Identification and Classification of Polymyalgia Rheumatica (PMR) and PMR-Like Syndromes Following Immune Checkpoint Inhibitors (ICIs) Therapy: Discussion Points and Grey Areas Emerging from a Systematic Review of Published Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction
2.5. Quality and Bias Risk Assessment
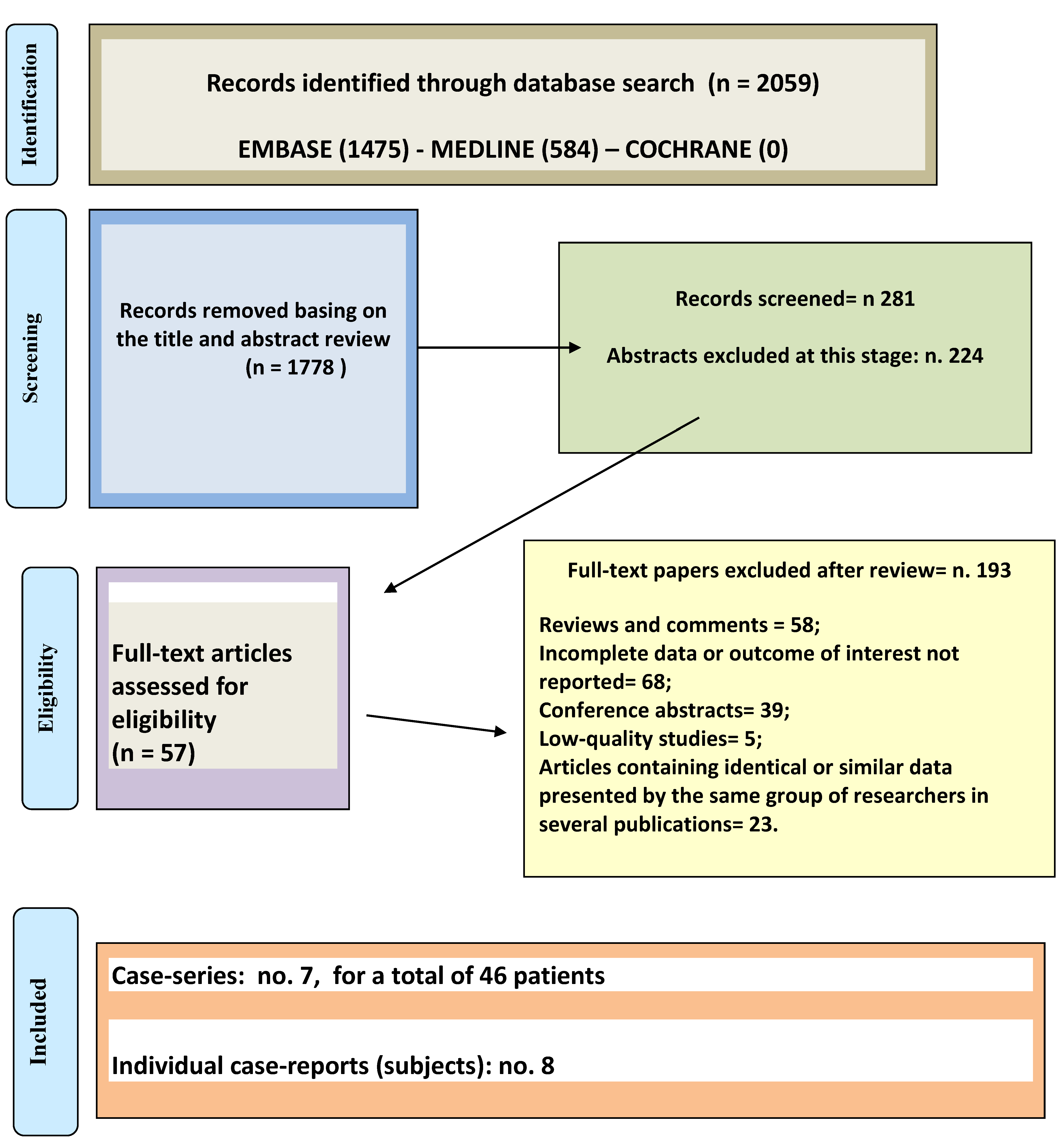
3. Results
3.1. Description of Included Studies
3.2. Case-Reports
3.3. Case-Series
3.4. Risk of Bias Assessment in Analysis of the Included Studies
3.5. ICIs in Patients with Preexisting PMR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Raheel, S.; Shbeeb, I.; Crowson, C.A.; Matteson, E.L. Epidemiology of polymyalgia rheumatica 2000–2014 and examination of incidence and survival trends over 45 years: A population-based study. Arthritis Care Res. (Hoboken) 2017, 69, 1282–1285. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, M.A.; Zaccaria, A. Epidemiology of polymyalgia rheumatica. Clin. Exp. Rheumatol. 2000, 18, S9–S11. [Google Scholar] [PubMed]
- Manzo, C. Incidence and prevalence of polymyalgia rheumatica (PMR): The importance of the epidemiological context. The Italian case. Med. Sci. 2019, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Gay, M.A.; Vazquez-Rodriguez, T.R.; Lopez-Diaz, M.J.; Miranda-Filloy, J.A.; Gonzales-Juanatey, C.; Martin, J.; Llorca, J. Epidemiology of giant cell arteritis and polymyalgia rheumatica. Arthritis Rheum. 2009, 61, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Partington, R.J.; Muller, S.; Helliwell, T.; Mallen, C.D.; Abdul Sultan, A. Incidence, Prevalence and Treatment Burden of Polymyalgia Rheumatica in the UK Over Two Decades: A Population-Based Study. Ann. Rheum. Dis. 2018, 77, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Sobrero, A.; Manzo, C.; Stimamiglio, A. The role of general practictioner and out-of-hospital public rheumatologist in the diagnosis and follow-up of the patient with polymyalgia rheumatica. Reumatismo 2018, 70, 44–50. [Google Scholar] [CrossRef]
- Manzo, C. Polymyalgia rheumatica (PMR) with normal values of both erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) concentration at the time of diagnosis in a centenarian man: A case report. Diseases 2018, 6, 84. [Google Scholar] [CrossRef]
