A Double-Blind Randomized Placebo-Controlled Study Assessing the Safety, Tolerability and Efficacy of a Herbal Medicine Containing Pycnogenol Combined with Papain and Aloe vera in the Prevention and Management of Pre-Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Design of This Study
2.3. Outcome Measures
2.4. Power and Sample Calculation
2.5. Statistical Analysis
3. Results
3.1. Efficacy Evaluation
3.1.1. Impaired Fasting Glucose (IFG) (Primary Endpoint)
3.1.2. Impaired Glucose Tolerance (Secondary Endpoint)
3.2. Safety and Tolerability
3.3. Quality of Life
3.4. Tertiary Endpoints
3.5. Physical Activity
3.6. Additional Physiological Measures
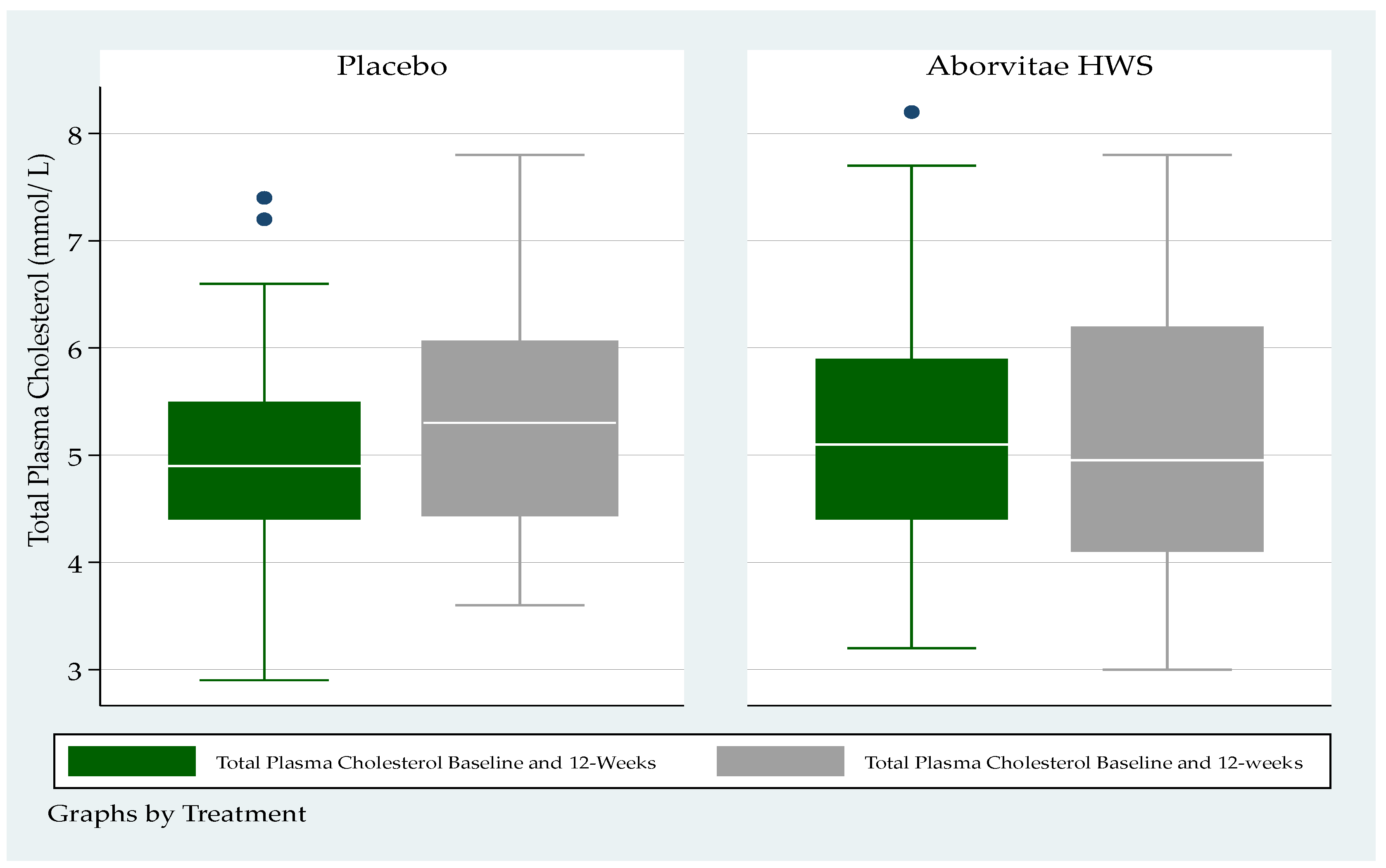
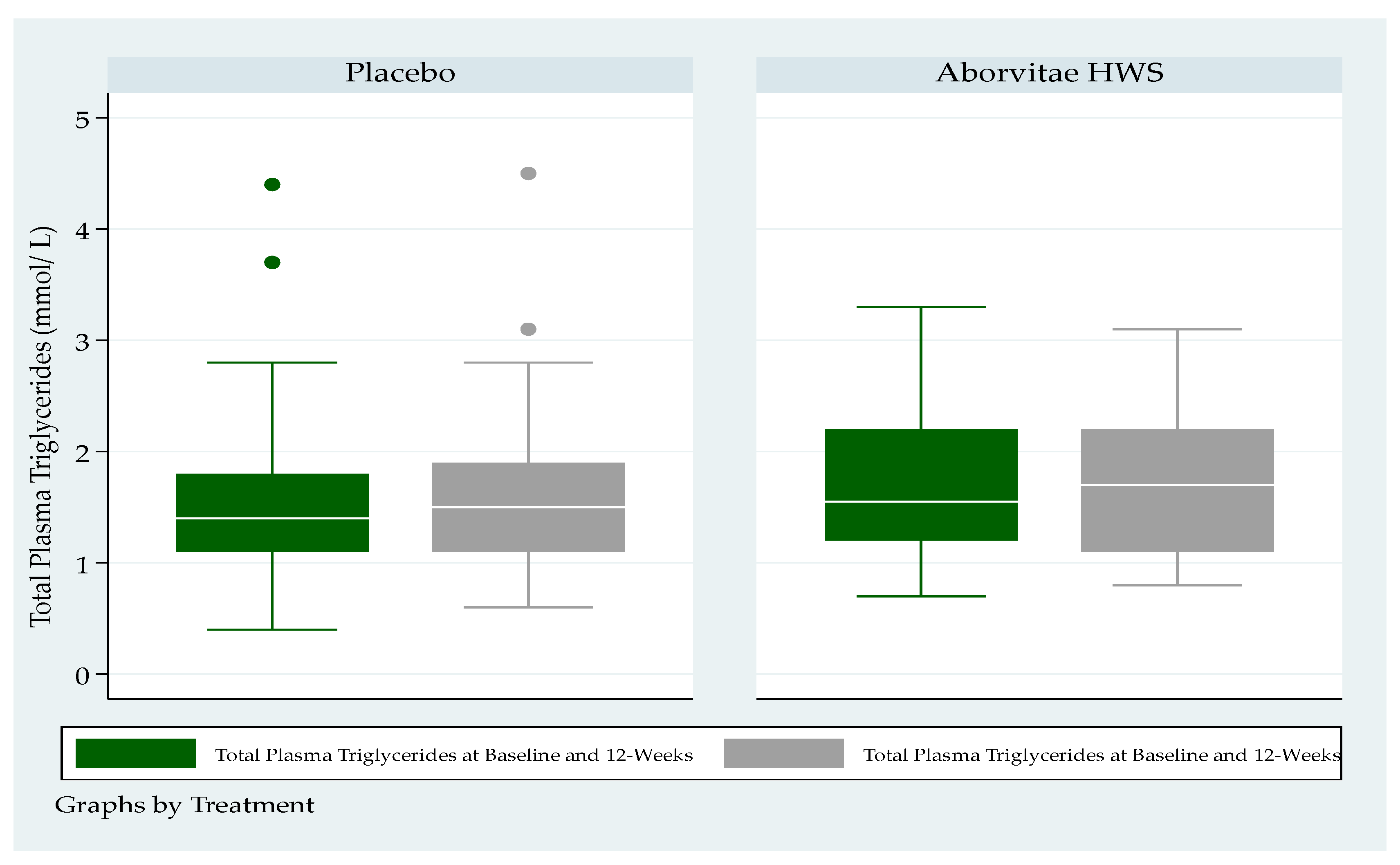
3.7. Blood Lipid Levels
4. Discussion
5. Conclusions
Author Contributions
Funding and Clinical Trial Sponsor
Conflicts of Interest
References
- Twigg, S.M.; Kamp, M.C.; Davis, T.M.; Neylon, E.K.; Flack, J.R. Prediabetes: A position statement from the Australian Diabetes Society and Australian Diabetes Educators Association. Med. J. Aust. 2007, 186, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Neumiller, J.J.; Umpierrez, G.E. 2018 Standards of Care Update: Pharmacologic Approaches to Glycemic Management in People with Type 2 Diabetes. Diabetes Spectr. 2018, 31, 254–260. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dunstan, D.W.; Zimmet, P.Z.; Welborn, T.A.; De Courten, M.P.; Cameron, A.; Sicree, R.A.; Dwyer, T.; Colagiuri, S.; Jolley, D.; Knuiman, M.; et al. The rising prevalence of diabetes and impaired glucose tolerance: The Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care 2002, 25, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Hill, J. Setting up a community oral glucose tolerance testing service. Prim. Health Care 2010, 20, 28–31. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Tripathy, D.; DeFronzo, R.A. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006, 29, 1130–1139. [Google Scholar] [CrossRef]
- Magliano, D.J.; Barr, E.L.M.; Zimmet, P.Z.; Cameron, A.; Dunstan, D.W.; Colagiuri, S.; Jolley, D.; Owen, N.; Phillips, P.; Tapp, R.J.; et al. Glucose Indices, Health Behaviors, and Incidence of Diabetes in Australia: The Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care 2007, 31, 267–272. [Google Scholar] [CrossRef]
