The Scientific and Cultural Journey to Ovarian Rejuvenation: Background, Barriers, and Beyond the Biological Clock
Abstract
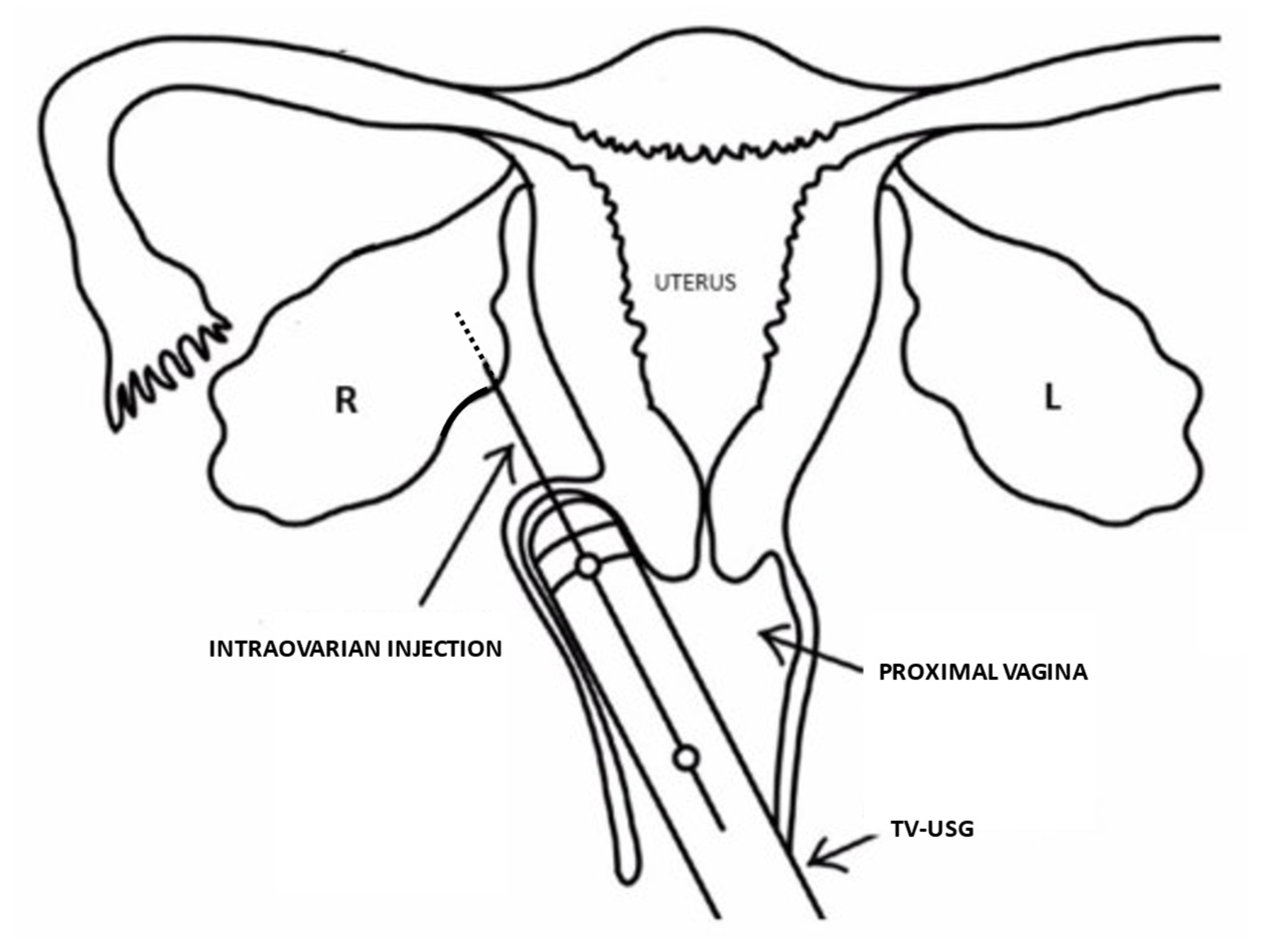
1. Introduction
2. Cultural Contexts
3. New Directions
4. Conclusion
Funding
Conflicts of Interest
References
- Polonio, A.M.; Chico-Sordo, L.; Córdova-Oriz, I.; Medrano, M.; García-Velasco, J.A.; Varela, E. Impact of ovarian aging in reproduction: From telomeres and mice models to ovarian rejuvenation. Yale J. Biol. Med. 2020, 93, 561–569. [Google Scholar] [PubMed]
- Sills, E.S.; Alper, M.M.; Walsh, A.P. Ovarian reserve screening in infertility: Practical applications and theoretical directions for research. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 146, 30–36. [Google Scholar] [CrossRef]
- Nugent, D.; Meirow, D.; Brook, P.F.; Aubard, Y.; Gosden, R.G. Transplantation in reproductive medicine: Previous experience, present knowledge and future prospects. Hum. Reprod. Update 1997, 3, 267–280. [Google Scholar] [CrossRef]
- Hosni, W.; Bastu, E. Ovarian stem cells and aging. Climacteric 2012, 15, 125–132. [Google Scholar] [CrossRef]
- Sills, E.S.; Rickers, N.S.; Li, X.; Palermo, G.D. First data on in vitro fertilization and blastocyst formation after intraovarian injection of calcium gluconate-activated autologous platelet rich plasma. Gynecol. Endocrinol. 2018, 34, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Monniaux, D.; Clément, F.; Dalbiès-Tran, R.; Estienne, A.; Fabre, S.; Mansanet, C.; Monget, P. The ovarian reserve of primordial follicles and the dynamic reserve of antral growing follicles: What is the link? Biol. Reprod. 2014, 90, 85. [Google Scholar] [CrossRef]
- Sills, E.S.; Li, X.; Rickers, N.S.; Wood, S.H.; Palermo, G.D. Metabolic and neurobehavioral response following intraovarian administration of autologous activated platelet rich plasma: First qualitative data. Neuroendocrinol. Lett. 2019, 39, 427–433. [Google Scholar]
- Dietl, J. Thomas Mann’s last novella “The Black Swan”: The tragic story of a post-menopausal woman. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 113, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Turner, C. Vasectomania and other cures for sloth—Better living through monkey glands. Cabinet 2008; Spring issue (#29): CABINET/Vasectomania, and Other Cures for Sloth. Available online: https://www.cabinetmagazine.org/ (accessed on 1 February 2021).
