Worldwide Use of RUCAM for Causality Assessment in 81,856 Idiosyncratic DILI and 14,029 HILI Cases Published 1993–Mid 2020: A Comprehensive Analysis
Abstract
1. Introduction
2. Literature Search and Source
3. Definitions
3.1. RUCAM Based Liver Injury
3.1.1. Liver Test Thresholds
3.1.2. Liver Injury Pattern
3.2. Idiosyncratic Versus Intrinsic Liver Injury
4. Worldwide Publications of DILI
5. Worldwide Publications of RUCAM Based Idiosyncratic DILI
5.1. Countries and Regions
5.2. Hospital and Other Sources
5.3. Top Ranking Countries
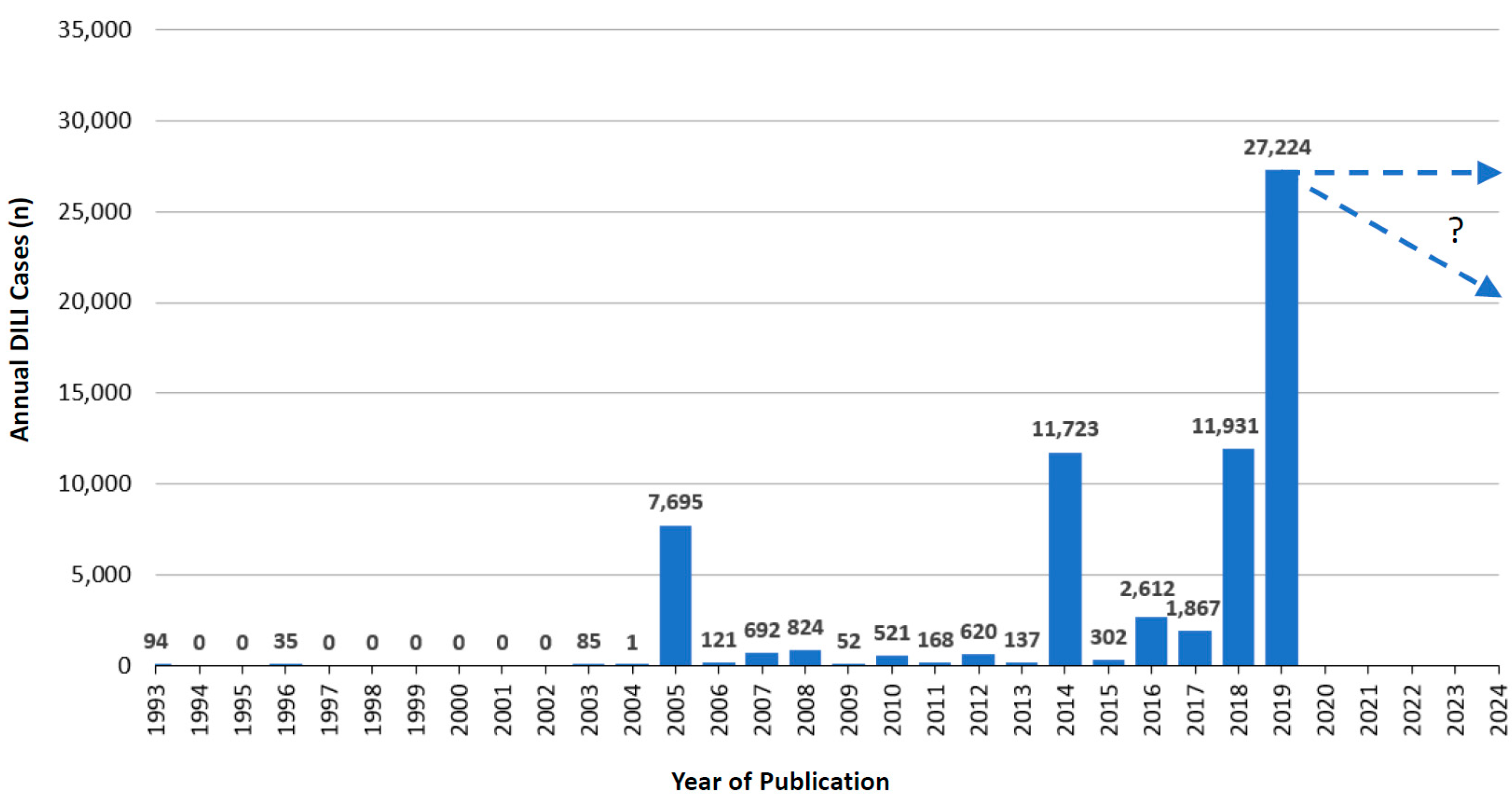
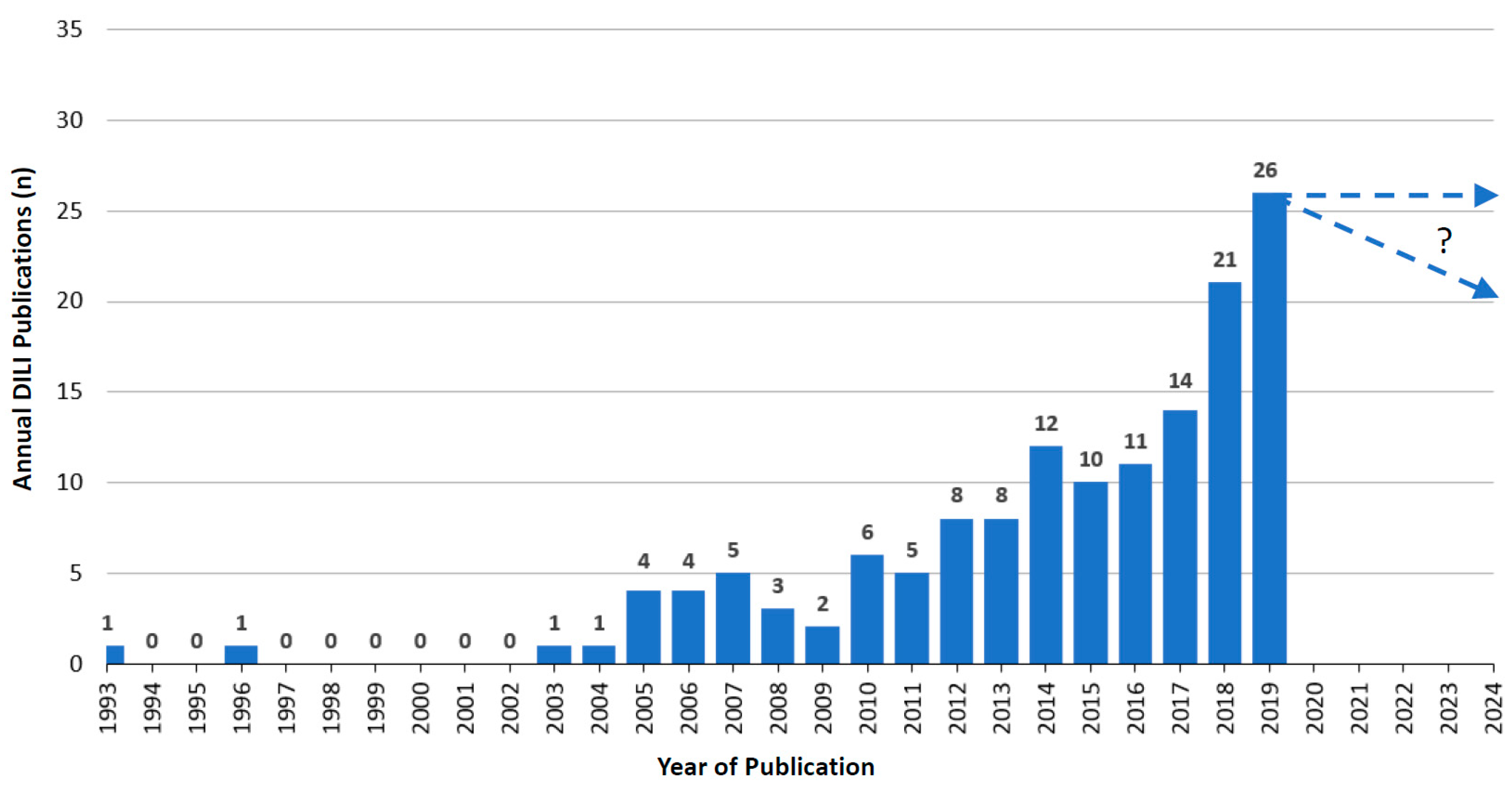
5.4. Annual Growth Trends of RUCAM Based DILI Case Publications
5.4.1. Published Annual RUCAM Based DILI Cases
5.4.2. Annual RUCAM Based DILI Publications and Growth Trend
5.5. Specificities of DILI Case Evaluation
5.6. Worldwide Top Ranking of Drugs Causing DILI
6. Worldwide Publications of HILI Cases Assessed for Causality Using RUCAM
6.1. Countries and Regions
6.2. Hospital and Other Sources
6.3. Top Ranking Countries
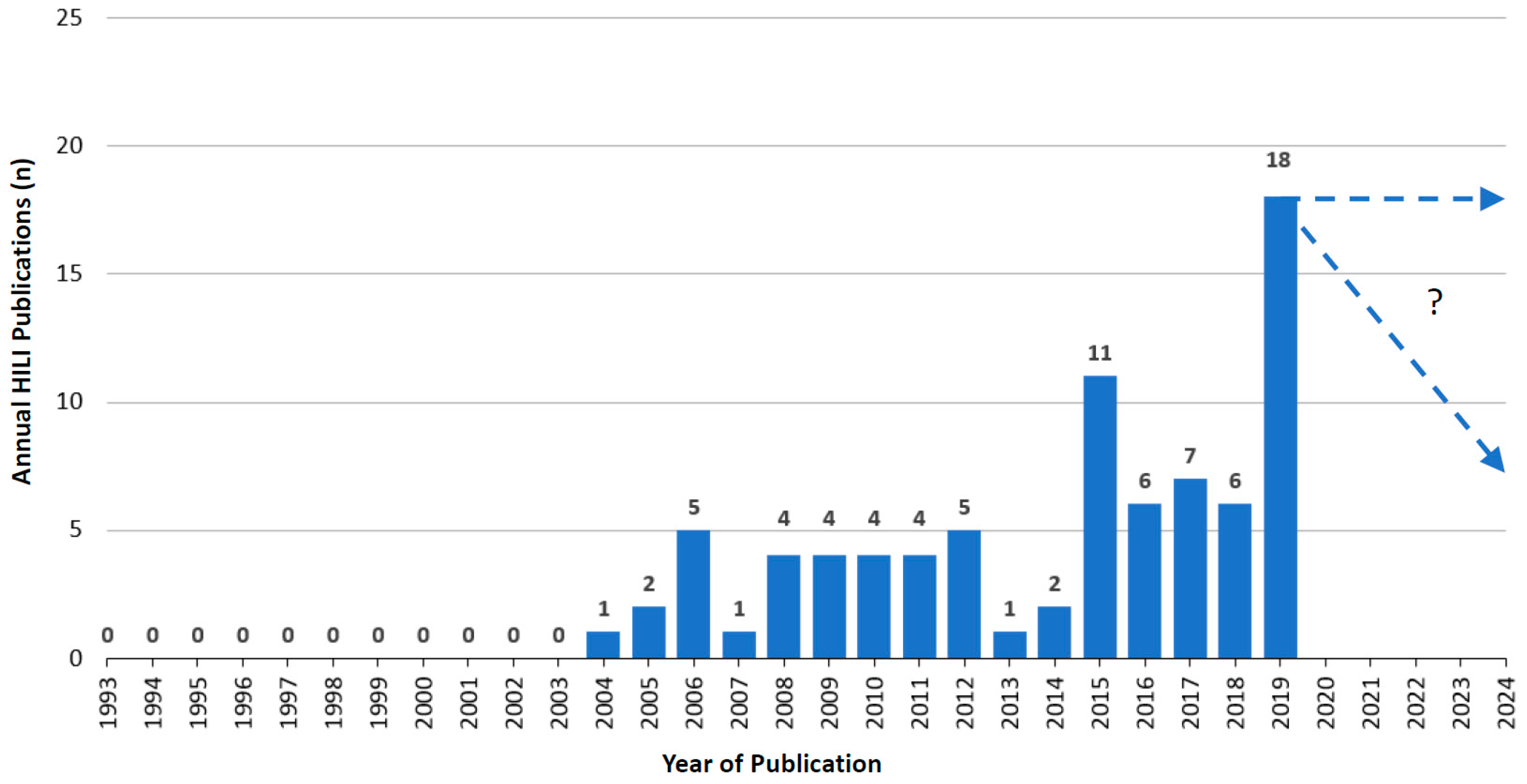
6.3.1. Published Annual RUCAM Based HILI Cases
6.3.2. Annual RUCAM Based HILI Publications and Growth Trend
6.4. Specificities of HILI Cases
7. Utility of RUCAM
8. Other CAMs
9. Limitation of the Analysis
10. Outlook
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ke, L.; Lu, C.; Shen, R.; Lu, T.; Ma, B.; Hua, Y. Knowledge mapping of drug-induced liver injury: A scientometric investigation (2010–2019). Front. Pharmacol. 2020, in press. [Google Scholar] [CrossRef]
- Uetrecht, J. Mechanistic studies of idiosyncratic DILI: Clinical implications. Front. Pharmacol. 2019, 10, 837, In: Special issue: Clinical drug induced liver injury: Current diagnostic and mechanistic challenges, guest editors: Rolf Teschke, Gaby Danan & James H. Lewis. [Google Scholar] [CrossRef]
- Teschke, R.; Uetrecht, J. Mechanism of idiosyncratic drug induced liver injury (DILI): Unresolved basic issues. Ann. Transl. Med. 2020. In special issue: Unresolved basic issues in hepatology, guest editors Ralf Weiskirchen & Wolfgang Stremmel. [Google Scholar] [CrossRef]
- Chen, M.; Will, Y. Drug-Induced Liver Toxicity; Series: Methods in Pharmacology and Toxicology; James, K., David, C., Eds.; Springer Protocols, Springer Nature: Berlin, Germany, 2018. [Google Scholar]
- Teschke, R.; Danan, G. Causality assessment methods in drug-induced liver injury. In Drug-Induced Liver Toxicity; Series: Methods in Pharmacology and Toxicology/Y; James, K., David, C.C., Minjun, C., Yvonne, W., Eds.; Springer Nature: Berlin, Germany, 2018; Chapter 27; pp. 555–594. [Google Scholar] [CrossRef]
- Teschke, R.; Danan, G.; Lewis, J.H. Special issue: Clinical drug induced liver injury: Current diagnostic and mechanistic challenges, guest editors: Rolf Teschke, Gaby Danan & James H. Lewis. Front. Pharmacol. 2019. Available online: https://www.frontiersin.org/research-topics/9104/clinical-drug-induced-liver-injury-current-diagnostic-and-mechanistic-challenges (accessed on 30 June 2020).
