Abstract
A current research topic of great interest is the study of the therapeutic properties of plants and of their bioactive secondary metabolites. Plants have been used to treat all types of health problems from allergies to cancer, in addition to their use in the perfumery industry and as food. Hedychium species are among those plants used in folk medicine in several countries and several works have been reported to verify if and how effectively these plants exert the effects reported in folk medicine, studying their essential oils, extracts and pure secondary metabolites. Hedychium coronarium and Hedychium spicatum are the most studied species. Interesting compounds have been identified like coronarin D, which possesses antibacterial, antifungal and antitumor activities, as well as isocoronarin D, linalool and villosin that exhibit better cytotoxicity towards tumor cell lines than the reference compounds used, with villosin not affecting the non-tumor cell line. Linalool and α-pinene are the most active compounds found in Hedychium essential oils, while β-pinene is identified as the most widespread compound, being reported in 12 different Hedychium species. Since only some Hedychium species have been investigated, this review hopes to shed some light on the uncharted territory that is the Hedychium genus.
1. Introduction
Since the beginning of the history of mankind there was always a connection between plants and human health, as they were used as food and medicines [1]. The traditional herbal medicine outlined the foundations from which modern medicine developed and is still largely practiced around the world [2], particularly in Asian and developing countries [3,4]. This popular knowledge, also known as folk medicine, gives a good indication to scientists looking for sources of new compounds with pharmaceutical potential. Thus, medicinal plants and their derived natural compounds have become an increasing topic of investigation and interest [5,6].
According to “The Plant List” database [7], the genus Hedychium (Zingiberaceae family) comprises 93 species with accepted scientific plant names that, with the exception of Hedychium peregrinum N.E.Br. that is endemic to Madagascar [8], are native to wooded habitats in tropical and temperate Asia (i.e., China, Indian subcontinent and Southeast Asia) [8,9,10]. Members of this genus are well distributed worldwide, being easily found particularly throughout tropical Asia, Australia, Fiji, New Caledonia, New Guinea, New Hebrides, Samoa and the Solomon Islands [8,10,11], with some species being considered invasive in some places: e.g., Hedychium coronarium J. Koenig in Brazil [12] and Hedychium gardnerianum Sheppard ex Ker-Gawl. in Azores Archipelago [13] and Hawaii [14].
Hedychium species are medium-size rhizomatous perennial monocotyledonous plants that can be easily recognized by their characteristic striking foliage and terminal spikes that produce diversified numerous short-lived flamboyant flowers with several hues and fragrances varying depending on the species [15]. These features give them a high ornamental value, being cultivated worldwide mostly for this purpose and for its use in the perfumery industry, since, besides the aromatic flowers, Hedychium species rhizomes also originate strongly scented oils [16,17].
The use of Hedychium species in folk medicine is common in several countries since they are easily harvested directly from nature or obtained at local markets [18]. These plants are reported to possess analgesic, antimicrobial, antidiabetic, anti-inflammatory, antitumor, anti-allergic, anthelmintic and antioxidant properties [19,20,21,22]. In Table 1, it is summarized the different Hedychium species with reported traditional medicinal use in literature over different geographic areas.

Table 1.
Hedychium species with reported traditional medicinal use.
In addition to the traditional medicinal uses stated in Table 1, Hedychium species are also included in the diet of some populations, like in Thailand where the flowers of Hedychium forrestii Diels can be boiled to become a beverage [45] or in India where the fruit of H. spicatum may be cooked and eaten with lentils in savory dishes [42]. Moreover, the rhizome of H. coronarium is also included in the diet of some populations of South East Asia, being consumed as a vegetable or as a food flavoring spice [46].
The traditional uses mentioned above show that several Hedychium species are used to treat a wide spectrum of diseases. These uses also show that Hedychium species should be considered as promising sources of new bioactive natural compounds and that is why these species have been the target of research by the scientific community. In recent years, several studies have been published on the phytochemical characterization of Hedychium species, as well as on the evaluation of the biological activities exhibited by their organic extracts, essential oils and pure compounds, with some of them showing very interesting results. Recently, literature reviews have been published focusing only on specific species, i.e., H. coronarium [20,47] and H. spicatum [21,48]. This work aims to update the available information that were not mentioned in the previous reviews, as well as involving all the other Hedychium species, their bioactivities and their bioactive isolated compounds. The research for this review was made combining the terms Hedychium, phytochemical and biological activities in the databases Web of Science, PubMed and Scopus and were considered only the published works involving Hedychium species whose binominal Latin name is an accepted name on the The Plant List database [7].
2. In Vitro and In Vivo Activities of Hedychium Extracts and Essential Oils
Taking into account the traditional uses of Hedychium species, several works have been carried out to elucidate how effectively plants can exert the reported biological effects. The following is a compilation and discussion of the most current works on this subject, in which essential oils and extracts of Hedychium species are studied and their biological activities are ascertained.
2.1. Anti-Acetylcholinesterase
The inhibition of the enzyme acetylcholinesterase (AChE) is one of the pathways to countering the cholinergic deficit associated with cognitive dysfunction diseases like in Alzheimer’s disease [49]. Arruda and colleagues [50] showed that the leaf essential oil of H. gardnerianum collected from four different locations could inhibit AChE action, mainly mixed inhibition, presenting IC50 values ranging from 1.03 ± 0.14 mg/mL to 1.37 ± 0.27 mg/mL, a value not statistically different from the value displayed by the AChE inhibitor standard compound α-pinene that presented an IC50 value of 1.43 ± 0.07 mg/mL. This work showed no statistically significant difference between the activity of samples taken in different geographical areas [50].
2.2. Antidiabetic
Deficiency in insulin secretion, insulin action or both, results in chronic hyperglycemia, the main characteristic of diabetes mellitus [51], the main treatment to this condition being the use of anti-diabetic drugs that can control glucose levels in the blood [52].
An in vivo study [53] was carried out to assess the effect of H. coronarium aqueous extract to lower blood glucose level in induced-type 2 diabetes mellitus (T2DM) animal models (streptozotocin (STZ)-induced T2DM Wistar rats and C57BKSdb/db mice, a mice model with a mutation that results in chronic hyperglycemia, pancreatic beta cell atrophy, low insulin level and obesity). After 28 days, the daily dose of H. coronarium aqueous extract (8.928 mg/kg for the STZ-induced T2DM rats and 17.71 mg/kg for the C57BKSdb/db mice) significantly increased glucose tolerance in both diabetic models, when compared with the group treated with distilled water (control group). In addition, the treatment also helped to maintain optimal β-cell structure, moderately increased insulin, improved the lipid profile and decreased aldosterone level in STZ-induced T2DM model.
In another in vivo assay [54], after 14 days of treatment, using an oral dose of 0.3 mL of essential oil from rhizomes of H. spicatum, was observed the reduction of blood glucose and urea levels in rats with diabetes induced by intraperitoneal injection of a solution of alloxan monohydrate (150 mg/kg). This result is similar to those obtained in the group of rats treated with the reference drug glibenclemide. Furthermore, it was noticed that the Islets of Langerhans regained their normal shape after the treatment period [54].
2.3. Anti-Inflammatory
Inflammation is a vital defense mechanism that works to ensure good health [55], but uncontrolled inflammation may lead to serious repercussions [56] and so it is important to continue research into products that can help in its control.
An in vivo study [57] with rats demonstrated the anti-inflammatory effect of a single oral dose (200 mg/kg) of aqueous and ethanolic extracts of H. spicatum rhizome against carrageenan-induced paw edema. Measurements of the edema volume were taken in a successive interval of 1 h, 2 h and 3 h and significant decrease in paw edema volume was detected since the beginning, with the aqueous extract reporting a 28.10% decrease in inflammation and the ethanolic extract a 25.62% decrease in inflammation. Although none of the extracts performed as well as the positive control compound indomethacin (41.32% decrease in inflammation), they both proved to present no acute toxicity in a concentration as high as 2000 mg/kg, with the rats never showing secondary toxic effects like coma, convulsion, salivation, increased motor activity or death. This dose of 2000 mg/kg was previously utilized in a similar work [58] where the ethanolic extract of H. spicatum reported a 55.54% of anti-inflammatory activity inhibition against carageenan-induced edema in rats.
2.4. Antimicrobial
A healthy human body is a symbiosis between human and microbial components [59]. However, sometimes that symbiotic balance can be disturbed, and human health can be impaired by pathogenic microorganisms (i.e., bacteria, fungi, parasites or viruses), the use of effective antimicrobial drugs being needed to restore health normality [60,61].
Noriega et al. [62] showed that, among five different plants, the essential oil of H. coronarium rhizome exhibited the most relevant antibacterial activity against Listeria grayi (MIC value = 0.45 mg/mL) and Streptococcus mutans (MIC value = 0.18 mg/mL) and even against the Gram-negative bacteria Klebsiell oxytoca (MIC value = 0.90 mg/mL). The authors point out the compounds 1,8-cineole and terpinen-4-ol as responsible for the reported activity [62]. In another work [63], H. coronarium leaves essential oil was also pointed out to have antibacterial activity against different bacterial strains, i.e., Escherichia coli (MIC value = 3.90 μL/mL), Staphylococcus aureus (MIC value = 7.81 μL/mL) and Pseudomonas aeruginosa (MIC value = 15.62 μL/mL). These two works are presented here also as examples of two constraints which are common in a variety of scientific papers. First, no work reports, as a comparative term, the activity exhibited by a standard antibacterial compound, determined under the same experimental conditions as the essential oil samples. Without these data it is very difficult to assess the true potential of the samples tested. Second, the MIC values are expressed in non-comparable units. Fortunately, one of the works [62] presents the density of the essential oil, making it possible to convert one of the sets of results [against Listeria grayi (MIC value = 0.50 μL/mL), Streptococcus mutans (MIC value = 0.20 μL/mL), Klebsiell oxytoca (MIC value = 1.0 μL /mL)], allowing to conclude that the essential oil from rhizome is more active as antibacterial agent than leaves essential oil. Regrettably, some papers do not present enough experimental data to allow a unit conversion. Additionally, Ray et al. [64] reported that the essential oil extracted from the rhizome of H. coronarium is an effective antifungal agent since it exhibited activity against Candida albicans (MIC = 3.12 μg/mL), Aspergillus flavus and Fusarium oxysporum (MIC value of 6.25 μg/mL for both species), these MIC values being much lower than those reported for antibacterial activity by Noriega et al. [62].
