Autologous Biologic Treatment with Fat, Bone Marrow Aspirate and Platelet Rich Plasma Is an Effective Alternative to Total Knee Arthroplasty for Patients with Moderate Knee Arthrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Injections
2.1.1. Bone Marrow
2.1.2. Autologous Adipose Tissue
2.1.3. PRP
2.2. Treatment Regimen
2.3. Outcome Data
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Hess, S.R.; O’Connell, R.S.; Bednarz, C.P.; Waligora, A.C.; Golladay, G.J.; Jiranek, W.A. Association of rapidly destructive osteoarthritis of the hip with intra-articular steroid injections. Arthroplast. Today 2018, 4, 205–209. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; LaValley, M.P.; Harvey, W.F.; Price, L.L.; Driban, J.B.; Zhang, M.; Ward, R.J. Effect of Intra-articular Triamcinolone vs. Saline on Knee Cartilage Volume and Pain in Patients with Knee Osteoarthritis: A Randomized Clinical Trial. JAMA 2017, 317, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Schairer, W.W.; Nwachukwu, B.U.; Mayman, D.J.; Lyman, S.; Jerabek, S.A. Preoperative Hip Injections Increase the Rate of Periprosthetic Infection After Total Hip Arthroplasty. J. Arthroplast. 2016, 31 (Suppl. 9), 166–169. [Google Scholar] [CrossRef] [PubMed]
- Werner, B.C.; Cancienne, J.M.; Browne, J.A. The Timing of Total Hip Arthroplasty After Intraarticular Hip Injection Affects Postoperative Infection Risk. J. Arthroplast. 2016, 31, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Al-Omari, A.A.; Aleshawi, A.J.; Marei, O.A.; Younes, H.M.B.; Alawneh, K.Z.; Mohaidat, Z.M. Avascular necrosis of the femoral head after single steroid intra-articular injection. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Bali, K.; Meena, D.; Krishnan, V.; Chana, R.; Rawall, S.; Aggarwal, S. Steroid-induced stress fracture of medial tibial condyle: A case report. J. Knee Surg. 2013, 26 (Suppl. 1), S25–S29. [Google Scholar]