- Sarges, P.; Steinberg, J.M.; Lewis, J.H. Drug-induced liver injury: Highlights from a review of the 2015 literature. Drug Saf. 2016, 39, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, O.; Mahajan, S.; Lewis, J.H. Highlights of drug- and herb-induced liver injury in the literature from 2016, How best to translate new information into clinical practice? Exp. Opin. Drug Metab. Toxicol. 2017, 13, 935–951. [Google Scholar] [CrossRef]
- Real, M.; Barnhill, M.S.; Higley, C.; Rosenberg, J.; Lewis, J.H. Drug-induced liver injury: Highlights of the recent literature. Drug Saf. 2019, 42, 365–387. [Google Scholar] [CrossRef]
- Rosenberg, J.J.; Higley, C.; Lewis, J.H. Selected highlights from the recent literature of newly reported herbal and dietary supplement-induced liver injury. Adv. Res. Gastroenterol. Hepatol. 2020, 15, 555904. [Google Scholar] [CrossRef]
- Teschke, R. Idiosyncratic DILI: Analysis of 46, 266 cases assessed for causality by RUCAM and published from 2014 to early 2019. Front. Pharmacol. 2019, 10, 730, In: Special issue: Clinical drug induced liver injury: Current diagnostic and mechanistic challenges. Guest editors: Rolf Teschke, Gaby Danan & James H. Lewis. [Google Scholar] [CrossRef]
- Teschke, R.; Zhu, Y.; Jing, J. Herb induced liver injury (HILI) in the Asian region and current role of RUCAM for causality assessment in 11, 160 published cases: Analysis and outlook. J. Clin. Transl. Hepatol. 2020, 8, 1–15. [Google Scholar]
- Teschke, R.; Eickhoff, A.; Schulze, J.; Danan, G. Herb-induced liver injury (HILI) with 12,068 worldwide cases published with causality assessments by Roussel Uclaf Causality Assessment Method (RUCAM): An overview. Transl. Gastroenterol. Hepatol. 2020, in press. [Google Scholar] [CrossRef]
- Danan, G.; Bénichou, C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: Application to drug-induced liver injuries. J. Clin. Epidemiol. 1993, 46, 1323–1330. [Google Scholar] [CrossRef]
- Danan, G.; Teschke, R. RUCAM in drug and herb induced liver injury: The update. Int. J. Mol. Sci. 2016, 17, 14. [Google Scholar] [CrossRef]
- Danan, G.; Teschke, R. Drug-induced liver injury: Why is the Roussel Uclaf Causality Assessment Method (RUCAM) still used 25 years after its launch? Drug Saf. 2018, 41, 735–743. [Google Scholar] [CrossRef]
- Danan, G.; Teschke, R. Roussel Uclaf Causality Assessment Method for drug-induced liver injury: Present and Future. Front. Pharmacol. 2019, 10, 853, In: Special issue “Clinical drug induced liver injury: Current diagnostic and mechanistic challenges”, guest editors: Rolf Teschke, Gaby Danan & James H. Lewis. [Google Scholar] [CrossRef]
- Teschke, R. Editorial. DILI, HILI, RUCAM algorithm, and AI, the Artificial Intelligence: Provocative issues, progress, and proposals. Arch. Gastroenterol. Res. 2020, 1, 4–11. [Google Scholar]
- Hayashi, P.H. Drug-Induced Liver Injury Network causality assessment: Criteria and experience in the United States. Int. J. Mol. Sci. 2016, 17, 201. [Google Scholar] [CrossRef]
- Teschke, R.; Andrade, R.J. Drug-induced liver injury: Expanding our knowledge by enlarging population analysis with prospective and scoring causality assessment. Gastroenterology 2015, 148, 1271–1273. [Google Scholar] [CrossRef]
- Teschke, R.; Eickhoff, A.; Brown, A.C.; Neuman, M.G.; Schulze, J. Diagnostic biomarkers in liver injury by drugs, herbs, and alcohol: Tricky dilemma after EMA correctly and officially retracted Letter of Support. Int. J. Mol. Sci. 2020, 21, 212. [Google Scholar] [CrossRef]
- Teschke, R. Acetaminophen syn. paracetamol: Acute liver injury and acute on chronic liver failure with case analysis and causality assessment using RUCAM. In Liver Failure: Acute and Acute on Chronic; Nikolaos, T., Ed.; Springer Nature: Cham, Switzerland, 2020. [Google Scholar]
- Bessone, F.; Hermandez, N.; Lucena, M.I.; Andrade, R.J. The Latin American DILI registry experience: A successful ongoing collaborative strategic initiative. Int. J. Mol. Sci. 2016, 17, 313. [Google Scholar] [CrossRef]
- Bessone, F.; Hernandez, N.; Mendizabal, M.; Sanchez, A.; Paraná, R.; Arrese, M.; Tagle, M.; Girala, M.; Lizarzabal, M.; Carrera, E.; et al. When the creation of a consortium provides useful answers: Experience of The Latin American DILI Network (LATINDILIN). Clin. Liver Dis. 2019, 13, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Colaci, C.S.; Mendizaba, M.; Bessone, F. Idiosyncratic drug-induced acute liver failure: A challenging and distressing scenario. Curr. Drug Saf. 2019, 14, 94–101. [Google Scholar] [CrossRef] [PubMed]
- García, D.S.; Saturansky, E.I.; Poncino, D.; Martínez-Artola, Y.; Rosenberg, S.; Abritta, G.; Ascimani-Peña, C.; Cravero, A. Hepatic toxicity by methotrexate with weekly single doses associated with folic acid in rheumatoid and psoriatic arthritis. What is its real frequency? Ann. Hepatol. 2019, 18, 765–769. [Google Scholar] [CrossRef]
- Lin, J.; Moore, D.; Hockey, B.; Di Lernia, R.; Gorelik, A.; Liew, D.; Nicoll, A. Drug-induced hepatotoxicity: Incidence of abnormal liver function tests consistent with volatile anaesthetic hepatitis in trauma patients. Liver Int. 2014, 34, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Pandey, R.; Shah, R.; Black, J. Resolution of ipilimumab induced severe hepatotoxicity with triple immunosuppressants therapy. BMJ Case Rep. 2015, 2015, bcr2014208102. [Google Scholar] [CrossRef]
- Laube, R.; Liu, K. An unwanted complement: Rare case of potential liver injury induced by an interaction between ginseng and atorvastatin. Br. J. Clin. Pharm. 2019, 85, 1612–1613. [Google Scholar] [CrossRef]
- Worland, T.; Chin, K.L.; van Langenberg, D.; Garg, M.; Nicoll, A. Retrospective study of idiosyncratic drug-induced liver injury from infliximab in an inflammatory bowel disease cohort: The IDLE study. Ann. Gastroenterol. 2020, 33, 162–169. [Google Scholar] [CrossRef]
- Sridharan, K.; Al Daylami, A.; Ajjawi, R.; Al Ajooz, H.A.M. Drug-induced liver injury in critically ill children taking antiepileptic drugs: A retrospective study. Curr. Ther. Res. 2020, in press. [Google Scholar]
- Becker, M.W.; Muller Lunardelli, M.J.; Valle Tovo, C. Drug and herb-induced liver injury: A critical review of Brazilian cases with proposals for the improvement of causality assessment using RUCAM. Ann. Hepatol. 2019, 18, 742–750. [Google Scholar] [CrossRef]
- Yan, B.; Leung, Y.; Urbanski, S.J.; Myers, R.P. Rofecoxib-induced hepatotoxicity: A forgotten complication of the coxibs. Can. J. Gastroenterol. 2006, 20, 351–355. [Google Scholar] [CrossRef]
- Nhean, S.; Yoong, D.; Wong, D.K.; Gough, K.; Tseng, A.L. Probable hepatotoxicity with dolutegravir: Report of two cases and review of the literature. Aids 2019, 33, 1261–1263. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.Q.; Zeng, Z.; Wang, G.Q. Hospital admissions for drug-induced liver injury: Clinical features, therapy, and outcomes. Cell Biochem. Biophys. 2012, 64, 77–83. [Google Scholar] [CrossRef]
- Lv, X.; Tang, S.; Xia, Y.; Zhang, Y.; Wu, S.; Yang, Z.; Li, X.; Tu, D.; Chen, Y.; Deng, P.; et al. NAT2 genetic polymorphisms and anti-tuberculosis drug-induced hepatotoxicity in Chinese community population. Ann. Hepatol. 2012, 11, 700–707. [Google Scholar] [CrossRef]
- Hao, K.; Yu, Y.; He, C.; Wang, M.; Wang, S.; Li, X. RUCAM scale-based diagnosis, clinical features and prognosis of 140 cases of drug-induced liver injury. Chin. J. Hepatol. 2014, 22, 938–941, (Abstract in English, Article in Chinese). [Google Scholar] [CrossRef]
- Ou, P.; Chen, Y.; Li, B.; Zhang, M.; Liu, X.; Li, F.; Li, Y.; Chen, C.; Mao, Y.; Chen, J. Causes, clinical features and outcomes of drug-induced liver injury in hospitalized patients in a Chinese tertiary care hospital. SpringerPlus 2015, 4, 802. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Y.G.; Wang, J.B.; Liu, S.H.; Wang, L.F.; Zhao, Y.L.; Bai, Y.F.; Wang, Z.X.; Li, J.Y.; Xiao, X.H. Causes, features, and outcomes of drug-induced liver injury in 69 children from China. Gut Liver 2015, 9, 525–533. [Google Scholar] [CrossRef]
- Lu, R.J.; Zhang, Y.; Tang, F.L.; Zheng, Z.W.; Fan, Z.D.; Zhu, S.M.; Qian, X.F.; Liu, N.N. Clinical characteristics of drug-induced liver injury and related risk factors. Exp. Med. 2016, 12, 2606–2616. [Google Scholar] [CrossRef]
- Yang, J.; Yu, Y.L.; Jin, Y.; Zhang, Y.; Zheng, C.Q. Clinical characteristics of drug-induced liver injury and primary biliary cirrhosis. World J. Gastroenterol. 2016, 22, 7579–7586. [Google Scholar] [CrossRef]
- Zhu, Y.; Niu, M.; Chen, J.; Zou, Z.S.; Ma, Z.J.; Liu, S.H.; Wang, R.L.; He, T.T.; Song, H.B.; Wang, Z.X.; et al. Comparison between Chinese herbal medicine and Western medicine-induced liver injury of 1985 patients. J. Gastroenterol. Hepatol. 2016, 31, 1476–1482. [Google Scholar] [CrossRef]
- Naiqiong, W.; Liansheng, W.; Zhanying, H.; Yuanlin, G.; Chenggang, Z.; Ying, G.; Qian, D.; Dongchen, L.; Yanjun, Z.; Jianjun, L. A multicenter and randomized controlled trial of Bicyclol in the treatment of statin-induced liver injury. Med. Sci. Monit. 2017, 23, 5760–5766. [Google Scholar] [CrossRef]
- Li, X.L.; Liu, X.; Song, Y.; Hong, R.T.; Shi, H. Suspected drug-induced liver injury associated with iguratimod: A case report and review of the literature. BMC Gastroenterol. 2018, 18, 130. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.W.; Panx, Y.C.; Huang, Z.C.; Liu, W.X.; Zhao, R.S.; Jing, H.M.; Dong, F. Posaconazole-associated severe hyperbilirubinemia in acute myeloid leukemia following chemotherapy: A case report. World J. Clin. Cases 2018, 6, 1206–1209. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.; Chen, S.; Lin, G.; Yang, M.; Lu, L.; He, X.; Pan, H.; Tang, S. Genetic polymorphisms of UGT 1A1 and susceptibility to anti-tuberculosis drug-induced liver injury: A RUCAM-based case-control study. Int. J. Immunopathol. Pharm. 2018, 32, 1–6. [Google Scholar] [CrossRef]
- Liao, P.F.; Wu, Y.K.; Huang, K.L.; Chen, H.Y. A rare case of cefapime-induced cholestatic liver injury. Tzu Chi Med. J. 2019, 31, 124–128. [Google Scholar]
- Shen, T.; Liu, Y.; Shang, J.; Xie, Q.; Li, J.; Yan, M.; Xu, J.; Niu, J.; Liu, J.; Watkins, P.B.; et al. Incidence and etiology of drug-induced liver injury in Mainland China. Gastroenterology 2019, 156, 2230–2241. [Google Scholar] [CrossRef]
- Xing, M.; Zhai, L.; Li, J.; Li, Q.; Gao, M.; Wen, J.; Xu, Z. Assessment of cholestasis in drug-induced liver injury by different methods. Medicine 2019, 98, e14399. [Google Scholar] [CrossRef]
- Ma, S.; Liu, S.; Wang, Q.; Chen, L.; Yang, P.; Sun, H. Fenofibrate-induced hepatotoxicity: A case with a special feature that is different from those in the LiverTox database. J. Clin. Pharm. 2020, 45, 204–207. [Google Scholar] [CrossRef]
- Tao, B.; Yang, M.; Chen, H.; Pan, H.; Liu, W.; Yi, H.; Tang, S. Association of ABO blood group and antituberculosis drug-induced liver injury: A case-control study from a Chinese Han population. J. Clin. Pharm. 2020. [Google Scholar] [CrossRef]
- Wang, S.; Shangguan, Y.; Ding, C.; Li, P.; Ji, Z.; Shao, J.; Fang, H.; Yang, M.; Shi, P.; Wu, J.; et al. Risk factors for acute liver failure among inpatients with anti-tuberculosis drug-induced liver injury. J. Int. Med. Res. 2020, 48. [Google Scholar] [CrossRef]
- Yang, H.; Guo, D.; Xu, Y.; Zhu, M.; Yao, C.; Chen, C.; Jia, W. Comparison of different liver test thresholds for drug-induced liver injury: Updated RUCAM versus other methods. Front. Pharmacol. 2019, 10, 816, In: Special issue: Clinical drug induced liver injury: Current diagnostic and mechanistic challenges, guest editors Rolf Teschke, Gaby Danan, James H. Lewis. [Google Scholar] [CrossRef]
- Ríos, D.; Restrepo, J.C. Abendazole-induced liver injury: A case report. Colomb. Med. 2013, 44, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Cano-Paniagua, A.; Amariles, P.; Angulo, N. Epidemiology of drug-induced liver injury in a university hospital from Colombia: Updated RUCAM being used for prospective causality assessment. Ann. Hepatol. 2019, 18, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Alhaddad, O.; Elsabaawy, M.; Abdelsameea, E.; Abdalla, A.; Shabaan, A.; Ehsan, N.; Elrefaey, A.; Elsabaawy, D.; Salama, M. Presentations, causes and outcomes of drug-induced liver injury in Egypt. Sci. Rep. 2020, 10, 5124. [Google Scholar] [CrossRef] [PubMed]
- Bénichou, C.; Danan, G.; Flahault, A. Causality assessment of adverse reactions to drugs—II. An original model for validation of drug causality assessment methods: Case reports with positive rechallenge. J. Clin. Epidemiol. 1993, 46, 1331–1336. [Google Scholar]
- Arotcarena, R.; Bigué, J.P.; Etcharry, F.; Pariente, A. Pioglitazone-induced acute severe hepatitis. Gastroenterol. Clin. Biol. 2004, 28, 609–618. (In French) [Google Scholar]
- Moch, C.; Rocher, F.; Lainé, P.; Lacotte, J.; Biour, M.; Gouraud, A.; Bernard, N.; Descotes, J.; Vial, T. Etifoxine-induced acute hepatitis: A case series. Clin. Res. Hepatol. Gastroenterol. 2012, 36, e85–e88. [Google Scholar] [CrossRef]
- Carrier, P.; Godet, B.; Crepin, S.; Magy, L.; Debette-Gratien, M.; Pillegand, B.; Jacques, J.; Sautereau, D.; Vidal, E.; Labrousse, F.; et al. Acute liver toxicity due to methylprednisolone: Consider this diagnosis in the context of autoimmunity. Clin. Res. Hepatol. Gastroenterol. 2013, 37, 100–104. [Google Scholar] [CrossRef]
- Ripault, M.P.; Pinzani, V.; Fayolle, V.; Pageaux, G.P.; Larrey, D. Crizotinib-induced acute hepatitis: First case with relaps after reintroduction with reduced dose. Clin. Res. Hepatol. Gastroenterol. 2013, 37, e21–e23. [Google Scholar] [CrossRef]
- Dumortier, J.; Cottin, J.; Lavie, C.; Guillaud, O.; Hervieu, V.; Chambon-Augoyard, C.; Scoazec, J.Y.; Vukusic, S.; Vital, T. Methylprednisolone liver toxicity: A new case and a French regional pharmacovigilance survey. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 497–501. [Google Scholar] [CrossRef]
- Meunier, L.; Larrey, D. Recent advances in hepatotoxicity of non steroidal anti-inflammatory drugs. Ann. Hepatol. 2018, 17, 187–191. [Google Scholar] [CrossRef]
- Stammschulte, T.; Treichel, U.; Pachl, H.; Gundert-Remy, U. Cases of Liver Failure in Association with Flupirtine in the German Spontaneous Reporting System. Available online: http://www.akdae.de/Kommission/Organisation/Aufgaben/Publikationen/PDF/Stammschulte2012.pdf (accessed on 30 June 2020).