Another work [65] found that 20 μL of Hedychium matthewii S. Thomas, B. Mani & S. J. Britto rhizome essential oil could be as effective as 30 μg of the standard antibiotic amoxicillin, since it exerted nearly the same growth inhibition effect against several strains of Gram- positive and Gram-negative bacteria (viz. Bacillus cereus, Staphylococcus aureus, Enterobacter aerogens, Salmonella paratyphi, Salmonella typhii, Escherichia coli, Vibrio parahaemolyticus, Proteus vulgaris, Klebsiella pneumoniae and Pseudomonas aeruginosa). Furthermore, it could be pointed out that Streptococcus haemolyticus and Vibrio cholerae were more susceptible towards the essential oil (20 μL) than towards amoxicillin (30 μg).
The activity of H. spicatum flowers essential oil was evaluated against the Gram-negative bacteria Borrelia burgdorferi in stationary phase cycle and it was found out that a 0.1% (v/v) essential oil concentration could eradicate B. burgdorferi (100 μL) with no regrowth [66]. This is one of the few published works that evaluates the antibacterial activity in the stationary-phase of growth.
A different work [67] found that a combination treatment using essential oil of H. spicatum rhizomes and γ-radiation was effective against Fusarium graminearum, inhibiting both the fungal growth in maize grains and the production of the toxic mycotoxins deoxynivalenol and zearalenone in a dose-dependent way, with a complete inhibition at the concentration of essential oil 1.89 mg/g and 4.1 kGy of γ-radiation. Combinational treatment proved to be better than individual treatment, since complete inhibition of F. graminearum required the essential oil concentration of 3.15 mg/g or 6 kGy of γ-radiation.
It is not just the essential oils of Hedychium species that have been evaluated concerning antimicrobial activity. Arora and Mazumder [68] evaluated the activity of H. spicatum rhizomes methanolic extract and the antibiotic ciprofloxacin against different bacterial strains (viz. Shigella boydii, Shigella soneii, Shigella flexneri, B. cereus, V. cholerae, E. coli, S. aureus, Ps. aeruginosa and K. pneumoniae) at the concentrations of 200 to 1200 μg/mL. The results showed a similar inhibition effect for both antibiotic and extract, B. subtilis being the bacteria with greater susceptibility to the extract and antibiotic.
Another work [69] evaluated the anthelmintic activity of methanolic, ethanolic, hydromethanolic, hydroethanolic and aqueous rhizome extracts of H. spicatum against Hemonchus contortus, with the results showing that the methanolic extract were as effective as the positive control compound thiabendazole on time taken for paralysis and time taken for death (tested concentrations 20, 40 and 60 mg/mL).
2.5. Antioxidant
Oxygen metabolism is fundamental for human life but its reaction products, like reactive oxygen species (ROS), can increase oxidative stress, causing damage to cells and tissues [70] that, with time, leads to the development or aggravation of several chronic diseases [71]. Thus, therapeutic antioxidant agents are key to mitigate the oxidative stress impact in human health, with natural plant-derived products being the main investigation focus of search [72].
Noriega et al. [62] evaluated the antioxidant activity of the essential oil extracted from the rhizome of H. coronarium, reporting IC50 values of 9.04 ± 0.55 mg/mL and 2.87 ± 0.17 mg/mL for 1,1-Diphenyl-2-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS) assays, respectively. In a similar work, Ray and colleagues [64] also evaluated the antioxidant activity of the essential oil from H. coronarium rhizome, but from ten distinct regions of India, obtaining activity values higher than those indicated in the work of Noriega et al. [62] (IC50 values range from 0.57 to 2.19 mg/mL for the DPPH assay; and 0.12 to 0.67 mg/mL for the ABTS assay), but lower than the positive control 2,6-di-tert-butyl-4-methylphenol (BHT) (IC50 = 0.12 ± 0.01 mg/mL on the DPPH assay, and 0.08 ± 0.01 mg/mL on the ABTS assay). It could be pointed out that Ray et al. [64] also demonstrate, very clearly, that the geographical origin of the samples is a relevant variable for the level of activity displayed. The same conclusion can be drawn from the results obtained by Arruda et al. [50], where the DPPH antioxidant activity of H. gardnerianum leaf essential oil collected from four different locations ranged from EC50 = 8.46 ± 0.90 μg/mL to 31.14 ± 2.70 μg/mL (EC50 = 31.00 ± 0.19 μg/mL for BHT). In a more recent work, Ray et al., [73] studied the antioxidant activity of Hedychium greenii W. W. Smith. and Hedychium gracile Roxb. rhizomes essential oils by the same methodology (DPPH and ABTS assays), with H. greenii showing higher antioxidant activity (IC50 values of 16.73 ± 0.19 μg/mL for DPPH and 12.18 ± 0.16 μg/mL for ABTS assays) than H. gracile sample (IC50 values of 46.94 ± 0.6 μg/mL for DPPH and 31.13 ± 0.29 μg/mL for ABTS assays), and slightly higher than the positive control BHT (IC50 = 18.94 ± 0.3 μg/mL and IC50 = 14.21 ± 0.27 μg/mL for DPPH and ABTS assays, respectively). These results [73], when compared with those obtained in the works mentioned above [50,64], show that the level of antioxidant activity of essential oils exhibits variability between different Hedychium species (IC50 values range from 8.46 to 2190 μg/mL) higher than geographical variability (IC50 values range from 0.57 to 2.19 mg/mL for the DPPH assay).
Zhao et al. [74] compared essential oils and ethanolic extracts from rhizomes of different species from the Zingiberaceae family in terms of its antioxidant capacity by DPPH assay. The ethanol extracts of H. coronarium and H. gardnerianum proved to be the best antioxidant samples presenting IC50 values of 0.94 μg/mL and 1.59 μg/mL, respectively, even better than the reference compounds trolox (IC50 = 10.19 μg/mL) or ascorbic acid (IC50 = 8.37 μg/mL). Essential oils of these plants were also tested but unfortunately the authors presented the results as a graphic which does not allow the reading of numerical values of antioxidant activity.
Usha et al. [75] compared the hydromethanolic rhizome extract of different species also from Zingiberaceae family in terms of its antioxidant capacity and found out that Hedychium sp. reported the best results, with the lowest IC50 value on DPPH assay (36.4 μg/mL). This activity was correlated with its high phenol and flavonoid content. Unfortunately, the authors do not specify neither the Hedychium species that was used nor the IC50 value of the ascorbic acid used as positive control, which makes impossible to compare with other published works.
Another work [69] evaluated, through ABTS, DPPH and nitric oxide (NO) free radical scavenging assays, the antioxidant activity of methanolic, ethanolic, hydromethanolic, hydroethanolic and aqueous rhizome extracts of H. spicatum. The results showed the methanolic extract as the most antioxidant extract, presenting the lowest EC50 values for all the assays (EC50 ABTS value = 24.93 mg/mL, EC50 DPPH value = 8.31 mg/mL and EC50 NO value = 3.57 mg/mL). However, this extract is much less active than the positive control ascorbic acid (EC50 = 1.63 mg/mL to ABTS assay, EC50 = 0.049 mg/mL to DPPH assay and EC50 = 0.10 mg/mL to NO assay) and since the extract EC50 values are very high, it should be considered an inactive extract.
In an in vivo study, Choudhary and Singh [76] demonstrated the antioxidant potential of H. spicatum rhizome, since an improvement in the oxidative stress state of white leghorn cockerels (Gallus gallus domesticus) was observed after the rhizome powder was added to the animal diet, following chronic exposure to indoxacarb.
2.6. Antitumor
Cancer is a complex disease that is a major cause of death worldwide [77], with several treatments but no cure [78]. In the light of the aggressive and not always effective treatments in current medicine, the demand for safer and better anticancer compounds have turned the search to natural products as another therapeutic approach to cancer [79].
Ray and colleagues [80] demonstrated the antiproliferative time-dependent effect of H. coronarium rhizome ethanol extract against human cervical carcinoma HeLa cells, without affecting the viability of non-tumor human umbilical vein endothelial cells (HUVEC). After 24, 48 and 72 h of incubation, the observed IC50 values were 17.18 ± 0.46, 15.32 ± 0.68 and 12.57 ± 0.32 μg/mL, respectively. Although the positive control drug camphothecin presented a far greater inhibitory effect against HeLa cells (IC50 values of 0.82 to 0.98 μg/mL), it is also more toxic to the HUVEC cells (IC50 value for 24 h = 10.13 ± 0.62 μg/mL) than the H. coronarium ethanol extract (IC50 value for 24 h > 320 μg/mL), which means that the extract presents a higher selective cytotoxicity. In addition, the same study shed some light on the mechanism whereby the extract exerts its antitumor activity. It denotes the modulation of the expression of proapoptotic and antiapoptotic protein levels together with an increase of ROS generation and consequent oxidative stress induction in HeLa cells that led to an apoptosis-mediated G1 phase cell arrest as the main cause of HeLa cells migratory capacity inhibition.
