Potency of Combining Eucalyptus camaldulensis subsp. camaldulensis with Low-Dose Cisplatin in A549 Human Lung Adenocarcinomas and MCF-7 Breast Adenocarcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Crude Extracts
2.3. Chemical Analysis
2.4. Chemical Quantifications of Secondary Products
2.4.1. Dry Matter Test
2.4.2. Soluble Sugar Test
2.4.3. Total Phenolic Content (TPC)
2.5. DPPH Scavenging Assay for Estimation of Antioxidant Activity
2.6. Evaluation of Antioxidant Activity
2.7. Culture and Treatment of Cancer Cell Line
2.7.1. A549 Cell Line
2.7.2. MCF-7 Cell Line
2.7.3. Peripheral Blood Mononuclear Cells (PBMC)
2.7.4. Preparation of the Tested Concentrations
2.7.5. Treatment of the Cells
2.7.6. Isolation of Peripheral Blood Mononuclear Cells (PBMC), Ficoll Pague Isolation
2.8. Viability Tests
2.8.1. Neutral Red Uptake Assay
2.8.2. MTT Assay
2.9. Statistical Analysis
3. Results
3.1. Chemical Testing Results
Extraction Yields of E. camaldulensis Crude Extracts
3.2. Chemical Quantifications of Secondary Products
Total Phenolic Content
3.3. Antioxidant Activity (AA)
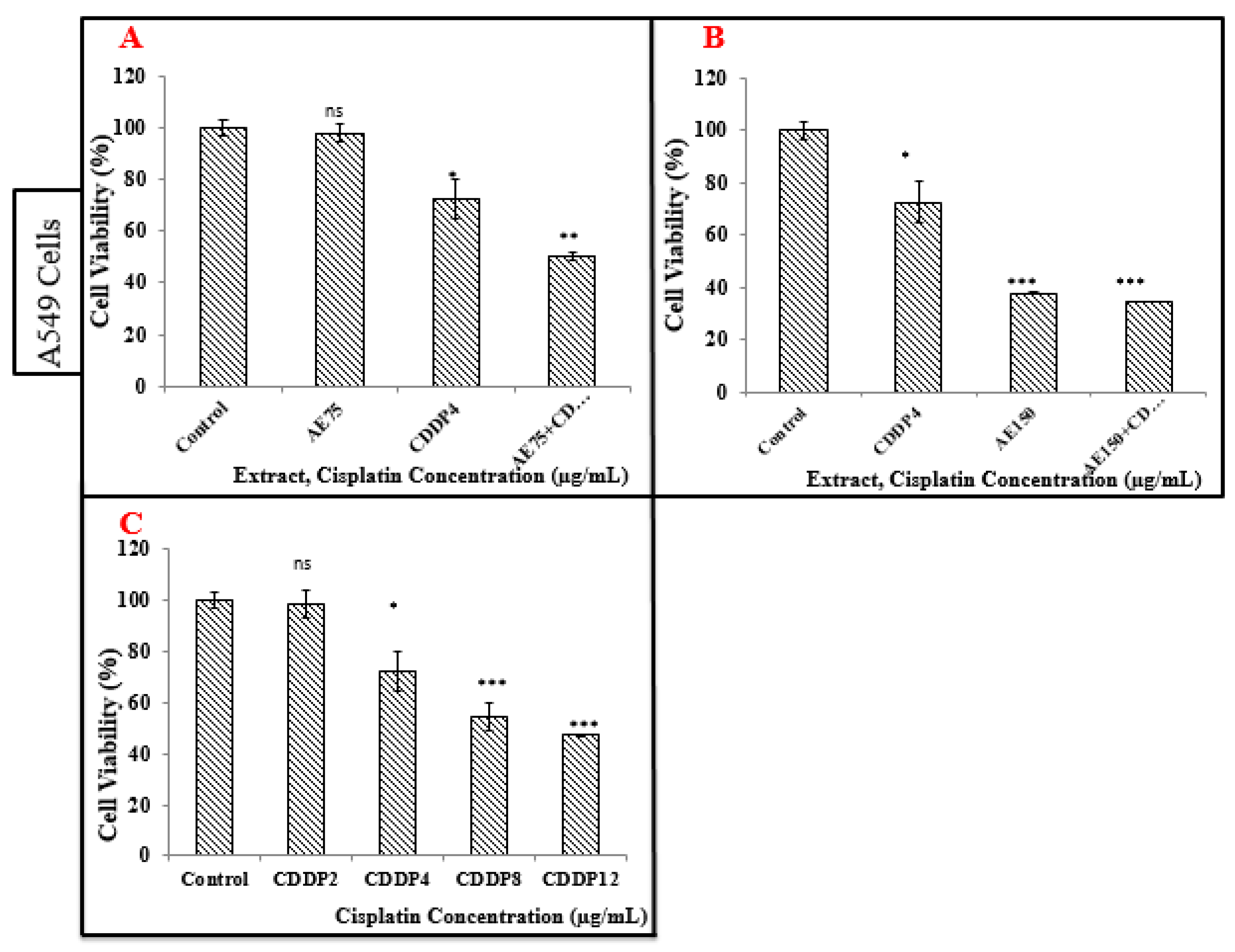
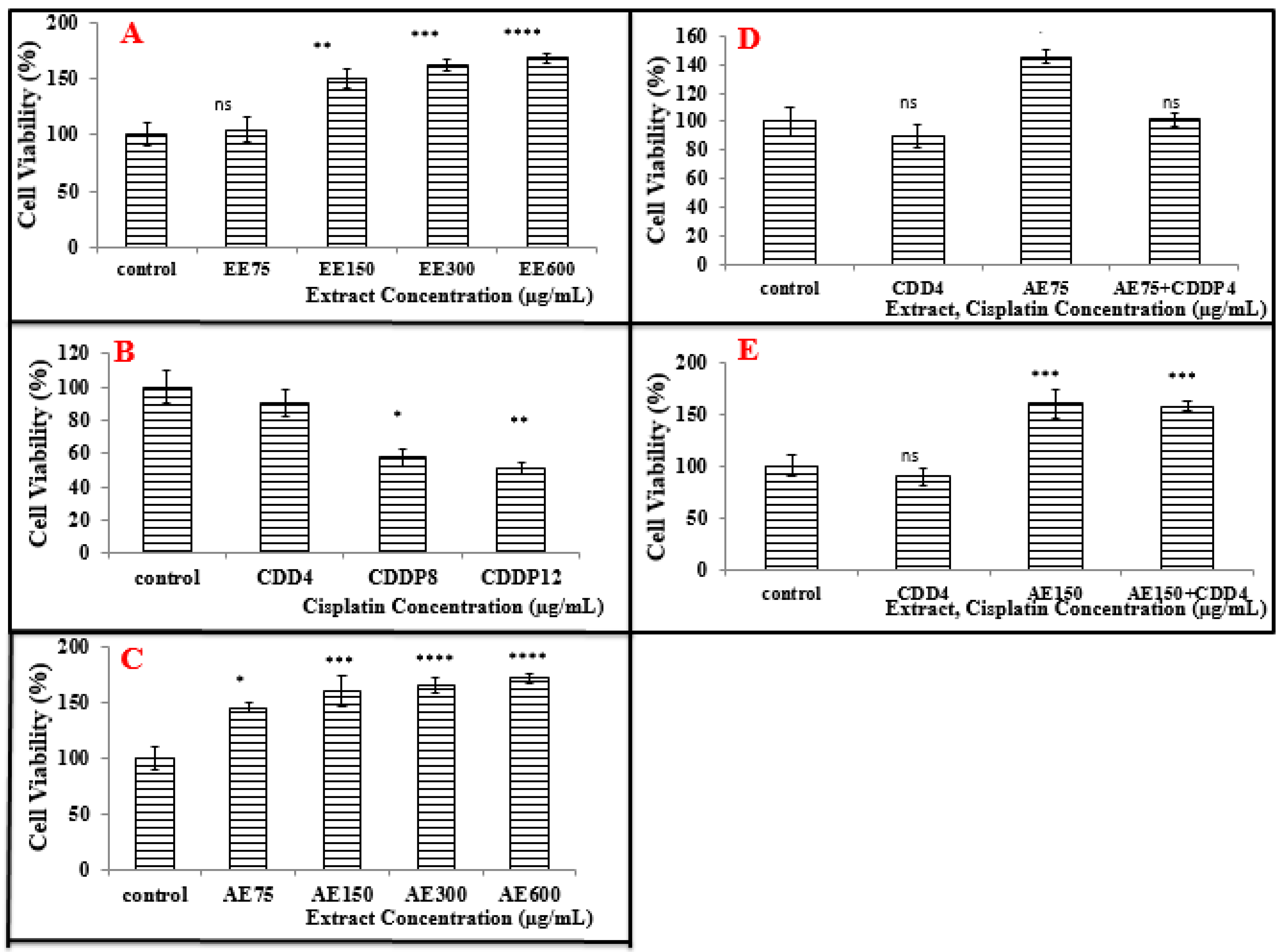
3.4. Cell Viability Tests on A549 and MCF-7 Cell Lines (Neutral Red Assay)
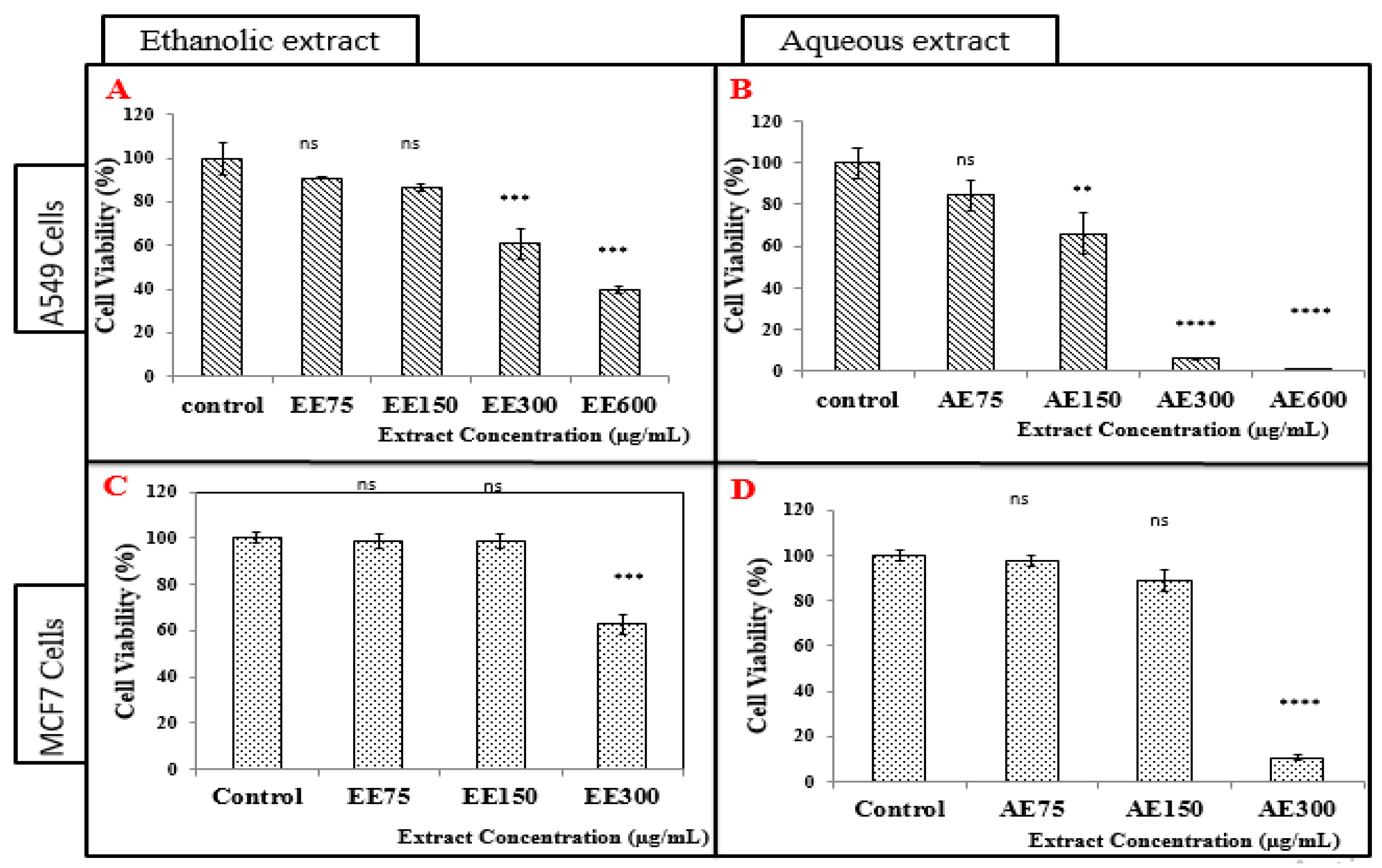
3.4.1. Treatment of A549 Cell Line with the Ethanolic Extract (EE)
3.4.2. Treatment of A549 Cell Line with the Aqueous Extract (AE)
3.4.3. Treatment of MCF-7 Cells with the Ethanolic Extract (EE)
3.4.4. Treatment of MCF-7 Cells with the Aqueous Extract (AE)
3.5. Treatment of A549 and MCF-7 Cell Lines with CDDP