- Gonzalez-Gay, M.A.; Matteson, E.L.; Castaneda, S. Polymyalgia rheumatica. Lancet 2017, 390, 1700–1712. [Google Scholar] [CrossRef]
- Matteson, E.L.; Dejaco, C. Polymyalgia rheumatica. Ann. Intern. Med. 2017, 166, ITC65–ITC80. [Google Scholar] [CrossRef]
- Milchert, M.; Brzosko, M. Diagnosis of polymyalgia rheumatica usually means a favourable outcome for your patient. Indian J. Med. Res. 2017, 145, 593–600. [Google Scholar] [PubMed]
- Manzo, C.; Camellino, D. La polimialgia reumatica: Difficoltà diagnostiche e terapeutiche per una malattia apparentemente “banale”. Recenti Progress. Med. 2017, 108, 221–231. [Google Scholar]
- Manzo, C.; Milchert, M. Polymyalgia rheumatica with normal values of both erythrocyte sedimentation rate and C-reactive protein concentration at the time of diagnosis: A four-point guidance. Reumatologia 2018, 56, 1–2. [Google Scholar] [CrossRef]
- Manzo, C.; Milchert, M.; Natale, M.; Brzosko, M. Polymyalgia rheumatica with normal values of both erythrocyte sedimentation rate and C-reactive protein concentration at the time of diagnosis. Rheumatology (Oxford) 2019, 5, 921–923. [Google Scholar] [CrossRef]
- Dasgupta, B.; Cimmino, M.A.; Kremers, H.M.; Schmidt, A.; Schirmer, M.; Salvarani, C.; Bachta, A.; Dejaco, C.; Duftner, C.; Slott Jensen, H.; et al. 2012 provisional classification criteria for polymyalgia rheumatica: A European League Against Rheumatism/American College of Rheumatology collaborative initiative. Arthritis Rheum. 2012, 64, 943–954. [Google Scholar] [CrossRef]
- Bird, H.A.; Leeb, B.F.; Montecucco, C.M.; Misiuniene, N.; Nesher, G.; Pai, S.; Pease, C.; Rovensky, J.; Rozman, B. A comparison of sensitivity of diagnostic criteria for polymyalgia rheumatica. Ann. Rheum. Dis. 2005, 64, 626–629. [Google Scholar] [CrossRef]
- Graziani, G.; Tentori, L.; Navarra, P. Ipilimumab: A novel immunostimulatory monoclonal antibody for the treatment of cancer. Pharmacol. Res. 2012, 65, 9–22. [Google Scholar] [CrossRef]
- Hosseini, A.; Gharibi, T.; Marofi, F.; Babaloo, Z.; Baradaran, B. CTLA-4: From mechanism to autoimmune therapy. Int. Immunopharmacol. 2020, 80, 106221. [Google Scholar] [CrossRef]
- Sharpe, A.H. Introduction to checkpoint inhibitors and cancer immunotherapy. Immunol. Rev. 2017, 276, 5–8. [Google Scholar] [CrossRef]