In another study [81], the methanolic extract of H. spicatum rhizomes was described as possessing a dose-dependent cytotoxicity activity against human liver hepatocellular carcinoma cell line HepG2, testing concentrations in the range of 25 to 3000 μg/mL. The concentrations tested and the IC50 value (281.917 μg/mL) are very high, and the authors do not provide the cytotoxicity of a positive control nor do they evaluate the effects of such concentrations on non-tumor cells. The results obtained in the studies performed in these conditions, should be considered with many reservations as the effects observed using such high concentrations are non-specific. On the other hand, the researchers should take into account that 20 µg/mL is the limit established by the National Cancer Institute to consider an extract active enough to justify continuing its study [82], so the tested extract should be considered inactive against HepG2 cells line.
The in vitro cytotoxicity, by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, of H. spicatum rhizome chloroform extract was assessed against colorectal adenocarcinoma (Colo-205) cell line, human epidermoid carcinoma (A-431) cell line, human breast adenocarcinoma (MCF-7) cell line, human lung adenocarcinoma (A549) and Chinese hamster ovary (CHO) cell lines [83]. The results show that the extract presented cytotoxicity against all cell lines exhibiting IC50 values ranging from 37.45 ± 0.90 µg/mL to 63.21 ± 1.19 µg/mL, including against non-tumor cell line CHO (39.52 ± 0.06 µg/mL), indicating that the H. spicatum rhizome chloroform extract have small potential as a good anticancer drug since it affected in a similar way both tumor and non-tumor cell lines. Results like these shows how difficult it is to find an ideal anti-tumor drug that affect only the tumor cells, leaving the non-tumor cells undamaged. In addition, it would have been interesting if the authors had also tested a reference compound, since it would have enriched their work.
2.7. Hepatoprotective
The liver is a vital organ, capable of detoxifying the body from endogenous and/or exogenous substances detrimental to the organism, and which is responsible for the regulation of diverse functions and physiological processes, such as the metabolism of carbohydrates and fats and the secretion of bile [84]. Exposure to drugs and chemicals can cause liver injury which, taking into account all the functions inherent to the liver, is a major health problem [85]. Thus, compounds that can protect the liver, stimulate hepatic function or help to regenerate hepatic cells, while simultaneously being less toxic and more effective are of great interest, with natural sources being identified as good search option [86].
A study [87] indicated that H. spicatum possess hepatoprotective properties since its three rhizome extracts (methanolic, ethanolic and aqueous) exerted protection on HepG2 cells against paracetamol-induced toxicity. The IC50 values were 282, 356 and 515 μg/mL for the methanolic, ethanolic and aqueous extracts, respectively, which translates in a cytoprotection percentage of 16%, 13% and 9%, respectively. Compared to the 19% cytoprotection provided by the control substance silymarin (IC50 = 110 μg/mL), the hepatoprotective effect of the extracts is not huge but it is worth mentioning at least the methanolic extract.
A study which was also carried out to evaluate the potential hepatoprotective effect was the in vivo study [88]. where cockerels were fed for 16 weeks with rhizome powder of H. spicatum, while simultaneously receiving a dose of indoxacarb intended to cause chronic toxicity. The results of the liver analysis show that, when compared with the control group (indoxacarb administration without the added H. spicatum rhizome powder to the cockerels diet), H. spicatum rhizome ameliorated the damages caused in cockerels by indoxacarb in the duration of the experiment. Apparently, the treatment with H. spicatum modulated the expression levels of several different hepatic genes, such as those involved in metabolization of indoxacarb (cytochrome P450 1A1), in the immune system (interleukin 6 (IL-6)) and in antioxidant function (catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GPx)).
2.8. Insecticide
Control of mosquito population is crucial, particularly in developing countries, since they act as vectors of several pathogens and parasites responsible for various worrisome diseases, e.g., dengue, filariasis, malaria, West Nile or yellow fever [89,90]. In order to reduce or eliminate the human contact with the vector, a wide range of methods exists with insecticides being a top choice in case of mosquitoes [91]. However, with insecticide resistance being a problem in recent years [92], the search for better substances with insecticide potential is imperative.
Kalimuthu and colleagues [93] carried out an interesting work where H. coronarium-synthesized silver nanoparticles (AgNPs) were produced and their toxicity towards larvae and pupae of the dengue vector Aedes aegypti was assessed, as well as their synergy with Mesocyclops formosanus predation over A. aegypti larvae. The toxicity of aqueous H. coronarium rhizome extract was also assessed. The results indicate that both H. coronarium formulations tested, aqueous rhizome extract and AgNPs, were toxic against A. aegypti in a dose-dependent manner. Aqueous H. coronarium rhizome extract caused toxicity with LC50 values from 0.688% against larval instar I to 1.882% dose against pupae stage of A. aegypti, while AgNPs demonstrated its toxicity with LC50 values varying from 24.264 ppm for larval instar I till 348.68 ppm for pupae of A. aegypti. Once again, we are faced with a work whose authors express results in non-comparable units and do not provide the necessary data for their conversion, significantly reducing the impact of this work. Nevertheless, AgNPs were found to be stable over time in aquatic environment and since a positive synergy was reported with M. formosanus predation on young A. aegypti larvae, its combined use could lead to a higher efficacy in removing the larval population of dengue mosquitoes from aquatic areas.
In another work [94], Hedychium larsenii M. Dan and C. Sathish Kumar rhizomes essential oil was evaluated regarding its toxicity against larvae of mosquito vectors of diseases, namely Anopheles stephensi (malaria), A. aegypti (dengue) and Culex quinquefasciatus (St. Louis encephalitis). The results demonstrate that the essential oil exerted larvicidal activity over the different larvae with the LC50 values of 82.02, 88.60 and 96.40 μg/mL for A. stephensi, A. aegypti and C. quinquefasciatus, respectively. Again, the lack of a tested reference compound impairs any conclusion taken from these results.
3. Secondary Metabolites from Hedychium Species and Its Activities
The diverse bioactivities observed on different Hedychium species/extracts are intrinsically linked to the compounds present in each one, so the need and interest in the phytochemical study of these extracts/species becomes clear. Several relevant works managed to isolate compounds from Hedychium extracts and carried out different assays to ascertain the bioactive potentials of those compounds. In Table 2 the compounds isolated from Hedychium extracts are gathered, as well as their bioactivities and the Hedychium species where they have already been identified. A figure with the chemical structures of the compounds (Figure 1) listed in this table is present after Table 2. It should be clarified that, for each compound in Table 2, only the highest activity value for each activity from each reference is presented, with some values converted from µg/mL to µM to facilitate comprehension and comparison of the different activities.

Table 2.
Secondary metabolites isolated from Hedychium extracts with proven activities.
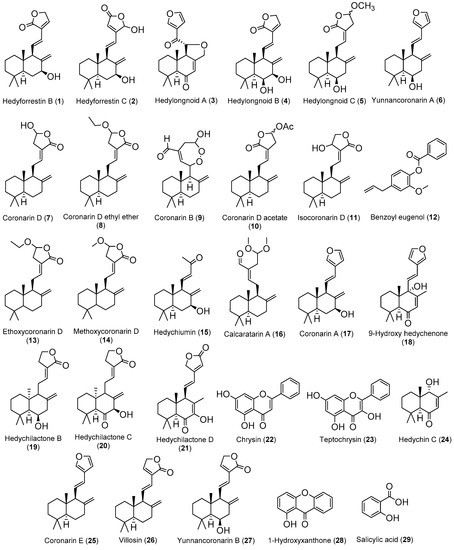
Figure 1.
Chemical structure of the compounds referred on Table 2.
Taking the information of Table 2 into account, it is possible to identify that H. coronarium provided the highest number of isolated compounds and that the antitumor activity is the most reported bioactivity in the above-mentioned studies. On the other hand, the labdane-type diterpene is the most frequent family of compounds in the genus Hedychium, and some flavonoids and simple phenolic compounds are also identified.
Villosin (26) can be pointed out as the most promising antitumor compound, since it presented a highest and selective cytotoxicity against NCI-H187 cell line with an IC50 value of 0.40 µM, without toxicity against the non-tumor Vero cell line at 166.42 μM and presenting better results than the positive control compound ellipticine (i.e., IC50 value against NCI-H187 of 1.79 µM and IC50 value against Vero of 7.47 µM). Coronarin D (7) appears also as one interesting compound, since recent works report its antibacterial activity against B. cereus to be better than the positive control oxacillin.
In addition to these compounds, hedyforrestin B (1) and hedyforrestin C (2) should also be noted, since their antitumor activities against the NCI-H187 cell line are slightly lower (less than 1.7 times) than that shown by the reference compound ellipticine and with selectivity indices of 14.5 and 4.8, respectively.
On the other hand, compound isocoronarin D (11) should be highlighted since it exhibits activity against a broad spectrum of tumor cell lines (i.e., A549, human cervical carcinoma (HeLa), human hepatocellular carcinoma (HepG2), human acute promyelocytic leukemia (HL-60), human cholangiocarcinoma (HuCCA-1), human epidermoid carcinoma (KB), human breast adenocarcinoma (MDA-MB-231), human acute lymphoblastic leukemia T-lymphoblasts (MOLT-3), mouse lymphoma neoplasm (P388), human hepatocellular carcinoma (S102) and human hormone-dependent breast cancer (T-47D)), with IC50 values between 2.14 to 36.1 µM, better than etoposide or doxorubicin which are toxic only to some of these cell lines, and being more active against HepG2 (IC50 = 16.6 µM) than the reference compound etoposide (IC50 = 23.8 µM) [98].
Bearing in mind that all these compounds have hydroxyl groups and double bonds in their chemical structure, it is suggested that these compounds could be lead compounds, and researchers in the field of medicinal chemistry should use these labile functional groups to carry out structural modifications, in order to obtain more active derivatives and to determine the structure/activity relationships.