- Sengoopta, C. ‘Dr Steinach coming to make old young!’: Sex glands, vasectomy and the quest for rejuvenation in the roaring twenties. Endeavour 2003, 27, 122–126. [Google Scholar] [CrossRef]
- Nobus, D. The madness of Princess Alice: Sigmund Freud, Ernst Simmel, and Alice of Battenberg at Kurhaus Schloß Tegel. Hist. Psychiatry 2020, 31, 147–162. [Google Scholar] [CrossRef]
- Mishra, B.; Ortiz, L.; Luderer, U. Charged iron particles, components of space radiation, destroy ovarian follicles. Hum. Reprod. 2016, 31, 1816–1826. [Google Scholar] [CrossRef] [PubMed]
- Sharpey-Schafer, E. Endocrine physiology; 1st John Mallet Purser Lecture, Trinity College Dublin 26th June 1931. Ir. J. Med. Sci. 1931, 9, 483–505. [Google Scholar] [CrossRef]
- Cagnacci, A.; Venier, M. The controversial history of hormone replacement therapy. Medicina 2019, 55, 602. [Google Scholar] [CrossRef] [PubMed]
- Bolognese, M.A. SERMs and SERMs with estrogen for postmenopausal osteoporosis. Rev. Endocr. Metab. Disord. 2010, 11, 253–259. [Google Scholar] [CrossRef]
- Tatsioni, A.; Siontis, G.C.; Ioannidis, J.P. Partisan perspectives in the medical literature: A study of high frequency editorialists favoring hormone replacement therapy. J. Gen. Intern. Med. 2010, 25, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.A.; Etzioni, R.; Waters, T.M.; Pettinger, M.; Rossouw, J.E.; Anderson, G.L.; Chlebowski, R.T.; Manson, J.E.; Hlatky, M.; Johnson, K.C.; et al. Economic return from the Women’s Health Initiative estrogen plus progestin clinical trial: A modeling study. Ann. Intern. Med. 2014, 160, 594–602. [Google Scholar] [CrossRef]
- Hunter, M.M.; Huang, A.J.; Wallhagen, M.I. “I’m going to stay young”: Belief in anti-aging efficacy of menopausal hormone therapy drives prolonged use despite medical risks. PLoS ONE 2020, 15, e0233703. [Google Scholar] [CrossRef] [PubMed]
- Goltz, F.; Freusberg, A. Ueber den Einfluss des Nervensystems auf die Vorgängewährend der Schwangerschaft und des Gebärakts. Arch. Gesamte Physiol. Menschen Tiere 1874, 9, 552–565. [Google Scholar] [CrossRef]
- Varner, E. Transcending Gender: Assimilation, Identity, and Roman Imperial Portraits. In Memoirs of the American Academy in Rome; University of Michigan Press: Ann Arbor, MI, USA, 2008; Volume 7, pp. 200–201. [Google Scholar]
- Blackman, H.J. Women, Savages, and Other Animals: The Comparative Physiology of Reproduction, 1850–1914. Ph.D. Thesis, University Manchester, Manchester, UK, 2001. Available online: https://isni.org/isni/0000000426936675 (accessed on 1 February 2021).