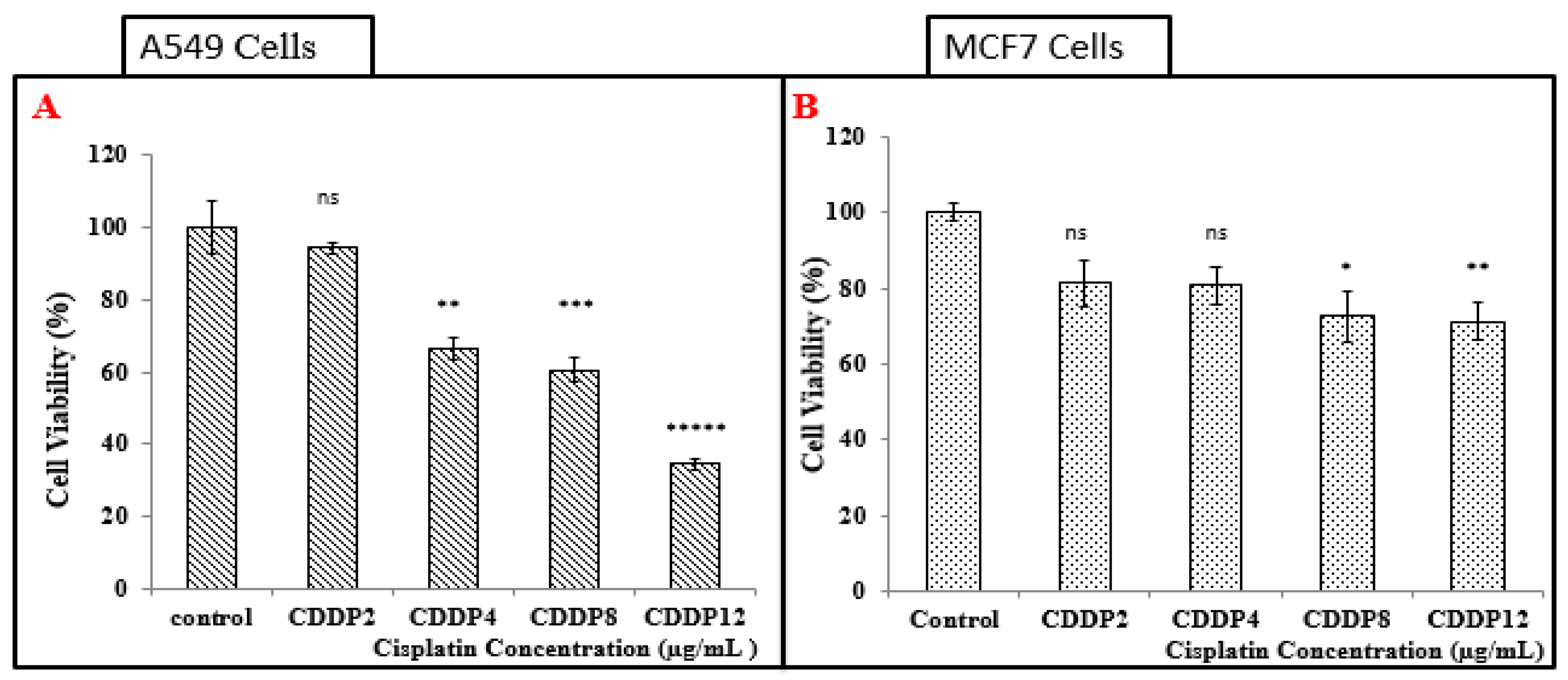
3.5.1. Treatment of A549 Cell Line with CDDP
3.5.2. Treatment of MCF-7 Cells with CDDP
3.6. Treatment of A549 and MCF-7 Cell Lines with the Combination of Aqueous Extract (AE) and CDDP
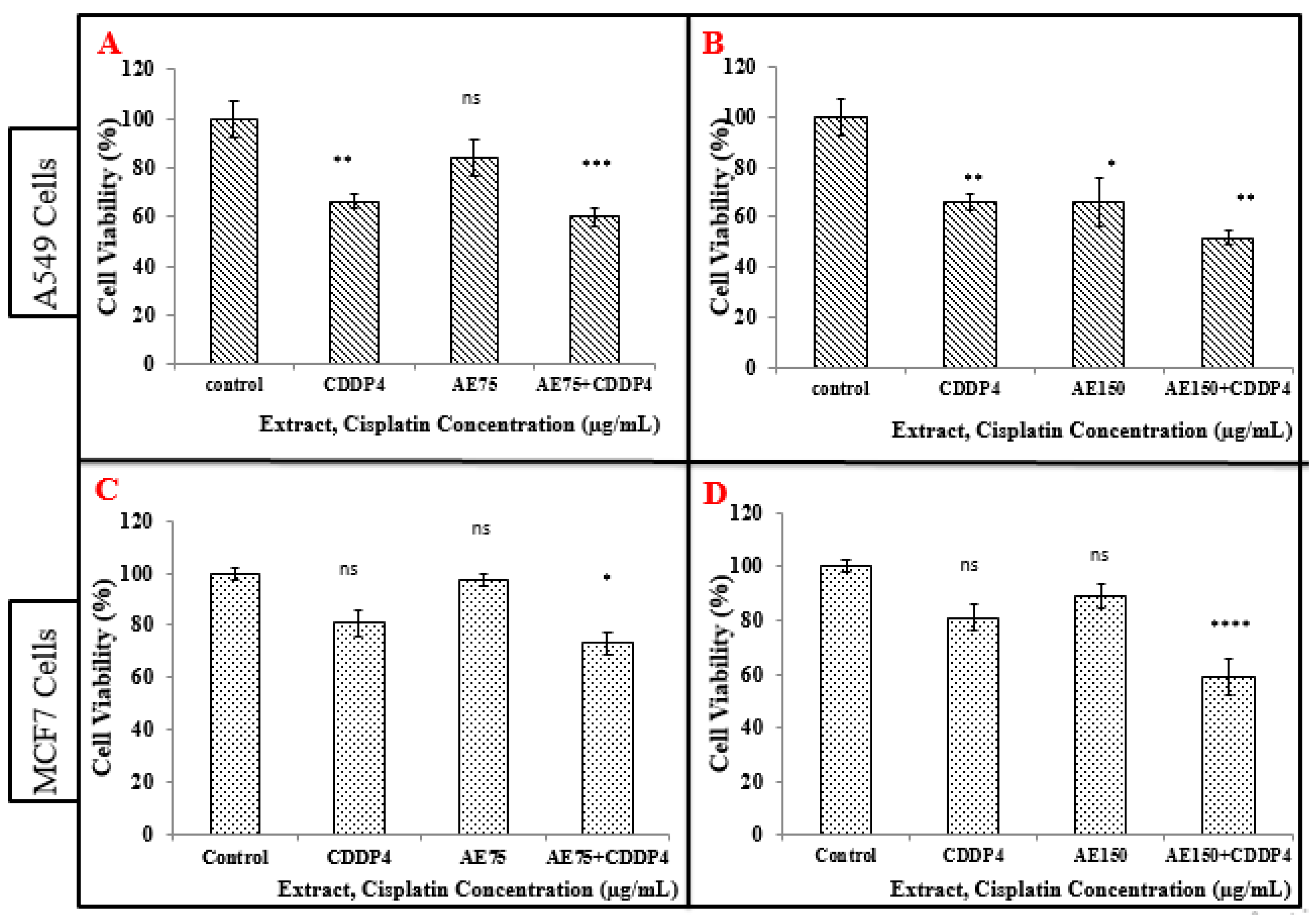
3.6.1. Treatment of A549 Cell Line with the Combination of Aqueous Extract (AE) and CDDP
3.6.2. Treatment of MCF-7 Cells with the Combination of Aqueous Extract (AE) and CDDP
3.7. Cell Viability Tests on A549 Cell Line (MTT Assay)
3.8. Cell Viability Tests on Peripheral Blood Mononuclear Cells (PBMC) (Neutral Red Assay)
3.8.1. Treatment of PBMC with the Ethanolic Extract (EE)
3.8.2. Treatment of PBMC with the Aqueous Extract (AE)
3.8.3. Treatment of PBMC with CDDP
3.8.4. Treatment of PBMC with the Combination of Aqueous Extract (AE) and CDDP
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mao, Y.; Yang, D.; He, J.; Krasna, M.J. Epidemiology of Lung Cancer. Surg. Oncol. Clin. N. Am. 2016, 25, 439–445. [Google Scholar] [CrossRef]
- Vinnitsky, V. The development of a malignant tumor is due to a desperate asexual self-cloning process in which cancer stem cells develop the ability to mimic the genetic program of germline cells. Intrinsically Disord. Proteins 2014, 2, e29997. [Google Scholar] [CrossRef][Green Version]
- Chan, B.A.; Coward, J.I. Chemotherapy advances in small-cell lung cancer. J. Thorac. Dis. 2013, 5, 565–578. [Google Scholar] [CrossRef]