- Kontovazenitis, P.I.; Starantzis, K.A.; Soucacos, P.N. Major complication following minor outpatient procedure: Osteonecrosis of the knee after intraarticular injection of cortisone for treatment of knee arthritis. J. Surg. Orthop. Adv. 2009, 18, 42–44. [Google Scholar]
- Simeone, F.J.; Vicentini, J.R.T.; Bredella, M.A.; Chang, C.Y. Are patients more likely to have hip osteoarthritis progression and femoral head collapse after hip steroid/anesthetic injections? A retrospective observational study. Skelet. Radiol. 2019, 48, 1417–1426. [Google Scholar] [CrossRef]
- Wolfe, M.M.; Lichtenstein, D.R.; Singh, G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N. Engl. J. Med. 1999, 340, 1888–1899. [Google Scholar] [CrossRef]
- Aweid, O.; Haider, Z.; Saed, A.; Kalairajah, Y. Treatment modalities for hip and knee osteoarthritis: A systematic review of safety. J. Orthop. Surg. 2018, 26, 2309499018808669. [Google Scholar] [CrossRef] [PubMed]
- Carey, K.; Morgan, J.R. Payments for outpatient joint replacement surgery: A comparison of hospital outpatient departments and ambulatory surgery centers. Health Serv. Res. 2020, 55, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Iorio, R.; Barnes, C.L.; Vitale, M.P.; Huddleston, J.I.; Haas, D.A. Total Knee Replacement: The Inpatient-Only List and the Two Midnight Rule, Patient Impact, Length of Stay, Compliance Solutions, Audits, and Economic Consequences. J. Arthroplast. 2020, 35, S28–S32. [Google Scholar] [CrossRef] [PubMed]
- Kremers, H.M.; Larson, D.R.; Crowson, C.S.; Kremers, W.K.; Washington, R.E.; Steiner, C.A.; Jiranek, W.A.; Berry, D.J. Prevalence of Total Hip and Knee Replacement in the United States. J. Bone Jt. Surg. Am. 2015, 97, 1386–1397. [Google Scholar] [CrossRef]