- Douros, A.; Bronder, E.; Andersohn, F.; Klimpel, A.; Thomae, M.; Orzechowski, H.D.; Kreutz, R.; Garbe, E. Flupirtine-induced liver injury-Seven cases from the Berlin Case-control Surveillance Study and review of the German spontaneous adverse drug reaction reporting database. Eur. J. Clin. Pharm. 2014, 70, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Douros, A.; Bronder, E.; Andersohn, F.; Klimpel, A.; Thomae, M.; Sarganas, G.; Kreutz, R.; Garbe, E. Drug-induced liver injury: Results from the hospital-based Berlin Case-Control Surveillance Study. Br. J. Clin. Pharm. 2014, 79, 988–999. [Google Scholar] [CrossRef] [PubMed]
- Buechter, M.; Manka, P.; Heinemann, F.M.; Lindemann, M.; Baba, H.A.; Schlattjan, M.; Canbay, A.; Gerken, G.; Kahraman, A. Potential triggering factors of acute liver failure as a first manifestation of autoimmune hepatitis-a single center experience of 52 adult patients. World J. Gastroenterol. 2018, 24, 1410–1418. [Google Scholar] [CrossRef]
- Dragoi, D.; Benesic, A.; Pichler, G.; Kulak, N.A.; Bartsch, H.S.; Gerbes, A.L. Proteomics analysis of monocyte-derived hepatocyte-like cells identifies integrin beta 3 as a specific biomarker for drug-induced liver injury by diclofenac. Front. Pharmacol. 2018, 9, 699. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Danan, G. Review: Drug induced liver injury with analysis of alternative causes as confounding variables. Br. J. Clin. Pharm. 2018, 84, 1467–1477. [Google Scholar] [CrossRef]
- Teschke, R. Review. Top-ranking drugs out of 3312 drug-induced liver injury cases evaluated by the Roussel Uclaf Causality Assessment Method. Expert Opin. Drug Metab. Toxicol. 2018, 14, 1169–1187. [Google Scholar] [CrossRef]
- Weber, S.; Benesic, A.; Rotter, I.; Gerbes, A.L. Early ALT response to corticosteroid treatment distinguishes idiosyncratic drug-induced liver injury from autoimmune hepatitis. Liver Int. 2019, 39, 1906–1917. [Google Scholar] [CrossRef]
- Björnsson, E.; Jacobsen, E.I.; Kalaitzakis, E. Hepatotoxicity associated with statins: Reports of idiosyncratic liver injury post-marketing. J. Hepatol. 2012, 56, 374–380. [Google Scholar] [CrossRef]
- Björnsson, E.S.; Bergmann, O.M.; Björnsson, H.K.; Kvaran, R.B.; Olafsson, S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013, 144, 1419–1425. [Google Scholar] [CrossRef]
- Björnsson, E.S.; Hoofnagle, J.H. Categorization of drugs implicated in causing liver injury: Critical assessment based on published case reports. Hepatology 2016, 63, 590–603. [Google Scholar] [CrossRef]
- Harugeri, A.; Parthasarathi, G.; Sharma, J.; D’Souza, G.A.; Ramesh, M. Montelukast induced acute hepatocellular injury. J. Postgrad. Med. 2009, 55, 141–142. [Google Scholar]
- Devarbhavi, H.; Dierkhising, R.; Kremers, W.K.; Sandeep, M.S.; Karanth, D.; Adarsh, C.K. Single center experience with drug-induced liver injury from India: Causes, outcome, prognosis, and predictors of mortality. Am. J. Gastroenterol. 2010, 105, 2396–2404. [Google Scholar] [CrossRef] [PubMed]
- Rathi, C.; Pipaliya, N.; Patel, R.; Ingle, M.; Phadke, A.; Sawant, P. Drug induced liver injury at a tertiary hospital in India: Etiology, clinical features and predictors of mortality. Ann. Hepatol. 2017, 16, 442–450. [Google Scholar] [CrossRef]
- Taneja, S.; Kumar, P.; Rathi, S.; Duseja, A.; Singh, V.; Dhiman, R.K.; Chawla, Y.K. Acute liver failure due to Etodolac, a selective cycloxygenase- 2 (COX-2) inhibitor non-steroidal anti-inflammatory drug established by RUCAM-based causality assessment. Ann. Hepatol. 2017, 16, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Behera, S.K.; Xavier, A.S.; Velupula, S.; Dkkar, S.A.; Selvarajan, S. Agreement among different scales for causality assessment in drug-induced liver injury. Clin. Drug Investig. 2018, 38, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.K.; Chakravarty, P.J.; Borah, A.J. Haloperidol induced hepatotoxicity: A case report. Ann. Clin. Case Rep. 2020, 5, 1827. [Google Scholar]
- Kulkarni, A.V.; Kumar, P.; Talukdar, R.; Rao, N. Steroids as rescue therapy for vitamin A-induced acute liver failure. BMJ Case Rep. CP 2020, 13, e233902. [Google Scholar] [CrossRef] [PubMed]
- Gluck, N.; Fried, M.; Porat, R. Acute amiodarone liver toxicity likely due to ischemic hepatitis. Isr. Med. Assoc. J. 2011, 13, 748–752. [Google Scholar]
- Rigato, I.; Cravatari, M.; Avellini, C.; Ponte, E.; Crocè, S.L.; Tiribelli, C. Drug-induced acute cholestatic liver damage in a patient with mutation of UGT1A1. Nat. Clin. Pr. Gastroenterol. Hepatol. 2007, 4, 43–408. [Google Scholar] [CrossRef]
- Licata, A.; Calvaruso, V.; Capello, M.; Craxi, A.; Almasio, P.L. Clinical course and outcomes of drug-induced liver injury: Nimesulide as the first implicated medication. Dig. Liver Dis. 2010, 42, 143–148. [Google Scholar] [CrossRef]
- Abenavoli, L.; Milic, N.; Beaugrand, M. Severe hepatitis by cyproterone acetate: Role of corticosteroids. A case report. Ann. Hepatol. 2013, 1, 152–155. [Google Scholar] [CrossRef]
- Ferrajolo, C.; Verhamme, K.M.; Trifirò, G.; W‘t Jong, G.; Picelli, G.; Giaquinto, C.; Mazzaglia, G.; Stricker, B.H.; Rossi, F.; Capuano, A.; et al. Antibiotic-induced liver injury in paediatric outpatients: A case-control study in primary care databases. Drug Saf. 2017, 40, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Licata, A.; Minissale, M.G.; Calvaruso, V.; Craxi, A. A focus on epidemiology of drug-induced liver injury; analysis of a prospective cohort. Eur. Rev. Pharm. Sci. 2017, 21, 112–121. [Google Scholar]
- Giacomelli, A.; Rusconi, S.; Falvella, F.S.; Oreni, M.L.; Cattaneo, D.; Cozzi, V.; Renisi, G.; Monge, E.; Cheli, S.; Clementi, E.; et al. Clinical and genetic determinants of nevirapine plasma through concentration. SAGE Open Med. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Licata, A.; Puccia, F.; Lombardo, V.; Serruto, A.; Minissale, M.G.; Morreale, I.; Giannitrapani, L.; Soresi, M.; Montalto, G.; Almasio, P.L. Rivaroxaban-induced hepatotoxicity: Review of the literature and report of new cases. Eur. J. Gastroenterol. Hepatol. 2018, 30, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Lovero, R.; Losurdo, G.; Mastromauro, M.; Castellaneta, N.M.; Mongelli, A.; Gentile, A.; Di Leo, A.; Principi, M. A case of severe transaminase elevation following a single Ustekinumab dose with remission after drug withdrawal. Curr. Drug Saf. 2018, 13, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Masumoto, T.; Horiike, N.; Abe, M.; Kumaki, T.; Matsubara, H.; Fazle Akbar, S.M.; Michitaka, K.; Hyodo, I.; Onji, M. Diagnosis of drug-induced liver injury in Japanese patients by criteria of the Consensus Meetings in Europe. Hepatol. Res. 2003, 25, 1–7. [Google Scholar] [CrossRef]
- Hanatani, T.; Sai, K.; Tohkin, M.; Segawa, K.; Kimura, M.; Hori, K.; Kawakami, J.; Saito, Y. A detection algorithm for drug-induced liver injury in medical information databases using the Japanese diagnostic scale and its comparison with the Council for International Organizations of Medical Sciences/the Roussel Uclaf Causality Assessment Method scale. Pharm. Drug Saf. 2014, 23, 984–988. [Google Scholar] [CrossRef]
- Niijima, K.; Niijima, Y.; Okada, S.; Yamada, M. Drug-induced liver injury caused by Ipragliflozin administration with causality established by a positive lymphocyte transformation test (LTT) and the Roussel Uclaf Causality Assessment Method (RUCAM): A case report. Ann. Hepatol. 2017, 16, 308–311. [Google Scholar] [CrossRef]
- Ji, H.; Yue, F.; Song, J.; Zhou, X. A rare case of methimazole-induced cholestatic jaundice in an elderly man of Asian ethnicity with hyperthyroidism: A case report. Medicine 2017, 96, e9093. [Google Scholar] [CrossRef]
- Aiso, M.; Takikawa, H.; Tsuji, K.; Kagawa, T.; Watanabe, M.; Tanaka, A.; Sato, K.; Sakisaka, S.; Hiasa, Y.; Takei, Y.; et al. Analysis of 307 cases with drug-induced liver injury between 2010 and 2018 in Japan. Hepatol. Res. 2019, 49, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, M.; Adachi, M.; Takahashi, K.; Washizaki, K. Clonazepam-induced liver dysfunction, severe hyperlipidaemia, and hyperglycaemic crisis: A case report. SAGE Open Med. Cases Rep. 2019, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, M.; Tanaka, T.; Koga, F.; Saito, A.; Oza, N.; Ikeda, O.; Manabe, T.; Miyoshi, A.; Kitahara, K.; Sato, S.; et al. An unusual case of acute liver failure caused by adjuvant oral Tegafur-Uracil with folinate therapy for colon cancer patient: A case report. J. Gastrointest. Cancer 2020, 51, 296–299. [Google Scholar] [CrossRef]
- Kakisaka, K.; Suzuki, Y.; Jinnouchi, Y.; Kanazawa, J.; Sasaki, T.; Yonezawa, T.; Yoshida, Y.; Kuroda, H.; Takikawa, Y. Unfavorable prognosis of patients with acute liver injury due to drug-induced liver injury and acute exacerbation of hepatitis B virus infection. Hepatol. Res. 2019, 49, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.Y.; Yang, H.W.; Cho, S.H.; Kang, D.W.; Go, H.; Lee, W.C.; Lee, Y.J.; Jung, S.H.; Kim, A.N.; Cha, S.W. Acute drug-induced hepatitis caused by albendazole. J. Korean Med. Sci. 2008, 23, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Suk, K.T.; Kim, D.J.; Kim, C.H.; Park, S.H.; Yoon, J.H.; Kim, Y.S.; Baik, G.H.; Kim, J.B.; Kweon, Y.O.; Kim, B.I.; et al. A prospective nationwide study of drug-induced liver injury in Korea. Am. J. Gastroenterol. 2012, 107, 1380–1387. [Google Scholar] [CrossRef]
- Son, C.G. Drug-induced liver injury by Western medication. J. Int. Korean Med. 2015, 36, 69–75. [Google Scholar]
- Woo, H.J.; Kim, H.Y.; Choi, E.S.; Cho, Y.; Kim, Y.; Lee, J.H.; Jang, E. Drug-induced liver injury: A 2-year retrospective study of 1169 hospitalized patients in a single medical center. Phytomedicine 2015, 22, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Byeon, J.H.; Kil, J.H.; Ahn, Y.C.; Son, C.S. Systematic review of published data on herb induced liver injury. J. EthnoPharmacol. 2019, 233, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Kim, S.; Yoo, H.; Lee, E. Nimesulide-induced hepatotoxicity: A systemic review and meta-analysis. PLoS ONE 2019, 14, e0209264. [Google Scholar] [CrossRef] [PubMed]
- Thalha, A.M.; Mahadeva, S.; Tan, A.T.B.; Mun, K.S. Kombiglyze (metformin and saxagliptin)-induced hepatotoxicity in a patient with non-alcoholic fatty liver disease. JGH Open 2018, 2, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Lammel-Lindemann, J.A.; Clores-Villalba, E.; Martagón, A.J.; DeObeso-Gonzalez, E.; Puente-Gallegos, F. Non-cholestatic acute hepatitis following Candesartan administration. Br. J. Clin. Pharm. 2018, 84, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Essaid, A.; Timraz, A. Cholestatic acute hepatitis induced by tadalafil (Cialis®). Gastroenterol. Clin. Biol. 2010, 34, e1–e2. (In French) [Google Scholar] [CrossRef] [PubMed]
- Abid, A.; Subhani, F.; Kayani, F.; Awan, S.; Abid, S. Drug induced liver injury is associated with high mortality-A study from a tertiary care hospital in Pakistan. PLoS ONE 2020, 15, e0231398. [Google Scholar] [CrossRef]
- Costa-Moreira, P.; Gaspar, R.; Pereira, P.; Lopes, S.; Canao, P.; Lopes, J.; Carneiro, F.; Macedo, G. Role of liver biopsy in the era of clinical prediction scores for “drug-induced liver injury” (DILI): Experience of a tertiary referral hospital. Virchows Arch. 2020. [Google Scholar] [CrossRef]
- Alqrinawi, S.H.; Akbar, N.; AlFaddag, H.; Akbar, S.; Akbar, L.; Butt, S.A.; Aljawad, M. Menotrophin induced autoimmune hepatitis. Case Rep. Gastrointest. Med. 2019. [Google Scholar] [CrossRef]
- Miljkovic, M.M.; Dobric, S.; Dragojevic-Simic, V. Consistency between causality assessments obtained with two scales and their agreement with clinical judgments in hepatotoxicity. Pharm. Drug Saf. 2011, 20, 272–285. [Google Scholar] [CrossRef]
- Miljkovic, M.M.; Dobric, S.; Dragojevic-Simic, V. Accuracy and reproducibility of two scales in causality assessment of unexpected hepatotoxicity. J. Clin. Pharm. 2012, 37, 196–203. [Google Scholar] [CrossRef]