- Yu, N.; Xiong, Y.; Wang, C. The water extract of chinese traditional herbal medicine, enhances cisplatin cytotoxicity in a549/ddp cells through induction of apoptosis and autophagy. Biomed. Res. Int. 2017, 2017, 3692797. [Google Scholar] [CrossRef] [PubMed]
- Chang, A. Chemotherapy, chemoresistance and the changing treatment landscape for NSCLC. Lung Cancer 2011, 71, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Dmitrenko, V.V. Pitfalls of the MTT assay: Direct and off-target effects of inhibitors can result in over/underestimation of cell viability. Gene 2015, 574, 193–203. [Google Scholar] [CrossRef]
- Suresh, S.; Ramalingam, T.K.O.; Fadlo, R. Lung cancer: New biological insights and recent therapeutic advances. ACS J. 2011, 61, 91–112. [Google Scholar] [CrossRef]
- Barrett-Lee, P.J.; Dixon, J.M.; Farrell, C.; Jones, A.; Leonard, R.; Murray, N.; Palmieri, C.; Plummer, C.J.; Stanley, A.; Verrill, M.W. Expert opinion on the use of anthracyclines in patients with advanced breast cancer at cardiac risk. Ann. Oncol. 2009, 20, 816–827. [Google Scholar] [CrossRef]
- Sulaiman, S.; Arafat, K.; Iratni, R.; Attoub, S. PTC-209 Anti-Cancer Effects Involved the Inhibition of STAT3 Phosphorylation. Front. Pharmacol. 2019, 10, 1199. [Google Scholar] [CrossRef]
- Li, F.; Wang, B.; He, M.; Chang, J.; Li, J.; Shan, L.; Wang, H.; Hong, W.; Luo, D.; Song, Y.; et al. Pilot study of docetaxel combined with lobaplatin or gemcitabine for recurrent and metastatic breast cancer. Medicine (Baltimore) 2019, 98, e18513. [Google Scholar] [CrossRef] [PubMed]
- Greenwell, M.; Rahman, P.K. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef]
