 weeks | ↓ Body weight, liver weight, food intake | ↓ IL-1β, IL-6, TNF-α, MCP-1 | ↓ MDA; ↑ SOD | ↓ AST, ALT | ||
| Santamarina 2015 [25] | HFD (M) | 50 mg/kg (DW) | 16 weeks | ↓ Body weight, WAT, ectopic fat, MAT; ↔ Liver weight, EAT, RAT | ↓ Glucose, insulin, IR | ↔ TNF-α, IL-6, IL-10, IL-6R, IL-10Rα | |||
| Mi 2017 [26] | HFD (M) | 2 g/L (DW) | 16 weeks | ↓ Body weight, liver weight, BAT | ↓ TG, TC, LDL; ↑ HDL, PPAR-γ, ACC, SIRT-I, FAS, SREBP-1C, CPT-II, CPT-Iα | ↓ Glucose, insulin, IR; ↑ Glucose tolerance, insulin sensitivity | |||
| Huang 2018 [27] | HFD (M) | 3.2 g/kg (CD) | 33 weeks | ↔ Body weight, liver weight, food intake | ↓ LDL; ↑ HDL, HMGCR, PPARα; ↔ TG, FAS | ↓ Glucose | ↑ CYP7A1, CYP27A1 | ↓ ALT | |
| Yang 2018 [28] | HFD (R) | 160 mg/kg (OG) | 11 weeks | ↓ Body weight, WAT, energy intake | ↓ TC, LDL, HDL, TG, NEFA | ↓ ALT, AST | |||
| Li 2018 [29] | HFD (R) | 25–100 mg/kg (CD) | 4 weeks | ↓ Liver weight | ↓ TC, LDL, TG, FFA, SREBP-II; ↑ HDL, SIRT-I, FOXO-I; ↔ HMGCR | ↓ MDA | ↓ ALT, AST | ||
| Sheng 2018 [30] | HFD (M) | 100 μg/g (CD) | 8 weeks | ↓ Body weight | ↓ TC, TG; ↔ LPL | ↓ ALP, ALT | |||
| Li 2018 [31] | HFD (m) | 50–100 mg/kg (IG) | 20 weeks | ↓ EAT; ↔ Body weight | ↓ LDL, TC, TG, CPT1α; ↑ HDL, ACC, FAS, ATGL | ↓ PPARα, ACO2; ↑ PPARγ, SREBP1 | ↓ UCP2 | ↑ HSL | |
| Ushiroda 2019 [32] | HFD (M) | 0.32% (CD) | 24 weeks | ↓ Body weight; ↔ Food intake | ↓ TG; ↔ LDL, HDL, TC, NEFA | ↓ ALT, AST | |||
| Hou 2020 [33] | HFD (R) | 0.32% (CD) | 16 weeks | ↔ Body weight | ↓ FFA, TG, IR | ↓ IR | ↓ TNF-α, p-NF-κb, TRAF6, IKKβ, p-IKKβ, TLR4 | ||
| Dey 2020 [34] | HFD (M) | 0.3% (CD) | 8 weeks | ↓ Body weight, liver weight, steatosis, ballooning; ↑ Energy intake | ↓ TC, TG; ↔ NEFA | ↓ Glucose, insulin, IR | ↓ TLR4, NF-κb, MCP-1, TNF-α | ↓ MDA | ↓ ALT |
| Ning 2020 [35] | MCDD (M) | 50 mg/kg (IP/OG) | 2 weeks | ↔ Body weight | ↔ LDL, HDL, TC, TG | ↓ Glucose | ↓ ALT; ↔ AST | ||
| Yuan 2020 [36] | HFD (R) | 50 mg/kg (DW) | 92 weeks | ↓ Body weight; ↔ Food intake | ↓ TC, TG, LDL, FFA; ↔ HDL; ↑ CPT-II, FOXO1, SIRT1, FAS, ACC | ↓ Glucose, insulin | ↓ IL-6, TNF-α; ↑ NF-κB | ↓ ROS; ↑ CAT, SOD; ↔ MDA | ↓ ALT, AST |
| Huang 2020 [37] | HFD (M) | 0.4% (CD) | 14 weeks | ↓ Body weight, EAT, PAT, MAT; ↔ Food intake | ↓ TC, LDL | ↓ Glucose | ↓ TNF-α, IL-6, LPS, MMP-3, COX-2, TLR4 | ↓ ALT, AST | |
| Du 2021 [38] | HFD (M) | 25–50 mg/kg (CD) | 16 weeks | ↓ Body weight, liver weight, steatosis | ↓ TG, HDL, TC; ↔ LDL | ↓ AST, ALT |
| Study (Ref) | Study Design | Duration (Number of Participants) | Green Tea Component Daily Intake | Clinical/Pathological Outcome | Lipid Metabolism | Carbohydrate Metabolism | Inflammatory Markers | Oxidative Stress Markers | Liver Injury Enzymes |
|---|---|---|---|---|---|---|---|---|---|
| Chantre 2002 [40] | Open study | 12 weeks (70) | 375 mg catechin | ↓ Body weight, WC | ↔TC | ||||
| Kovacs 2003 [41] | (R/P/PC) | 13 weeks (104) | 323 mg EGCG | ↔ Body weight, BMI, REE, RQ | ↔ TG, NEFA | ↔ Glucose, insulin | |||
| Nagao 2004 [42] | (DB) | 12 weeks (38) | 690 mg catechin | ↓ Body weight, BMI, WC, HC | ↓ LDL; ↑ FFA; ↔ HDL, TG | ↑ Glucose, insulin | ↓ MDA | ||
| Nagao 2006 [43] | (R/DB) | 12 weeks (240) | 583 mg catechin | ↓ Body weight, BMI, WC, HC; ↔ Energy intake | ↓ LDL; ↔ HDL, TC, TG, FFA | ↔ Glucose | ↔ ALP | ||