In addition, there are some works which require a critical analysis. Zhao and colleagues [96] isolated six labdanes from H. longipetalum rhizome that exhibited NO production inhibitory effects in lipopolysaccharides (LPS) and interferon gamma (IFN-γ)-induced murine macrophages RAW 264.7 cell line. The most active compound is yunnancoronarin A (6) (IC50 = 1.86 µM), but less active than the positive control carbobenzoxy-Leu-Leu-leucinal (MG132) (IC50 = 0.17 µM). Unfortunately, the authors do not mention the extraction and chromatographic procedures they carried out to isolate these compounds, which would have been a valuable information.
Another study that looks promising, but which actually shows very questionable results, is the one carried out by Kiem and colleagues [37]. They isolated compounds from rhizomes of H. coronarium methanol extract and investigated their anti-inflammatory potential through inhibition of pro-inflammatory cytokines production in LPS-stimulated bone marrow-derived dendritic cells (BMDC). The results are not acceptable and do not allow to infer conclusions since they are presented with associated standard errors greater than 20% (e.g., IC50 IL-6 inhibition value = 7.57 ± 2.02 µM) and in some cases close to 100% (e.g., IC50 IL-12p40 inhibition value = 0.19 ± 0.11 µM). This work [37] was only mentioned here to point out to all authors the need to present reliable data in their works, aiming always to show results with standard error less than 10%.
In other lines of work, several studies (e.g., Reddy et al. [83], Chimnoi et al. [98] and Endringer et al. [101]) assessed the antitumor potential of isolated compounds from Hedychium extracts without following the best guidelines for evaluating the cytotoxic potential of compounds. In fact, the authors did not test a reference compound in the same experimental conditions, and did not test the isolated compounds against a non-tumor cell line, which makes it difficult to draw conclusions. Regrettably, without these results, it is not possible to conclude about the efficacy and selectivity of the isolated compound compared to the drugs already available on the market.
In addition to compounds 28 and 29 (Figure 1), Carvalho and colleagues [104] also isolated the compounds 3-(2-hydroxyethoxy)xanthone (30) and oplopanone (31) from H. gardenerianum rhizome acetone extract, but the two compounds (Figure 2) do not present any reported activity and, therefore, were not included in Table 2. Since they belong to families of organic compounds well-known for their broad spectrum of activities (flavonoids and terpenes) [107], it would be worth investigating the biological activity of these compounds.
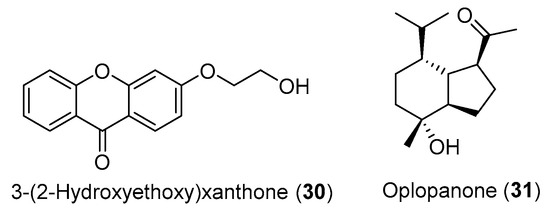
Figure 2.
Chemical structure of the compounds 30 and 31.
It is a fact that the availability of a specific compound in a plant can depend on several factors, like the geographic location where the plant developed [108] and/or the season when it was harvested [109]. Thus, different studies can present different percentages of the total content of the same compound which makes it difficult sometimes to make comparisons between the same plants. This fact is particularly relevant with regard to essential oils, where the majority of published studies refers to quantitative chemical analysis. These studies reveal a complex composition and a huge variability in the content of each compound, depending on geographic, seasonal and species factors, which is reflected in the variability of the biological activity level of the respective essential oils, already highlighted in point 2.
Hedychium species are not different, with several compounds being identified with distinct percentages on its essential oils. However, a deeper analysis of the published works allows to identify some compounds that, with some slight differences, appear repeatedly as the most abundant compounds in their essential oils. In Table 3 are gathered the five most abundant compounds identified in essential oils from Hedychium species as well as their activities and the species where they have already been identified. The respective structures are presented on Figure 3.

Table 3.
The five most frequent and abundant chemical compounds identified in essential oils from Hedychium species.
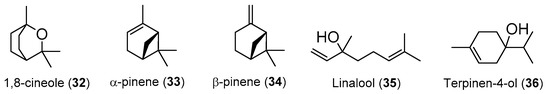
Figure 3.
Chemical structure of the compounds referred on Table 3.
As it is possible to see on Table 3, linalool (35) proved to have promising antitumor potential since it presented cytotoxicity against U937 cell line (i.e., IC50 = 2.59 µM), better than the positive control 5-FU against the same cell line (i.e., IC50 = 4.86 µM). It would have been interesting if the authors had tested the compounds cytotoxicity against a non-tumor cell line, but unfortunately that was not the case.
From the five most abundant and most frequent present compounds in essential oils of Hedychium species, β-pinene (34) is the most widespread compound among species being identified in 12 Hedychium species, mainly rhizomes but also in some cases from flower and leaf essential oil. The compounds α-pinene (33) and linalool (35), exhibit a broad range of bioactivities, being anti-acetylcholinesterase, anti-allergic, antidepressive, antidiabetic, anti-inflammatory, antimicrobial, antitumor, fumigant and neuroprotective agents.
The antimicrobial activity of α-pinene (33), β-pinene (34) reported by Leite et al. [115] presented in Table 3 should be noted, which appears as µL/mL and the authors do not provide the necessary data to convert it to µM or µg/mL. Thus, it is impossible to compare the exhibited activity with other published results and even to compare with the positive control used in this study.
Despite not being so abundant as the compounds referred in Table 3, the isolation of two compounds from H. larsenii rhizomes essential oil could be mentioned, i.e., ar-curcumene (37) and epi-β-bisabolol (38) (Figure 4), that presented insecticide properties against diseases mosquito vectors larvae A. stephensi, A. aegypti and C. quinquefasciatus [94]. The results show that the most affected vector was A. stephensi with compounds 37 and 38 presenting a LC50 values of 51.65 and 66.02 µM, respectively. Unfortunately, the lack of a tested reference compound is a handicap in this work.
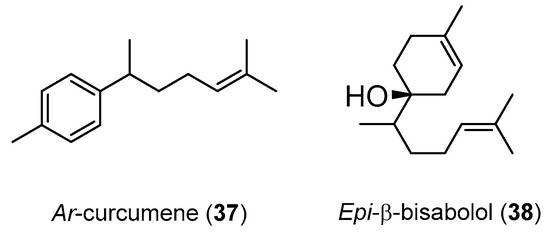
Figure 4.
Chemical structure of the compounds 37 and 38.
Taking together, Table 2 and Table 3 offer a summary view point of the works carried out in recent years that permitted the isolation of some compounds from Hedychium genus, being ascertained their bioactivities. This allows to easily identify where there is work already successfully developed and which paths have not yet been explored.
4. Conclusions
Hedychium genus is undoubtedly proven to be a valuable group of medicinal plants, being present in several folk medicines around the world where it is known to treat allergies, cancer, diabetes, inflammation, rheumatism and skin problems, as well as being also used as an analgesic, antimicrobial, anti-helminthic, antioxidant and insect repellent. In addition, some Hedychium species are part of human diet, being cooked as a vegetable, used as a spice or drunk as a beverage.
Several works explored Hedychium species in order to confirm if and how effectively these plants exert the reported biological effects on folk medicine, studying their essential oils, extracts and their isolated compounds. Taking into account the results of the literature in recent years, Hedychium species have been proven to possess interesting pharmaceutical activities, i.e., anti-acetylcholinesterase, antidiabetic, anti-inflammatory, antimicrobial, antioxidant, antitumor and hepatoprotective, as well as having potential to develop insecticides.
Phytochemical works have been carried out in Hedychium, mainly on H. coronarium and H. spicatum, but also on other less known species, leading to the isolation of interesting compounds that, in some cases, proved to be better than reference compounds. An example is coronarin D (7), possessing antifungal, antitumor and antibacterial properties, being more effective than the positive control oxacillin against B. cereus in antibacterial assays. Isocoronarin D (11), villosin (26) and linalool (35) can be pointed out as very promising antitumor compounds since they exhibited better cytotoxicity towards tumor cell lines than the reference compounds used, and in case of villosin (26) without toxicity on non-tumor cell line. Furthermore, the most bioactive compounds found in Hedychium essential oils can be highlighted as α-pinene (33) and linalool (35), since they are reported as presenting a wide spectrum of bioactivities. In addition, being identified in 12 different Hedychium species to this date, β-pinene (34) is the most widespread compound in Hedychium essential oils.
Hedychium species as proved to be a very rich genus that can still have a lot to offer to the scientific community. Moreover, the discovery in recent years of four new Hedychium species (i.e., Hedychium chingmeianum N.Odyuo and D.K.Roy [128], Hedychium putaoense Y.H.Tan and H.B.Ding [129], Hedychium viridibracteatum X.Hu [130] and Hedychium ziroense V.Gowda and Ashokan [131], may bring new compounds with pharmaceutical potential to the equation.
Author Contributions
M.d.C.B. and A.M.L.S. conceptualized and revised the paper; W.R.T. conducted the research and wrote the first draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by project MACBIOPEST (MAC2/1.1a/289), program Interreg MAC 2014–2020 co-financed by DRCT (Azores Regional Government), supporting W.R. Tavares’s grant, as well as by FCT—Fundação para a Ciência e Tecnologia, the European Union, QREN, FEDER, and COMPETE, through funding the cE3c center (UIDB/00329/2020) and the LAQV-REQUIMTE (UIDB/50006/2020).