- Nasser, M.; Cheikh-Ali, H.; Hijazi, A.; Merah, O.; Al-Rekaby, A.; Awada, R. Phytochemical Profile, Antioxidant and Antitumor Activities of Green Grape Juice. Processes 2020, 8, 507. [Google Scholar] [CrossRef]
- Silva, J.; Abebe, W.; Sousa, S.M.; Duarte, V.G.; Machado, M.I.; Matos, F.J. Analgesic and anti-inflammatory effects of essential oils of Eucalyptus. J. Ethnopharmacol. 2003, 89, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Sefidkon, F. The effect of distillation methods and stage of plant growth on the essential oil content and composition of Satureja rechingeri Jamzad. Food Chem. 2007, 100, 1054–1058. [Google Scholar] [CrossRef]
- Aleksic, V.; Knezevic, S.P. Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: A review. Ind. Crops Prod. 2019, 132, 413–429. [Google Scholar] [CrossRef]
- Herzi, N.; Bouajila, J.; Camy, S.; Cazaux, S.; Romdhane, M.; Condoret, J.S. Comparison between supercritical CO2 extraction and hydrodistillation for two species of eucalyptus: Yield, chemical composition, and antioxidant activity. J. Food Sci. 2013, 78, C667–C672. [Google Scholar] [CrossRef]
- Huang, H.-C.; Ho, Y.-C.; Lim, J.-M.; Chang, T.-Y.; Ho, C.-L.; Chang, T.M. Investigation of the Anti-Melanogenic and Antioxidant Characteristics of Eucalyptus camaldulensis Flower Essential Oil and Determination of Its Chemical Composition. Int. J. Mol. Sci. 2015, 16, 10470–10490. [Google Scholar] [CrossRef]
