Greater Height Is Associated with a Larger Carotid Lumen Diameter
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurements
2.2.1. Weight and Height
2.2.2. Adiposity
2.2.3. Blood Pressure
2.2.4. Blood Studies
2.2.5. Ultrasound
2.3. Statistical Methods
2.3.1. Exploratory Analyses
2.3.2. Main Model Development
2.3.3. Sensitivity Analyses
3. Results
3.1. Descriptive Analyses
3.2. Exploratory Analyses
3.3. Prediction Models
with an R2 of 0.20 (SE, ± 0.77, p < 0.001; CVE, ± 0.04), and
with an R2 of 0.24 (SE, ± 0.18, p < 0.001).
3.4. Sensitivity Analyses
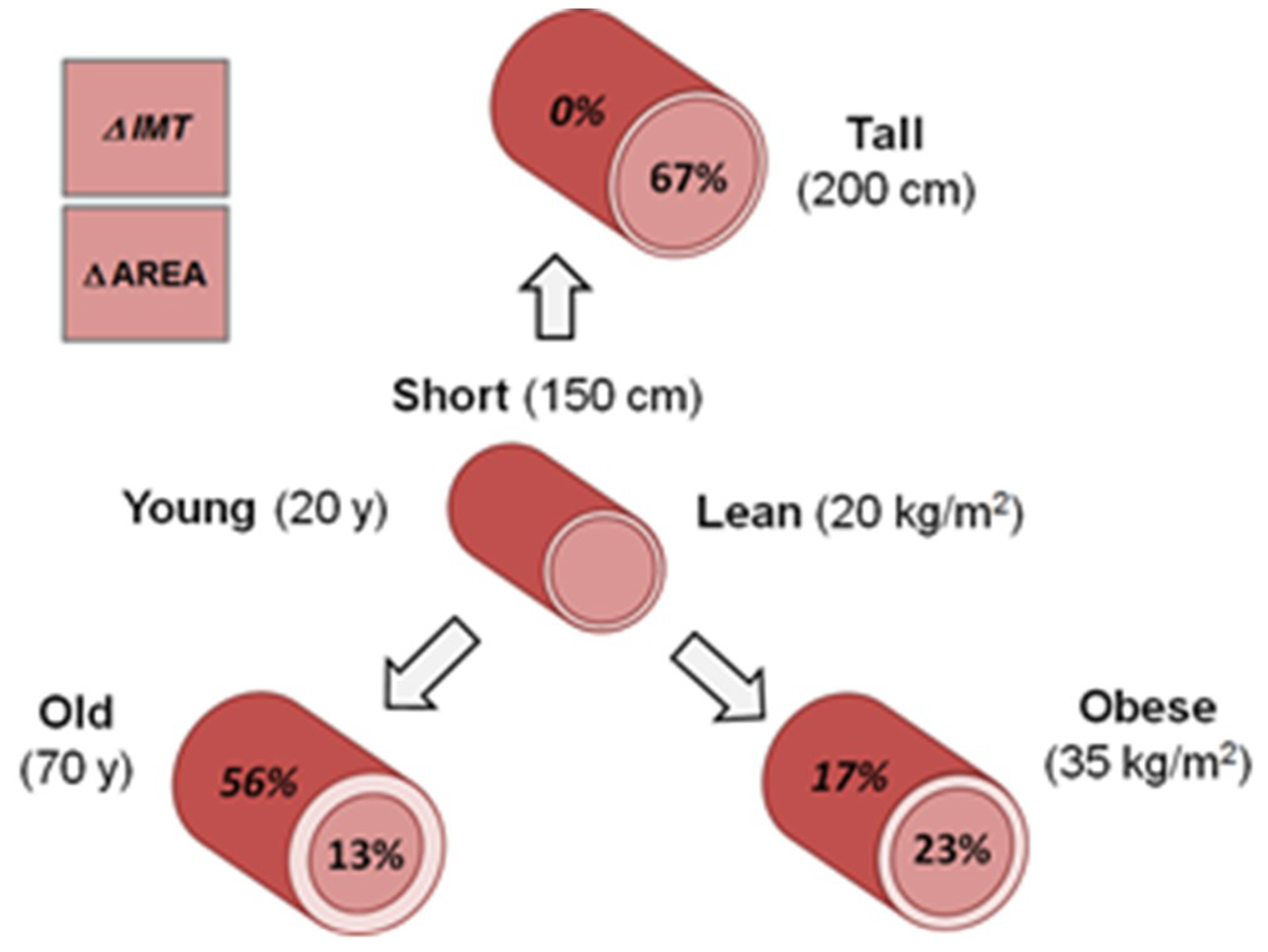
3.5. Model Integration
4. Discussion
4.1. Height and Carotid Artery Structure
4.2. Height and Other Vascular Structures
4.3. Age and Adiposity Effects on Carotid Structure
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Emerging Risk Factors Collaboration. Adult height and the risk of cause-specific death and vascular morbidity in 1 million people: Individual participant meta-analysis. Int. J. Epidemiol. 2012, 41, 1419–1433. [Google Scholar] [CrossRef]
- Goldbourt, U.; Tanne, D. Body height is associated with decreased long-term stroke but not coronary heart disease mortality? Stroke 2002, 33, 743–748. [Google Scholar] [CrossRef]
- McCarron, P.; Greenwood, R.; Ebrahim, S.; Elwood, P.; Smith, G.D. Adult height is inversely associated with ischaemic stroke. The Caerphilly and Speedwell collaborative studies. J. Epidemiol. Community Health 2000, 54, 239–240. [Google Scholar] [CrossRef][Green Version]
- Njolstad, I.; Arnesen, E.; Lund-Larsen, P.G. Body height, cardiovascular risk factors, and risk of stroke in middle-aged men and women: A 14-year follow-up of the Finnmark Study. Circulation 1996, 94, 2877–2882. [Google Scholar] [CrossRef]
- Nuesch, E.; Dale, C.; Palmer, T.M.; White, J.; Keating, B.J.; van Iperen, E.P.; Goel, A.; Padmanabhan, S.; Asselbergs, F.W.; Investigators, E.P.-N.; et al. Adult height, coronary heart disease and stroke: A multi-locus Mendelian randomization meta-analysis. Int. J. Epidemiol. 2016, 45, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, B.; Watts, K.; Thomas, D.M.; Carmichael, O.; Hu, F.B.; Heo, M.; Hall, J.E.; Heymsfield, S.B. Associations between height and blood pressure in the United States population. Medicine 2017, 96, e9233. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.P.; Hamby, S.E.; Saleheen, D.; Hopewell, J.C.; Zeng, L.; Assimes, T.L.; Kanoni, S.; Willenborg, C.; Burgess, S.; Amouyel, P.; et al. Genetically determined height and coronary artery disease. N. Engl. J. Med. 2015, 372, 1608–1618. [Google Scholar] [CrossRef]
- Korhonen, P.E.; Kautiainen, H.; Eriksson, J.G. The shorter the person, the higher the blood pressure: A birth cohort study. J. Hypertens. 2017, 35, 1170–1177. [Google Scholar] [CrossRef]
- Langenberg, C.; Hardy, R.; Kuh, D.; Wadsworth, M.E. Influence of height, leg and trunk length on pulse pressure, systolic and diastolic blood pressure. J. Hypertens. 2003, 21, 537–543. [Google Scholar] [CrossRef]
- Polanczyk, A.; Podgorski, M.; Wozniak, T.; Stefanczyk, L.; Strzelecki, M. Computational fluid dynamics as an engineering tool for the reconstruction of hemodynamics after carotid artery stenosis operation: A case Study. Medicina 2018, 54, 42. [Google Scholar] [CrossRef] [PubMed]
- Polanczyk, A.; Klinger, M.; Nanobachvili, J.; Huk, I.; Neumayer, C. Artificial circulatory model for analysis of human and artificial vessels. Appl. Sci. 2018, 8, 1017. [Google Scholar] [CrossRef]
- Polanczyk, A.; Piechota-Polanczyk, A.; Domenig, C.; Nanobachvili, J.; Huk, I.; Neumayer, C. Computational fluid dynamic accuracy in mimicking changes in blood hemodynamics in patients with acute type IIIb aortic dissection treated with TEVAR. Appl. Sci. 2018, 8, 1309. [Google Scholar] [CrossRef]
- Messe, S.R.; Kasner, S.E.; Mehta, Z.; Warlow, C.P.; Rothwell, P.M. Effect of body size on operative risk of carotid endarterectomy. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1759–1761. [Google Scholar] [CrossRef][Green Version]
- Nwasokwa, O.N.; Weiss, M.; Gladstone, C.; Bodenheimer, M.M. Higher prevalence and greater severity of coronary disease in short versus tall men referred for coronary arteriography. Am. Heart J. 1997, 133, 147–152. [Google Scholar] [CrossRef]
- Krejza, J.; Arkuszewski, M.; Kasner, S.E.; Weigele, J.; Ustymowicz, A.; Hurst, R.W.; Cucchiara, B.L.; Messe, S.R. Carotid artery diameter in men and women and the relation to body and neck size. Stroke 2006, 37, 1103–1105. [Google Scholar] [CrossRef]