Acknowledgments
Thanks are due to the University of Azores and University of Aveiro.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| A-431 | Human epidermoid carcinoma |
| A549 | Human lung adenocarcinoma |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) |
| AChE | Acetylcholinesterase |
| AgNPs | H. coronarium-synthesized silver nanoparticles |
| BHT | 2,6-di-tert-butyl-4-methylphenol |
| BMDC | Bone marrow-derived dendritic cells |
| C57BKSdb/db | Strain of laboratory mouse with a mutation that results in chronic hyperglycemia, pancreatic beta cell atrophy, low insulin level and obesity |
| CAT | Catalase |
| CHO | Chinese hamster ovary cells |
| Colo-205 | Colorectal adenocarcinoma |
| COX-1 | Cyclooxygenase 1 |
| DLD-1 | Human colorectal carcinoma |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl |
| EC50 | Half maximal effective concentration |
| ED50 | Half maximal effective dose |
| GPx | Glutathione peroxidase |
| HeLa | Human cervical carcinoma |
| HepG2 | Human hepatocellular carcinoma |
| HL-60 | Human acute promyelocytic leukemia |
| HuCCA-1 | Human cholangiocarcinoma |
| HUVEC | Human umbilical vascular endothelial cells |
| IC50 | Half maximal inhibitory concentration |
| IFN-γ | Interferon gamma |
| IL-6 | Interleukin 6 |
| Il-12p40 | Interleukin-12 subunit p40 |
| KB | Human epidermoid carcinoma |
| KT50 | Knockdown time 50% |
| LC50 | Lethal concentration that kills 50% of exposed organisms |
| LNCaP | Human prostate adenocarcinoma |
| LPS | Lipopolysaccharide |
| MAO-A | Monoamine oxidase A |
| MCF-7 | Human breast adenocarcinoma |
| MDA-MB-231 | Human breast adenocarcinoma |
| MG132 | Carbobenzoxy-Leu-Leu-leucinal |
| MIC | Minimum inhibitory concentration |
| MOLT-3 | Human acute lymphoblastic leukemia T-lymphoblasts |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NCI-H187 | Human classic small cell lung carcinoma |
| NF-κB | Nuclear factor kappa-B |
| NO | Nitric oxide |
| OHSC | Organotypic hippocampal slice cultures |
| P388 | Mouse lymphoma neoplasm |
| P388D1 | Murine macrophage-like lymphoma |
| RAW 264.7 | Murine macrophage |
| ROS | Reactive oxygen species |
| S102 | Human hepatocellular carcinoma |
| SI | Selectivity index |
| SOD | Superoxide dismutase |
| STZ | Streptozotocin |
| T2DM | Type 2 diabetes mellitus |
| T-47D | Human hormone-dependent breast cancer |
| TNF-α | Tumor necrosis factor α |
| U937 | Human histiocytic lymphoma |
| Vero | African green monkey kidney epithelial cells |
| XWLC-05 | Human lung adenocarcinoma |
References
- Schaal, B. Plants and people: Our shared history and future. Plants People Planet 2019, 1, 14–19. [Google Scholar] [CrossRef]
- Lemonnier, N.; Zhou, G.-B.; Prasher, B.; Mukerji, M.; Chen, Z.; Brahmachari, S.K.; Noble, D.; Auffray, C.; Sagner, M. Traditional knowledge-based medicine: A review of history, principles, and relevance in the present context of P4 systems medicine. Prog. Prev. Med. 2017, 2, e0011. [Google Scholar] [CrossRef]
- Kumar, A.; Aswal, S.; Chauhan, A.; Semwal, R.B.; Kumar, A.; Semwal, D.K. Ethnomedicinal investigation of medicinal plants of Chakrata region (Uttarakhand) used in the traditional medicine for diabetes by Jaunsari tribe. Nat. Prod. Bioprospecting 2019, 9, 175–200. [Google Scholar] [CrossRef] [PubMed]
- Wyk, B.-E.V. A family-level floristic inventory and analysis of medicinal plants used in Traditional African Medicine. J. Ethnopharmacol. 2020, 249, 112351. [Google Scholar] [CrossRef] [PubMed]
- Belokurov, S.S.; Narkevich, I.A.; Flisyuk, E.V.; Kaukhova, I.E.; Aroyan, M.V. Modern extraction methods for medicinal plant raw material (Review). Pharm. Chem. J. 2019, 53, 559–563. [Google Scholar] [CrossRef]
- Kharbach, M.; Marmouzi, I.; Jemli, M.E.; Bouklouze, A.; Heyden, Y.V. Recent advances in untargeted and targeted approaches applied in herbal-extracts and essential-oils fingerprinting—A review. J. Pharm. Biomed. Anal. 2020, 177, 112849. [Google Scholar] [CrossRef] [PubMed]
- The Plant List. Available online: http://www.theplantlist.org/browse/A/Zingiberaceae/Hedychium/ (accessed on 20 February 2020).
- Branney, T.M.E. Hardy Gingers: Including Hedychium, Roscocea, and Zingiber, Royal Horticultural Society Plant Collector Guide; Timber Press: Portland, OR, USA, 2005; p. 267. ISBN 0881926779. [Google Scholar]
- Basak, S.; Ramesh, A.M.; Kesari, V.; Parida, A.; Mitra, S.; Rangan, L. Genetic diversity and relationship of Hedychium from Northeast India as dissected using PCA analysis and hierarchical clustering. Meta Gene 2014, 2, 459–468. [Google Scholar] [CrossRef]
- Tanaka, N.; Ohi-Toma, T.; Aung, M.M.; Murata, J. Systematic notes on the genus Hedychium (Zingiberaceae) in Myanmar. Bull. Natl. Mus. Nat. Sci. Ser. B 2016, 42, 57–66. [Google Scholar]
- Vanchhawng, L.; Lalramnghinglova, H. Notes on the genus Hedychium J. Koen. (Zingiberaceae) in Mizoram, north east India. Int. J. Waste Resour. 2016, 6, 1000234. [Google Scholar] [CrossRef]
- Costa, R.O.; Batisteli, A.F.; Espindola, E.L.G.; Matos, D.M.S. Invasive Hedychium coronarium inhibits native seedling growth through belowground competition. Flora 2019, 261, 151479. [Google Scholar] [CrossRef]
- Costa, H.; Bettencourt, M.J.; Silva, C.M.N.; Teodósio, J.; Gil, A.; Silva, L. Invasive alien plants in the azorean protected areas: Invasion status and mitigation actions. In Plant Invasions in Protected Areas Patterns, Problems and Challenges; Foxcroft, L.C., Pyšek, P., Richardson, D.M., Genovesi, P., Eds.; Invading Nature-Springer Series in Invasion Ecology; Springer Science + Business Media: Dordrecht, The Netherlands, 2013; Volume 7, Chapter 17; pp. 375–394. ISBN 978-94-007-7750-7. [Google Scholar]
- Minden, V.; Hennenberg, K.J.; Porembski, S.; Boehmer, H.J. Invasion and management of alien Hedychium gardnerianum (kahili ginger, Zingiberaceae) alter plant species composition of a montane rainforest on the island of Hawai’i. Plant Ecol. 2010, 206, 321–333. [Google Scholar] [CrossRef]
- Ashokan, A.; Gowda, V. Describing terminologies and discussing records: More discoveries of facultative vivipary in the genus Hedychium J.Koenig (Zingiberaceae) from Northeast India. PhytoKeys 2018, 96, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.; Wong, S.K. Phytochemistry and pharmacology of ornamental gingers, Hedychium coronarium and Alpinia purpurata: A review. J. Integr. Med. 2015, 13, 368–379. [Google Scholar] [CrossRef]
- Yue, Y.; Yu, R.; Fan, Y. Transcriptome profiling provides new insights into the formation of floral scent in Hedychium coronarium. BMC Genom. 2015, 16, 470. [Google Scholar] [CrossRef]
- Badola, H.K. Hedychium spicatum—A commercial Himalayan herb needs entrepreneurship at local level. Non-Wood News 2009, 19, 26–27. [Google Scholar]
- Hartati, R.; Suganda, A.G.; Fidrianny, I. Botanical, phytochemical and pharmacological properties of Hedychium (Zingiberaceae)—A review. Procedia Chem. 2014, 13, 150–163. [Google Scholar] [CrossRef]
- Pachurekar, P.; Dixit, A.K. A review on pharmacognostical phytochemical and ethnomedicinal properties of Hedychium coronarium J. Koenig an Endangered Medicine. IJCM 2017, 1, 49–61. [Google Scholar] [CrossRef]
- Rawat, S.; Jugran, A.K.; Bhatt, I.D.; Ranbeer, S.; Rawal, R.S. Hedychium spicatum: A systematic review on traditional uses, phytochemistry, pharmacology and future prospectus. J. Pharm. Pharmacol. 2018, 70, 687–712. [Google Scholar] [CrossRef]
- Kamble, K.G.; Dale, A.V. A review on pharmacognostic and pharmacological approach of different species of Hedychium. Indo Am. J. Phamacol. Sci. 2018, 5, 6030–6036. [Google Scholar]
- Ong, H.G.; Ling, S.M.; Win, T.T.M.; Kang, D.-H.; Lee, J.-H.; Kim, Y.-D. Ethnomedicinal plants and traditional knowledge among three Chin indigenous groups in Natma Taung National Park (Myanmar). J. Ethnopharmacol. 2018, 225, 136–158. [Google Scholar] [CrossRef]