- Wai, C.T. Presentation of drug-induced liver injury in Singapore. Singap. Med. J. 2006, 47, 116–120. [Google Scholar]
- Rodríguez, L.A.; Stricker, B.H.; Zimmerman, H.J. Risk of acute liver injury associated with the combination of amoxicillin and clavulanic acid. Arch. Int. Med. 1996, 156, 1327–1332. [Google Scholar] [CrossRef]
- Andrade, R.J.; Lucena, M.I.; Fernández, M.C.; Pelaez, G.; Pachkoria, K.; García-Ruiz, E.; García-Muñoz, B.; Gonzalez-Grande, R.; Pizarro, A.; Durán, J.A.; et al. Spanish Group for the Study of Drug-induced Liver Disease. Drug-induced liver injury: An analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology 2005, 129, 512–521. [Google Scholar] [CrossRef]
- Andrade, R.J.; Lucena, M.I.; Kaplowitz, N.; García-Muñoz, B.; Borraz, Y.; Pachkoria, K.; García-Cortés, M.; Fernández, M.C.; Pelaez, G.; Rodrigo, L.; et al. Outcome of acute idiosyncratic drug-induced liver injury: Long term follow-up in a hepatotoxicity registry. Hepatology 2006, 44, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- García-Cortés, M.; Lucena, M.I.; Pachkoria, K.; Borraz, Y.; Hidalgo, R.; Andrade, R.J. Evaluation of Naranjo Adverse Drug Reactions Probability Scale in causality assessment of drug-induced liver injury. Aliment. Pharm. 2008, 27, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Lucena, M.I.; Molokhia, M.; Shen, Y.; Urban, T.J.; Aithal, G.P.; Andrade, R.J.; Day, C.P.; Ruiz-Cabello, F.; Donaldson, P.T.; Stephens, C.; et al. Susceptibility to amoxicillin-clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology 2011, 141, 338–347. [Google Scholar] [CrossRef]
- Lucena, M.I.; Kaplowitz, N.; Hallal, H.; Castiella, A.; García-Bengoechea, M.; Otazua, P.; Berenguer, M.; Fernandez, M.C.; Planas, R.; Andrade, R.J. Recurrent drug-induced liver injury (DILI) with different drugs in the Spanish Registry: The dilemma of the relationship to autoimmune hepatitis. J. Hepatol. 2011, 55, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Robles-Diaz, M.; Gonzalez-Jimenez, A.; Medina-Caliz, I.; Stephens, C.; García-Cortes, M.; García-Muñoz, B.; Ortega-Alonso, A.; Blanco-Reina, E.; Gonzalez-Grande, R.; Jimenez-Perez, M.; et al. on behalf of the Spanish DILI Registry and the SLatinDILI Network. Distinct phenotype of hepatotoxicity associated with illicit use of anabolic androgenic steroids. Aliment. Pharm. 2015, 41, 116–125. [Google Scholar] [CrossRef]
- Tong, H.Y.; Díaz, C.; Collantes, E.; Medrano, N.; Borobia, A.M.; Jara, P.; Ramírez, E. Liver transplant in a patient under methylphenidate therapy: A case report and review of the literature. Case Rep. Pediatr. 2015, 2015, 437298. [Google Scholar] [CrossRef]
- Medina-Caliz, I.; Robles-Diaz, M.; Garcia-Muñoz, B.; Stephens, C.; Ortega-Alonso, A.; Garcia-Cortes, M.; González-Jimenez, A.; Sanabria-Cabrera, J.A.; Moreno, I.; Fernandez, M.C.; et al. Spanish DILI registry. Definition and risk factors for chronicity following acute idiosyncratic drug-induced liver injury. J. Hepatol. 2016, 65, 532–542. [Google Scholar] [CrossRef]
- López-Riera, M.; Conde, I.; Quitas, G.; Pedrola, L.; Zaragoza, Á.; Perez-Rojas, J.; Saledo, M.; Benlloch, S.; Castell, J.V.; Jover, R. Non-invasive prediction of NAFLD severity: A comprehensive, independent validation of previously postulated serum microRNA biomarkers. Sci. Rep. 2018, 8, 10606. [Google Scholar] [CrossRef]
- Machlab, S.; Mireia, M.; Vergara, M.; Escoda, M.R.; Casas, M. Apixaban-induced liver injury. Rev. Esp. Enferm. Dig. 2019, 111, 161–163. [Google Scholar] [CrossRef]
- Zoubek, M.E.; Pinazo-Bandera, J.; Ortega-Alonso, A.; Hernández, N.; Crespo, J.; Contreras, F.; Medina-Cáliz, I.; Sanabria-Cabrera, J.; Sanjuan-Jiménez, R.; González-Jiménez, A.; et al. Liver injury after methylprednisolone pulses: A disputable cause of hepatotoxicity. A case series and literature review. United Eur. Gastroenterol. J. 2019, 7, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, E.; Olsson, R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology 2005, 42, 481–489. [Google Scholar] [CrossRef] [PubMed]
- De Valle, M.B.; Klinteberg, A.V.; Alem, N.; Olsson, R.; Björnsson, E. Drug-induced liver injury in a Swedish University hospital out-patient hepatology clinic. Aliment. Pharm. 2006, 24, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, E.; Kalaitzakis, E.; Klinteberg, V.A.V.; Alem, E.; Olsson, R. Long-term follow-up of patients with mild to moderate drug-induced liver injury. Aliment. Pharm. 2007, 26, 79–85. [Google Scholar] [CrossRef]
- Björnsson, E.S. Epidemiology and risk factors for idiosyncratic drug-induced liver injury. Semin Liver Dis. 2014, 34, 115–122. [Google Scholar] [CrossRef]
- Goossens, N.; Spahr, L.; Rubbia-Brandt, L. Severe immune-mediated drug-induced liver injury linked to ibandronate: A case report. J. Hepatol. 2013, 59, 1139–1142. [Google Scholar] [CrossRef]
- Russmann, S.; Niedrig, D.F.; Budmiger, M.; Schmidt, C.; Stieger, B.; Hürlimann, S.; Kullak-Ublick, G.A. Rivaroxaban postmarketing risk of liver injury. J. Hepatol. 2014, 61, 293–300. [Google Scholar] [CrossRef]
- Scalfaro, E.; Streefkerk, H.J.; Merz, M.; Meier, C.; Lewis, D. Preliminary results of a novel algorithmic method aiming to support initial causality assessment of routine pharmacovigilance case reports for medication-induced liver injury: The PV-RUCAM. Drug Saf. 2017, 40, 715–727. [Google Scholar] [CrossRef]
- Schneider, J.S.; Montani, M.; Stickel, F. Drug-induced autoimmune hepatitis following treatment with Zoledronic acid. Case Rep. Gastroenterol. 2017, 11, 440–445. [Google Scholar] [CrossRef]
- Terziroli Beretta-Piccoli, B.; Mieli-Vergani, G.; Bertoli, R.; Mazzucchelli, L.; Nofziger, C.; Paulmichl, M.; Vergani, D. Atovaquone/proguanil-induced autoimmune-like hepatitis. Hepatol. Commun. 2017, 1, 293–298. [Google Scholar] [CrossRef]
- Visentin, M.; Lenggenhager, D.; Gai, Z.; Kullak-Ublick, G.A. Drug-induced bile duct injury. BBA Mol. Basis Dis. 2018, 1864, 1498–1506. [Google Scholar] [CrossRef]
- Treeprasertsuk, S.; Huntrakul, J.; Ridtitid, W.; Kullavanijaya, P.; Björnsson, E.S. The predictors of complications in patients with drug-induced liver injury caused by antimicrobial agents. Aliment. Pharm. 2010, 11, 1200–1207. [Google Scholar]
- Sobhonslidsuk, A.; Poovorawan, K.; Soonthornworasiri, N.; Pangnum, W.; Phaosawasdi, K. The incidence, presentation, outcomes, risk of mortality and economic data of drug-induced liver injury from a national database in Thailand: A population-base study. BMC Gastroenterol. 2016, 16, 135. [Google Scholar] [CrossRef] [PubMed]
- Chayanupatkul, M.; Schiano, T.D. Acute liver failure secondary to drug-induced liver injury. Clin. Liver Dis. 2020, 24, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Duzenli, T.; Tanoglu, A.; Akyol, T.; Kara, M.; Yazgan, Y. Drug-induced liver injury caused by Phenprobamate: Strong probability due to repeated toxicity. Eur. J. HepatoGastroenterol. 2019, 9, 49–51. [Google Scholar] [CrossRef]
- Hussaini, S.H.; O’Brien, C.S.; Despott, E.J.; Dalton, H.R. Antibiotic therapy: A major cause of drug-induced jaundice in southwest England. Eur. J. Gastroenterol. Hepatol. 2007, 19, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.K.; Donaldson, P.T.; Bhatnagar, P.; Shen, Y.; Pe’er, I.; Floratos, A.; Daly, M.J.; Goldstein, D.B.; John, S.; Nelson, M.R.; et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat. Genet. 2009, 41, 816–819. [Google Scholar]
- Spraggs, C.F.; Budde, L.R.; Briley, L.P.; Bing, N.; Cox, C.J.; King, K.S.; Whittaker, J.C.; Mooser, V.E.; Preston, A.J.; Stein, S.H.; et al. HLA-DQA1*02, 01 is a major risk factor for Lapatinib-induced hepatotoxicity in women with advanced breast cancer. J. Clin. Oncol. 2011, 29, 667–673. [Google Scholar] [CrossRef]
- Islam, M.; Wright, G.; Tanner, P.; Lucas, R. A case of anastrazole-related drug-induced autoimmune hepatitis. Clin. J. Gastroenterol. 2014, 7, 414–417. [Google Scholar] [CrossRef]
- Dyson, J.K.; Hutchinson, J.; Harrison, L.; Rotimi, O.; Tiniakos, D.; Foster, G.R.; Aldersley, M.A.; McPherson, S. Liver toxicity associated with sofosbuvir, an NS5A inhibitor and ribavirin use. J. Hepatol. 2016, 64, 234–238. [Google Scholar] [CrossRef]
- Abbara, A.; Chitty, S.; Roe, J.K.; Ghani, R.; Collin, S.M.; Ritchie, A.; Kon, O.M.; Dzvova, J.; Davidson, H.; Edwards, T.E.; et al. Drug-induced liver injury from antituberculous treatment: A retrospective study from a large TB centre in the UK. BMC Infect. Dis. 2017, 17, 231. [Google Scholar] [CrossRef] [PubMed]
- Vliegenthart, A.D.B.; Berends, C.; Potter, C.M.J.; Kersaudy-Kerhoas, M.; Dear, J.W. MicroRNA-122 can be measured in capillary blood which facilitates point-of-care testing for drug-induced liver injury. Br. J. Clin. Pharm. 2017, 83, 2027–2033. [Google Scholar] [CrossRef] [PubMed]
- Fontana, R.J.; Shakil, O.; Greenson, J.K.; Boyd, I.; Lee, W.M. Acute liver failure due to amoxicillin and amoxicillin/clavulanate. Dig. Dis. Sci. 2005, 10, 1785–1790. [Google Scholar] [CrossRef]
- Lee, W.M.; Larrey, D.; Olsson, R.; Lewis, J.H.; Keisu, M.; Auclert, L.; Sheth, S. Hepatic findings in long-term clinical trials of ximelagatran. Drug Saf. 2005, 28, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Stojanovski, S.D.; Casavant, M.J.; Mousa, H.M.; Baker, P.; Nahata, M.C. Atomoxetine-induced hepatitis in a child. Clin. Toxicol. 2007, 45, 51–55. [Google Scholar] [CrossRef]
- Lammert, C.; Einarsson, S.; Saha, C.; Niklasson, A.; Bjornsson, E.; Chalasani, N. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: Search for signals. J. Hepatol. 2008, 47, 2003–2009. [Google Scholar] [CrossRef]
- Singla, A.; Hammad, H.T.; Hammoud, G.M. Uncommon cause of acute drug-induced liver injury following mammoplasty. Gastroenterol. Res. 2010, 3, 171–172. [Google Scholar] [CrossRef]
- Nabha, L.; Balba, G.P.; Tuanzon, C.; Kumar, P.N. Etravirine induced severe hpersensitivity reaction and fulminant hepatitis: A case report and review of the literature. J. Aids Clin. Res. 2012, 3, 005. [Google Scholar] [CrossRef]
- Sprague, D.; Bamha, K. Drug-induced liver injury due to varenicline. BMC Gastroenterol. 2012, 12, 65. [Google Scholar] [CrossRef]
- Markova, S.M.; De Marco, T.; Bendjilali, N.; Kobashigawa, E.A.; Mefford, J.; Sodhi, J.; Le, H.; Zhang, C.; Halladay, J.; Rettie, A.E.; et al. Association of CYP2C9*2 with bosentan-induced liver injury. Clin. Pharm. 2013, 94, 678–686. [Google Scholar] [CrossRef]
- Marumoto, A.; Roytman, M.M.; Tsai, N.C.S. Trial and error: Investigational drug induced liver injury, a case series report. Hawaii J. Med. Public Health 2013, 72, 30–33. [Google Scholar] [PubMed]
- Bohm, N.; Bohm, N.; Makowski, C.; Machado, M.; Davie, A.; Seabrook, N.; Wheless, L.; Bevill, B.; Clark, B.; Kyle, T.R., III. Case report and cohort analysis of drug-induced liver injury associated with daptomycin. Antimicrob. Agents Chemother. 2014, 58, 4902–4903. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheetham, T.C.; Lee, J.; Hunt, C.M.; Niu, F.; Reisinger, S.; Murray, R.; Powell, G.; Papay, J. An automated causality assessment algorithm to detect drug-induced liver injury in electronic medical record data. Pharm. Drug Saf. 2014, 23, 601–608. [Google Scholar] [CrossRef]
- Lim, R.; Choundry, H.; Conner, K.; Karnsakul, W. A challenge for diagnosing acute liver injury with concomitant/sequential exposure to multiple drugs: Can causality assessment scales be utilized to identify the offending drug? Case Rep. Pediatr. 2014, 2014, 156389. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.W.; Hoofnagle, J.H.; Gu, J.; Fontana, R.J.; Barnhart, H.; Kleiner, D.E.; Chalasani, N.; Bonkovsky, H.L. Spectrum of statin hepatotoxicity: Experience of the drug-induced liver injury network. Hepatology 2014, 60, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Veluswamy, R.R.; Ward, S.C.; Yum, K.; Abramovitz, R.B.; Isola, L.M.; Jagannath, S.; Parekh, S. Adverse drug reaction: Pomalidomide-induced liver injury. Lancet 2014, 383, 2125–2126. [Google Scholar] [CrossRef]