| Auvichayapat 2007 [44] | (R) | 12 weeks (60) | 750 mg green tea | ↓ Body weight, BMI; ↑ REE; ↔ Food intake, physical activity, RQ | |||||
| Hill 2007 [45] | (R/PC) | 12 weeks (38) | 300 mg EGCG | ↓ Total body fat, WC; ↔ Body weight, energy intake, EE, BMI, HC | ↔ Glucose, insulin | ||||
| Hsu 2008 [46] | (R/DB/PC) | 12 weeks (78) | 1200 mg GTE | ↓ WC, HC; ↔ Body weight, BMI | ↓ LDL, TG; ↑ HDL; ↔ TC | ↔ Insulin, IR | ↔ AST | ||
| Matsuyama 2008 [47] | (R/DB) | 36 weeks (40) | 75–576 mg catechins | ↔ Body weight, BMI, HC | ↓ TG, FFA | ↓ Glucose | ↑ CRP | ↓ AST, ALT | |
| Maki 2008 [48] | (R/DB/C) | 12 weeks (107) | 625 mg EGCG | ↓ Body weight; ↔ Physical activity, energy intake, WC | ↓ TG, FFA; ↔ LDL, HDL | ↔ Glucose, insulin | ↔ CRP | ↔ MDA | |
| Brown 2009 [49] | (R/DB/PC/P) | 8 weeks (88) | 800 mg EGCG | ↔ BMI, WC | ↔ TC, HDL, LDL, TG | ↔ Insulin, IR | |||
| Pierro 2009 [50] | (R) | 90 days (100) | 300 mg GTE | ↓ Body weight, BMI | ↓ TG, LDL, TC; ↑ HDL | ↓ Glucose, insulin | |||
| Basu 2010 [51] | (R/C) | 8 weeks (35) | 440 mg EGCG | ↔ WC | ↔ TG, HDL | ↔ Glucose | ↔ IL-6, IL-1β, sVCAM-1, CRP | ↔ AST, ALT | |
| Basu 2010 [52] | (R/C/SB) | 8 weeks (35) | 900 mg EGCG in capsule | ↔ Body weight, BMI, WC | ↓ TC, LDL; ↔ TG | ↔ Glucose, IR | ↓ MDA | ||
| Thielecke 2010 [53] | (R/DB/PC/X) | 3 days (12) | 300–600 mg EGCG in capsule | ↔ EE, RQ | ↔ NEFA | ↔ Glucose, insulin | |||
| Brown 2011 [54] | (R/PC/X) | 6 weeks (70) | 800 mg catechins | ↑ Energy intake; ↔ Body weight | ↓ LDL; ↔ HDL, TG | ↔ Glucose, insulin | |||
| Bogdanski 2011 [55] | (DB/PC) | 3 months (56) | 379 mg GTE | ↔ BMI, WC | ↓ TC, LDL, TG; ↑ HDL | ↓ Glucose, insulin, IR | ↓ CRP, TNF-α | ↑ TAS | |
| Suliburska 2012 [56] | (R/DB/PC/C) | 3 months (46) | 379 mg GTE | ↓ BMI, WC | ↓TC, LDL, TG; ↔ HDL | ↔ Glucose | ↑ TAS | ||
| Mielgo-Ayuso 2013 [57] | (R/DB/PC) | 12 weeks (88) | 300 mg EGCG | ↓ Body weight, BMI, WC | ↓ TC, LDL, HDL; ↔ TG | ↓ Insulin, IR | ↓ AST; ↔ ALT | ||
| Pezeshki 2016 [58] | (R/DB/PC) | 90 days (80) | 500 mg GTE | ↓ Body weight, BMI | ↓ AST, ALT, ALP | ||||
| Hussain 2017 [59] | (R/PC) | 91 days (80) | 500 mg GTE | ↓ Body weight, BMI | ↓ TC, LDL, TG; ↑ HDL | ↓ IR | ↓ CRP | ↓ AST, ALT | |
| Roberts 2021 [60] | (R/DB/PC) | 8 weeks (27) | 580 mg GTE | ↔ Body weight, BMI, EE, WC | ↔ TC, TG, LDL, HDL, FFA | ↔ALT, AST, ALP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abunofal, O.; Mohan, C. Salubrious Effects of Green Tea Catechins on Fatty Liver Disease: A Systematic Review. Medicines 2022, 9, 20. https://doi.org/10.3390/medicines9030020
Abunofal O, Mohan C. Salubrious Effects of Green Tea Catechins on Fatty Liver Disease: A Systematic Review. Medicines. 2022; 9(3):20. https://doi.org/10.3390/medicines9030020
Chicago/Turabian StyleAbunofal, Omar, and Chandra Mohan. 2022. "Salubrious Effects of Green Tea Catechins on Fatty Liver Disease: A Systematic Review" Medicines 9, no. 3: 20. https://doi.org/10.3390/medicines9030020
APA StyleAbunofal, O., & Mohan, C. (2022). Salubrious Effects of Green Tea Catechins on Fatty Liver Disease: A Systematic Review. Medicines, 9(3), 20. https://doi.org/10.3390/medicines9030020