- Panmei, R.; Gajurel, P.R.; Singh, B. Ethnobotany of medicinal plants used by the Zeliangrong ethnic group of Manipur, northeast India. J. Ethnopharmacol. 2019, 235, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Tribess, B.; Pintarelli, G.M.; Bini, L.A.; Camargo, A.; Funez, L.A.; Gasper, A.L.; Zeni, A.L.B. Ethnobotanical study of plants used for therapeutic purposes in the Atlantic Forest region, Southern Brazil. J. Ethnopharmacol. 2015, 164, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Yazbek, P.B.; Matta, P.; Passero, L.F.; Santos, G.; Braga, S.; Assunção, L.; Sauini, T.; Cassas, F.; Garcia, R.J.F.; Honda, S.; et al. Plants utilized as medicines by residents of Quilombo da Fazenda, Núcleo Picinguaba, Ubatuba, São Paulo, Brazil: A participatory survey. J. Ethnopharmacol. 2019, 244, 112123. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Fernandes, V.F.; Lata, S.; Ayub, A. Indo-Amazonian ethnobotanic connections-Similar uses of some common plants. Ethnobotany 1995, 7, 29–37. [Google Scholar]
- Vásquez, J.; Alarcón, J.C.; Jiménez, S.L.; Jaramillo, G.I.; Gómez-Betancur, I.C.; Rey-Suárez, J.P.; Jaramillo, K.M.; Muñoz, D.C.; Marín, D.M.; Romero, J.O. Main plants used in traditional medicine for the treatment of snake bites n the regions of the department of Antioquia, Colombia. J. Ethnopharmacol. 2015, 170, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Parida, R.; Mohanty, S.; Nayak, S. Chemical composition of essential oil from leaf and rhizome of micropropagated and conventionally grown Hedychium coronarium Koen from Eastern India. J. Essent. Oil Bear. Plants 2015, 18, 161–167. [Google Scholar] [CrossRef]
- Ray, A.; Dash, B.; Sahoo, S.; Sahoo, A.; Jena, S.; Kar, B.; Chatterjee, T.; Ghosh, B.; Nayak, S. Development and validation of an HPTLC method for estimation of coronarin D in Hedychium coronarium rhizome. Acta Chromatogr. 2017, 29, 1–12. [Google Scholar] [CrossRef]
- Shah, A.; Bharati, K.A.; Ahmad, J.; Sharma, M.P. New ethnomedicinal claims from Gujjar and Bakerwals tribes of Rajouri and Poonch districts of Jammu and Kashmir, India. J. Ethnopharmacol. 2015, 166, 119–128. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lim, Y.Y.; Wong, L.F.; Lianto, F.S.; Wong, S.K.; Lim, K.K.; Joe, C.E.; Lim, T.Y. Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem. 2008, 109, 477–483. [Google Scholar] [CrossRef]
- Suroowan, S.; Pynee, K.B.; Mahomoodally, M.F. A comprehensive review of ethnopharmacologically important medicinal plant species from Mauritius. S. Afr. J. Bot. 2019, 122, 189–213. [Google Scholar] [CrossRef]
- Coe, F.G.; Anderson, G.J. Snakebite ethnopharmacopoeia of eastern Nicaragua. J. Ethnopharmacol. 2005, 96, 303–323. [Google Scholar] [CrossRef] [PubMed]
- Valadeau, C.; Castillo, J.A.; Sauvain, M.; Lores, A.F.; Bourdy, G. The rainbow hurts my skin: Medicinal concepts and plants uses among the Yanesha (Amuesha), an Amazonian Peruvian ethnic group. J. Ethnopharmacol. 2010, 127, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Tawatsin, A.; Asavadachanukorn, P.; Thavara, U.; Wongsinkongman, P.; Bansidhi, J.; Boonruad, T.; Chavalittumrong, P.; Soonthornchareonnon, N.; Komalamisra, N.; Mulla, M.S. Repellency of essential oils extracted from plants in Thailand against four mosquito vectors (Diptera: Culicidae) and oviposition deterrent effects against Aedes aegypti (Diptera: Culicidae). Southeast Asian J. Trop. Med. Public Health 2006, 37, 915–931. [Google Scholar] [PubMed]
- Kiem, P.V.; Thuy, N.T.K.; Anh, H.L.T.; Nhiem, N.X.; Minh, C.V.; Yen, P.H.; Ban, N.K.; Hang, D.T.; Tai, B.H.; Tuyen, N.V.; et al. Chemical constituents of the rhizomes of Hedychium coronarium and their inhibitory effect on the pro-inflammatory cytokines production LPS-stimulated in bone marrow-derived dendritic cells. Bioorganic Med. Chem. Lett. 2011, 21, 7460–7465. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Jantan, I.; Jalil, J. Constituents of the rhizome oil of Hedychium cylindricum Ridl. J. Essent. Oil Res. 2004, 16, 299–301. [Google Scholar] [CrossRef]
- Malla, B.; Gauchan, D.P.; Chhetri, R.B. An ethnobotanical study of medicinal plants used by ethnic people in Parbat district of western Nepal. J. Ethnopharmacol. 2015, 165, 103–117. [Google Scholar] [CrossRef]
- Ranjarisoa, L.N.; Razanamihaja, N.; Rafatro, H. Use of plants in oral health care by the population of Mahajanga, Madagascar. J. Ethnopharmacol. 2016, 193, 179–194. [Google Scholar] [CrossRef]
- Sirirugsa, P. Thai Zingiberaceae: Species diversity and their uses. The International Conference on Biodiversity and Bioresource: Conservation and Utilization. Phuket, Thailand, 1998. Available online: http://old.iupac.org/symposia/proceedings/phuket97/sirirugsa.html (accessed on 27 April 2020).
- Bhatt, V.P.; Negi, V.; Purohit, V.K. Hedychium spicatum Buch.-Ham.: A high valued skin glowing and curing medicinal herb needs future attention on its conservation. N. Y. Sci. J. 2010, 3, 86–88. [Google Scholar]
- Savithramma, N.; Sulochana, C.; Rao, K.N. Ethnobotanical survey of plants used to treat asthma in Andhra Pradesh, India. J. Ethnopharmacol. 2007, 113, 54–61. [Google Scholar] [CrossRef]
- Upasani, M.S.; Upasani, S.V.; Beldar, V.G.; Beldar, C.G.; Gujarathi, P.P. Infrequent use of medicinal plants from India in snakebite treatment. Integr. Med. Res. 2018, 7, 9–26. [Google Scholar] [CrossRef]
- Rachkeeree, A.; Kantadoung, K.; Suksathan, R.; Puangpradab, R.; Page, P.A.; Sommano, S.R. Nutritional compositions and phytochemical properties of the edible flowers from selected Zingiberaceae found in Thailand. Front. Nutr. 2018, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Rajput, M.; Kumar, M.; Pant, A.K. Chemical composition and antibacterial activity of rhizome oils from Hedychium coronarium Koenig and Hedychium spicatum Buch-Ham. J. Essent. Oil Bear. Plants 2010, 13, 250–259. [Google Scholar] [CrossRef]
- Chaithra, B.; Satish, S.; Hegde, K.; Shabaraya, A.R. Pharmacological review on Hedychium coronarium Koen: The white ginger lily. Int. J. Pharm. Chem. Res. 2017, 3, 831–836. [Google Scholar]
- Rasool, S.; Maqbool, M. An overview about Hedychium spicatum: A review. J. Drug Deliv. Ther. 2019, 9, 476–480. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Arruda, M.; Viana, H.; Rainha, N.; Neng, N.R.; Rosa, J.S.; Nogueira, J.M.F.; Barreto, M.C. Anti-acetylcholinesterase and antioxidant activity of essential oils from Hedychium gardnerianum Sheppard ex Ker-Gawl. Molecules 2012, 17, 3082–3092. [Google Scholar] [CrossRef]
- Kharroubi, A.T.; Darwish, H.M. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850–867. [Google Scholar] [CrossRef]
- Dowarah, J.; Singh, V.P. Anti-diabetic drugs recent approaches and advancements. Bioorganic Med. Chem. 2020, 28, 115263. [Google Scholar] [CrossRef]
- Tse, L.-S.; Liao, P.-L.; Tsai, C.-H.; Li, C.-H.; Liao, J.-W.; Kang, J.-J.; Cheng, Y.-W. Glycemia lowering effect of an aqueous extract of Hedychium coronarium leaves in diabetic rodent models. Nutrients 2019, 11, 629. [Google Scholar] [CrossRef]
- Kaur, H.; Richa, R. Antidiabetic activity of essential oil of Hedychium spicatum. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 853–857. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H.; Schradin, C. Chronic inflammatory systemic diseases: An evolutionary trade-off between acutely beneficial but chronically harmful programs. Evol. Med. Public Health 2016, 2016, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Ghildiyal, S.; Gautam, M.K.; Joshi, V.K.; Goel, R.K. Pharmacological evaluation of extracts of Hedychium spicatum (Ham-ex-Smith) rhizome. Anc. Sci. Life 2012, 31, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Chachad, D.; Shimpi, S. Anti-inflammatory activity of “Kapurkachari”. Electron. J. Pharmacol. Ther. 2008, 1, 25–27. [Google Scholar]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.; Knight, R.; Gordon, J.I. The human microbiome project: Exploring the microbial part of ourselves in a changing world. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb. Pathog. 2018, 123, 505–526. [Google Scholar] [CrossRef]
- Watkins, K. Emerging infectious diseases: A review. Curr. Emerg. Hosp. Med. Rep. 2018, 6, 86–93. [Google Scholar] [CrossRef]
- Noriega, P.; Guerrini, A.; Sacchetti, G.; Grandini, A.; Ankuash, E.; Manfredini, S. Chemical composition and biological activity of five essential oils from the ecuadorian Amazon rain forest. Molecules 2019, 24, 1637. [Google Scholar] [CrossRef]
- Rath, C.C.; Priyadarshanee, M. Evaluation of in-vitro antibacterial activity of selected essential oils. J. Essent. Oil Bear. Plants 2017, 20, 359–367. [Google Scholar] [CrossRef]