- DECODE Study Group; European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: Comparison of fasting and 2-hour diagnostic criteria. Arch. Intern. Med. 2001, 161, 397–405. [Google Scholar] [CrossRef]
- Gillies, C.; Abrams, K.; Lambert, P.; Cooper, N.J.; Sutton, A.J.; Hsu, R.T.; Khunti, K. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: Systematic review and meta-analysis. BMJ 2007, 334, 299. [Google Scholar] [CrossRef]
- Liu, X.; Wei, J.; Tan, F.; Zhou, S.; Würthwein, G.; Rohdewald, P. Antidiabetic effect of Pycnogenol® French maritime pine bark extract in patients with diabetes type II. Life Sci. 2004, 75, 2505–2513. [Google Scholar] [CrossRef]
- Zibadi, S.; Rohdewald, P.J.; Park, D.; Watson, R.R. Reduction of cardiovascular risk factors in subjects with type 2 diabetes by Pycnogenol supplementation. Nutr. Res. 2008, 28, 315–320. [Google Scholar] [CrossRef]
- Belcaro, G.; Cornelli, U.; Luzzi, R.; Cesarone, M.R.; Dugall, M.; Feragalli, B.; Errichi, S.; Ippolito, E.; Grossi, M.G.; Hosoi, M.; et al. Pycnogenol® Supplementation Improves Health Risk Factors in Subjects with Metabolic Syndrome. Phytother. Res. 2013, 27, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Gulati, O.P. Pycnogenol(r) in metabolic syndrome and related disorders. Phytother. Res. 2015, 29, 949–968. [Google Scholar] [CrossRef] [PubMed]
- Mazorra-Manzano, M.A.; Ramírez-Suarez, J.C.; Yada, R.Y. Plant proteases for bioactive peptides release: A review. Crit. Rev. Food Sci. Nutr. 2017, 58, 2147–2163. [Google Scholar] [CrossRef] [PubMed]
- Badgujar, S.; Patel, V.; Bandivdekar, A.H.; Mahajan, R.T. Traditional uses, phytochemistry and pharmacology of Ficus carica: A review. Pharm. Boil. 2014, 52, 1487–1503. [Google Scholar] [CrossRef]
- Shakib, Z.; Shahraki, N.; Razavi, B.M.; Hosseinzadeh, H. Aloe vera as an herbal medicine in the treatment of metabolic syndrome: A review. Phytother. Res. 2019, 33, 2649–2660. [Google Scholar] [CrossRef]