- Raddaoui, K.; Khedhri, W.; Zoghlami, K.; Radhouani, M.; Trigui, E.; Kaabachi, O. Perioperative morbidity in total knee arthroplasty. Pan Afr. Med. J. 2019, 33, 233. [Google Scholar] [CrossRef]
- Richardson, S.S.; Kahlenberg, C.A.; Blevins, J.L.; Goodman, S.M.; Sculco, T.P.; Figgie, M.P.; Sculco, P.K. Complications associated with staged versus simultaneous bilateral total knee arthroplasty: An analysis of 7747 patients. Knee 2019, 26, 1096–1101. [Google Scholar] [CrossRef]
- Inacio, M.C.S.; Paxton, E.W.; Graves, S.E.; Namba, R.S.; Nemes, S. Projected increase in total knee arthroplasty in the United States—An alternative projection model. Osteoarthr. Cartil. 2017, 25, 1797–1803. [Google Scholar] [CrossRef]
- Singh, J.A.; Lewallen, D.G. Time trends in the characteristics of patients undergoing primary total knee arthroplasty. Arthritis Care Res. 2014, 66, 897–906. [Google Scholar] [CrossRef]
- Fodor, P.B.; Paulseth, S.G. Adipose Derived Stromal Cell (ADSC) Injections for Pain Management of Osteoarthritis in the Human Knee Joint. Aesthet. Surg. J. 2016, 36, 229–236. [Google Scholar] [CrossRef]
- Hudetz, D.; Borić, I.; Rod, E.; Jeleč, Ž.; Radić, A.; Vrdoljak, T.; Skelin, A.; Lauc, G.; Trbojević-Akmačić, I.; Plečko, M.; et al. The Effect of Intra-articular Injection of Autologous Microfragmented Fat Tissue on Proteoglycan Synthesis in Patients with Knee Osteoarthritis. Genes 2017, 8, 270. [Google Scholar] [CrossRef]
- Roato, I.; Belisario, D.C.; Compagno, M.; Lena, A.; Bistolfi, A.; Maccari, L.; Mussano, F.; Genova, T.; Godio, L.; Perale, G.; et al. Concentrated adipose tissue infusion for the treatment of knee osteoarthritis: Clinical and histological observations. Int. Orthop. 