- Timeline of Progress in Immunotherapy–Cancer Research Institute. Available online: https://www.canceresearch.org/immunotherapy/timeline-of-progress (accessed on 12 June 2020).
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Le Burel, S.; Champiat, S.; Mateus, C.; Marabelle, A.; Michot, J.M.; Robert, C. Prevalence of immune-related systemic adverse events in patients treated with anti-Programmed cell Death 1/anti-Programmed cell Death-Ligand 1 agents: A single-centre pharmacovigilance database analysis. Eur. J. Cancer 2017, 82, 34–44. [Google Scholar] [CrossRef]
- Schoenfeld, S.R.; Aronow, M.E.; Leaf, R.K.; Dougan, M.; Reynolds, K.L. Diagnosis and Management of Rare Immune-Related Adverse Events. Oncologist 2020, 25, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Kumar, V.; Chaudhary, N.; Garg, M.; Floudas, C.A.; Soni, P.; Chandra, A.B. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitory therapy. Front. Pharmacol. 2017, 8, 49. [Google Scholar] [CrossRef]
- Kostine, M.; Rouxel, L.; Barnetche, T.; Veillon, R.; Martin, F.; Dutriaux, C.; Dousset, L.; Pham-Ledard, A.; Prey, S.; Beylot-Barry, M.; et al. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer-clinical aspects and relationship with tumour response: A single-centre prospective cohort study. Ann. Rheum. Dis. 2018, 77, 393–398. [Google Scholar] [CrossRef]
- Salem, J.-E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
- Gediz, F.; Kobak, S. Immune Checkpoint Inhibitors-related Rheumatic Diseases: What Rheumatologist Shoul Know? Curr. Rheumatol. Rev. 2019, 15, 201–208. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Salvarani, C.; Cantini, F.; Hunder, G.G. Polymyalgia rheumatica and giant-cell arteritis. Lancet 2008, 372, 234–245. [Google Scholar] [CrossRef]
- Dejaco, C.; Brouwer, E.; Mason, J.C.; Buttgereit, F.; Matteson, E.L.; Dasgupta, B. Giant cell arteritis and polymyalgia rheumatica: Current challenges and opportunities. Nat. Rev. Rheumatol. 2017, 13, 578–592. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of non-randomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions. Available online: http://handbook.cochrane.org (accessed on 26 August 2020).