- Döll-Boscardin, P.M.; Sartoratto, A.; Sales Maia, B.H.L.d.N.; Padilha de Paula, J.; Nakashima, T.; Farago, P.V.; Kanunfre, C.C. In Vitro Cytotoxic Potential of Essential Oils of Eucalyptus benthamii and Its Related Terpenes on Tumor Cell Lines. Evid. Based Complement. Altern. Med. 2012, 2012, 342652. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Dlzar, A.; Omer, A.; Gianluca, G.; Giovanni, V. Components of Volatile Fractions from Eucalyptus camaldulensis Leaves from Iraqi–Kurdistan and Their Potent Spasmolytic Effects. Molecules 2020, 25, 804. [Google Scholar] [CrossRef]
- Brand, W.; Cuvelier, W.; Berset, M.E. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Shamseddine, A.; Saleh, A.; Charafeddine, M.; Seoud, M.; Mukherji, D.; Temraz, S.; Sibai, A.M. Cancer trends in Lebanon: A review of incidence rates for the period of 2003–2008 and projections until 2018. Popul. Health Metr. 2014, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Naeimeh, M.; Monireh, R. Study of cytotoxic effects of Ethanolic extract of Eucalyptus camaldulensis leaf on the cells k562 of human chronic Myelogenous leukemia (CML) under in Vitro conditions. Bull. Environ. Pharmacol. Life Sci. 2014, 3, 186–190. [Google Scholar]
- Ashour, H.M. Antibacterial, antifungal, and anticancer activities of volatile oils and extracts from stems, leaves, and flowers of Eucalyptus sideroxylon and Eucalyptus torquata. Cancer Biol. Ther. 2007, 7, 399–403. [Google Scholar] [CrossRef]
- Mulugeta, M.; Fazlurrahman, K.; Gizachew, M.; Archana, P. Phytochemical Profile and Antimicrobial Effects of Different Medicinal Plant: Current Knowledge and Future Perspectives. Curr. Tradit. Med. 2020, 6, 24–42. [Google Scholar] [CrossRef]