2018, 43, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Screpis, D.; Di Donato, S.L.; Bonetti, S.; Piovan, G.; Zorzi, C. Autologous micro-fragmented adipose tissue for the treatment of diffuse degenerative knee osteoarthritis: An update at 3 year follow-up. J. Exp. Orthop. 2018, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Dall’Oca, C.; Breda, S.; Elena, N.; Valentini, R.; Samaila, E.M.; Magnan, B. Mesenchymal Stem Cells injection in hip osteoarthritis: Preliminary results. Acta Biomed. 2019, 90, 75–80. [Google Scholar] [PubMed]
- Emadedin, M.; Labibzadeh, N.; Liastani, M.G.; Karimi, A.; Jaroughi, N.; Bolurieh, T.; Hosseini, S.E.; Baharvand, H.; Aghdami, N. Intra-articular implantation of autologous bone marrow-derived mesenchymal stromal cells to treat knee osteoarthritis: A randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy 2018, 20, 1238–1246. [Google Scholar] [CrossRef]
- Garay-Mendoza, D.; Villarreal-Martínez, L.; Garza-Bedolla, A.; Pérez-Garza, D.M.; Acosta-Olivo, C.; Vilchez-Cavazos, F.; Diaz-Hutchinson, C.; Gómez-Almaguer, D.; Jaime-Pérez, J.C.; Mancías-Guerra, C. The effect of intra-articular injection of autologous bone marrow stem cells on pain and knee function in patients with osteoarthritis. Int. J. Rheum. Dis. 2018, 21, 140–147. [Google Scholar] [CrossRef]
- Li, J.; Shao, Q.; Zhu, X.; Sun, G. Efficacy of autologous bone marrow mesenchymal stem cells in the treatment of knee osteoarthritis and their effects on the expression of serum TNF-alpha and IL-6. J. Musculoskelet Neuronal Interact. 2020, 20, 128–135. [Google Scholar]
- Migliorini, F.; Rath, B.; Colarossi, G.; Driessen, A.; Tingart, M.; Niewiera, M.; Eschweiler, J. Improved outcomes after mesenchymal stem cells injections for knee osteoarthritis: Results at 12-months follow-up: A systematic review of the literature. Arch. Orthop. Trauma Surg. 2020, 140, 853–868. [Google Scholar] [CrossRef]