- Polak, J.F.; Kronmal, R.A.; Tell, G.S.; O’Leary, D.H.; Savage, P.J.; Gardin, J.M.; Rutan, G.H.; Borhani, N.O. Compensatory increase in common carotid artery diameter: Relation to blood pressure and artery intima-media thickness in older adults. Stroke 1996, 27, 2012–2015. [Google Scholar] [CrossRef]
- Litwin, M.; Trelewicz, J.; Wawer, Z.; Antoniewicz, J.; Wierzbicka, A.; Rajszys, P.; Grenda, R. Intima-media thickness and arterial elasticity in hypertensive children: Controlled study. Pediatr. Nephrol. 2004, 19, 767–774. [Google Scholar] [CrossRef]
- Sturm, W.; Sandhofer, A.; Engl, J.; Laimer, M.; Molnar, C.; Kaser, S.; Weiss, H.; Tilg, H.; Ebenbichler, C.F.; Patsch, J.R. Influence of visceral obesity and liver fat on vascular structure and function in obese subjects. Obesity 2009, 17, 1783–1788. [Google Scholar] [CrossRef]
- Chien, C.Y.; Liu, C.C.; Po, H.L.; Yen, C.H.; Hou, C.J.; Kuo, J.Y.; Hung, C.L.; Wang, S.S.; Yeh, H.I.; Lam, C.S. The Relationship among carotid artery remodeling, cardiac geometry, and serum N-Terminal pro-B-Type natriuretic peptide level in asymptomatic asians: Sex-differences and longitudinal GEE study. PLoS ONE 2015, 10, e0131440. [Google Scholar] [CrossRef]
- Samijo, S.K.; Willigers, J.M.; Barkhuysen, R.; Kitslaar, P.J.; Reneman, R.S.; Brands, P.J.; Hoeks, A.P. Wall shear stress in the human common carotid artery as function of age and gender. Cardiovasc. Res. 1998, 39, 515–522. [Google Scholar] [CrossRef]
- Kozakova, M.; Palombo, C.; Morizzo, C.; Hojlund, K.; Hatunic, M.; Balkau, B.; Nilsson, P.M.; Ferrannini, E. Obesity and carotid artery remodeling. Nutr. Diabetes 2015, 5, e177. [Google Scholar] [CrossRef]
- Bella, J.N.; Cole, S.A.; Laston, S.; Almasy, L.; Comuzzie, A.; Lee, E.T.; Best, L.G.; Fabsitz, R.R.; Howard, B.V.; Maccluer, J.W.; et al. Genome-wide linkage analysis of carotid artery lumen diameter: The strong heart family study. Int. J. Cardiol. 2013, 168, 3902–3908. [Google Scholar] [CrossRef] [PubMed][Green Version]
- North, K.E.; MacCluer, J.W.; Devereux, R.B.; Howard, B.V.; Welty, T.K.; Best, L.G.; Lee, E.T.; Fabsitz, R.R.; Roman, M.J. Heritability of carotid artery structure and function: The Strong Heart Family Study. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1698–1703. [Google Scholar] [CrossRef]