- Ray, A.; Jena, S.; Dash, B.; Kar, B.; Halder, T.; Chatterjee, T.; Ghosh, B.; Panda, P.C.; Nayak, S.; Mahapatra, N. Chemical diversity, antioxidant and antimicrobial activities of the essential oils from Indian populations of Hedychium coronarium Koen. Ind. Crops Prod. 2018, 112, 353–362. [Google Scholar] [CrossRef]
- Thomas, S.; Mani, B. Composition, antibacterial and anti-oxidant potentials of the essential oil of Hedychium matthewii. Bangladesh J. Pharmacol. 2017, 12, 173–179. [Google Scholar] [CrossRef]
- Feng, J.; Shi, W.; Miklossy, J.; Tauxe, G.M.; McMeniman, C.J.; Zhang, Y. Identification of essential oils with strong activity against stationary phase Borrelia burgdorferi. Antibiotics 2018, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Kalagatur, N.K.; Kamasani, J.R.; Siddaiah, C.; Gupta, V.K.; Krishna, K.; Mudili, V. Combinational inhibitory action of Hedychium spicatum L. essential oil and γ-radiation on growth rate and mycotoxins content of Fusarium graminearum in maize: Response surface methodology. Front. Microbiol. 2018, 9, 1511. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Mazumder, A. Phytochemical screening and antimicrobial activity of rhizomes of Hedychium spicatum. Pharmacog. J. 2017, 9, s64–s68. [Google Scholar] [CrossRef]
- Choudhary, G.K.; Singh, S.P.; Kumar, R.R. In vitro antioxidant and anthelmintic properties of rhizome extracts of Hedychium spicatum. Indian J. Anim. Sci. 2018, 88, 300–303. [Google Scholar]
- Chandel, N.S.; Budinger, G.R.S. The cellular basis for diverse responses to oxygen. Free Radic. Biol. Med. 2007, 42, 165–174. [Google Scholar] [CrossRef]
- Tavares, W.R.; Seca, A.M.L. Inula L. Secondary metabolites against oxidative stress-related human diseases. Antioxidants 2019, 8, 122. [Google Scholar] [CrossRef]
- Wright, G.D. Unlocking the potential of natural products in drug discovery. Microb. Biotechnol. 2019, 12, 55–57. [Google Scholar] [CrossRef]
- Ray, A.; Jena, S.; Kar, B.; Patnaik, J.; Panda, P.C.; Nayak, S. Chemical composition and antioxidant activities of essential oil of Hedychium greenii and Hedychium gracile from India. Nat. Prod. Res. 2019, 33, 1482–1485. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, L.; Yang, Z.; Wei, J.; Zhang, K.; Zheng, X.; Fang, Y.; Lin, L.; Tang, J.; Wu, F.; et al. Antioxidative activities of essential oils and ethanol extrations from ornamental Zingiberaceae species. J. Essent. Oil Bear. Plants 2017, 20, 215–222. [Google Scholar] [CrossRef]
- Usha, T.; Pradhan, S.; Goyal, A.K.; Dhivya, S.; Kumar, H.P.P.; Singh, M.K.; Joshi, N.; Basistha, B.C.; Murthy, K.R.S.; Selvaraj, S.; et al. Molecular simulation-based combinatorial modeling and antioxidant activities of Zingiberaceae family rhizomes. Pharmacogn. Mag. 2017, 13, S715–S722. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, G.K.; Singh, S.P. Ameliorating potential of Hedychium spictum on oxidative stress following chronic exposure to indoxacarb in WLH cockerels. Indian J. Anim. Res. 2017, 51, 832–836. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Bidram, E.; Esmaeili, Y.; Ranji-Burachaloo, H.; Al-Zaubai, N.; Zarrabi, A.; Stewart, A.; Dunstan, D.E. A concise review on cancer treatment methods and delivery systems. J. Drug Deliv. Sci. Technol. 2019, 54, 101350. [Google Scholar] [CrossRef]
- Dutta, S.; Mahalanobish, S.; Saha, S.; Ghosh, S.; Sil, P.C. Natural products: An upcoming therapeutic approach to cancer. Food Chem. Toxicol. 2019, 128, 240–255. [Google Scholar] [CrossRef]
- Ray, A.; Jena, S.; Dash, B.; Sahoo, A.; Kar, B.; Patnaik, J.; Panda, P.C.; Nayak, S.; Mahapatra, N. Hedychium coronarium extract arrests cell cycle progression, induces apoptosis, and impairs migration and invasion in HeLa cervical cancer cells. Cancer Manag. Res. 2019, 11, 483–500. [Google Scholar] [CrossRef]
- Choudhary, G.K.; Singh, S.P. Cytotoxic potential of rhizome extracts of Hedychium spicatum L. in HepG2 cell line using MTT. Indian J. Anim. Sci. 2017, 87, 313–315. [Google Scholar]
- Geran, R.I.; Greenberg, N.H.; McDonald, M.M.; Schumacher, A.M.; Abbott, B.J. Protocols for screening chemical agents and natural products against animal tumour and other biological systems. Cancer Chemother. Rep. 1972, 3, 1–103. [Google Scholar]
- Reddy, P.P.; Rao, R.R.; Rekha, K.; Babu, K.S.; Shashidhar, J.; Shashikiran, G.; Lakshmi, V.V.; Rao, J.M. Two new cytotoxic diterpenes from the rhizomes of Hedychium spicatum. Bioorganic Med. Chem. Lett. 2009, 19, 192–195. [Google Scholar] [CrossRef]
- Cornu, R.; Béduneau, A.; Martin, H. Influence of nanoparticles on liver tissue and hepatic functions: A review. Toxicology 2020, 430, 152344. [Google Scholar] [CrossRef]
- Chen, S.; Melchior, W.B., Jr.; Wu, Y.; Guo, L. Autophagy in drug-induced liver toxicity. J. Food Drug Anal. 2014, 22, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Santillán, E.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Sumaya-Martínez, M.T.; Gutiérrez-Salinas, J.; Bautista, M.; Morales-González, Á.; González-Rubio, M.G.-L.; Aguilar-Faisal, J.L.; Morales-González, J.A. Review of natural products with hepatoprotective effects. World J. Gastroenterol. 2014, 20, 14787–14804. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, G.K.; Singh, S.P. In vitro hepatoprotective efficacy of extract of Hedychium spicatum rhizome in paracetamol induced toxicity in HepG2 cell line. Indian J. Anim. Sci. 2018, 88, 546–549. [Google Scholar]
- Choudhary, G.K.; Singh, S.P.; Kumar, A. Effects of GandhPaalashi (Hedychium spicatum) on the expression of hepatic genes associated with biotransformation, antioxidant and immune systems in WLH cockerels fed indoxacarb. Indian J. Anim. Sci. 2018, 88, 786–790. [Google Scholar]
- Jamison, A.; Tuttle, E.; Jensen, R.; Bierly, G.; Gonser, R. Spatial ecology, landscapes, and the geography of vector-borne disease: A multi-disciplinary review. Appl. Geogr. 2015, 63, 418–426. [Google Scholar] [CrossRef]
- Flores, H.A.; O’Neill, S.L. Controlling vector-borne diseases by releasing modified mosquitoes. Nat. Rev. Microbiol. 2018, 16, 508–518. [Google Scholar] [CrossRef]
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.J.; Lindsay, S.W. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis. 2020, 14, e0007831. [Google Scholar] [CrossRef]
- Dusfour, I.; Vontas, J.; David, J.-P.; Weetman, D.; Fonseca, D.M.; Corbel, V.; Raghavendra, K.; Coulibaly, M.B.; Martins, A.J.; Kasai, S.; et al. Management of insecticide resistance in the major Aedes vectors of arboviruses: Advances and challenges. PLoS Negl. Trop. Dis. 2019, 13, e0007615. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Panneerselvam, C.; Chou, C.; Tseng, L.-C.; Murugan, K.; Tsai, K.-H.; Alarfaj, A.A.; Higuchi, A.; Canale, A.; Hwang, J.-S.; et al. Control of dengue and Zika virus vector Aedes aegypti using the predatory copepod Megacyclops formosanus: Synergy with Hedychium coronarium-synthesized silver nanoparticles and related histological changes in targeted mosquitoes. Proces Saf. Environ. Prot. 2017, 109, 82–96. [Google Scholar] [CrossRef]
- AlShebly, M.M.; AlQahtani, F.S.; Govindarajan, M.; Gopinath, K.; Vijayan, P.; Benelli, G. Toxicity of ar-curcumene and epi-β-bisabolol from Hedychium larsenii (Zingiberaceae) essential oil on malaria, chikungunya and St. Louis encephalitis mosquito vectors. Ecotoxicol. Environ. Saf. 2017, 137, 149–157. [Google Scholar] [CrossRef]
- Kumrit, I.; Suksamrarn, A.; Meepawpan, P.; Songsri, S.; Nuntawong, N. Labdane-type diterpenes from Hedychium gardnerianum with potent cytotoxicity against human small cell lung cancer cells. Phytother. Res. 2010, 24, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zeng, G.; Zhao, S.; Xu, J.; Kong, L.; Li, Y.; Tan, N.; Yang, S. Cytotoxic labdane-type diterpenes from Hedychium longipetalum inhibiting production of nitric oxide. Bioorganic Med. Chem. Lett. 2015, 25, 4572–4575. [Google Scholar] [CrossRef] [PubMed]
- Chimnoi, N.; Sarasuk, C.; Khunnawutmanotham, N.; Intachote, P.; Seangsai, S.; Saimanee, B.; Pisutjaroenpong, S.; Mahidol, C.; Techasakul, S. Phytochemical reinvestigation of labdane-type diterpenes and their cytotoxicity from the rhizomes of Hedychium coronarium. Phytochem. Lett. 2009, 2, 184–187. [Google Scholar] [CrossRef]