- Vega, A.; Martín-Ferrero, M.A.; Del Canto, F.; Alberca, M.; García, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.J.; Huguet, M.; et al. Treatment of Knee Osteoarthritis With Allogeneic Bone Marrow Mesenchymal Stem Cells: A Randomized Controlled Trial. Transplantation 2015, 99, 1681–1690. [Google Scholar] [CrossRef]
- Shapiro, S.A.; Kazmerchak, S.E.; Heckman, M.G.; Zubair, A.C.; O’Connor, M.I. A Prospective, Single-Blind, Placebo-Controlled Trial of Bone Marrow Aspirate Concentrate for Knee Osteoarthritis. Am. J. Sports Med. 2016, 45, 82–90. [Google Scholar] [CrossRef]
- Centeno, C.; Pitts, J.; Al-Sayegh, H.; Freeman, M. Efficacy of autologous bone marrow concentrate for knee osteoarthritis with and without adipose graft. BioMed Res. Int. 2014, 2014, 370621. [Google Scholar] [CrossRef]
- Kim, J.D.; Lee, G.W.; Jung, G.H.; Kim, C.K.; Kim, T.; Park, J.H.; Cha, S.S.; You, Y.B. Clinical outcome of autologous bone marrow aspirates concentrate (BMAC) injection in degenerative arthritis of the knee. Eur. J. Orthop. Surg. Traumatol. 2014, 24, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.A.; Saltzman, B.M.; Mascarenhas, R.; Khair, M.M.; Verma, N.N.; Bach, B.R.J.; Cole, B.J. Does Intra-articular Platelet-Rich Plasma Injection Provide Clinically Superior Outcomes Compared With Other Therapies in the Treatment of Knee Osteoarthritis? A Systematic Review of Overlapping Meta-analyses. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 31, 2213–2221. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli, M.; Janmohammadi, N.; Hosseini, A.; Khafri, S.; Esmaeilnejad-Ganji, S.M. Single- and double-dose of platelet-rich plasma versus hyaluronic acid for treatment of knee osteoarthritis: A randomized controlled trial. World J. Orthop. 2019, 10, 310–326. [Google Scholar] [CrossRef]