- Liu, Y.; Cotillard, A.; Vatier, C.; Bastard, J.P.; Fellahi, S.; Stevant, M.; Allatif, O.; Langlois, C.; Bieuvelet, S.; Brochot, A.; et al. A dietary supplement containing cinnamon, chromium and carnosine decreases fasting plasma glucose and increases lean mass in overweight or obese pre-diabetic subjects: A randomized, placebo-controlled trial. PLoS ONE 2015, 10, e0138646. [Google Scholar] [CrossRef]
- Li, Y.; Feng, J.; Zhang, X.-L.; Cui, Y.-Y. Pine Bark Extracts: Nutraceutical, Pharmacological, and Toxicological Evaluation. J. Pharmacol. Exp. Ther. 2015, 353, 9–16. [Google Scholar] [CrossRef]
- Krishna, K.L.; Patel, J. Review on nutritional, medicinal and pharmacological properties of papaya (carica papaya linn). Nat. Prod. Rad. 2008, 7, 364–373. [Google Scholar]
- Bansal, N. Prediabetes diagnosis and treatment: A review. World J. Diabetes 2015, 6, 296–303. [Google Scholar] [CrossRef]
- Wu, S.; McCormick, J.B.; E Curran, J.; Fisher-Hoch, S.P. Transition from pre-diabetes to diabetes and predictors of risk in Mexican-Americans. Diabetes Metab. Syndr. Obes. Targets Ther. 2017, 10, 491–503. [Google Scholar] [CrossRef]
- Hostalek, U. Global epidemiology of prediabetes—Present and future perspectives. Clin. Diabetes Endocrinol. 2019, 5, 5. [Google Scholar] [CrossRef]
- Shaw, J.E.; Zimmet, P.Z.; Alberti, K.G.M. Point: Impaired Fasting Glucose: The Case for the New American Diabetes Association Criterion. Diabetes Care 2006, 29, 1170–1172. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Li, Q.; Wu, X.; Zhao, X.; Zhao, L.; Tong, X. New Insights into the Mechanisms of Chinese Herbal Products on Diabetes: A Focus on the “Bacteria-Mucosal Immunity-Inflammation-Diabetes” Axis. J. Immunol. Res. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jerez, M.; Selga, A.; Sineiro, J.; Torres, J.L.; Nuñez, M.J. A comparison between bark extracts from Pinus pinaster and Pinus radiata: Antioxidant activity and procyanidin composition. Food Chem. 2007, 100, 439–444. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Liu, D.; Zhao, T.; Tian, H. Efficacy of Aloe Vera Supplementation on Prediabetes and Early Non-Treated Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 388. [Google Scholar] [CrossRef]