- De Simone, G.; Daniels, S.R.; Devereux, R.B.; Meyer, R.A.; Roman, M.J.; de Divitiis, O.; Alderman, M.H. Left ventricular mass and body size in normotensive children and adults: Assessment of allometric relations and impact of overweight. J. Am. Coll. Cardiol. 1992, 20, 1251–1260. [Google Scholar] [CrossRef]
- Katori, R. Normal cardiac output in relation to age and body size. Tohoku. J. Exp. Med. 1979, 128, 377–387. [Google Scholar] [CrossRef]
- Lundell, B.P.; Casas, M.L.; Wallgren, C.G. Oxygen consumption in infants and children during heart catheterization. Pediatr. Cardiol. 1996, 17, 207–213. [Google Scholar] [CrossRef]
- Fujishiro, K.; Yoshimura, S. Haemodynamic changes in carotid blood flow with age. Jikeikai. Med. J. 1982, 29, 125–138. [Google Scholar]
- O’Connor, N.J.; Morton, J.R.; Birkmeyer, J.D.; Olmstead, E.M.; O’Connor, G.T. Effect of coronary artery diameter in patients undergoing coronary bypass surgery. Circulation 1996, 93, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.D.; Kennedy, J.W.; Davis, K.B.; Maynard, C.; Fritz, J.K.; Kaiser, G.; Myers, W.O. Association of sex, physical size, and operative mortality after coronary artery bypass in the Coronary Artery Surgery Study (CASS). J. Thorac. Cardiovasc. Surg. 1982, 84, 334–341. [Google Scholar] [PubMed]
- Reed, C.M.; Richey, P.A.; Pulliam, D.A.; Somes, G.W.; Alpert, B.S. Aortic dimensions in tall men and women. Am. J. Cardiol. 1993, 71, 608–610. [Google Scholar] [CrossRef]
- Svensson, L.G.; Kim, K.H.; Lytle, B.W.; Cosgrove, D.M. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection in patients with bicuspid aortic valves. J. Thorac. Cardiov. Sur. 2003, 126, 892–893. [Google Scholar] [CrossRef]
- Vasan, R.S.; Larson, M.G.; Benjamin, E.J.; Levy, D. Echocardiographic reference values for aortic root size: The framingham heart study. J. Am. Soc. Echocardiogr. 1995, 8, 793–800. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Picard, M.H.; Triulzi, M.O.; Thomas, J.D.; Newell, J.; King, M.E.; Weyman, A.E. New perspectives in the assessment of cardiac chamber dimensions during development and adulthood. J. Am. Coll. Cardiol. 1992, 19, 983–988. [Google Scholar] [CrossRef]