- Muschler, G.F.; Boehm, C.; Easley, K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: The influence of aspiration volume. J. Bone Jt. Surg. Am. 1997, 79, 1699–1709. [Google Scholar] [CrossRef]
- DeLong, J.M.; Russell, R.P.; Mazzocca, A.D. Platelet-rich plasma: The PAW classification system. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 998–1009. [Google Scholar] [CrossRef]
- Davies, M.A.; Kerr, Z.Y.; DeFreese, J.D.; Arden, N.K.; Marshall, S.W.; Guskiewicz, K.M.; Padua, D.A.; Pietrosimone, B. Prevalence of and Risk Factors for Total Hip and Knee Replacement in Retired National Football League Athletes. Am. J. Sports Med. 2019, 47, 2863–2870. [Google Scholar] [CrossRef] [PubMed]
- Eymard, F.; Chevalier, X.; Conrozier, T. Obesity and radiological severity are associated with viscosupplementation failure in patients with knee osteoarthritis. J. Orthop. Res. 2017, 35, 2269–2274. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, F.; Shafiq, B.; Zamir, M.; Noor, S.; Memon, N.; Memon, N.; Dina, T.K. Micro-organisms and risk factors associated with prosthetic joint infection following primary total knee replacement-our experience in Pakistan. Int. Orthop. 2020, 44, 283–289. [Google Scholar] [CrossRef]
- Kulkarni, K.; Karssiens, T.; Kumar, V.; Pandit, H. Obesity and osteoarthritis. Maturitas 2016, 89, 22–28. [Google Scholar] [CrossRef]
- Wang, C.T.; Lin, J.; Chang, C.J.; Lin, Y.T.; Hou, S.M. Therapeutic effects of hyaluronic acid on osteoarthritis of the knee. A meta-analysis of randomized controlled trials. J. Bone Jt. Surg. Am. 2004, 86, 538–545. [Google Scholar] [CrossRef]
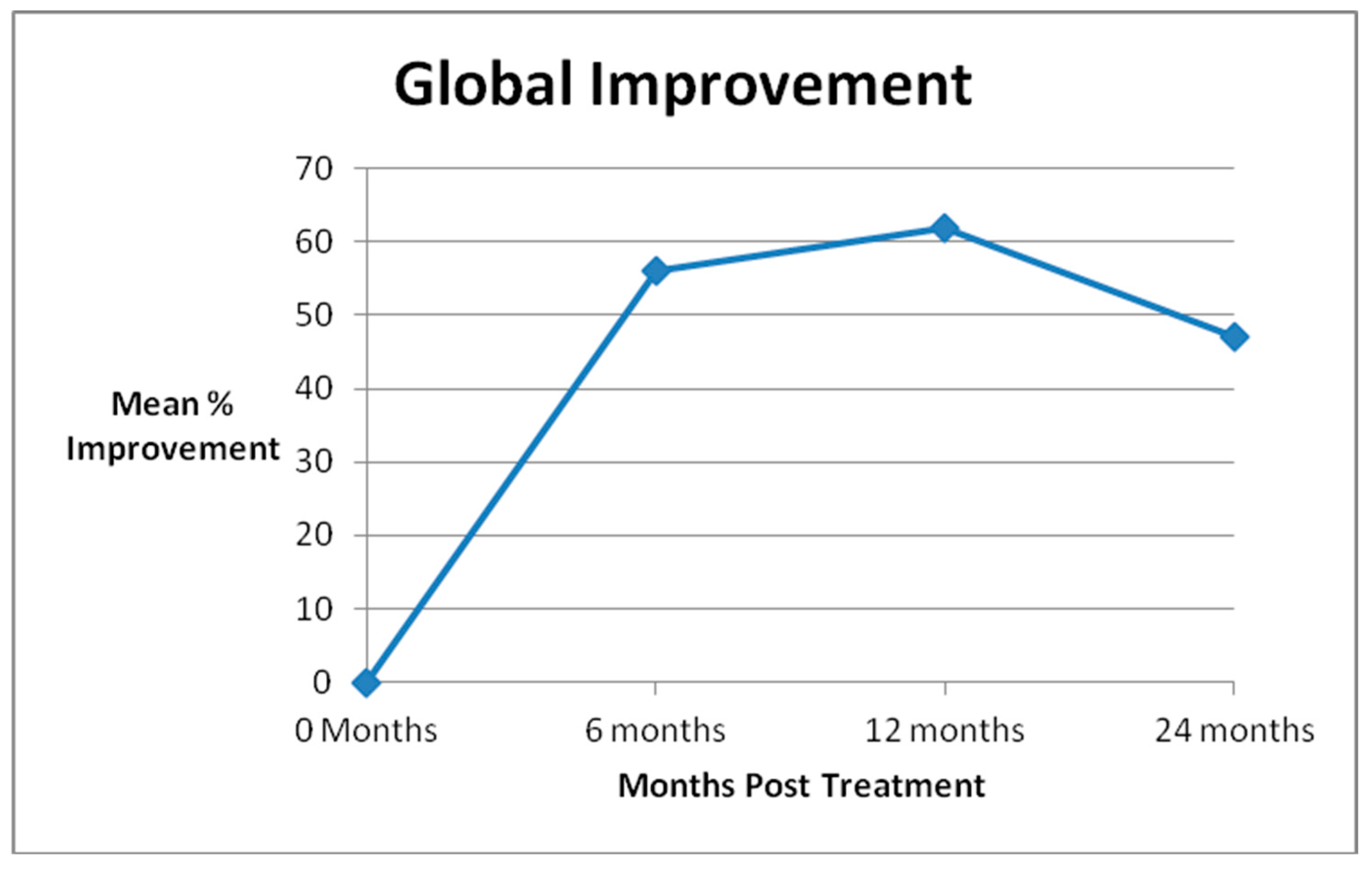
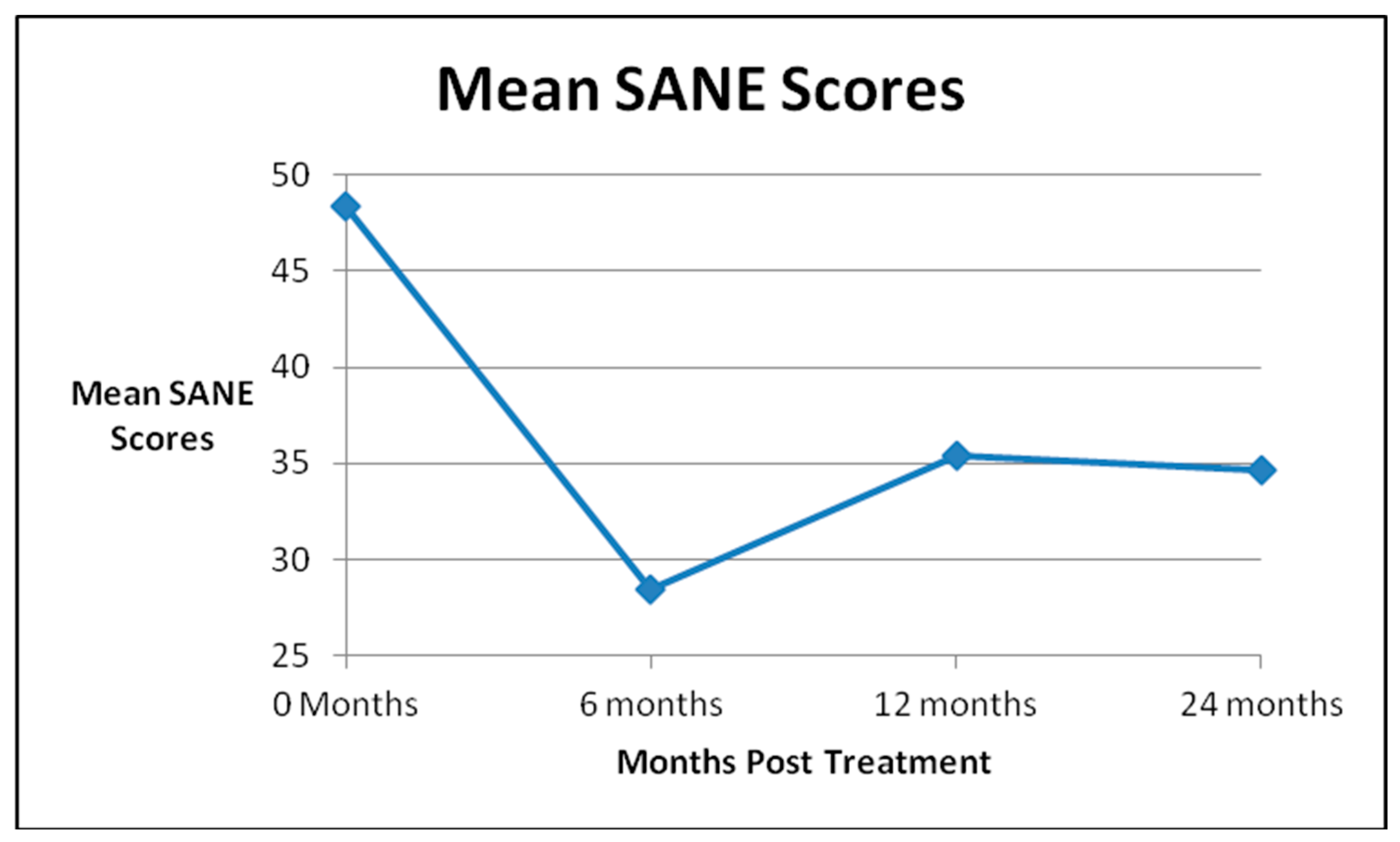
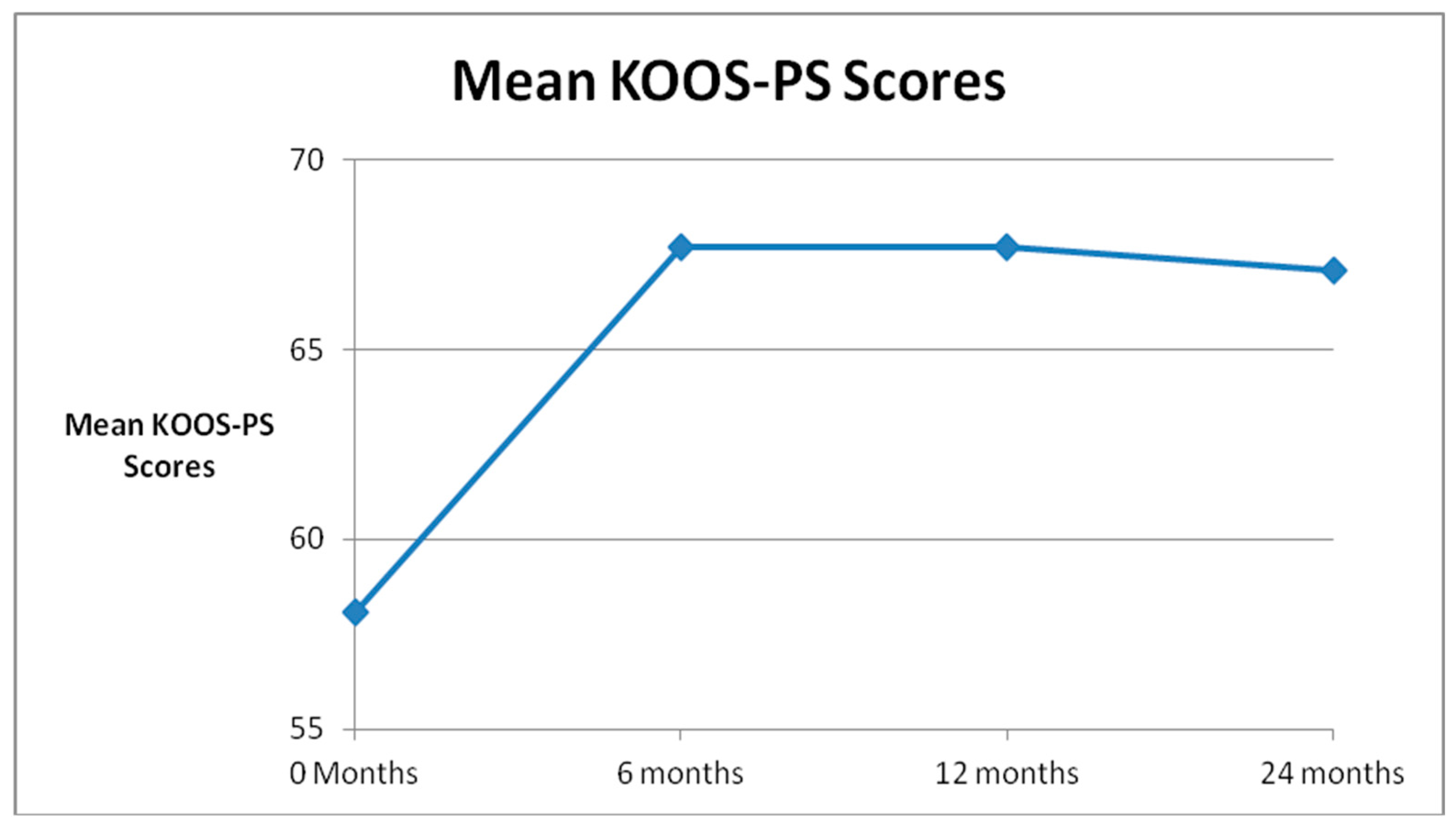
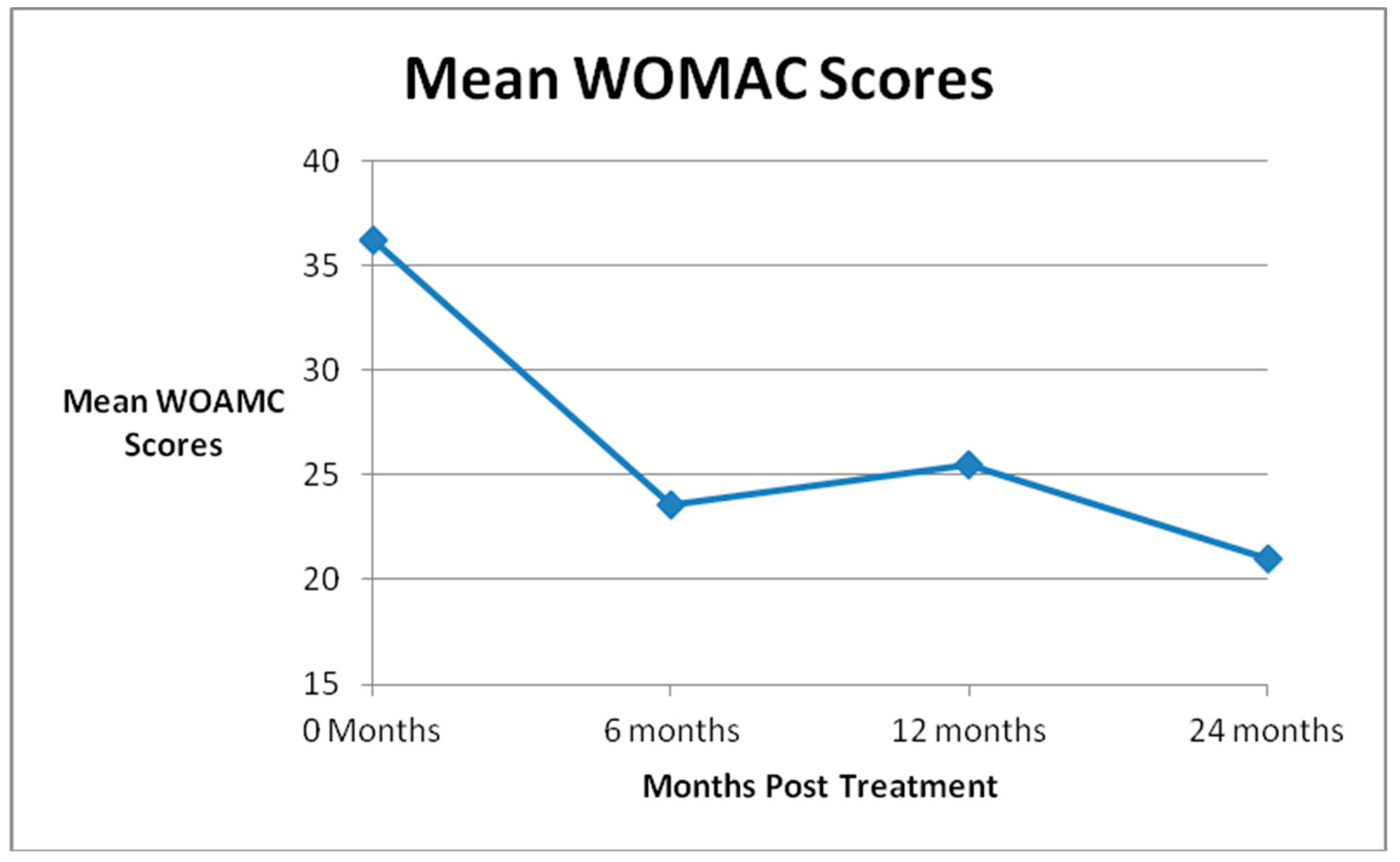