- Bernier, M.; Guillaume, C.; Leon, N.; Alexandre, J.; Hamel-Senecal, L.; Chretien, B.; Lecaignec, F.; Humbert, X.; Fedrizzi, S.; Madeleine, J.; et al. Nivolumab Causing a Polymyalgia Rheumatica in a Patient with a Squamous Non-Small Cell Lung Cancer. J Immunother. 2017, 40, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.K.; Bass, A.R. Checkpoint inhibitor-induced polymyalgia rheumatica controlled by cobimetinib, a MEK ½ inhibitor. Ann. Rheum. Dis. 2019, 78, e70. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Tanaka, M.; Fujii, R.; Uchitani, K.; Okazaki, K. Effectiveness of a Low-dose Corticosteroid in a Patient with Polymyalgia Rheumatica Associated with Nivolumab Treatment. J. Pharm. Soc. Jpn. 2019, 139, 491–495. [Google Scholar] [CrossRef]
- Garel, B.; Kramkimel, N.; Trouvin, A.P.; Frantz, C.; Dupin, N. Pembrolizumab-induced polymyalgia rheumatica in two patients with metastatic melanoma. Jt. Bone Spine 2017, 84, 233–234. [Google Scholar] [CrossRef]
- Iskandar, A.; Hwang, A.; Dasanu, C.A. Polymyalgia rheumatica due to pembrolizumab therapy. J. Oncol. Pharm. Pract. 2019, 25, 1282–1284. [Google Scholar] [CrossRef]
- Nakamagoe, K.; Moriyama, T.; Maruyama, H.; Yokosawa, M.; Hara, T.; Tanaka, S.; Fujimoto, M.; Tamaoka, A. Polymyalgia rheumatica in a melanoma patient due to nivolumab treatment. J. Cancer Res. Clin. Oncol. 2017, 143, 1357–1358. [Google Scholar] [CrossRef]
- Maniu, C.; Kobe, C.; Schlaak, M.; Mauch, C.; Eming, S.A. Polymyalgia rheumatic occurring during treatment with ipilimumab. Eur. J. Derm. 2016, 26, 413–414. [Google Scholar]
- Calabrese, C.; Cappelli, L.C.; Kostine, M.; Kirchner, E.; Braaten, T.; Calabrese, L. Polymyalgia rheumatica-like syndrome from checkpoint inhibitor therapy: Case series and systematic review of the literature. RMD Open 2019, 5, E000906. [Google Scholar] [CrossRef]
- Richter, M.D.; Crowson, C.; Kottschade, L.A.; Finnes, H.D.; Markovic, S.N.; Thanarajasingam, U. Rheumatic syndromes associated with immune checkpoint inhibitors: A single-center cohort of sixty-one patients. Arthritis Rheumatol. 2019, 71, 468–475. [Google Scholar] [CrossRef]
- Kostine, M.; Finckh, A.; Bingham, C.O., 3rd; Visser, K.; Leipe, J.; Schulze-Koops, H.; Choy, E.H.; Benesova, C.; Radstake, T.R.D.J.; Cope, A.P.; et al. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Ann. Rheum. Dis. 2020, 1–13. [Google Scholar] [CrossRef]
- Belkhir, R.; Le Burel, S.; Dunogeant, L.; Marabelle, A.; Hollebecque, A.; Besse, B.; Leary, A.; Voisin, A.L.; Ponoizeau, C.; Coutte, L.; et al. Rheumatoid arthritris and polymyalgia rheumatica occurring after immune checkpoint inhibitor treatment. Ann. Rheum. Dis. 2017, 76, 1747–1750. [Google Scholar] [CrossRef] [PubMed]
- Kuswanto, W.F.; MacFarlane, L.A.; Gedmintas, L.; Mulloy, A.; Choueiri, T.K.; Bermas, B.L. Rheumatologic symptoms in oncologic patients on PD-1 inhibitors. Semin. Arthritis Rheum. 2018, 47, 907–910. [Google Scholar] [CrossRef]