- Kroger, K.; Nettelrodt, J.; Muntsches, C.; Neudorf, U.; Feuersenger, A.; Rudofsky, G.; Schmalz, A.A. Impact of age, height, and body mass index on arterial diameters in infants and children: A model for predicting femoral artery diameters prior to cardiovascular procedures. J. Endovasc. Ther. 2004, 11, 419–423. [Google Scholar] [CrossRef]
- Sandgren, T.; Sonesson, B.; Ahlgren, R.; Lanne, T. The diameter of the common femoral artery in healthy human: Influence of sex, age, and body size. J. Vasc. Surg. 1999, 29, 503–510. [Google Scholar] [CrossRef]
- Ussavarungsi, K.; Whitlock, J.P.; Lundy, T.A.; Carabenciov, I.D.; Burger, C.D.; Lee, A.S. The significance of pulmonary artery size in pulmonary hypertension. Diseases 2014, 2, 243–259. [Google Scholar] [CrossRef]
- Sandgren, T.; Sonesson, B.; Ahlgren, A.R.; Lanne, T. Factors predicting the diameter of the popliteal artery in healthy humans. J. Vasc. Surg. 1998, 28, 284–289. [Google Scholar] [CrossRef]
- Tanaka, H.; Dinenno, F.A.; Monahan, K.D.; DeSouza, C.A.; Seals, D.R. Carotid artery wall hypertrophy with age is related to local systolic blood pressure in healthy men. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 82–87. [Google Scholar] [CrossRef]
- Roman, M.J.; Saba, P.S.; Pini, R.; Spitzer, M.; Pickering, T.G.; Rosen, S.; Alderman, M.H.; Devereux, R.B. Parallel cardiac and vascular adaptation in hypertension. Circulation 1992, 86, 1909–1918. [Google Scholar] [CrossRef]
- Bonithon-Kopp, C.; Touboul, P.J.; Berr, C.; Leroux, C.; Mainard, F.; Courbon, D.; Ducimetiere, P. Relation of intima-media thickness to atherosclerotic plaques in carotid arteries: The Vascular Aging (EVA) Study. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 310–316. [Google Scholar] [CrossRef]
- Irace, C.; Carallo, C.; De Franceschi, M.S.; Scicchitano, F.; Milano, M.; Tripolino, C.; Scavelli, F.; Gnasso, A. Human common carotid wall shear stress as a function of age and gender: A 12-year follow-up study. Age 2012, 34, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Cecelja, M.; Jiang, B.; Keehn, L.; Hussain, T.; Vieira, M.S.; Phinikaridou, A.; Greil, G.; Spector, T.D.; Chowienczyk, P. Arterial stiffening is a heritable trait associated with arterial dilation but not wall thickening: A longitudinal study in the twins UK cohort. Eur. Heart J. 2018, 39, 2282–2288. [Google Scholar] [CrossRef]