| Global Improvement | SANE Scores | KOOS-PS Scores | WOMAC Scores | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| # | Mean % Improvement | # | Mean Score | p Value | # | Mean Score | p Value | # | Mean Score | p Value | |
| Pre Treatment | 46 | 48.4 | 45 | 58.1 | 33 | 36.2 | |||||
| 6 Month FU (N = 45) | 41 | 56 | 41 | 28.4 | p = 0.0002 | 37 | 67.7 | p = 0.0004 | 37 | 23.6 | p = 0.002 |
| 1 Year FU (N = 45) | 38 | 62 | 37 | 35.4 | p = 0.02 | 32 | 67.7 | p = 0.004 | 31 | 25.5 | p = 0.02 |
| 2 Years FU (N = 31) | 22 | 47 | 18 | 34.6 | p = 0.05 | 19 | 67.1 | p = 0.02 | 20 | 21.0 | p = 0.002 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prodromos, C.; Finkle, S. Autologous Biologic Treatment with Fat, Bone Marrow Aspirate and Platelet Rich Plasma Is an Effective Alternative to Total Knee Arthroplasty for Patients with Moderate Knee Arthrosis. Medicines 2020, 7, 37. https://doi.org/10.3390/medicines7060037
Prodromos C, Finkle S. Autologous Biologic Treatment with Fat, Bone Marrow Aspirate and Platelet Rich Plasma Is an Effective Alternative to Total Knee Arthroplasty for Patients with Moderate Knee Arthrosis. Medicines. 2020; 7(6):37. https://doi.org/10.3390/medicines7060037
Chicago/Turabian StyleProdromos, Chadwick, and Susan Finkle. 2020. "Autologous Biologic Treatment with Fat, Bone Marrow Aspirate and Platelet Rich Plasma Is an Effective Alternative to Total Knee Arthroplasty for Patients with Moderate Knee Arthrosis" Medicines 7, no. 6: 37. https://doi.org/10.3390/medicines7060037
APA StyleProdromos, C., & Finkle, S. (2020). Autologous Biologic Treatment with Fat, Bone Marrow Aspirate and Platelet Rich Plasma Is an Effective Alternative to Total Knee Arthroplasty for Patients with Moderate Knee Arthrosis. Medicines, 7(6), 37. https://doi.org/10.3390/medicines7060037