- Devaraj, S.; Yimam, M.; Brownell, L.A.; Jialal, I.; Singh, S.; Jia, Q. Effects of Aloe vera Supplementation in Subjects with Prediabetes/Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2013, 11, 35–40. [Google Scholar] [CrossRef]
- Somanah, J.; Bourdon, E.; Rondeau, P.; Bahorun, T.; Aruoma, O.I. Relationship between fermented papaya preparation supplementation, erythrocyte integrity and antioxidant status in pre-diabetics. Food Chem. Toxicol. 2014, 65, 12–17. [Google Scholar] [CrossRef]
- Somanah, J.; Aruoma, O.I.; Gunness, T.K.; Kowelssur, S.; Dambala, V.; Murad, F.; Googoolye, K.; Daus, D.; Indelicato, J.; Bourdon, E.; et al. Effects of a short term supplementation of a fermented papaya preparation on biomarkers of diabetes mellitus in a randomized Mauritian population. Prev. Med. 2012, 54, S90–S97. [Google Scholar] [CrossRef]
- Cabreiro, F. Metformin Joins Forces with Microbes. Cell Host Microbe 2016, 19, 1–3. [Google Scholar] [CrossRef]
- Chávez-Carbajal, A.; Pizano-Zárate, M.L.; Hernández-Quiroz, F.; Ortiz-Luna, G.; Morales-Hernández, R.; De Sales-Millán, A.; Hernández-Trejo, M.; García-Vite, A.; Beltrán-Lagunes, L.; Hoyo-Vadillo, C.; et al. Characterization of the Gut Microbiota of Individuals at Different T2D Stages Reveals a Complex Relationship with the Host. Microorganisms 2020, 8, 94. [Google Scholar] [CrossRef] [PubMed]
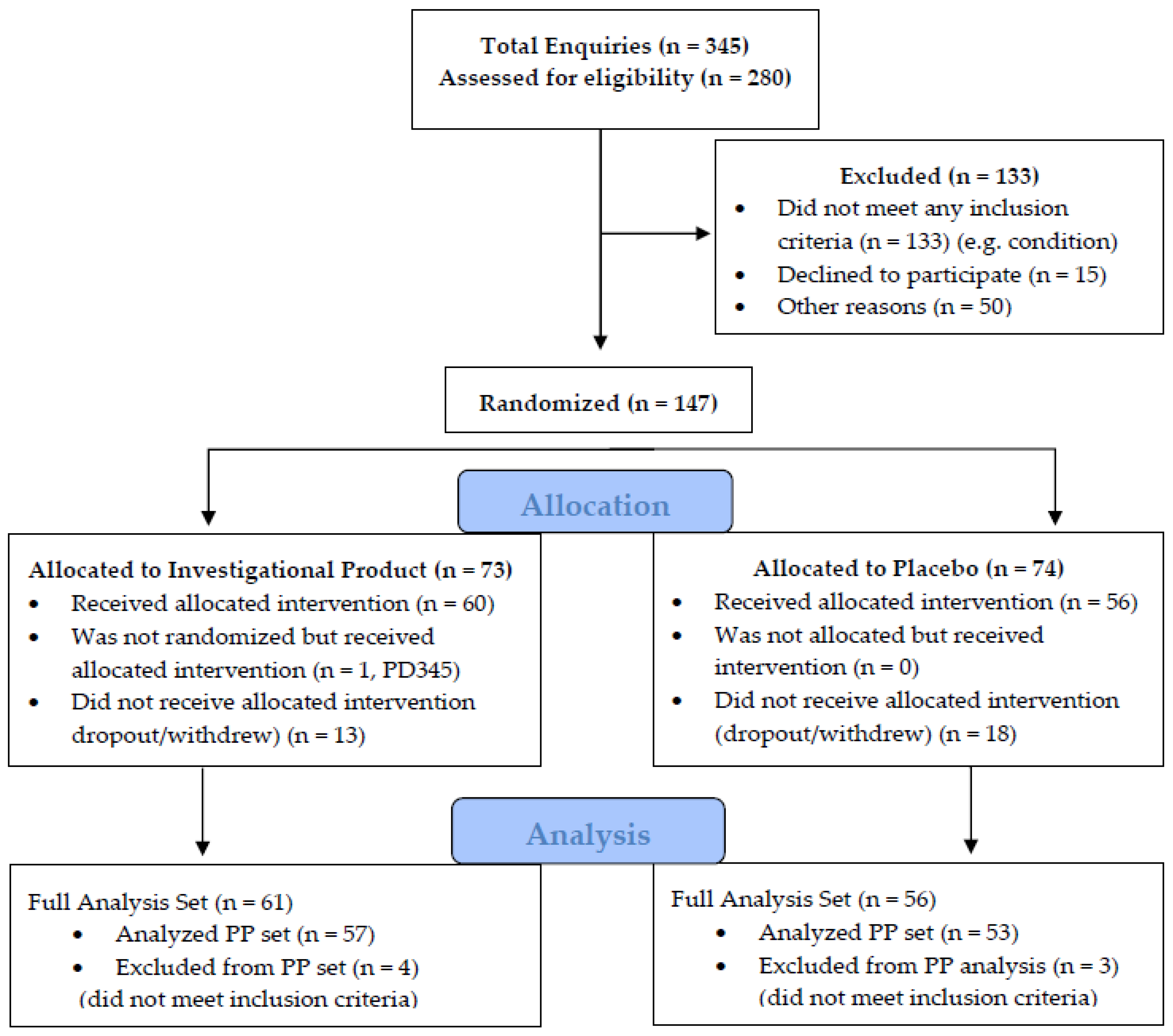
| Intervention Arm | Placebo Arm | p–Value | |
|---|---|---|---|
| FACTORS | n = 61 | n = 56 | |
| Sex | |||
| M [%] (n): F [%] (n) | 57% (35): 43% (26) | 48% (27): 52% (29) | - |
| Age [mean (SD years) | |||
| M: F | 61.1 (12.6): 60.8 (8.6) | 63.7 (11.4): 60.1 (9.0) | - |
| Height [mean (SD) cm] | 169.6 (10.8) | 168.5 (10.5) | 0.55 |
| Weight [mean (SD) kg] | 90.7 (19.1) | 90.0 (18.9) | 0.84 |
| BMI ≥ 25 [%] | 89% (54) | 95% (53) | 0.68 |
| FPG [5.5–6.9 mmol/L] [%] | 66% (40) | 62% (35) | 0.73 |
| FPG, mean (SD) | 5.9 (0.9) mmol/L | 5.9 (0.8) mmol/L | 0.94 |
| OGTT 1 h ≥ 8.6 mmol/L [%] | 93% (57) | 95% (53) | - |
| 1 h post OGTT mean (SD) | 11.3 (2.4) mmol/L | 10.7 (3.1) mmol/L | 0.25 |
| 2 h post OGTT mean (SD) | 8.2 (3.1) mmol/L | 7.8 (3.0) mmol/L | 0.49 |
| Insulin mU/L fast mean (SD) | 16.6 (8.1) | 17.6 (11.4) | 0.60 |
| Insulin mU/L 1 h mean (SD) | 130.0 (76.7) | 130.9 (71.9) | 0.95 |