- Mannami, T.; Baba, S.; Ogata, J. Potential of carotid enlargement as a useful indicator affected by high blood pressure in a large general population of a Japanese city: The Suita study. Stroke 2000, 31, 2958–2965. [Google Scholar] [CrossRef] [PubMed]
- Kappus, R.M.; Fahs, C.A.; Smith, D.; Horn, G.P.; Agiovlasitis, S.; Rossow, L.; Jae, S.Y.; Heffernan, K.S.; Fernhall, B. Obesity and overweight associated with increased carotid diameter and decreased arterial function in young otherwise healthy men. Am. J. Hypertens. 2014, 27, 628–634. [Google Scholar] [CrossRef]
- Boutouyrie, P.; Bussy, C.; Lacolley, P.; Girerd, X.; Laloux, B.; Laurent, S. Association between local pulse pressure, mean blood pressure, and large-artery remodeling. Circulation 1999, 100, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
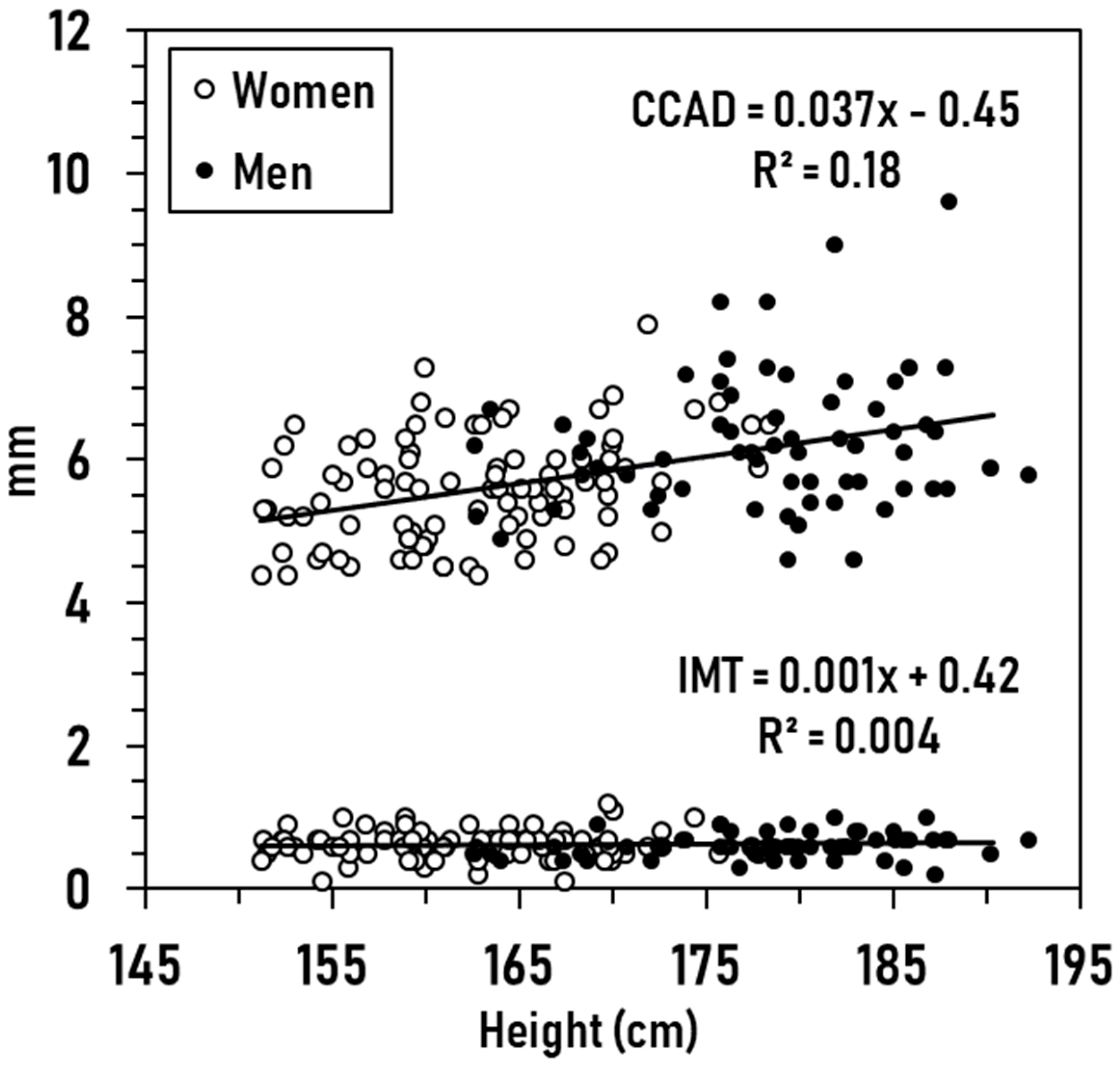
| Men | Women | Total | |
|---|---|---|---|
| N | 101 | 130 | 231 |
| Age (years) | 45.7 ± 17.7 | 47.7 ± 17.0 | 46.8 ± 17.3 |
| Weight (kg) | 90.2 ± 21.8 | 72.3 ± 19.2 ‡ | 80.1 ± 22.2 |
| Height (cm) | 177.3 ± 6.9 | 162.4 ± 6.6 ‡ | 168.9 ± 10.0 |
| BMI (kg/m2) | 28.6 ± 6.3 | 27.4 ± 7.1 | 27.9 ± 6.8 |
| %Fat | 26.3 ± 6.3 | 37.9 ± 7.5 ‡ | 32.9 ± 9.1 |
| CCAD (mm) | 6.16 ± 0.88 | 5.72 ± 0.79 ‡ | 5.92 ± 0.85 |
| IMT (mm) | 0.66 ± 0.20 | 0.64 ± 0.20 | 0.65 ± 0.20 |
| Plasma Glucose (mg/dL) | 96.3 ± 20.2 | 94.7 ± 18.8 | 95.4 ± 19.4 |
| TG (mg/dL) | 97.5 ± 63.0 | 86.6 ± 46.6 | 91.32 ± 54.5 |
| LDL (mg/dL ) | 104.9 ± 30.1 | 112.7 ± 30.3 | 109.3 ± 30.4 |
| HDL (mg/dL) | 53.4 ± 14.3 | 63.5 ± 16.7 ‡ | 59.1 ± 16.5 |
| TC (mg/dL) | 177.8 ± 37.4 | 193.8 ± 38.3 ‡ | 186.8 ± 38.7 |
| SBP (mmHg) | 119.2 ± 12.8 | 116.6 ± 15.2 | 117.7 ± 14.2 |
| DBP (mmHg) | 77.9 ± 8.3 | 73.9 ± 8.8 ‡ | 75.6 ± 8.8 |
| PP (mmHg) | 41.3 ± 9.6 | 42.7 ± 11.4 | 42.1 ± 10.7 |
| MBP (mmHg) | 91.6 ± 0.89 | 88.1 ± 0.87 † | 89.7 ± 0.64 |
| Medical History | Total | ||
| Blood Pressure (BP) | 47 | ||
| Only BP | 22 | ||
| +Chol | 11 | ||
| +Diabetes | 8 | ||
| +Chol and Diabetes | 4 | ||
| Cholesterol (Chol) | 28 | ||
| Only Chol | 8 | ||
| +Diabetes | 2 | ||
| Type II Diabetes | 26 | ||
| Only Diabetes | 7 | ||
| Heart Disease | 5 | ||
| Only Heart Disease | 0 | ||
| +Diabetes | 3 | ||
| +BP and Chol | 1 | ||