- Baig, M.; Wool, K.J.; Halalnych, J.H.; Sarmad, R.A. Acute liver failure after initiation of rivaroxaban: A case report and review of the literature. N. Am. J. Med. Sci. 2015, 7, 407–410. [Google Scholar] [CrossRef]
- Hammerstrom, A.E. Possible Amlodipine-induced hepatotoxicity after stem cell transplant. Ann. Pharm. 2015, 49, 135–139. [Google Scholar] [CrossRef]
- Stine, J.G.; Intagliata, N.; Sha, N.L.; Argo, C.K.; Caldwell, S.H.; Lewis, J.H.; Northup, P.G. Hepatic decompensation likely attributable to Simeprevir in patients with advanced cirrhosis. Dig. Dis. Sci. 2015, 60, 1031–1035. [Google Scholar] [CrossRef]
- Tang, D.M.; Koh, C.; Twaddell, W.S.; von Rosenvinge, E.C.; Han, H. Acute hepatocellular drug-induced liver injury from bupropion and doxycycline. ACG Case Rep. J. 2015, 3, 66–68. [Google Scholar] [CrossRef]
- Unger, C.; Al-Jashaami, L. Ciprofloxacin exposure leading to fatal hepatotoxicity: An unusual correlation. Am. J. Case Rep. 2016, 17, 676–681. [Google Scholar] [CrossRef]
- Gharia, B.; Seegobin, K.; Maharaj, S.; Marji, N.; Deutch, A.; Zuberi, L. Letrozole-induced hepatitis with autoimmune features: A rare adverse drug reaction with review of the relevant literature. Oxf. Med. Case Rep. 2017, 2017, omx074. [Google Scholar] [CrossRef]
- Nicoletti, P.; Aithal, G.P.; Bjornsson, E.S.; Andrade, R.J.; Sawle, A.; Arrese, M.; Barnhart, H.X.; Bondon-Guitton, E.; Hayashi, P.H.; Bessone, F.; et al. International Drug-Induced Liver Injury Consortium, Drug-Induced Liver Injury Network Investigators, and International Serious Adverse Events Consortium. Association of liver injury from specific drugs or group of drugs with polymorphisms in HLA and other genes in a Genome-wide Association Study. Gastroenterology 2017, 152, 1078–1089. [Google Scholar]
- Gayam, V.; Khalid, M.; Shrestha, B.; Rajib Hossain, M.; Dahal, S.; Garlapati Gill, P.A.; Kumar Mandal, A.; Sangha, R. Drug-Induced Liver Injury: An institutional case series and review of literature. J. Investig. Med. High Impact Case Rep. 2018, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, P.H.; Björnsson, E.S. Long-term outcomes after drug-induced liver injury. Curr. Hepatol. Rep. 2018, 17, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Mendler, M.H.; Valasek, M.A.; Tsunoda, S.M. Drug-induced liver injury associated with the use of Everolimus in a liver transplantant patient. Case Rep. Transpl. 2018. [Google Scholar] [CrossRef]
- Shamberg, L.; Vaziri, H. Hepatotoxicity of inflammatory bowel disease medications. J. Clin. Gastroenterol. 2018, 52, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Cirulli, E.T.; Nicoletti, P.; Abramson, K.; Andrade, R.J.; Bjornsson, E.S.; Chalasani, N.; Fontana, R.J.; Hallberg, P.; Li, Y.J.; Lucena, M.I.; et al. Drug-Induced Liver Injury Network (DILIN) investigators; International DILI consortium (iDILIC). A missense variant in PTPN22 is a risk factor for drug-induced liver injury. Gastroenterology 2019, 56, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, P.; Aithal, G.P.; Chamberlain, T.C.; Coulthard, S.; Alshabeeb, M.; Grove, J.I.; Andrade, R.J.; Björnsson, E.; Dillon, J.F.; Hallberg, P.; et al. International Drug-Induced Liver Injury Consortium (iDILIC). Drug-induced liver injury due to Flucloxacillin: Relevance of multiple human leukocyte antigen alleles. Clin. Pharm. 2019, 106, 245–253. [Google Scholar] [CrossRef]
- Sandritter, T.L.; Goldman, J.L.; Habiger, C.J.; Daniel, J.F.; Lowry, J.; Fisher, R.T. An electronic medical records-based approach to identify idiosyncratic drug-induced liver injury in children. Sci. Rep. 2019, 9, 18090. [Google Scholar] [CrossRef]
- Shumar, J.; Ordway, S.; Junga, Z.; Sadowski, B.; Torres, D. Memantine-induced liver injury with probable causality as assessed using the Roussel Uclaf Causality Assessment Method (RUCAM). ACG Case Rep. J. 2019, 6, e00184. [Google Scholar] [CrossRef]
- Tsung, I.; Dolan, R.; Lao, C.D.; Fecher, L.; Riggenbach, K.; Yeboah-Korang, A.; Fontana, R.J. Liver injury is most commonly due to hepatic metastases rather than drug hepatotoxicity during pembrolizumab immunotherapy. Aliment. Pharm. 2019, 50, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Abdullah, H.; Abdallah, M.; Quist, E.; Niazi, M. Anastrozole-induced liver injury after a prolonged latency: A very rare complication of a commonly prescribed medication. BMJ Case Rep. 2019, 12, e231741. [Google Scholar] [CrossRef] [PubMed]
- Ghabril, M.; Gu, J.; Yoder, L.; Corbito, L.; Dakhoul, L.; Ringel, A.; Beyer, C.D.; Vuppalanchi, R.; Barnhart, H.; Hayashi, P.H.; et al. Significant medical comorbidities are associated with lower causality scores in patients presenting with suspected drug-induced liver injury. Clin. Transl. Gastroenterol. 2020, 11, e00141. [Google Scholar] [CrossRef]
- Mehershahi, S.; Mantri, N.; Kumar, A.; Danial, S.; Harish, P. Enoxaparin-induced liver injury. Case Rep. Gastroenterol. 2020, 14, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Mullins, C.; Beaulac, K.; Sylvia, L. Drug-induced liver injury (DILI) with Micafungin: The importance of causality assessment. Ann. Pharm. 2020, 54, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.J.; Bertilone, C.; Robertson, A.G. Fulminant liver failure and transplantation after use of dietary supplements. Med. J. Aust. 2016, 204, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, V.; Fickert, P.; Lackner, C.; Schmerlaib, J.; Krisper, P.; Trauner, M.; Stauber, E.R. Hepatotoxicity of NONI juice: Report of two cases. World J. Gastroenterol. 2005, 11, 4758–4760. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, S.T.A.; Dall’Oglio, V.M.; de Araújo, A.; Schmidt Cerski, C.T. Sinusoidal obstruction syndrome secondary the intake of Senecio brasiliensis: A case report. Ann. Hepatol. 2019, in press. [Google Scholar] [CrossRef]
- Yuen, M.F.; Tam, S.; Fung, J.; Wong, D.K.H.; Wong, B.C.Y.; Lai, C.L. Traditional Chinese Medicine causing hepatotoxicity in patients with chronic hepatitis B infection: A 1-year prospective study. Aliment. Pharm. 2006, 24, 1179–1186. [Google Scholar] [CrossRef]
- Cheung, W.I.; Tse, M.L.; Ngan, T.; Lin, J.; Lee, W.K.; Poon, W.T.; Mak, T.W.; Leung, V.K.S.; Chau, T.N. Liver injury associated with the use of Fructus Psoraleae (Bol-gol-zhee or Bu-gu-zhi) and its related proprietary medicine. Clin. Toxicol. 2009, 47, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Chau, T.N.; Cheung, W.I.; Ngan, T.; Lin, J.; Lee, K.W.S.; Poon, W.T.; Leung, V.K.S.; Mak, T.; Tse, M.L.; the Hong Kong Herb-Induced Liver Injury Network (HK-HILIN). Causality assessment of herb-induced liver injury using multidisciplinary approach and the Roussel Uclaf Causality Assessment Method (RUCAM). Clin. Toxicol. 2011, 49, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Wang, J.Y.; Li, N.; Li, M.; Gao, H.; Ji, Y.; Zhang, F.; Wang, H.; Zhou, Y.; Ye, Y.; et al. Hepatic sinusoidal obstruction syndrome associated with consumption of Gynura segetum. J. Hepatol. 2011, 54, 666–673. [Google Scholar] [CrossRef]
- Gao, H.; Li, N.; Wang, J.Y.; Zhang, S.C.; Lin, D. Definitive diagnosis of hepatic sinusoidal obstruction syndrome induced by pyrrolizidine alkaloids. J. Dig. Dis. 2012, 13, 33–39. [Google Scholar] [CrossRef]
- Lai, R.T.; Wang, H.; Gui, H.L.; Ye, M.Z.; Dai, W.J.; Xiang, X.; Zhao, G.D.; Wang, W.J.; Xie, Q. Clinical and pathological features in 138 cases of drug-induced liver injury. Zhonghua Gan Zang Bing Za ZhiZhonghua Ganzangbing Zazhi Chin. J. Hepatol. 2012, 20, 185–189. [Google Scholar] [CrossRef]
- Dong, H.; Slain, D.; Cheng, J.; Ma, W.; Liang, W. Eighteen cases of liver injury following ingestion of Polygonum multiflorum. Complement. Med. 2014, 22, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Ruan, J.Q.; Chen, J.; Li, N.; Ke, C.Q.; Ye, Y.; Lin, G.; Wang, J.Y. Blood pyrrole-protein adducts as diagnostic and prognostic index in pyrrolizidine alkaloid-hepatic sinusoidal obstruction syndrome. Drug Des. Dev. 2015, 9, 4861–4868. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, Z.; Niu, M.; Li, N.; Meng, Y.; Li, Q.; Qin, L.; Teng, G.; Cao, J.; Li, B.; et al. Evidence chain-based causality identification in herb-induced liver injury: Exemplification of a well-known liver-restorative herb Polygonum multiflorum. Front. Med. 2015, 9, 457–467. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, S.H.; Wang, J.B.; Song, H.B.; Li, Y.G.; He, T.T.; Ma, X.; Wang, Z.X.; Wang, L.P.; Zhou, K.; et al. Clinical analysis of drug-induced liver injury caused by Polygonum multiflorum and its preparations. Zhongguo Zhong Xi Yi Jie He Za Zhi 2015, 35, 1442–1447, (Abstract in English, Article in Chinese). [Google Scholar]
- Zhang, P.; Ye, Y.; Yang, X.; Jiao, Y. Systematic review on Chinese herbal medicine induced liver injury. Evid.-Based Complement. Altern. Med. 2016. [Google Scholar] [CrossRef]
- Li, C.Y.; He, Q.; Gao, D.; Li, R.Y.; Zhu, Y.; Li, H.F.; Feng, W.W.; Yang, M.H.; Xiao, X.H.; Wang, J.B. Idiosyncratic drug-induced liver injury linked to Polygonum multiflorum: A case study by pharmacognosy. Chin. Integr. Med. 2017, 23, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.C.; So, T.H.; Choi, H.C.W.; Lam, K.O. Medicine herbs-induced liver injury from an oncological perspective with RUCAM. Integr. Cancer 2019, 18, 1–13. [Google Scholar]
- Jing, J.; Wang, R.L.; Zhao, X.Y.; Zhu, Y.; Niu, M.; Wang, L.F.; Song, X.A.; He, T.T.; Sun, Y.Q.; Xu, W.-T.; et al. Association between the concurrence of pre-existing chronic liver disease and worse prognosis in patients with an herb-Polygonum multiflorum thunbinduced liver injury: A case-control study from a specialised liver disease center in China. BMJ Open 2019, 9, e023567. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Gao, M.; Zhao, N.; Li, P.; Zhu, J.; Li, W. Acute liver failure associated with Fructus Psoraleae: A case report and literature review. BMC Complement. Altern. Med. 2019, 19, 84. [Google Scholar] [CrossRef]
- Ni, J.; Liu, Y.; Wang, W.; Sun, M.; Ma, B.; Pang, L.; Du, Y.; Dong, X.; Yin, X. Polygonum multiflorum-induced liver injury: Clinical characteristics, risk factors, material basis, action mechanism and current challenges. Front. Pharmacol. 2019, 10, 1467. [Google Scholar]
- Tan, Y.; Chen, H.; Zhou, X.; Sun, L. RUCAM-based assessment of liver injury by xian-tian-guo (Swietenia macrophylla) seeds, a plant used for treatment of hypertension and diabetes. Ann. Hepatol. 2019, 18, 403–407. [Google Scholar] [CrossRef]
- Zhu, Y.; Niu, M.; Wang, J.B.; Wang, R.L.; Li, J.Y.; Ma, Y.; Zhao, Y.L.; Zhang, Y.F.; He, T.T.; Yu, S.M.; et al. Predictors of poor outcomes in 488 patients with herb-induced liver injury. Turk. J. Gastroenterol. 2019, 30, 47–58. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Z.; Tang, J.; Liu, X.; Shi, W.; Qin, N.; Wang, X.; Pang, Y.; Li, R.; Zhang, Y.; et al. New incompatible pair of TCM: Epimedii Folium combined with Psoraleae Fructus induces idiosyncratic hepatotoxicity under immunological stress conditions. Front. Med. 2020, 28. [Google Scholar] [CrossRef]
- Xia, C.; Liu, Y.; Yao, H.; Zhu, W.; Ding, J.; Jin, J. Causality assessment of skyfruit-induced liver injury using the updated RUCAM: A case report and review of the literature. J. Int. Med. Res. 2020, 48, 300060520917569. [Google Scholar] [CrossRef]
- Cárdenas, A.; Restrepo, J.C.; Sierra, F.; Correa, G. Acute hepatitis due to shen-min: A herbal product derived from Polygonum multiflorum. J. Clin. Gastroenterol. 2006, 40, 629–632. [Google Scholar] [CrossRef]
- Parlati, L.; Voican, C.S.; Perlemuter, K.; Perlemuter, G. Aloe vera-induced acute liver injury: A case report and literature review. Clin. Res. Hepatol. Gastroenterol. 2017, 41, e39–e42. [Google Scholar] [CrossRef]
- Teschke, R.; Bahre, R. Severe hepatotoxicity by Indian Ayurvedic herbal products: A structured causality assessment. Ann. Hepatol. 2009, 8, 258–266. [Google Scholar] [CrossRef]