- Mitchell, E.L.; Lau, P.K.H.; Khoo, C.; Liew, D.; Leung, J.; Liu, B.; Rischin, A.; Frauman, A.G.; Kee, D.; Smith, K.; et al. Rheumatic immune-related adverse events secondary to anti-programmed death-1 antibodies and preliminary analysis on the impact of corticosteroids on anti-tumour response: A case series. Eur. J. Cancer 2018, 105, 88–102. [Google Scholar] [CrossRef]
- Calabrese, C.; Kirchner, E.; Kontzias, A.; Velcheti, V.; Calabrese, L.H. Rheumatic immune-related adverse events of checkpoint therapy for cancer: Case series of a new nosological entity. RMD Open 2017, 3, e000412. [Google Scholar] [CrossRef]
- Guggino, G.; Ferrante, A.; Macaluso, F.; Triolo, G.; Ciccia, F. Pathogenesis of polymyalgia rheumatica. Reumatismo 2018, 70, 10–17. [Google Scholar] [CrossRef]
- Camellino, D.; Cimmino, M.A. Are the new ACR/EULAR criteria the ultimate answer for polymyalgia rheumatica classification? J. Rheumatol. 2016, 43, 836–838. [Google Scholar] [CrossRef]
- Liew, D.F.L.; Leung, J.L.Y.; Liu, B.; Cebon, J.; Frauman, A.G.; Buchanan, R.R.C. Association of good oncological response to therapy with the development of rheumatic immune-related adverse events following PD-1 inhibitor therapy. Int. J. Rheum. Dis. 2019, 22, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Manzo, C.; Milchert, M.; Natale, M.; Brzosko, M. Polymyalgia rheumatica with normal inflammatory indices at the time of diagnosis: Can we just move a step forward? Reumatologia 2020, 58, 184–186. [Google Scholar] [CrossRef]
- Braaten, T.; Brahmer, J.R.; Forde, P.M.; Le, D.; Lipson, E.J.; Naidoo, J.; Schollenberger, M.; Zheng, L.; Bingham, C.O.; Shah, A.A.; et al. Immune checkpoint inhibitor-induced inflammatory arthritis persists after immunotherapy cessation. Ann. Rheum. Dis. 2020, 79, 332–338. [Google Scholar] [CrossRef]
- Lidar, M.; Giat, E.; Garelick, D.; Horowitz, Y.; Amital, H.; Steinberg-Silman, Y.; Schacther, J.; Shapira-Frommer, R.; Markel, G. Rheumatic manifestations among cancer patients treated with immune checkpoint inhibitors. Autoimmun. Rev. 2018, 17, 284–289. [Google Scholar] [CrossRef]
- Agbabiaka, T.B.; Savovic, J.; Ernst, E. Methods for causality assessment of adverse drug reactions. Drug Saf. 2008, 31, 21–37. [Google Scholar] [CrossRef]
- Srinivasan, R.; Ramya, G. Adverse drug reaction–Causality assessment. Int. J. Res. Pharm. Chem. 2011, 1, 606–612. [Google Scholar]
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Robert, E.A.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A method for estimating the probability of adverse drug reaction. Clin. Pharmacol. Ther. 1981, 30, 239–245. [Google Scholar] [CrossRef]
- Ernstoff, M.; Ghandi, S.; Pandey, M.; Puzanov, I.; Grivas, P.; Montero, A.; Velcheti, V.; Turk, M.J.; Diaz-Montero, C.M.; Lewis, L.D.; et al. Challenges faced when identifying patients for combination immunotherapy. Future Oncol. 2017, 153, 1162–1165. [Google Scholar] [CrossRef]
- Coury, M.A.; Bell, R.B.; Patel, A.A.; Romba, M.C.; Crittenden, M.R.; Curti, B.D.; Urba, W.J.; Leidner, R.S. Delayed immune-related events (dire) after discontinuation of immunotherapy: Diagnostic hazard of autoimmunity at a distance. J. Immunother. Cancer 2019, 7, 165. [Google Scholar]
| 1st Author and Year | ICI | PMR-Onset | PMR Diagnosis | GCs | ICI Stopped | W.a.r. |