| +Diabetes, BP, and Chol | 1 | ||
| Current Smokers | 17 | ||
| Only Smokers | 15 | ||
| +BP | 1 | ||
| +Diabetes | 1 |
| Model 1: CCAD | Model 2: IMT | |||||
|---|---|---|---|---|---|---|
| Covariate | ß | 95% CI | SE | ß | 95% CI | SE |
| Intercept | −0.62 | −4.25 to 3.00 | 1.84 | −0.29 | −1.09 to 0.51 | 0.41 |
| Height (cm) | 0.035 ‡ | 0.017 to 0.053 | 0.009 | 0.003 | −0.001 to 0.007 | 0.002 |
| BMI (kg/m2) | 0.047 † | 0.014 to 0.080 | 0.017 | 0.004 | −0.003 to 0.011 | 0.004 |
| Age (years) | 0.007 | −0.003 to 0.016 | 0.005 | 0.004 ‡ | 0.002 to 0.006 | 0.001 |
| Sex (0, F; 1, M) | 0.172 | −0.40 to 0.74 | 0.289 | -0.041 | −0.167 to 0.086 | 0.064 |
| %Fat | 0.009 | −0.020 to 0.039 | 0.015 | 0.0002 | −0.006 to 0.007 | 0.003 |
| PGlu (mg/dL) | −0.004 | −0.022 to 0.014 | 0.009 | 0.002 | −0.002 to 0.006 | 0.002 |
| TG (mg/dL) | 0.001 | −0.002 to 0.004 | 0.001 | 0.00008 | −0.0005 to 0.0006 | 0.0003 |
| LDL (mg/dL) | −0.005 | −0.010 to 0.0001 | 0.003 | 0.0003 | −0.001 to 0.001 | 0.0006 |
| HDL (mg/dL) | 0.005 | −0.004 to 0.013 | 0.004 | −0.0003 | −0.002 to 0.002 | 0.001 |
| SBP (mmHg) | −0.002 | −0.016 to 0.011 | 0.007 | 0.0003 | −0.003 to 0.003 | 0.002 |
| DBP (mmHg) | −0.008 | −0.029 to 0.013 | 0.011 | −0.002 | −0.007 to 0.003 | 0.002 |
| R2 | 0.27 ‡ | 0.77 | 0.13 ‡ | 0.17 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwaung, P.; Heo, M.; Bourgeois, B.; Kennedy, S.; Shepherd, J.; Heymsfield, S.B. Greater Height Is Associated with a Larger Carotid Lumen Diameter. Medicines 2019, 6, 57. https://doi.org/10.3390/medicines6020057
Hwaung P, Heo M, Bourgeois B, Kennedy S, Shepherd J, Heymsfield SB. Greater Height Is Associated with a Larger Carotid Lumen Diameter. Medicines. 2019; 6(2):57. https://doi.org/10.3390/medicines6020057
Chicago/Turabian StyleHwaung, Phoenix, Moonseong Heo, Brianna Bourgeois, Samantha Kennedy, John Shepherd, and Steven B. Heymsfield. 2019. "Greater Height Is Associated with a Larger Carotid Lumen Diameter" Medicines 6, no. 2: 57. https://doi.org/10.3390/medicines6020057
APA StyleHwaung, P., Heo, M., Bourgeois, B., Kennedy, S., Shepherd, J., & Heymsfield, S. B. (2019). Greater Height Is Associated with a Larger Carotid Lumen Diameter. Medicines, 6(2), 57. https://doi.org/10.3390/medicines6020057