| Insulin mU/L 2 h mean (SD) | 101.4 (73.8) | 102.7 (71.5) | 0.93 |
| HbA1c % fast mean (SD) | 5.7 (0.4) | 5.6 (0.5) | 0.25 |
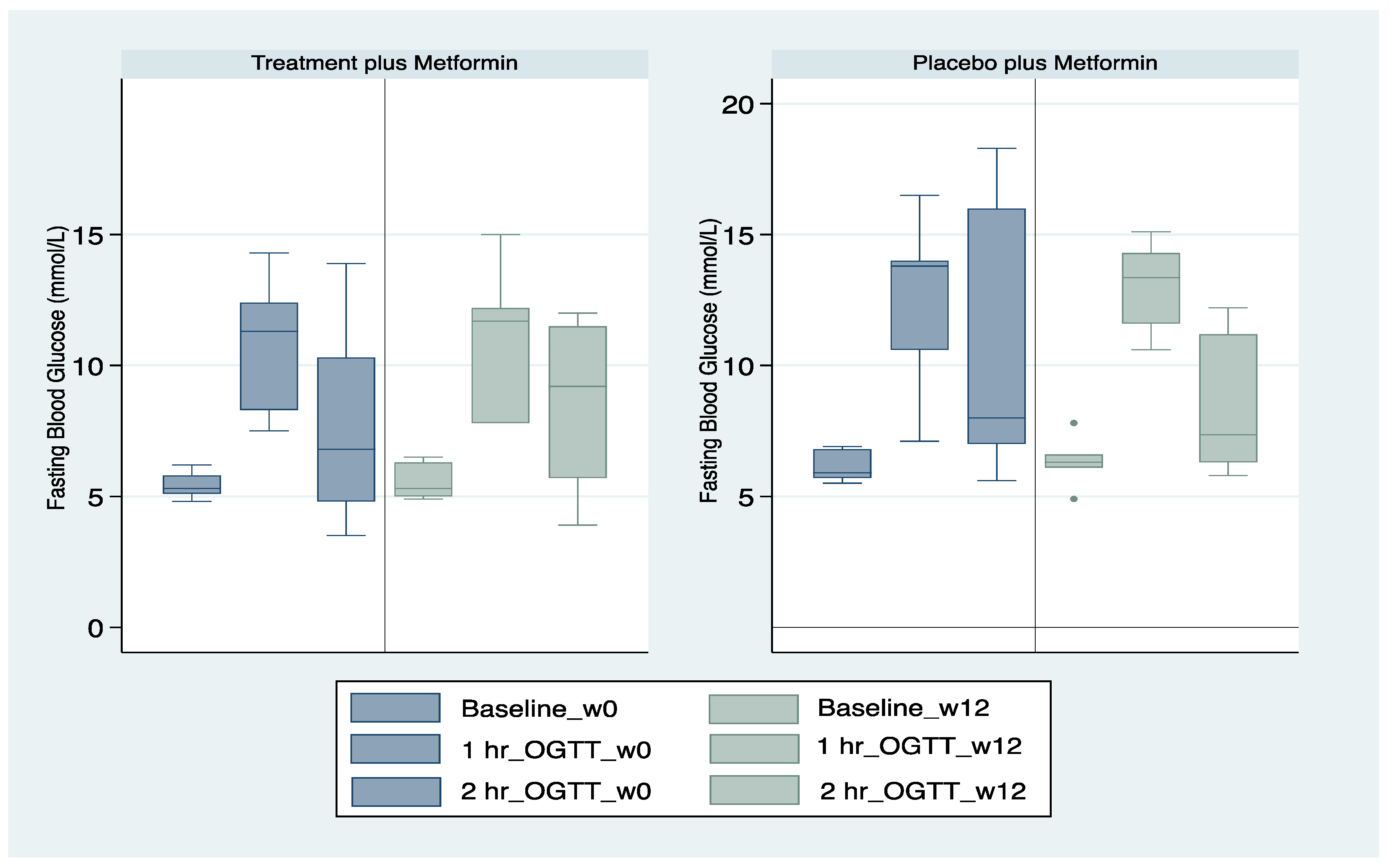
| With prescribed metformin FBG mmol/L 1 h post OGTT mean (SD) 2 h post OGTT mean (SD) | 7/61 (11.5%) 6.2 (0.6) m12.9 (3.1) 10.8 (5.0) | 10/56 (17.9%) 5.4 (0.5) 10.8 (3.1) 7.5 (3.4) | 0.35 |
| Proportion of Patients with IFG at Baseline and Week 12 (Screened Population) | |||
|---|---|---|---|
| Test Arm (n) % n = 61 | Placebo Arm (n) % n = 56 | p Value | |
| Baseline IFG | |||
| Yes | 42 (69) | 40 (71) | 0.76 |
| No | 19 (31) | 16 (29) | |
| Week 12 IFG | |||
| Yes | 40 (71) | 35 (69) | 0.75 |
| No | 16 (29) | 16 (31) | |
| Proportion of Patients with IFG at Baseline and Week 12 (Population Meeting Inclusion/Exclusion Criteria) | |||
| Baseline IFG | |||
| Yes | 20 (35) | 20 (38) | 0.77 |
| No | 37 (65) | 33 (62) | |
| Week 12 IFG | |||
| Yes | 39 (75) | 33 (69) | 0.49 |
| No | 13 (25) | 15 (31) | |
| Proportion of Patients with IFG at Week 12 Who Had IFG at Baseline (Screened Population) | |||
| Baseline IFG | |||
| 40 | 35 | ||
| Week 12 IFG | |||
| Yes | 14 (39) | 13 (43) | |
| No | 22 (61) | 17 (57) | 0.71 |
| Proportion of Patients with IFG at Week 12 Who Had IFG at Baseline (Population Meeting Inclusion/Exclusion Criteria) | |||
| Baseline IFG | |||
| 37 | 33 | ||
| Yes | 11 (33) | 12 (43) | |
| No | 22 (67) | 16 (57) | 0.44 |
| Proportion of Patients with IGT at Baseline and Week 12 (FAS Population) | ||||
| Test Arm (n) % | Placebo Arm (n) % | p Value | ||
| Baseline IGT | n = 61 | n = 56 | ||
| Yes | 23 (38) | 16 (29) | ||
| No | 37 (62) | 40 (71) | 0.27 | |
| Week 12 IGT | ||||
| Yes | 15 (27) | 11 (22) | ||
| No | 41 (73) | 40 (78) | 0.53 | |
| Proportion of Patients with IGT at Baseline and Week 12 (PP Population) | ||||
| Baseline IGT | n = 57 | n = 53 | ||
| Yes | 20 (35) | 15 (28) | ||
| No | 36 (64) | 38 (72) | 0.41 | |
| Week 12 IGT | ||||
| Yes | 13 (25) | 11 (23) | ||
| No | 39 (75) | 37 (77) | 0.81 | |
| Proportion of Patients with IFG at Week 12 Who had IGT at Baseline (FAS Population) | ||||
| Baseline IFG | ||||
| Yes | 23 | 16 | ||
| Week 12 IFG | ||||
| Yes | 12 (52) | 8 (53) | ||