- Abadio Finco, F.; Kloss, L.; Graeve, L. Bacaba (Oenocarpus bacaba) phenolic extract induces apoptosis in the MCF-7 breast cancer cell line via the mitochondria-dependent pathway. NFS J. 2016, 5. [Google Scholar] [CrossRef]
- Catanzaro, D.; Vianello, C.; Ragazzi, E.; Caparrotta, L.; Montopoli, M. Cell cycle control by natural phenols in cisplatin-resistant cell lines. Nat. Prod. Commun. 2014, 9, 1465–1468. [Google Scholar] [CrossRef]
- Sun, Q.; Heilmann, J.; König, B. Natural phenolic metabolites with anti-angiogenic properties—A review from the chemical point of view. Beilstein J. Org. Chem. 2015, 11, 249–264. [Google Scholar] [CrossRef]
| Plant Constituents | Extract | Reagent | Color |
|---|---|---|---|
| Reducing sugar | 0.5 mL | 1 mL water + 5–8 drops of Fehlings (A + B) + boil | Brick-red precipitate |
| Anthraquinones | 1 mL | 1 mL HCL (10%) + boil | Precipitate |
| Proteins and amino acids | 1 mL | 1 mL ninhydrin (0.25%) + boil | Blue color |
| Phlabotannins | 1 mL | 1 mL HCL (1%) + boil (5 min) + cooling | Red precipitate |
| Alkaloids | 1 mL | Five drops of Dragendorff | Reddish orange precipitate/reddish brown or turbidity |
| Tannins | 1 mL | Ferric chloride (FeCl3 1%) | Blue color |
| Resins | 1 mL | Acetone + small amount of water + agitation | Turbidity |
| Terpenoids | 1 mL | 2 mL chloroform + 3 mL concentrated sulphuric acid | Reddish brown color in the surface |
| Flavonoids | 1 mL | 5 mL potassium hydroxide (KOH 50%) | Yellow color |
| Quinones | 1 mL | HCL concentrated | Precipitate or yellow color |