- Chimnoi, N.; Pisutjaroenpong, S.; Ngiwsara, L.; Dechtrirut, D.; Chokchaichamnankit, D.; Khunnawutmanotham, N.; Mahidol, C.; Techasakul, S. Labdane diterpenes from the rhizomes of Hedychium coronarium. Nat. Prod. Res. 2008, 22, 1249–1256. [Google Scholar] [CrossRef]
- Ray, A.; Halder, T.; Jena, S.; Sahoo, A.; Ghosh, B.; Mohanty, S.; Mahapatra, N.; Nayak, S. Application of artificial neural network (ANN) model for prediction and optimization of coronarin D content in Hedychium coronarium. Ind. Crops Prod. 2020, 146, 112186. [Google Scholar] [CrossRef]
- Reuk-ngam, N.; Chimnoi, N.; Khunnawutmanotham, N.; Techasakul, S. Antimicrobial activity of coronarin D and its synergistic potential with antibiotics. Biomed Res. Int. 2014, 2014, 581985. [Google Scholar] [CrossRef]
- Endringer, D.C.; Taveira, F.S.N.; Kondratyuk, T.P.; Pezzuto, J.M.; Braga, F.C. Cancer chemoprevention activity of labdane diterpenes from rhizomes of Hedychium coronarium. Rev. Bras. Farmacogn. 2014, 24, 408–412. [Google Scholar] [CrossRef]
- Chen, L.-C.; Wen, Z.-H.; Sung, P.-J.; Shu, C.-W.; Kuo, W.-L.; Chen, P.-Y.; Jih-Jung Chen, J.-J. New labdane-type diterpenoid and cytotoxic constituents of Hedychium coronarium. Chem. Nat. Compd. 2017, 53, 72–76. [Google Scholar] [CrossRef]
- Wang, W.-H.; Gao, J.-J.; Zuo, X.-F.; Qin, X.-J.; Liu, H.-Y.; Zhao, Q. New diterpenoids from the rhizomes of Hedychium forrestii. Nat. Prod. Res. 2019, 1–7. [Google Scholar] [CrossRef]
- Carvalho, M.J.; Carvalho, L.M.; Ferreira, A.M.; Silva, A.M.S. A new xanthone from Hedychium gardnerianum. Nat. Prod. Res. 2003, 17, 445–449. [Google Scholar] [CrossRef]
- Gnerre, C.; Thull, U.; Gaillard, P.; Carrupt, P.-A.; Testa, B.; Fernandes, E.; Silva, F.; Pinto, M.; Pinto, M.M.M.; Wolfender, J.-L.; et al. Natural and synthetic xanthones as monoamine oxidase inhibitors: Biological assay and 3D-QSAR. Helv. Chim. Acta 2001, 84, 552–570. [Google Scholar] [CrossRef]
- Aung, H.T.; Nikai, T.; Niwa, M.; Takaya, Y. Benzenepolycarboxylic acids with potential anti-hemorrhagic properties and structure-activity relationships. Bioorganic Med. Chem. 2011, 19, 7000–7002. [Google Scholar] [CrossRef]
- Sülsen, V.P.; Lizarraga, E.; Mamadalieva, N.Z.; Lago, J.H.G. Potential of terpenoids and flavonoids from Asteraceae as anti-inflammatory, antitumor, and antiparasitic agents. Evid. Based. Complement. Altern. Med. 2017, 2017, 6196198. [Google Scholar] [CrossRef]
- Ray, A.; Jena, S.; Haldar, T.; Sahoo, A.; Kar, B.; Patnaik, J.; Ghosh, B.; Panda, P.C.; Mahapatra, N.; Nayak, S. Population genetic structure and diversity analysis in Hedychium coronarium populations using morphological, phytochemical and molecular markers. Ind. Crops Prod. 2019, 132, 118–133. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Rashmi; Parcha, V. Effect of seasonal variation on chemical composition and physicochemical properties of Hedychium spicatum rhizomes essential oil. J. Essent. Oil Bear. Plants 2019, 22, 1593–1600. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Ahd, A.A. Antimicrobial and antifungal properties of the essential oil and methanol extracts of Eucalyptus largiflorens and Eucalyptus intertexta. Pharmacogn. Mag. 2010, 6, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Sfara, V.; Zerba, E.N.; Alzogaray, R.A. Fumigant insecticidal activity and repellent effect of five essential oils and seven monoterpenes on first-instar nymphs of Rhodnius prolixus. J. Med. Entomol. 2009, 46, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Jena, S.; Kar, B.; Sahoo, A.; Pratap Chandra Panda, P.C.; Nayak, S.; Mahapatra, N. Volatile metabolite profiling of ten Hedychium species by gas chromatography mass spectrometry coupled to chemometrics. Ind. Crops Prod. 2018, 126, 135–142. [Google Scholar] [CrossRef]
- Nam, S.-Y.; Chung, C.-K.; Seo, J.-H.; Rah, S.-Y.; Kim, H.-M.; Jeong, H.-J. The therapeutic efficacy of α-pinene in an experimental mouse model of allergic rhinitis. Int. Immunopharmacol. 2014, 23, 273–282. [Google Scholar] [CrossRef]
- Özbek, H.; Yılmaz, B.S. Anti-inflammatory and hypoglycemic activities of alpha-pinene. Acta Pharm. Sci. 2017, 55, 7–14. [Google Scholar] [CrossRef]
- Leite, A.M.; Lima, E.O.; Souza, E.L.; Diniz, M.F.F.M.; Trajano, V.N.; Medeiros, I.A. Inhibitory effect of β-pinene, α-pinene and eugenol on the growth of potential infectious endocarditis causing Gram-positive bacteria. Braz. J. Pharm. Sci. 2007, 43, 121–126. [Google Scholar] [CrossRef]
- Yang, C.; Chen, H.; Chen, H.; Zhong, B.; Luo, X.; Chun, J. Antioxidant and anticancer activities of essential oil from Gannan navel orange peel. Molecules 2017, 22, 1391. [Google Scholar] [CrossRef] [PubMed]
- Parida, R.; Nayak, S. Chemical composition of Hedychium coronarium Koen. flowers from eastern India. Plant Sci. Today 2019, 6, 259–263. [Google Scholar] [CrossRef]
- Medeiros, J.R.; Campos, L.B.; Mendonça, S.C.; Davin, L.B.; Lewis, N.G. Composition and antimicrobial activity of the essential oils from invasive species of the Azores, Hedychium gardnerianum and Pittosporum undulatum. Phytochemistry 2003, 64, 561–565. [Google Scholar] [CrossRef]
- Ribeiro, S.S.; de Jesus, A.M.; dos Anjos, C.S.; da Silva, T.B.; Santos, A.D.C.; de Jesus, J.R.; Andrade, M.S.; Sampaio, T.S.; Gomes, W.F.; Alves, P.B.; et al. Evaluation of the cytotoxic activity of some Brazilian medicinal plants. Planta Med. 2012, 78, 1601–1606. [Google Scholar] [CrossRef]
- Kotan, R.; Kordali, S.; Cakir, A. Screening of antibacterial activities of twenty-one oxygenated monoterpenes. Z. Naturforsch. C. J. Biosci. 2007, 62, 507–513. [Google Scholar] [CrossRef]
- Coelho, V.; Mazzardo-Martins, L.; Martins, D.F.; Santos, A.R.S.; Brum, L.F.S.; Picada, J.N.; Pereira, P. Neurobehavioral and genotoxic evaluation of (-)-linalool in mice. J. Nat. Med. 2013, 67, 876–880. [Google Scholar] [CrossRef]
- Kim, M.-G.; Kim, S.-M.; Min, J.-H.; Kwon, O.-K.; Park, M.-H.; Park, J.-W.; Ahn, H.I.; Hwang, J.-Y.; Oh, S.-R.; Lee, J.-W.; et al. Anti-inflammatory effects of linalool on ovalbumin-induced pulmonary inflammation. Int. Immunopharmacol. 2019, 74, 105706. [Google Scholar] [CrossRef]
- Chang, M.-Y.; Shieh, D.-E.; Chen, C.-C.; Yeh, C.-S.; Dong, H.-P. Linalool induces cell cycle arrest and apoptosis in leukemia cells and cervical cancer cells through CDKIs. Int. J. Mol. Sci. 2015, 16, 28169–28179. [Google Scholar] [CrossRef]
- Kheloul, L.; Anton, S.; Gadenne, C.; Kellouche, A. Fumigant toxicity of Lavandula spica essential oil and linalool on different life stages of Tribolium confusum (Coleoptera: Tenebrionidae). J. Asia Pac. Entomol. 2020, 23, 320–326. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Hobbie, F.; Keerthi, A.; Oun, A.; Kortholt, A.; Boddeke, E.; Dolga, A. Linalool attenuates oxidative stress and mitochondrial dysfunction mediated by glutamate and NMDA toxicity. Biomed. Pharmacother. 2019, 118, 109295. [Google Scholar] [CrossRef] [PubMed]
- Brilhante, R.S.N.; Caetano, E.P.; Lima, R.A.C.; Marques, F.J.F.; Castelo-Branco, D.S.C.M.; Melo, C.V.S.; Guedes, G.M.M.; Oliveira, J.S.; Camargo, Z.P.; Moreira, J.L.B.; et al. Terpinen-4-ol, tyrosol, and β-lapachone as potential antifungals against dimorphic fungi. Braz. J. Microbiol. 2016, 47, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.M.; Ezzat, S.M. Effect of the method of preparation on the composition and cytotoxic activity of the essential oil of Pituranthos tortuosus. Z. Naturforsch. C. J. Biosci. 2011, 66, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Odyuo, N.; Roy, D.K. Hedychium chingmeianum (Zingiberaceae), a new species from Nagaland, India. Telopea 2017, 20, 193–199. [Google Scholar] [CrossRef]
- Ding, H.-B.; Bin, Y.; Zhou, S.-S.; Li, R.; Maw, M.B.; Kyaw, W.M.; Tan, Y.-H. Hedychium putaoense (Zingiberaceae), a new species from Putao, Kachin State, Northern Myanmar. PhytoKeys 2018, 94, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Huang, J.-Q.; Tan, J.-C.; Wu, Y.-Q.; Chen, J. Hedychium viridibracteatum X.Hu, a new species from Guangxi Autonomous Region, South China. PhytoKeys 2018, 110, 69–79. [Google Scholar] [CrossRef]
- Ashokan, A.; Gowda, V. Hedychium ziroense (Zingiberaceae), a new species of ginger lily from Northeast India. PhytoKeys 2019, 117, 73–84. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).