| No | 11 (48) | 7 (47) | 0.94 | |
| Proportion of Patients with IGT at Week 12 Who had IGT at Baseline (PP Population) | ||||
| Baseline IFG | ||||
| 20 | 15 | |||
| Yes | 10 (50) | 8 (57) | ||
| No | 10 (50) | 6 (43) | 0.68 | |
| FAS Population | Intervention Arm | Placebo Arm | p Value |
| n = 61 | n = 56 | ||
| Baseline HbA1c % (mean SD) | 7.7 (0.4) | 5.6 (0.5) | 0.25 |
| Week 12 HbA1c % (mean SD) | 5.8 (0.4) | 5.6 (0.6) | 0.15 |
| Change in HbA1c % (mean SD) | 0.0 (0.2) | 0.0 (0.3) | 0.88 |
| PP population | Intervention Arm | Placebo Arm | p value |
| n = 57 | n = 53 | ||
| Baseline HbA1c % (mean SD) | 5.7 (0.4) | 5.6 (0.5) | 0.24 |
| Week 12 HbA1c % (mean SD) | 5.7 (0.4) | 5.6 (0.6) | 0.23 |
| Change in HbA1c % (mean SD) | 0.0 (0.2) | 0.0 (0.3) | 0.46 |
| FAS Population | Intervention Arm | Placebo Arm | p Value |
| n = 61 | n = 56 | ||
| SF12 baseline physical score (mean SD) | 47.2 (9.0) | 47.0 (8.4) | 0.93 |
| SF12 baseline mental score (mean SD) | 50.5 (11.6) | 51.0 (9.3) | 0.86 |
| SF12 week 12 physical score (mean SD) | 46.1 (8.5) | 47.1 (8.5) | 0.60 |
| SF12 week 12 mental score (mean SD) | 50.4 (10.1) | 53.2 (8.2) | 0.20 |
| Change in SF12 physical score baseline to week 12, mean (SD) | –0.2 (9.0) | 0.1 (7.8) | 0.88 |
| Change in SF12 mental score baseline to week 12, mean (SD) | 0.3 (9.5) | 1.2 (9.2) | 0.69 |
| PP Population | Intervention Arm | Placebo Arm | p value |
| n = 57 | n = 53 | ||
| SF12 baseline physical score (mean SD) | 47.3 (9.1) | 47.3 (8.0) | 0.99 |
| SF12 baseline mental score (mean SD) | 51.1 (11.6) | 50.7 (9.3) | 0.86 |
| SF12 week 12 physical score (mean SD) | 46.3 (8.5) | 47.2 (8.8) | 0.67 |
| SF12 week 12 mental score (mean SD) | 50.0 (10.2) | 53.2 (8.4) | 0.91 |
| Change in SF12 physical score baseline to week 12, mean (SD) | –0.4 (9.1) | –0.2 (7.6) | 0.91 |
| Change in SF12 mental score baseline to week 12, mean (SD) | –0.9 (7.9) | 1.4 (9.6) | 0.30 |
| Factor | Intervention Arm | Control Arm | p Value |
|---|---|---|---|
| FAS population | n = 61 | n = 56 | |
| BMI baseline (mean SD) | 31.4 (6.3) | 31.8 (5.5) | 0.68 |
| BMI week 12 (mean SD) | 31.2 (5.2) | 32.4 (9.0) | 0.51 |
| Fasting plasma glucose baseline (mean SD) | 5.9 (0.9) | 5.9 (0.8) | 0.96 |