- Teschke, R.; Glass, X.; Schulze, J. Herbal hepatotoxicity by Greater Celandine (Chelidonium majus): Causality assessment of 22 spontaneous reports. Regul. Toxicol. Pharm. 2011, 61, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Glass, X.; Schulze, J.; Eickhoff, A. Suspected Greater Celandine hepatotoxicity: Liver specific causality evaluation of published case reports from Europe. Eur. J. Gastroenterol. Hepatol. 2012, 24, 270–280. [Google Scholar] [CrossRef]
- Douros, A.; Bronder, E.; Andersohn, F.; Klimpel, A.; Kreutz, R.; Garbe, E.; Bolbrinker, J. Herb-induced liver injury in the Berlin Case-Control Surveillance Study. Int. J. Mol. Sci. 2016, 17, 114. [Google Scholar] [CrossRef]
- Teschke, R.; Zhang, L.; Long, H.; Schwarzenboeck, A.; Schmidt-Taenzer, W.; Genthner, A.; Wolff, A.; Frenzel, C.; Schulze, J.; Eickhoff, A. Traditional Chinese Medicine and herbal hepatotoxicity: A tabular compilation of reported cases. Ann. Hepatol. 2015, 14, 7–19. [Google Scholar] [CrossRef]
- Melchart, D.; Hager, S.; Albrecht, S.; Dai, J.; Weidenhammer, W.; Teschke, R. Herbal Traditional Chinese Medicine and suspected liver injury: A prospective study. World J. Hepatol. 2017, 18, 1141–1157. [Google Scholar] [CrossRef]
- Diener, H.C.; Freitag, F.G.; Danesch, U. Safety profile of a special butterbur extract from Petasites hybridus in migraine prevention with emphasis on the liver. Ceph. Rep. 2018, 1, 1–8. [Google Scholar] [CrossRef]
- Anderson, N.; Borlak, J. Hepatobiliary events in migraine therapy with herbs—The case of Petadolex, a Petasites hybridus extract. J. Clin. Med. 2019, 8, 652. [Google Scholar] [CrossRef]
- Gerhardt, F.; Benesic, A.; Tillmann, H.L.; Rademacher, S.; Wittekind, C.; Gerbes, A.L.; Henker, R.; Berg, T.; Maidhof, H.; Trauer, H.; et al. Iberogast-induced acute liver failure-reexposure and in vitro assay support causality. Am. J. Gastroenterol. 2019, 114, 1358–1359. [Google Scholar] [CrossRef]
- Teschke, R.; Xuan, T.D. Suspected herb induced liver injury by green tea extracts: Critical review and case analysis applying RUCAM for causality assessment. Jpn. J. Gastroenterol. Hepatol. 2019, 1, 1–16. [Google Scholar]
- Philips, C.A.; Paramaguru, R.; Joy, A.K.; Anthony, K.L.; Augustine, P. Clinical outcomes, histopathological patterns, and chemical analysis of Ayurveda and herbal medicine associated with severe liver injury—A single-center experience from southern India. Indian J. Gastroenterol. 2018, 37, 9–17. [Google Scholar] [CrossRef]
- Philips, C.A.; Augustine, P.; Rajesh, S.; Kumar, P.; Madhu, D. Complementary and alternative medicine-related drug-induced liver injury in Asia. J. Clin. Transl. Hepatol. 2019, 7, 263. [Google Scholar] [CrossRef] [PubMed]
- Lapi, F.; Gallo, E.; Giocalliere, E.; Vietri, M.; Baronti, R.; Pieraccini, G.; Tafi, A.; Menniti-Ippolito, F.; Mugelli, A.; Firenzuoli, F.; et al. Acute liver damage due to Serenoa repens: A case report. Br. J. Clin. Pharm. 2010, 69, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, G.; Di Soto, A.; Vitalone, A. Hepatotoxicity of green tea: An update. Arch. Toxicol. 2015, 89, 1175–1191. [Google Scholar] [CrossRef] [PubMed]
- Sáez-González, E.; Conde, I.; Díaz-Jaime, F.C.; Benlloch, S.; Prieto, M.; Berenguer, M. Iberogast-induced severe hepatotoxicity leading to liver transplantation. Am. J. Gastroenterol. 2016, 111, 1364–1365. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, G.; Moro, P.A.; Raschi, E.; Da Cas, R.; Menniti-Ippolito, F. Adverse reactions to dietary supplements containing red yeast rice: Assessment of cases from the Italian surveillance system: Safety of red yeast rice dietary supplements. Br. J. Clin. Pharm. 2017, 83, 894–908. [Google Scholar] [CrossRef]
- Osborne, C.S.; Overstreet, A.N.; Rockey, D.C.; Shreiner, A.D. Drug-induced liver injury caused by Kratom use as an alternative pain treatment amid an ongoing opioid epidemic. J. Investig. Med. High Impact Case Rep. 2019, 7. [Google Scholar] [CrossRef]
- Tsuda, T.; Yashiro, S.; Gamo, Y.; Watanabe, K.; Hoshino, T.; Oikawa, T.; Hanawa, T. Discrepancy between clinical course and drug-induced lymphocyte stimulation tests in a case of Saireito-induced liver injury accompanied by Sjögren syndrome. J. Altern. Complement. Med. 2010, 16, 501–505. [Google Scholar] [CrossRef]
- Hisamochi, A.; Kage, M.; Arinaga, T.; Ide, T.; Miyajima, I.; Ogata, K.; Kuwhara, T.; Koga, Y.; Kumashiro, R.; Sata, M. Drug-induced liver injury associated with Agaricus blazei Murill which is very similar to autoimmune hepatitis. Clin. J. Gastroenterol. 2013, 6, 139–144. [Google Scholar] [CrossRef]
- Ahn, B.M. Herbal preparation-induced liver injury. Korean J. Gastroenterol. 2004, 44, 113–125. [Google Scholar] [PubMed]
- Seo, J.C.; Jeon, W.J.; Park, S.S.; Kim, S.H.; Lee, K.M.; Chae, H.B.; Park, S.M.; Youn, S.J. Clinical experience of 48 acute toxic hepatitis patients. Korean J. Hepatol. 2006, 12, 74–81, (Abstract in English, Article in Korean). [Google Scholar]
- Kang, S.H.; Kim, J.I.; Jeong, K.H.; Ko, K.H.; Ko, P.G.; Hwang, S.W.; Kim, E.M.; Kim, S.H.; Lee, H.Y.; Lee, B.S. Clinical characteristics of 159 cases of acute toxic hepatitis. Korean J. Hepatol. 2008, 14, 483–492, (Abstract in English, Article in Korean). [Google Scholar] [CrossRef]
- Sohn, C.H.; Cha, M.I.; Oh, B.J.; Yeo, W.H.; Lee, J.H.; Kim, W.; Lim, K.S. Liver transplantation for acute toxic hepatitis due to herbal medicines and preparations. J. Korean Soc. Clin. Toxicol. 2008, 6, 110–116, (Abstract in English, Article in Korean). [Google Scholar]
- Kang, H.S.; Choi, H.S.; Yun, T.J.; Lee, K.G.; Seo, Y.S.; Yeon, J.E.; Byun, K.S.; Um, H.S.; Kim, C.D.; Ryu, H.S. A case of acute cholestatic hepatitis induced by Corydalis speciosa Max. Korean Hepatol. 2009, 15, 517–523, (Abstract in English, article in Korean). [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Yim, H.J.; Ahn, J.H.; Kim, J.H.; Kim, J.N.; Yoon, I.; Kim, D.I.; Lee, H.S.; Lee, S.W.; Choi, J.H. Two cases of toxic hepatitis caused by arrowroot juice. Korean J. Hepatol. 2009, 15, 504–509, (Abstract in English, Article in Korean). [Google Scholar] [CrossRef]
- Bae, S.H.; Kim, D.H.; Bae, Y.S.; Lee, K.J.; Kim, D.W.; Yoon, J.B.; Hong, J.H.; Kim, S.H. Toxic hepatitis associated with Polygoni multiflori. Korean J. Hepatol. 2010, 16, 182–186, (Abstract in English, Article in Korean). [Google Scholar] [CrossRef]
- Yang, H.; Kim, D.J.; Kim, Y.M.; Kim, B.H.; Sohn, K.M.; Choi, M.J.; Choi, Y.H. Aloe-induced toxic hepatitis. J. Korean Med. Sci. 2010, 25, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.A.; Min, H.J.; Yoo, S.S.; Kim, H.J.; Choi, S.N.; Ha, C.Y.; Kim, H.J.; Kim, T.H.; Jung, W.T.; Lee, O.J. Drug-induced liver injury: Twenty five cases of acute hepatitis following ingestion of Polygonum multiflorum Thun. Gut Liver 2011, 5, 493–499. [Google Scholar] [CrossRef]
- Kim, Y.J.; Ryu, S.L.; Shim, J.W.; Kim, D.S.; Shim, J.Y.; Park, M.S.; Jung, H.L. A pediatric case of toxic hepatitis induced by Hovenia dulcis. Pediatr. Gastroenterol. Hepatol. Nutr. 2012, 15, 111–116. [Google Scholar] [CrossRef]
- Lee, J.; Shin, J.S.; Kim, M.R.; Byon, J.H.; Lee, S.Y.; Shin, Y.S.; Kim, H.; Park, K.B.; Shin, B.C.; Lee, M.S.; et al. Liver enzyme abnormalities in taking traditional herbal medicine in Korea: A retrospective large sample cohort study of musculoskeletal disorder patients. J. Ethnopharmacol. 2015, 169, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Kim, H.W.; Lee, H.Y.; Son, C.G. Systematic review on herb-induced liver injury in Korea. Food Chem. Toxicol. 2015, 84, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Oh, D.S.; Hong, S.H.; Ko, H.; Lee, N.H.; Park, S.E.; Han, C.W.; Kim, S.M.; Kim, Y.C.; Kim, K.S. A nationwide study of the incidence rate of herb-induced liver injury in Korea. Arch. Toxicol. 2017, 91, 4009–4015. [Google Scholar] [CrossRef] [PubMed]
- Teo, D.C.H.; Ng, P.S.L.; Tan, S.H.; Lim, A.T.; Toh, D.S.L.; Chan, S.Y.; Cheong, H.H. Drug-induced liver injury associated with Complementary and Alternative Medicine: A review of adverse event reports in an Asian community from 2009 to 2014. BMC Complement. Altern. Med. 2016, 16, 192. [Google Scholar] [CrossRef]
- Awortwe, C.; Makiwane, M.; Reuter, H.; Muller, C.; Louw, J.; Rosenkranz, B. Critical evaluation of causality assessment of herb-drug interactions in patients. Br. J. Clin. Pharm. 2018, 84, 679–693. [Google Scholar] [CrossRef]
- Jimenez-Saenz, M.; Martinez-Sanchez, C. Acute hepatitis associated with the use of green tea infusions. J. Hepatol. 2006, 44, 616–617. [Google Scholar] [CrossRef]
- García-Cortés, M.; Borraz, Y.; Lucena, M.I.; Paláez, G.; Salmerón, J.; Diago, M.; Martínez-Sierra, J.M.; Navarro, J.M.; Planas, M.J.; Bruguera, M.; et al. Liver injury induced by “natural remedies”: An analysis of cases submitted to the Spanish Liver Toxicity Registry. Rev. Esp. Enferm. Dig. 2008, 100, 688–695. [Google Scholar]
- Medina-Caliz, I.; Garcia-Cortes, M.; Gonzalez-Jimenez, A.; Cabello, M.R.; Robles-Diaz, M.; Sanabria-Cabrera, J.; Sanjuan-Jimenez, R.; Ortega-Alonso, A.; Garcia-Munoz, B.; Moreno, I.; et al. Herbal and dietary supplement-induced liver injuries in the Spanish DILI Registry. Clin. Gastroenterol. Hepatol. 2018, 16, 1495–1502. [Google Scholar] [CrossRef]
- Björnsson, E.; Olsen, R. Serious adverse liver reactions associated with herbal weight-loss supplements. J. Hepatol. 2007, 47, 295–302. [Google Scholar] [CrossRef]
- Ruperti-Repilado, F.J.; Haefliger, S.; Rehm, S.; Zweier, M.; Rentsch, K.M.; Blum, J.; Jetter, A.; Heim, M.; Leuppi-Taegtmeyer, A.; Terracciano, L.; et al. Danger of herbal tea: A case of acute cholestatic hepatitis due to Artemisia annua tea. Front. Med. 2019, 6, 221. [Google Scholar] [CrossRef]
- Yilmaz, B.; Yilmaz, B.; Aktaş, B.; Unlu, O.; Roach, E.C. Lesser celandine (pilewort) induced acute toxic liver injury: The first case report worldwide. World J. Hepatol. 2015, 7, 285–288. [Google Scholar] [CrossRef]
- Papafragkakis, C.; Ona, M.A.; Reddy, M.; Anand, S. Acute hepatitis after ingestion of a preparation of Chinese Skullcap and Black Catechu for joint pains. Case Rep. Hepatol. 2016. [Google Scholar] [CrossRef]
- Dalal, K.K.; Holdbrook, T.; Peikin, S.R. Ayurvedic drug induced liver injury. World J. Hepatol. 2017, 9, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Kesavarapu, K.; Kang, M.; Shin, J.J.; Rothstein, K. Yogi Detox Tea: A potential cause of acute liver failure. Case Rep. Gastrointest. Med. 2017, 2017, 3540756. [Google Scholar] [CrossRef] [PubMed]
- Kothadia, J.P.; Kaminski, M.; Samant, H.; Olivera-Martinez, M. Hepatotoxicity associated with use of the weight loss supplement Garcinia cambogia: A case report and review of the literature. Case Rep. Hepatol. 2018, 2018, 6483605. [Google Scholar] [CrossRef] [PubMed]
- Surapaneni, B.K.; Le, M.; Jakobovits, J.; Vinayek, R.; Dutta, S. A case of acute severe hepatotoxicity and mild constriction of common bile duct associated with ingestion of green tea extract: A clinical challenge. Clin. Med. Insights Gastroenterol. 2018, 11, 1–4. [Google Scholar] [CrossRef]
- Imam, Z.; Khasawneh, M.; Jomaa, D.; Iftikhar, H.; Sayedahmad, Z. Drug induced liver injury attributed to a curcumin supplement. Case Rep. Gastrointest. Med. 2019, 2019, 6029403. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, M.N.; Chaudhary, F.S.; Hodanazari, S.M.; Sittambalam, C.D. Hepatotoxicity associated with with Garcinia cambogia: A case report. World J. Hepatol. 2019, 11, 735–742. [Google Scholar] [CrossRef]
- Oketch-Rabah, H.A.; Roe, A.L.; Rider, C.V.; Bonkovsky, H.L.; Giancaspro, G.I.; Navarro, V.; Paine, M.F.; Betz, J.M.; Marles, R.J.; Casper, S.; et al. United States Pharmacopeia (USP) comprehensive review of the hepatotoxicity of green tea extracts. Toxicol. Rep. 2020, 7, 386–402. [Google Scholar] [CrossRef]
- Schimmel, J.; Dart, R.C. Kratom (Mitrogyna speciosa) Liver Injury: A Comprehensive Review. Drugs 2020. [Google Scholar] [CrossRef] [PubMed]
- Le Louet, H. Drug-Induced Liver Injury (Dili): Current Status and Future Directions for Drug Development and the Post-Marketing Setting. Available online: https://cioms.ch/wp-content/uploads/2020/06/CIOMS_DILI_Web_16Jun2020.pdf (accessed on 30 June 2020).