- Haupt, C.; Henke, M.; Kutschmar, A.; Hauser, B.; Baldinger, S.; Schreiber, G. Antiandrogen or estradiol treatment or both during hormone therapy in transitioning transgender women. Cochrane Database Syst. Rev. 2020, 11, CD013138. [Google Scholar] [CrossRef] [PubMed]
- Obituary for Eugen Steinach. In The New York Times; 16 May 1944; p. 20.
- Niikura, Y.; Niikura, T.; Tilly, J.L. Aged mouse ovaries possess rare premeiotic germ cells that can generate oocytes following transplantation into a young host environment. Aging 2009, 1, 971–978. [Google Scholar] [CrossRef]
- Niikura, Y.; Niikura, T.; Wang, N.; Satirapod, C.; Tilly, J.L. Systemic signals in aged males exert potent rejuvenating effects on the ovarian follicle reserve in mammalian females. Aging 2010, 2, 999–1003. [Google Scholar] [CrossRef]
- Puhm, F.; Boilard, E.; Machlus, K.R. Platelet extracellular vesicles: Beyond the blood. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.H.; Sills, E.S. Intraovarian vascular enhancement via stromal injection of platelet-derived growth factors: Exploring subsequent oocyte chromosomal status and in vitro fertilization outcomes. Clin. Exp. Reprod. Med. 2020, 47, 94–100. [Google Scholar] [CrossRef]
- Puhm, F.; Afonyushkin, T.; Resch, U.; Obermayer, G.; Rohde, M.; Penz, T.; Schuster, M.; Wagner, G.; Rendeiro, A.; Melki, I.; et al. Mitochondria are a subset of extracellular vesicles released by activated monocytes and induce type I IFN and TNF responses in endothelial cells. Circ. Res. 2019, 125, 43–52. [Google Scholar] [CrossRef]
- Islam, M.N.; Das, S.R.; Emin, M.T.; Wei, M.; Sun, L.; Westphalen, K.; Rowlands, D.J.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012, 18, 759–765. [Google Scholar] [CrossRef]
- Phinney, D.G.; Di Giuseppe, M.; Njah, J.; Sala, E.; Shiva, S.; Croix, C.M.S.; Stolz, D.B.; Watkins, S.C.; Di, Y.P.; Leikauf, G.; et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat. Commun. 2015, 6, 8472. [Google Scholar] [CrossRef] [PubMed]
- Sills, E.S.; Wood, S.H. Autologous activated platelet-rich plasma injection into adult human ovary tissue: Molecular mechanism, analysis, and discussion of reproductive response. Biosci. Rep. 2019, 39, BSR20190805. [Google Scholar] [CrossRef]
- García-Martínez, O.; Reyes-Botella, C.; Díaz-Rodríguez, L.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; Vallecillo-Capilla, M.F.; Ruiz, C. Effect of platelet-rich plasma on growth and antigenic profile of human osteoblasts and its clinical impact. J. Oral Maxillofac. Surg. 2012, 70, 1558–1564. [Google Scholar] [PubMed]
- Sfakianoudis, K.; Simopoulou, M.; Nitsos, N.; Rapani, A.; Pappas, A.; Pantou, A.; Chronopoulou, M.; Deligeoroglou, E.; Koutsilieris, M.; Pantos, K. Autologous platelet-rich plasma treatment enables pregnancy for a woman in premature menopause. J. Clin. Med. 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Sills, E.S.; Rickers, N.S.; Svid, C.S.; Rickers, J.M.; Wood, S.H. Normalized ploidy following 20 consecutive blastocysts with chromosomal error: Healthy 46, XY pregnancy with IVF after intraovarian injection of autologous enriched platelet-derived growth factors. Int. J. Mol. Cell. Med. 2019, 8, 84–90. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sills, E.S. The Scientific and Cultural Journey to Ovarian Rejuvenation: Background, Barriers, and Beyond the Biological Clock. Medicines 2021, 8, 29. https://doi.org/10.3390/medicines8060029
Sills ES. The Scientific and Cultural Journey to Ovarian Rejuvenation: Background, Barriers, and Beyond the Biological Clock. Medicines. 2021; 8(6):29. https://doi.org/10.3390/medicines8060029
Chicago/Turabian StyleSills, E. Scott. 2021. "The Scientific and Cultural Journey to Ovarian Rejuvenation: Background, Barriers, and Beyond the Biological Clock" Medicines 8, no. 6: 29. https://doi.org/10.3390/medicines8060029
APA StyleSills, E. S. (2021). The Scientific and Cultural Journey to Ovarian Rejuvenation: Background, Barriers, and Beyond the Biological Clock. Medicines, 8(6), 29. https://doi.org/10.3390/medicines8060029