| OGTT baseline 1 h (mean SD) | 11.3 (2.4) | 10.7 (3.1) | 0.25 |
| OGTT baseline 2 h (mean SD) | 8.2 (3.1) | 7.8 (3.0) | 0.49 |
| Fasting plasma glucose week 12 mean (SD) | 5.8 (0.7) | 6.0 (0.9) | 0.21 |
| 1 hr post OGTT week 12 (mean SD) | 11.1 (2.8) | 10.4 (3.3) | 0.20 |
| 2 hr post OGTT week 12 (mean SD) | 7.6 (2.9) | 7.9 (3.1) | 0.64 |
| Insulin levels mU/L baseline fasting (mean SD) | 16.6 (8.1) | 17.6 (11.4) | 0.60 |
| Insulin levels mU/L 1 h (mean SD) | 130.0 (76.7) | 130.9 (71.9) | 0.95 |
| Insulin levels mU/L 2 h (mean SD) | 101.4 (73.8) | 102.7 (72.5) | 0.93 |
| Insulin levels mU/L week 12 fasting (mean SD) | 15.7 (9.3) | 18.0 (9.8) | 0.21 |
| Insulin levels mU/L week 12 1 h (mean SD) | 121.7 (74.1) | 120.5 (67.0) | 0.56 |
| Insulin levels mU/L week 12 2 h (mean SD) | 97.5 (82.9) | 101.2 (67.6) | 0.82 |
| PP population | n = 57 | n = 53 | |
| BMI baseline (mean SD) | 31.9 (6.2) | 32.3 (5.2) | 0.77 |
| BMI week 12 (mean SD) | 31.5 (5.0) | 33.2 (8.7) | 0.32 |
| Fasting plasma glucose baseline (mean SD) | 5.9 (0.9) | 5.9 (0.9) | 0.94 |
| OGTT baseline 1 h (mean SD) | 11.3 (2.5) | 10.8 (3.0) | 0.30 |
| OGTT baseline 2 h (mean SD) | 8.0 (3.1) | 7.7 (3.0) | 0.62 |
| Fasting plasma glucose week 12 mean (SD) | 5.7 (0.7) | 6.0 (0.9) | 0.14 |
| 1 hr post OGTT week 12 (mean SD) | 11.0 (2.8) | 10.2 (3.4) | 0.23 |
| 2 hr post OGTT week 12 (mean SD) | 7.3 (2.7) | 7.7 (3.1) | 0.50 |
| Insulin levels mU/L baseline fasting (mean SD) | 16.9 (8.2) | 18.1 (11.5) | 0.56 |
| Insulin levels mU/L 1 h (mean SD) | 134.1 (74.8) | 134.7 (71.0) | 0.97 |
| Insulin levels mU/L 2 h (mean SD) | 99.9 (72.1) | 104.9 (72.2) | 0.74 |
| Insulin levels mU/L week 12 fasting (mean SD) | 16.1 (9.5) | 18.6 (9.9) | 0.20 |
| Insulin levels mU/L week 12 1 h (mean SD) | 120.8 (73.2) | 133.5 (67.1) | 0.41 |
| Insulin levels mU/L week 12 2 h (mean SD) | 90.2 (78.4) | 103.0 (69.6) | 0.44 |
| Factor | Intervention Arm | Placebo Arm | p Value |
|---|---|---|---|
| n = 16 | n = 15 | ||
| Gender M F | 12 4 | 8 7 | 0.21 |
| BMI (mean SD) BMI ≥ 25 yes | 29.2 (4.0) 15 of 16 | 35.1 (4.7) 15 of 15 | <0.001 0.32 |
| Height cm (mean SD) | 171.1 (9.1) | 168.3 (10.0) | 0.42 |