| Sterols and Steroids | 1 mL | 2 mL chloroform + concentrated sulphuric acid | Red color of the upper layer + greenish yellow fluorescence in the acid layer |
| Diterpenes | 1 mL dissolved in water | Few drops of copper sulphate | Green color |
| Anthocyanins | 1 mL | 1 mL NaOH (10%) | Blue color |
| Flavanones | 1 mL | 1 mL concentrated sulphuric acid | Purple red color |
| Lignines | 2 mL | Safranine | Pink color |
| Cardiac glycosides | 2 mL | 1 mL acetic acid glacial + 1 drop ferric chloride FeCl3 (5%) + 1 mL concentrated sulphuric acid | Purple ring + brown ring + green ring |
| Saponins | 2 mL | Vigorous shaking (5 min on Vortex) | Layer of foam |
| Phenols | 5 mL | 1 mL FeCl3 (1%) + 1 mL K3(Fe(CN)6) (1%) | Greenish blue color |
| Fixed oils and Fatty acids | Small amount of extract | On filter paper | Oil spot |
| Solvent | Initial Weight (g) | Final Weight (g) | Extraction Yield (%) |
|---|---|---|---|
| Ethanol | 20.0 | 3.3 | 16.5 |
| Water | 20.0 | 4.0 | 20.0 |
| Plant Constituents | Ethanol | Aqueous |
|---|---|---|
| Reducing sugar | + | + |
| Anthraquinones | + | + |
| Proteins and amino acids | - | - |
| Phlabotannins | - | - |
| Alkaloids | + | + |
| Tannins | + | + |
| Resins | + | - |
| Terpenoids | + | + |
| Flavonoids | + | + |
| Quinones | + | + |
| Sterols and Steroids | + | + |
| Diterpenes | + | + |
| Anthocyanins | - | - |
| Flavanones | + | + |
| Lignines | + | + |
| Cardiac glycosides | + | - |
| Saponins | - | + |
| Phenols | + | + |
| Fixed oils | + | + |
| Solvent | TPC (mg GAE/g of Extract) | Soluble Sugar Test (%) | Dry matter (%) | Antioxidant Activity (TE Umol/g of Extract) |
|---|---|---|---|---|