- Aithal, G.P.; Rawlins, M.D.; Day, C.P. Accuracy of hepatic adverse drug reaction reporting in one English health region. Br. Med. J. 1999, 319, 154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Teschke, R.; Frenzel, C.; Wolff, A.; Eickhoff, A.; Schulze, J. Drug induced liver injury: Accuracy of diagnosis in published reports. Ann. Hepatol. 2014, 13, 248–255. [Google Scholar] [CrossRef]

| Country/ DILI Cases, n | First Author/Year | DILI Cases, n | Drugs | Comments on RUCAM Based DILI Cases |
|---|---|---|---|---|
| Argentina n = 625 | Bessone, 2016 [23] | 197 | Various drugs | DILI caused by a variety of drugs, not allowing individual description of features |
| Bessone, 2019 [24] | 114 | Various drugs | Individual drugs not available for DILI feature characterization | |
| Colaci, 2019 [25] | 311 | Various drugs | DILI features for single drugs were not presented | |
| García, 2019 [26] | 3 | Methotrexate | Feature details provided for DILI by methotrexate | |
| Australia n = 106 | Lin, 2014 [27] | 47 | Various volatile anaesthetics | DILI by anesthetics without individual features of isoflurane, desflurane, or sevoflurane |
| Ahmed, 2015 [28] | 1 | Ipilimumab | Detailed features of the DILI case | |
| Laube, 2019 [29] | 1 | Atorvastatin | Good feature presentation of this DILI case | |
| Worland, 2020 [30] | 57 | Infliximab | Feature presentation of DILI by the drug | |
| Bahrain n = 25 | Sridharan, 2020 [31] | 25 | Various antiepileptic drugs | No feature details provided of DILI due to individual drugs |
| Brazil n = 4 | Becker, 2019 [32] | 4 | Various drugs | Features of DILI caused by some drugs |
| Canada n = 4 | Yan, 2006 [33] | 2 | Rofecoxib | Two well described case features of DILI caused by rofecoxib |
| Nhean, 2019 [34] | 2 | Dolutegravir | Careful described features of DILI | |
| China n = 35,825 | Hou, 2012 [35] | 300 | Various drugs | No feature details available for DILI by individual drugs |
| Lv, 2012 [36] | 89 | Various drugs | Specific features of DILI by individual drugs were not presented | |
| Hao, 2014 [37] | 140 | Anti-Tuberculotics | Lacking specific DILI features of any drug | |
| Ou, 2015 [38] | 231 | Various drugs | No feature specifics of DILI are available for individual drugs | |
| Zhu, 2015 [39] | 39 | Various drugs | Specific features of DILI caused by individual drugs were not provided | |
| Lu, 2016 [40] | 513 | Various drugs | Missing specific features of DILI caused by individual drugs | |
| Yang, 2016 [41] | 124 | Various drugs | Feature specifics of DILI caused by individual drugs were not provided | |
| Zhu, 2016 [42] | 870 | Various drugs | No specific features of DILI by individual drugs were presented | |
| Naqiong, 2017 [43] | 157 | Various statins | Cohort consisted of patients with DILI caused by atorvastatin, simvastatin, and rosuvastatin, but specific features were not provided for individual statins | |
| Li, 2018 [44] | 1 | Iguratmod | Detailed feature description of DILI | |
| Song, 2018 [45] | 1 | Posaconazole | Careful feature presentation of DILI by this drug | |
| Tao, 2018, [46] | 290 | Anti-Tuberculotics | Cohort included patients with DILI caused by isoniazid, rifampin, pyrazinamide, ethambutol, and streptomycin, but specific features were not presented for individual drugs | |
| Liao, 2019 [47] | 1 | Cefepime | Well described features of DILI by this drug | |
| Shen, 2019 [48] | 18,956 | Various drugs | Cohort comprized patients with DILI, but special DILI features related to individual drugs were not published. | |
| Xing, 2019 [49] | 133 | Various drugs | No specific feature presentation of DILI by individual drugs | |
| Ma, 2020 [50] | 1 | Fenofibrate | Specific feature of DILI by this drug presented | |
| Tao, 2020 [51] | 146 | Anti-Tuberculotics | Lacking feature data of DILI caused by individual drugs | |
| Wang, 2020 [52] | 155 | Anti-Tuberculotics | Cohort included patients with DILI due to not further identified anti-TB regimens, hence attributing specific DILI features to individual drugs was not possible | |
| Yang, 2020 [53] | 13,678 | Various drugs | No feature details of DILI by individual drugs | |
| Colombia n = 19 | Ríos, 2013 [54] | 1 | Albendazole | Detailed feature description of DILI caused by albendazole |
| Cano-Paniagua, 2019 [55] | 18 | Various drugs | Perfect feature description of DILI by drugs in this excellent prospective epidemiology study using the updated RUCAM for causality assessment | |
| Egypt n = 75 | Alhaddad, 2020 [56] | 75 | Various drugs | Feature details of DILI by individual drugs incompletely provided |
| France n = 170 | Bénichou, 1993 [57] | 94 | Various drugs | No detailed feature description of DILI by the drugs |
| Arotcarena, 2004 [58] | 1 | Pioglitazone | Feature description of the case | |
| Moch, 2012 [59] | 18 | Etifoxine | Detailed features of DILI due to etifoxine treatment | |
| Carrier, 2013 [60] | 1 | Methyl-prednisolone | Features of DILI well described for the drug | |
| Ripault, 2013 [61] | 1 | Crizotinib | Good feature details provided for DILI by this drug | |
| Dumortier, 2017 [62] | 5 | Methyl-prednisolone | Careful feature description of DILI caused by the drug | |
| Meunier, 2018 [63] | 50 | Nimesulide | No feature description of DILI by this drug | |
| Germany n = 10,907 | Stammschulte, 2012 [64] | 37 | Flupirtine | Carefully presented features of DILI caused by flupirtine |
| Douros, 2014 [65] | 7 | Flupirtine | Comprehensive feature presentation of DILI due to flupirtine | |
| Douros, 2014 [66] | 198 | Various drugs | Cohort of patients with DILI associated with the use of various drugs, but special features of DILI by individual drugs were not provided | |
| Buechter, 2018 [67] | 15 | Various drugs | No detailed feature presentation of DILI caused by individual drugs | |
| Dragoi, 2018 [68] | 16 | Diclofenac | Cohort of DILI patients with presentation of limited specific DILI features | |
| Teschke, 2018 [69] | 7278 | Various drugs | Cohort of DILI patients without feature specification for individual drugs | |
| Teschke, 2018 [70] | 3312 | Various drugs | Cohort of DILI cases not providing special features of DILI by individual drugs | |
| Weber, 2019 [71] | 44 | Various drugs | No specific features of DILI caused by individual drugs provided | |
| Iceland n = 367 | Björnsson, 2012 [72] | 73 | Statins | Specific feature details provided of DILI by statins |
| Björnsson, 2013 [73] | 72 | Various drugs | Cohort of DILI cases without providing typical features of DILI by single drugs | |
| Björnsson, 2016 [74] | 222 | Various drugs | The two assessed cohorts provided no typical features of DILI caused by the evaluated drugs | |
| India n = 424 | Harugeri, 2009 [75] | 1 | Montelukast | Good feature presentation of a patient with DILI caused by montelukast |
| Devarbhavi, 2010 [76] | 313 | Various drugs | No feature description of DILI due to individual drugs | |
| Rathi, 2017 [77] | 82 | Various drugs | Cohort of DILI cases but features of DILI by indivual drugs were not presented | |
| Taneja, 2017 [78] | 2 | Etodolac | Detailed feature presentation of DILI | |
| Das, 2018 [79] | 24 | Various drugs | Cohort with limited feature description of few patients with DILI caused by drugs assessed for causality by RUCAM or other CAMs | |
| Dutta, 2020 [80] | 1 | Haloperidol | Perfect presented feature details of DILI caused by this drug | |
| Kulkarni, 2020 [81] | 1 | Vitamin A | Perfect feature details of this DILI case | |
| Israel n = 1 | Gluck, 2011 [82] | 1 | Amiodarone | Careful feature description of a patient with DILI caused by a single drug |
| Italy n = 1562 | Rigato, 2007 [83] | 1 | Flavoxate | Good feature presentation of a patient with DILI caused by flavoxate |
| Licata, 2010 [84] | 46 | Various drugs including Nimesulide | Feature description of patients with DILI by nimesulide but no description for DILI by other drugs | |
| Abenavoli, 2013 [85] | 1 | Cyproterone acetate | Detailed feature description of a patient with DILI | |
| Ferrajolo, 2017 [86] | 938 | Various antibiotics | Combined feature presentation of all antibiotics causing DILI in paediatric patients | |
| Licata, 2017 [87] | 185 | Various drugs | Epidemiology study, hence no feature description of patients with DILI by any drug | |
| Giacomelli, 2018 [88] | 362 | Nevirapine | Detailed feature description of DILI by nevirapine observed in all patients | |
| Licata, 2018 [89] | 28 | Rivaroxaban | Perfect feature description of this DILI cohort | |
| Lovero, 2018 [90] | 1 | Ustekinumab | Careful feature description of DILI caused by this drug | |
| Japan n = 939 | Masumoto, 2003 [91] | 85 | Various drugs | No detailed feature description of DILI caused by drugs |
| Hanatani, 2014 [92] | 182 | Various drugs | Detailed features of DILI by individual drugs not provided | |
| Niijima, 2017 [93] | 1 | Ipragliflozin | Provided case features of DILI | |
| Ji, 2017 [94] | 1 | Methimazole | Perfect feature presentation of DILI caused by this drug | |
| Aiso, 2019 [95] | 270 | Various drugs | Global feature description of DILI by all drugs | |
| Kishimoto, 2019 [96] | 1 | Clonazepam | Detailed feature of DILI by this drug | |
| Hiraki, 2019 [97] | 1 | Tegafur-Uracil | Good feature presentation of DILI caused by the drug | |
| Kakisaki, 2019 [98] | 398 | Various drugs | Perfect feature description of the cohort | |
| Korea n = 6528 | Choi, 2008 [99] | 1 | Albendazole | Detailed feature description of DILI by albendazole in a case report of a single patient |
| Suk, 2012 [100] | 101 | Various drugs | Lacking detailed feature description of DILI by individual drugs | |
| Son, 2015 [101] | 1 | Various drugs | No specific feature description of DILI due to comedication | |
| Woo, 2016 [102] | 1 | Various comedicated drugs | Lacking specific feature description of DILI due to comedication | |
| Byeon, 2019 [103] | 6391 | Various drugs | Missing specific feature of DILI caused by individual drugs | |
| Kwon, 2019 [104] | 33 | Nimesulide | Detailed feature description of DILI caused by nimesulide, using a prospective study design in this perfect analysis | |
| Malaysia n = 1 | Thalha, 2018 [105] | 1 | Kombiglyze | Perfect feature description of DILI caused by the combination of metformin and saxagliptin |
| Mexico n = 1 | Lammel-Lindemann, 2018 [106] | 1 | Candesartan | Well described features of DILI by this drug |
| Morocco n = 1 | Essaid, 2010 [107] | 1 | Tadalafil | Feature description of DILI due to the drug |
| Pakistan n = 264 | Abid, 2020 [108] | 264 | Various drugs | No specific feature details presented for DILI caused by individual drugs |
| Portugal n = 53 | Costa-Moreira, 2020 [109] | 53 | Various drugs | Specific feature details of DILI caused by individual drugs were not provided |
| Saudi Arabia n = 1 | Alqrinawi, 2020 [110] | 1 | Menotropin | Perfect feature details of DILI by this specific drug |
| Serbia n = 99 | Miljkovic, 2010 [111] | 80 | Various drugs | No detailed feature description of DILI by individual drugs |
| Miljkovic, 2011 [112] | 19 | Various drugs | Lacking detailed feature presentation of DILI caused by individual drugs | |
| Singapore n = 14 | Wai, 2006 [113] | 14 | Various drugs | Limited feature description of DILI |
| Spain n = 1181 | Rodríguez, 1996 [114] | 35 | Various drugs | No feature details of DILI cases provided for individual drugs |
| Andrade, 2005 [115] | 461 | Various drugs | Limited feature description of DILI case details | |
| Andrade, 2006 [116] | 28 | Various drugs | Partial feature description of DILI by few drugs | |
| García-Cortés, 2008 [117] | 225 | Various drugs | Limited feature description of DILI by few drug groups | |
| Lucena, 2011 [118] | 78 | Amoxicillin Clavulanate | Careful feature description of DILI by the drug combination | |
| Lucena, 2011 [119] | 9 | Various drugs | Feature description of DILI caused by individual drugs | |
| Robles-Diaz, 2015 [120] | 25 | Anabolic and androgenetic steroids | Limited feature description of DILI | |
| Tong, 2015 [121] | 1 | Methylphenidate | Careful evaluation of feature details provided for this DILI case | |
| Medina-Calitz, 2016 [122] | 298 | Various drugs | No specific feature description for DILI by any drug | |
| López-Riera, 2018 [123] | 17 | Various drugs | Lack of specific feature presentation of DILI by any drug | |
| Machlab, 2019 [124] | 1 | Apixabam | Careful feature description of the DILI case caused by the drug | |
| Zoubek, 2019 [125] | 3 | Methyl-prednisolone | Perfect feature presentation of DILI | |
| Sweden n = 1508 | Björnsson, 2005 [126] | 784 | Various drugs | Detailed feature description of DILI by few drugs |
| De Valle, 2006 [127] | 77 | Various drugs | Limited feature description of DILI by a few drugs | |
| Björnsson, 2007 [128] | 77 | Various drugs | Lacking substantial feature description of DILI caused by few drugs | |
| Björnsson, 2007 [129] | 570 | Various drugs | Feature details of DILI presented | |
| Switzerland n = 68 | Goossens, 2013 [130] | 1 | Ibandronate | Detailed description of immune DILI features by ibandronate, a biphosphonate |
| Russmann, 2014 [131] | 14 | Rivaroxaban | Perfect individual feature presentation of each DILI case | |
| Scalfaro, 2017 [132] | 49 | Sacubitril Valsartan | No feature details of DILI caused by the drugs | |
| Schneider, 2017 [133] | 1 | Zoledronic acid | Feature details provided for DILI by this drug | |
| Terziroli Beretta-Piccoli, 2018 [134] | 1 | Atovaquon/Proguanil | Detailed feature description of DILI | |
| Visentin, 2018 [135] | 2 | NSAID Amoxicillin/Clavulanate | Lack of feature details of DILI by individual drugs | |
| Thailand n = 509 | Treeprasertsuk, 2010 [136] | 80 | Various antibiotics | No feature details provided for DILI caused by individual drugs in the context of this epidemiology study |
| Sobhonslidsuk, 2016 [137] | 383 | Various drugs | Missing feature details of DILI caused by individual drugs | |
| Chayanupatkul, 2020 [138] | 46 | Various drugs | Feature details of DILI due to individual drugs not provided | |
| Duzenli, 2019 [139] | 1 | Phenprobamate | Detailed feature description of DILI by this drug | |
| Hussaini, 2007 [140] | 43 | Various antibiotics | No feature details of DILI caused by individual drugs | |
| Daly, 2009 [141] | 51 | Flucloxacillin | Excellent feature details presented for DILI cases | |
| Turkey n = 1 | Spraggs, 2011 [142] | 61 | Laptinib | Excellent feature description of DILI case |
| United Kingdom n = 263 | Islam, 2014 [143] | 1 | Anastrazole | Feature presentation of a patient with DILI due to anastrazole |
| Dyson, 2016 [144] | 1 | Sofosbuvir | Features of DILI by this drug provided | |
| Abbara, 2017 [145] | 105 | Various anti-Tuberculotics | Feature presentation of all DILI cases due to various anti-tuberculosis drugs | |
| Vliegenthart, 2017 [146] | 1 | Nitrofurantoin | Feature description of DILI by the drug | |
| United States n = 20,311 | Fontana, 2005 [147] | 2 | Amoxicillin, Amoxicillin/Clavulanate | Well described features of DILI caused by the drugs |
| Lee, 2005 [148] | 6448 | Ximelagatran | Perfect presentation of DILI features | |
| Stojanovski, 2007 [149] | 1 | Atomoxetine | Good DILI case feature presentation | |
| Lammert, 2008 [150] | 598 | Various drugs | No feature presentation of DILI by individual drugs | |
| Singla, 2010 [151] | 1 | Cephalexin | DILI features of a single case | |
| Nabha, 2012 [152] | 1 | Etravirine | Presentation of DILI feature | |
| Sprague, 2012 [153] | 1 | Varenicline | Careful DILI feature description | |
| Markova, 2013 [154] | 56 | Bosentan | Some global feature description | |
| Marumoto, 2013 [155] | 4 | NSAID | Limited feature details of DILI cases | |
| Bohm, 2014 [156] | 1 | Daptomycin | DILI feature description in this case | |
| Cheetham, 2014 [157] | 11,109 | Various drugs | No specific feature presentation of any drug under consideration | |
| Lim, 2014 [158] | 1 | Various drugs | No presentation of specific features of DILI by 4 drugs used concomitantly or sequentially | |