|---|---|---|---|---|---|---|
| Bernier, 2017 [33] | nivolumab | after 13 cycles | n.c. | Yes | Yes | No |
| Chan, 2019 [34] | atezolizumab | after 6 months | n.c. | Yes | No | n.e. |
| Imay, 2019 [35] | nivolumab | after 12 cycles | clear | Yes | Yes | n.e. |
| Garel, 2017 [36] | pembrolizumab | after 1 day | clear | Yes | Yes | No |
| Garel, 2017 [36] | pembrolizumab | after 3 cycles | clear | Yes | No | n.e. |
| Iskandar, 2019 [37] | pembrolizumab | n.c. | n.c. | Yes | No | n.e. |
| Nakamagoe, 2017 [38] | nivolumab | n.c. | n.c. | Yes | Yes | n.e. |
| Maniu, 2016 [39] | ipilimumab | n.c. | n.c. | Yes | Yes | n.e. |
| 1st Author, Year | Study | PMR(No.) | Onset (Median) | Diagnosis | GCs | ICI Stopped | W.a.r. |
|---|---|---|---|---|---|---|---|
| Calabrese, 2019 [40] | retrospective | 20 | 12 week | ACR/EULAR | 7.5–60 | n.c. | n.r. |
| Richter, 2019 [41] | retrospective | 4 * | 4–8 weeks | n.r. | 15–60 | in a minority | n.r. |
| Kostine, 2018 [42] | observational | 11 | n.c | 7.5–60 | Yes | n.c. | |
| Belkhir, 2017 [43] | retrospective | 4 | n.c. | yes | n.c. | ||
| Kuswanto, 2018 [44] | retrospective | 4 | n.c. | n.c. | yes | Yes | n.r. |
| Le Burel, 2017 [21] | retrospective | 1 | n.c. | n.r. | yes | Not | n.c. |
| Mitchell, 2018 [45] | retrospective | 2 ** | n.c. | n.r. | yes | Not | n.c. |
| First Author | Selection | Comparability | Outcome |
|---|---|---|---|
| Calabrese | 3 | 1 | 2 |
| Richter | 2 | 1 | 2 |
| Kostine | 2 | 1 | 2 |
| Belkhir | 2 | 1 | 2 |
| Kuswanto | 2 | 1 | 2 |
| Le Burel | 1 | 1 | 2 |
| Mitchell | 1 | 1 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzo, C.; Isetta, M.; Natale, M.; Castagna, A. Identification and Classification of Polymyalgia Rheumatica (PMR) and PMR-Like Syndromes Following Immune Checkpoint Inhibitors (ICIs) Therapy: Discussion Points and Grey Areas Emerging from a Systematic Review of Published Literature. Medicines 2020, 7, 68. https://doi.org/10.3390/medicines7110068
Manzo C, Isetta M, Natale M, Castagna A. Identification and Classification of Polymyalgia Rheumatica (PMR) and PMR-Like Syndromes Following Immune Checkpoint Inhibitors (ICIs) Therapy: Discussion Points and Grey Areas Emerging from a Systematic Review of Published Literature. Medicines. 2020; 7(11):68. https://doi.org/10.3390/medicines7110068
Chicago/Turabian StyleManzo, Ciro, Marco Isetta, Maria Natale, and Alberto Castagna. 2020. "Identification and Classification of Polymyalgia Rheumatica (PMR) and PMR-Like Syndromes Following Immune Checkpoint Inhibitors (ICIs) Therapy: Discussion Points and Grey Areas Emerging from a Systematic Review of Published Literature" Medicines 7, no. 11: 68. https://doi.org/10.3390/medicines7110068
APA StyleManzo, C., Isetta, M., Natale, M., & Castagna, A. (2020). Identification and Classification of Polymyalgia Rheumatica (PMR) and PMR-Like Syndromes Following Immune Checkpoint Inhibitors (ICIs) Therapy: Discussion Points and Grey Areas Emerging from a Systematic Review of Published Literature. Medicines, 7(11), 68. https://doi.org/10.3390/medicines7110068