| Ethanol | 15.33 ± 0.54 | 9.95 | 95.06 | 211.89 ± 0.39 |
| Aqueous | 19.52 ± 0.22 | 9.95 | 95.06 | 214.01 ± 1.66 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasser, M.; Damaj, R.; Merah, O.; Hijazi, A.; Trabolsi, C.; Wehbe, N.; Nasser, M.; Al-Khatib, B.; Damaj, Z. Potency of Combining Eucalyptus camaldulensis subsp. camaldulensis with Low-Dose Cisplatin in A549 Human Lung Adenocarcinomas and MCF-7 Breast Adenocarcinoma. Medicines 2020, 7, 40. https://doi.org/10.3390/medicines7080040
Nasser M, Damaj R, Merah O, Hijazi A, Trabolsi C, Wehbe N, Nasser M, Al-Khatib B, Damaj Z. Potency of Combining Eucalyptus camaldulensis subsp. camaldulensis with Low-Dose Cisplatin in A549 Human Lung Adenocarcinomas and MCF-7 Breast Adenocarcinoma. Medicines. 2020; 7(8):40. https://doi.org/10.3390/medicines7080040
Chicago/Turabian StyleNasser, Mohamad, Raghida Damaj, Othmane Merah, Akram Hijazi, Christine Trabolsi, Nour Wehbe, Malak Nasser, Batoul Al-Khatib, and Ziad Damaj. 2020. "Potency of Combining Eucalyptus camaldulensis subsp. camaldulensis with Low-Dose Cisplatin in A549 Human Lung Adenocarcinomas and MCF-7 Breast Adenocarcinoma" Medicines 7, no. 8: 40. https://doi.org/10.3390/medicines7080040
APA StyleNasser, M., Damaj, R., Merah, O., Hijazi, A., Trabolsi, C., Wehbe, N., Nasser, M., Al-Khatib, B., & Damaj, Z. (2020). Potency of Combining Eucalyptus camaldulensis subsp. camaldulensis with Low-Dose Cisplatin in A549 Human Lung Adenocarcinomas and MCF-7 Breast Adenocarcinoma. Medicines, 7(8), 40. https://doi.org/10.3390/medicines7080040