| Weight kg (mean SD) | 88.4 (15.5) | 100.2 (17.3) | 0.05 |
| Baseline blood glucose (mean SD) | 6.5 (0.2) | 6.5 (0.3) | 0.89 |
| Blood glucose 1 h post OGTT (mean SD) | 12.3 (2.5) | 12.7 (2.2) | 0.67 |
| Blood glucose 2 h post OGTT (mean SD) | 10.1 (3.6) | 9.0 (2.7) | 0.35 |
| Insulin levels mU/L baseline fasting (mean SD) | 16.1 (8.5) | 27.1 (16.1) | 0.022 |
| Insulin levels mU/L baseline 1 h (mean SD) | 103.9 (47.2) | 167.0 (82.5) | 0.022 |
| Insulin levels mU/L baseline 2 h (mean SD) | 110.5 (58.6) | 158.2 (88.3) | 0.10 |
| Baseline HbA1c% (mean SD) | 5.9 (0.6) | 5.9 (0.3) | 0.65 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitetta, L.; Butcher, B.; Dal Forno, S.; Vitetta, G.; Nikov, T.; Hall, S.; Steels, E. A Double-Blind Randomized Placebo-Controlled Study Assessing the Safety, Tolerability and Efficacy of a Herbal Medicine Containing Pycnogenol Combined with Papain and Aloe vera in the Prevention and Management of Pre-Diabetes. Medicines 2020, 7, 22. https://doi.org/10.3390/medicines7040022
Vitetta L, Butcher B, Dal Forno S, Vitetta G, Nikov T, Hall S, Steels E. A Double-Blind Randomized Placebo-Controlled Study Assessing the Safety, Tolerability and Efficacy of a Herbal Medicine Containing Pycnogenol Combined with Papain and Aloe vera in the Prevention and Management of Pre-Diabetes. Medicines. 2020; 7(4):22. https://doi.org/10.3390/medicines7040022
Chicago/Turabian StyleVitetta, Luis, Belinda Butcher, Serena Dal Forno, Gemma Vitetta, Tessa Nikov, Sean Hall, and Elizabeth Steels. 2020. "A Double-Blind Randomized Placebo-Controlled Study Assessing the Safety, Tolerability and Efficacy of a Herbal Medicine Containing Pycnogenol Combined with Papain and Aloe vera in the Prevention and Management of Pre-Diabetes" Medicines 7, no. 4: 22. https://doi.org/10.3390/medicines7040022
APA StyleVitetta, L., Butcher, B., Dal Forno, S., Vitetta, G., Nikov, T., Hall, S., & Steels, E. (2020). A Double-Blind Randomized Placebo-Controlled Study Assessing the Safety, Tolerability and Efficacy of a Herbal Medicine Containing Pycnogenol Combined with Papain and Aloe vera in the Prevention and Management of Pre-Diabetes. Medicines, 7(4), 22. https://doi.org/10.3390/medicines7040022