| Russo, 2014 [159] | 22 | Statins | No individual feature description for DILI caused by various statins | |
| Veluswamy, 2014 [160] | 1 | Polamidomide | Feature presentation of DILI | |
| Baig, 2015 [161] | 1 | Rivaroxaban | Good feature decription of this DILI case | |
| Hammerstrom, 2015 [162] | 1 | Amblodipine | Feature presentation of DILI caused by this drug | |
| Stine, 2015 [163] | 2 | Simeprevir | Detailed feature presentation of the DILI cases | |
| Tang, 2015 [164] | 1 | Bupropion, doxycycline | Complex feature presentation of DILI due to comedication | |
| Unger, 2016 [165] | 1 | Ciprofloxacin | Detailed feature description of DILI by this single drug | |
| Gharia, 2017 [166] | 1 | Letrozole | Perfect feature presentation of DILI | |
| Nicoletti, 2017 [167] | 339 | Various drugs | No specific feature details of DILI caused by individual drugs | |
| Gayam, 2018 [168] | 3 | Various drugs | Feature details of DILI by the drugs | |
| Hayashi, 2018 [169] | 493 | Various drugs | Lacking specific feature details of DILI by individual drugs | |
| Patel, 2018 [170] | 1 | Everolimus | Specific features described for DILI by this drug | |
| Shamberg, 2018 [171] | 34 | Various drugs | No specific features of DILI caused by individual drugs presented | |
| Cirulli, 2019 [172] | 268 | Various drugs | Feature details of DILI by individual drugs not provided | |
| Nicoletti, 2019 [173] | 197 | Flucloxacillin | Specific feature details of DILI by flucloxacillin were not provided | |
| Sandritter, 2019 [174] | 1 | Various drugs | No feature description of DILI by individual drugs | |
| Shumar, 2019 [175] | 1 | Memantine | Detailed feature description of DILI by this drug | |
| Tsung, 2019 [176] | 70 | Pembrolizumab | Features described in detail for DILI caused by this drug | |
| Xie, 2019 [177] | 1 | Anastrozole | Feature details of DILI presented | |
| Ghabril, 2020 [178] | 551 | Various drugs | No feature details of DILI by individual drugs | |
| Mullins, 2020 [179] | 99 | Micafungin | Feature details presented of DILI by this drug |
| Top Ranking Countries | Cases, n | References |
|---|---|---|
| 1. China | 35,825 | [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53] |
| 2. United States | 20,311 | [147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179] |
| 3. Germany | 10,907 | [64,65,66,67,68,69,70,71] |
| 4. Korea | 6528 | [99,100,101,102,103,104] |
| 5. Italy | 1562 | [83,84,85,86,87,88,89,90] |
| 6. Sweden | 1508 | [126,127,128,129] |
| 7. Spain | 1181 | [114,115,116,117,118,119,120,121,122,123,124,125] |
| 8. Japan | 939 | [91,92,93,94,95,96,97,98] |
| 9. Argentina | 625 | [23,24,25,26] |
| 10.Thailand | 509 | [136,137,138] |
| 11. India | 424 | [75,76,77,78,79,80,81] |
| 12. Iceland | 367 | [72,73,74] |
| 13. Pakistan | 264 | [108] |
| 14. UK | 263 | [140,141,142,143,144,145,146] |
| 15. France | 170 | [57,58,59,60,61,62,63] |
| 16. Australia | 106 | [27,28,29,30] |
| 17. Serbia | 99 | [111,112] |
| 18. Egypt | 75 | [56] |
| 19. Switzerland | 68 | [130,131,132,133,134,135] |
| 20. Portugal | 53 | [109] |
| 21. Bahrain | 25 | [31] |
| 22. Colombia | 19 | [54] |
| 23. Singapore | 14 | [113] |
| 24. Brazil | 4 | [32] |
| 25. Canada | 4 | [33,34] |
| 26. Israel | 1 | [82] |
| 27. Malaysia | 1 | [105] |
| 28. Mexico | 1 | [106] |
| 29. Morocco | 1 | [107] |
| 30. Saudi Arabia | 1 | [110] |
| 31. Turkey | 1 | [139] |
| Drug | RUCAM Based DILI Cases (n) |
|---|---|
| 1. Amoxicillin-clavulanate | 333 |
| 2. Flucloxacilllin | 130 |
| 3. Atorvastatin | 50 |
| 4. Disulfiram | 48 |
| 5. Diclofenac | 46 |
| 6. Simvastatin | 41 |
| 7. Carbamazepine | 38 |
| 8. Ibuprofen | 37 |
| 9. Erythromycin | 27 |
| 10. Anabolic steroids | 26 |
| 11. Phenytoin | 22 |
| 12. Sulfamethoxazole/Trimethoprim | 21 |
| 13. Isoniazid | 19 |
| 14. Ticlopidine | 19 |
| 15. Azathioprine/6-Mercaptopurine | 17 |
| 16. Contraceptives | 17 |
| 17. Flutamide | 17 |
| 18. Halothane | 15 |
| 19. Nimesulide | 13 |
| 20. Valproate | 13 |
| 21. Chlorpromazine | 11 |
| 22. Nitrofurantoin | 11 |
| 23. Methotrexate | 8 |
| 24. Rifampicin | 7 |
| 25. Sulfazalazine | 7 |
| 26. Pyrazinamide | 6 |
| 27. Gold salts | 5 |
| 28. Sulindac | 5 |
| 29. Amiodarone | 4 |
| 30. Interferon beta | 3 |
| 31. Propylthiouracil | 2 |
| 32. Allopurinol | 1 |
| 33. Hydralazine | 1 |
| 34. Infliximab | 1 |
| 35. Interferon alpha/Peginterferon | 1 |
| 36. Ketaconazole | 1 |
| 37. Busulfan | 0 |
| 38. Dantrolene | 0 |
| 39. Didanosine | 0 |
| 40. Efavirenz | 0 |
| 41. Floxuridine | 0 |
| 42. Methyldopa | 0 |
| 43. Minocycline | 0 |
| 44. Telithromycin | 0 |
| 45. Nevirapine | 0 |
| 46. Quinidine | 0 |
| 47. Sulfonamides | 0 |
| 48. Thioguanine | 0 |
| Country/ HILI Cases, n | First Author/ Year | HILI Cases, n | Herbal Products | Comments on RUCAM Based HILI Cases |
|---|---|---|---|---|
| Australia n = 2 | Smith, 2016 [181] | 1 | Garcinia Cambogia | Careful feature detail description of HILI by this herb |
| Laube, 2019 [29] | 1 | Ginseng | Feature presentation of this single HILI case | |
| Austria n = 2 | Stadlbauer, 2005 [182] | 2 | Various herbs contained in Tahitian NONI juice | Features described for these HILI cases |
| Brazil n = 1 | Barcelos, 2019 [183] | 1 | Senecio brasiliensis | Complete feature description of HSOS caused by this herb |
| China n = 10,914 | Yuen, 2006 [184] | 7 | Various herbs | No specific feature description of HILI by individual herbs |
| Cheung, 2009 [185] | 3 | Psoralea corylifolia | Well described features of HILI cases | |
| Chau, 2011 [186] | 27 | Various herbs | Lacking feature presentation of HILI by individual herbs | |
| Lin, 2011 [187] | 1 | Gynura segetum | Perfect feature presentation of HSOS | |
| Gao, 2012 [188] | 5 | Gynura segetum | Excellent feature description of HSOS | |
| Lai, 2012 [189] | 74 | Various herbs Polygonum multiflorum | Missing feature presentation of HILI by individual herbs | |
| Dong, 2014 [190] | 18 | Various herbs | Good feature presentation of HILI | |
| Hao, 2014 [37] | 8 | PA containing herbs | Lacking feature presentation of HILI by individual herbs | |
| Gao, 2015 [191] | 23 | Various herbs | Perfect feature presentation of HSOS cases | |
| Ou, 2015 [38] | 130 | Polygonum multiflorum | No feature description of HILI caused by the herb | |
| Wang, 2015 [192] | 40 | Polygonum multiflorum | Comprehensive feature description of HILI cases | |
| Zhu, 2015 [193] | 158 | Polygonum multiflorum | Detailed feature presentation of HILI cases | |
| Zhang, 2016 [194] | 54 | Various herbs | No feature details described of HILI by individual herbs | |
| Zhu, 2016 [42] | 866 | Various herbs | Missing feature details of HILI caused by individual herbs | |
| Li, 2017 [195] | 1 | Polygonum multiflorum | Excellent description of feature details provided for HILI case | |
| Chow, 2019 [196] | 1552 | Various herbs | No feature presentation of HILI by individual herbs but excellent listings | |
| Jing, 2019 [197] | 145 | Polygonum multiflorum | Missing feature presentation of HILI caused by individual herbs | |
| Li, 2019 [198] | 1 | Psoralea corylifolia | Perfect feature presentation of HILI | |
| Ni, 2019 [199] | 331 | Polygonum multiflorum | Feature description of HILI cases | |
| Shen, 2019 [48] | 6971 | Various herbs | No feature details presented for HILI by individual herbs | |
| Tan, 2019 [200] | 3 | Swietenia macrophylla | Perfect presentation of feature details for these HILI cases | |
| Zhu, 2019 [201] | 488 | Various herbs | Lacking feature details of HILI caused by individual herbs | |
| Gao, 2020 [202] | 1 | Psoralea | Feature description of this HILI case | |
| Xia, 2020 [203] | 7 | Swietenia macrophylla, syn skyfruit | Careful description of HILI features | |
| Colombia n = 1 | Cárdenas, 2006 [204] | 1 | Polygomun multiflorum | Features well described for this HILI case |
| France n = 10 | Parlati, 2017 [205] | 10 | Aloe vera | Excellent feature presentation of the HILI cases |
| Germany n = 170 | Teschke, 2009 [206] | 1 | Ayurveda herbs | Complete feature presentation of the HILI case |
| Teschke, 2011 [207] | 22 | Chelidonium majus syn. Greater Celandine | Complete feature description provided for HILI cases | |
| Teschke, 2012 [208] | 21 | Chelidonium majus syn. Greater Celandine | Thorough features presented of HILI cases | |
| Douros, 2015 [209] | 10 | Various herbs | No detailed features reported of HILI caused by individual herbs | |
| Teschke, 2015 [210] | 12 | Camellia sinensis, syn. Green tea, or Lu Cha | Feature details presented of HILI cases | |
| Melchart, 2017 [211] | 26 | Herbal TCMs | Well described features of HILI caused by individual TCM herbs | |
| Diener, 2018 [212] | 10 | Petasites hybridus | Provided features of HILI by this herb | |
| Anderson, 2019 [213] | 48 | Petasites hybridus | Description of HILI features | |
| Gerhardt, 2019 [214] | 1 | Chelidonium majus, syn. Greater Celandine | HILI features described | |
| Teschke, 2019 [215] | 19 | Camellia sinensis | Careful feature presentation of HILI cases | |
| India n = 117 | Philips, 2018 [216] | 94 | Ayurvedic and other herbs | Features not individually described for HILI by various herbs |
| Philips, 2019 [217] | 17 | Various herbs | No detailed features presented for HILI cases by individual herbs | |
| Italy n = 77 | Lapi, 2010 [218] | 1 | Serenoa repens | Detailed feature presentation of HILI |
| Mazzanti, 2015 [219] | 19 | Camellia sinensis, syn. green tea | Careful feature description of HILI cases | |
| Sáez-González, 2016 [220] | 1 | Chelidonium majus | Feature details of the HILI case | |
| Mazzanti, 2017 [221] | 55 | Red yeast rice | Thorough feature presentation of HILI | |
| Osborne, 2019 [222] | 1 | Mitragyna speciosa, syn. Kraton | Individual feature details not provided for the HILI case | |
| Japan n = 3 | Tsuda, 2010 [223] | 1 | Saireito | Perfect feature details presented for the HILI case |
| Hisamochi, 2013 [224] | 2 | Agaricus blazei Murill | Excellent presentation of HILI features | |
| Korea n = 2507 | Ahn, 2004 [225] | 64 | Various herbs | Missing feature presentation of HILI by individual herbs |
| Seo, 2006 [226] | 17 | Various herbs | No individual feature description of HILI by specific herbs | |
| Kang, 2008 [227] | 66 | Various herbs | Lacking feature details of HILI by individual herbs | |
| Sohn, 2008 [228] | 24 | Various herbs | Feature details of HILI by individual herbs not provided | |
| Kang, 2009 [229] | 1 | Corydalis speciosa | Perfect feature details provided for this single HILI | |
| Kim, 2009 [230] | 2 | Arrowroot, syn. ge Gen | Excellent presentation of features for these HILI cases | |
| Bae, 2010 [231] | 1 | Polygonum multiflorum | Careful feature details presented for this HILI case | |
| Yang, 2010 [232] | 3 | Aloe vera or arborescens | Thorough description of features of these HILI cases | |
| Jung, 2011 [233] | 25 | Polygonum | Excellent feature presentation of the HILI cases | |
| Kim, 2012 [234] | 1 | multiflorum | Perfect feature description for this HILI case | |
| Suk, 2012 [100] | 149 | Hovenia dulcis, syn. Juguju | No feature description of HILI caused by individual herbs | |
| Lee, 2015 [235] | 27 | Various herbs | Lacking feature details of HILI caused by individual herbs | |
| Lee, 2015 [236] | 97 | Various herbs | Feature details of HILI cases caused by individual herbs were not provided | |
| Woo, 2015 [102] | 5 | Various herbs | No feature details presented for HILI by individual herbs | |
| Cho, 2017 [237] | 6 | Various herbs | Missing feature details of HILI cases by individual herbs | |
| Byeon, 2019 [103] | 2019 | Various herbs | No detailed feature description of HILI by individual herbs | |
| Singapore n = 25 | Wai, 2006 [113] | 15 | Various herbs | No detailed features presented for HILI cases by individual herbs |
| Teo, 2016 [238] | 10 | Various herbs | Missing feature details of HILI cases | |
| South Africa n = 47 | Awortwe, 2018 [239] | 47 | Various herbs | Features were not provided for cases of HILI caused by individual herbs |
| Spain n = 46 | Andrade, 2005 [115] | 9 | Various herbs | No feature details of HILI by individual herbs |
| Jimenez-Saenz, 2006 [240] | 1 | Camellia sinensis | Feature details presented for this HILI case | |
| García-Cortés, 2008 [241] | 13 | Various herbs | Lacking feature details of HILI caused by individual herbs | |
| García-Cortés, 2008 [117] | 5 | Various herbs | Specific feature details of HILI by individual herbs not provided | |
| Medina-Caliz, 2018 [242] | 18 | Camellia sinensis and other herbs | No specific feature details provided for HILI | |
| Sweden n = 5 | Björnsson, 2007 [243] | 5 | Camellia sinensis | Feature details provided for HILI cases |
| Switzerland n = 1 | Ruperti-Repilado, 2019 [244] | 1 | Artemisia annua | Careful feature presentation of this HILI case |
| Turkey n = 1 | Yilmaz, 2015 [245] | 1 | Lesser Celandine, syn. Pilewort | Excellent feature presentation of the HILI case |
| United States n = 100 | Papafragkakis, 2016 [246] | 1 | Chinese skullcap plus Black catechu | Perfect feature presentation of this HILI case |
| Dalal, 2017 [247] | 1 | Ayurvedic herb | Specific case features described | |
| Kesavarapu, 2017 [248] | 1 | Yogi Detox tea with multiple herbs | Individual specific features not provided for this HILI case caused specifically by a single herb | |
| Kothadia, 2018 [249] | 19 | Garcinia Cambogia | Careful feature presentation of HILI cases | |
| Surapaneni, 2018 [250] | 19 | Camellia sinensis | Feature details provided for the HILI case | |
| Imam, 2019 [251] | 1 | Curcumin | Thorough feature description of the HILI case | |
| Yousaf, 2019 [252] | 9 | Garcinia Cambogia | Excellent feature description of the HILI case | |
| Oketch-Rabah, 2020 [253] | 29 | Camellia sinensis extract | Perfect feature presentation of HILI by this herb | |
| Schimmel, 2020 [254] | 20 | Mitragyna speciosa, syn. Kraton | Feature details provided for HILI cases |
| Top Ranking Countries | Cases, n | References |
|---|---|---|
| 1. China | 10,914 | [37,38,42,48,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203] |
| 2. Korea | 2507 | [100,101,102,103,225,226,227,228,229,230,231,232,233,234,235,236,237] |
| 3. Germany | 170 | [206,207,208,209,210,211,212,213,214,215] |
| 4. India | 117 | [216,217] |
| 5. US | 100 | [247,248,249,250,251,252,253,254,255] |
| 6. Italy | 77 | [218,219,220,221,222] |
| 7. South Africa | 47 | [239] |
| 8. Spain | 46 | [115,117,240,241,242] |
| 9. Singapore | 25 | [113,238] |
| 10. France | 10 | [205] |
| 11. Sweden | 5 | [244] |
| 12. Japan | 3 | [223,224] |
| 13. Australia | 2 | [29,181] |
| 14. Austria | 2 | [182] |
| 15. Brazil | 1 | [183] |
| 16. Colombia | 1 | [204] |
| 17. Switzerland | 1 | [245] |
| 18. Turkey | 1 | [246] |
| RUCAM Specificities |
|---|
| Basic features |
|
|
|
|
|
|
|
|
|
|
|
| Clearly defined and scored key elements |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other important specificities |
|
|
|
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teschke, R.; Danan, G. Worldwide Use of RUCAM for Causality Assessment in 81,856 Idiosyncratic DILI and 14,029 HILI Cases Published 1993–Mid 2020: A Comprehensive Analysis. Medicines 2020, 7, 62. https://doi.org/10.3390/medicines7100062
Teschke R, Danan G. Worldwide Use of RUCAM for Causality Assessment in 81,856 Idiosyncratic DILI and 14,029 HILI Cases Published 1993–Mid 2020: A Comprehensive Analysis. Medicines. 2020; 7(10):62. https://doi.org/10.3390/medicines7100062
Chicago/Turabian StyleTeschke, Rolf, and Gaby Danan. 2020. "Worldwide Use of RUCAM for Causality Assessment in 81,856 Idiosyncratic DILI and 14,029 HILI Cases Published 1993–Mid 2020: A Comprehensive Analysis" Medicines 7, no. 10: 62. https://doi.org/10.3390/medicines7100062
APA StyleTeschke, R., & Danan, G. (2020). Worldwide Use of RUCAM for Causality Assessment in 81,856 Idiosyncratic DILI and 14,029 HILI Cases Published 1993–Mid 2020: A Comprehensive Analysis. Medicines, 7(10), 62. https://doi.org/10.3390/medicines7100062






