Green Nanotechnology: Advancement in Phytoformulation Research
Abstract
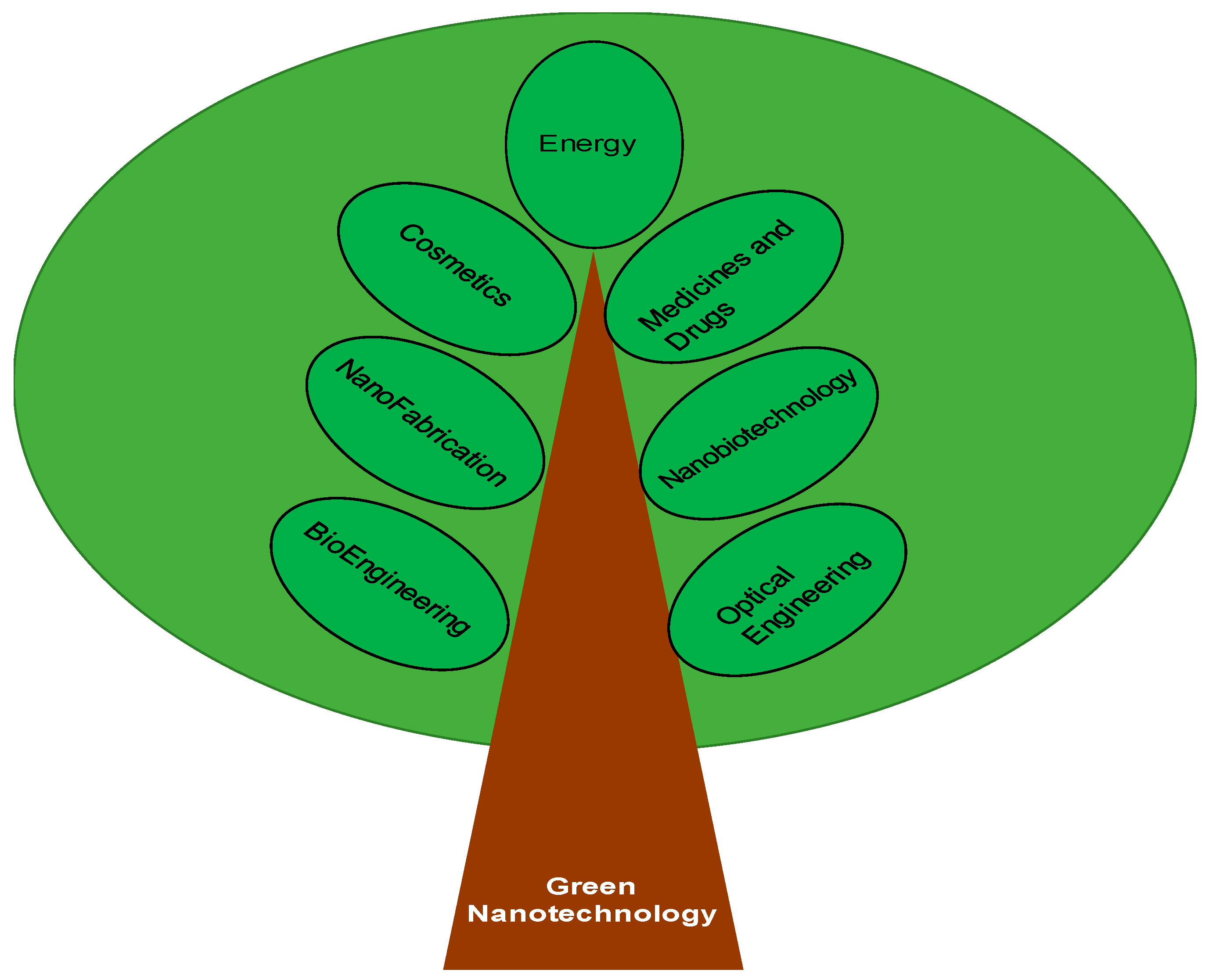
1. Introduction
2. Herbal Approach for Developing Nanoparticles
3. Nanoparticles Synthesized from Plant Extracts
3.1. Gold and Silver Nanoparticles
3.2. Copper and Copper Oxide Nanoparticles
3.3. Palladium and Platinium Nanoparticles
3.4. Titanium Dioxide and Zinc Oxide Nanoparticles
3.5. Indium Oxide (In2O3), Iron Oxide, Lead, and Selenium Nanoparticles
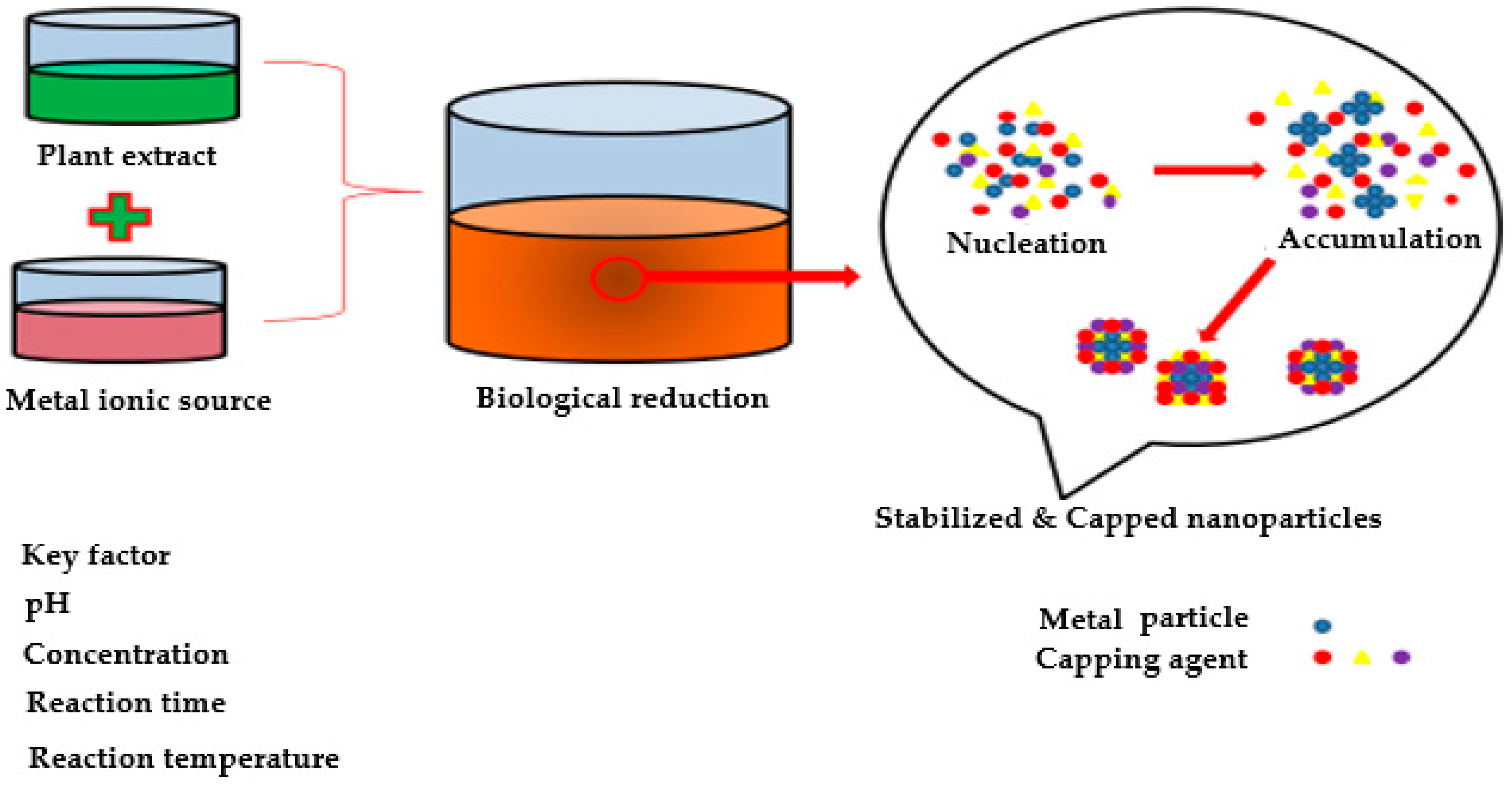
4. Green Synthesis of Metal Nanoparticles
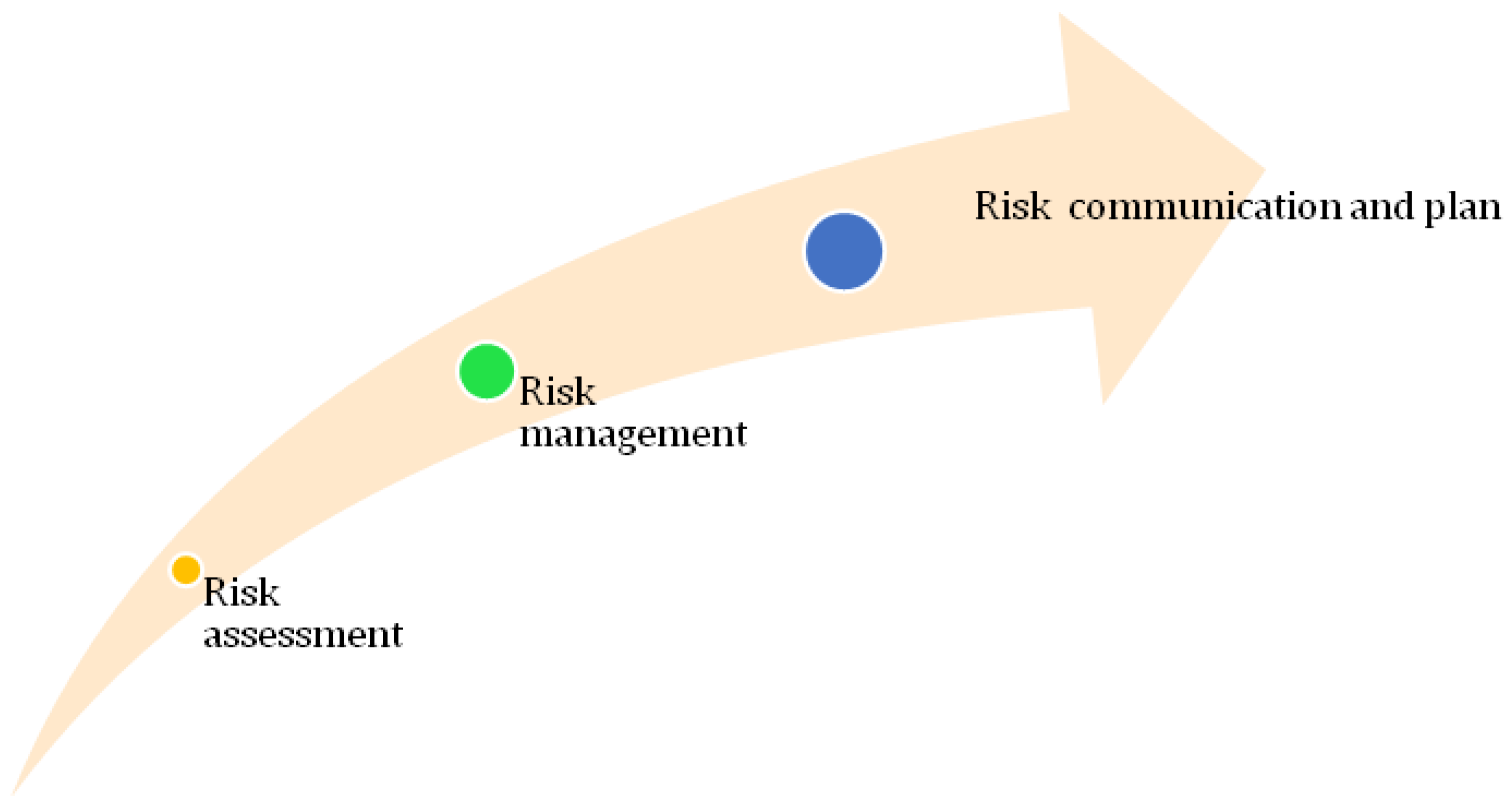
5. Green Nanotechnology: Risk Aspects
6. Risk Assessment
7. Risk Management
8. Risk Communication
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hullmann, A.; Meyer, M. Publications and patents in nanotechnology. Scientometrics 2003, 58, 507–527. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/silica nanocomposites: Preparation, characterization, properties, and applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef]
- Balbus, J.M.; Florini, K.; Denison, R.A.; Walsh, S.A. Protecting workers and the environment: An environmental NGO’s perspective on nanotechnology. J. Nanopart. Res. 2007, 9, 11–22. [Google Scholar] [CrossRef]
- Benn, T.M.; Westerhoff, P. Nanoparticle Silver Released into Water from Com mercially Available Sock Fabrics. Environ. Sci. Technol. 2008, 42, 4133–4139. [Google Scholar] [CrossRef] [PubMed]
- Besley, J.C.; Kramer, V.L.; Priest, S.H. Expert opinion on nanotechnology: Risks, benfits, and regulation. J. Nanopart. Res. 2008, 10, 549–558. [Google Scholar] [CrossRef]
- Guo, L.; Lui, X.; Sanchez, V.; Vaslet, C.; Kane, A.B.; Hurt, R.H. Window of Opportunity: Designing Carbon Nanomaterials for Environmental Safety and Health. Mater. Sci. Forum 2007, 511–516, 544–545. [Google Scholar]
- Hristozov, D.; Ertel, J. Nanotechnology and Sustainability: Benefits and Risk of Nanotechnology for Environmental Sustainability. Forum der Forschung 2009, 22, 161–168. [Google Scholar]
- Jayapalan, A.R.; Lee, B.Y.; Kurtis, K.E. Can nanotechnology be green? Comparing efficacy of nano and microparticles in cementitious materials. Cem. Concr. Compos. 2013, 36, 16–24. [Google Scholar] [CrossRef]
- Som, C.; Wick, P.; Krug, H.; Nowack, B. Environmental and health effects of nanomaterials in nanotextiles and façade coatings. Environ. Int. 2011, 37, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Rickerby, D.G.; Morrison, M. Nanotechnology and the environment: A European perspective. Sci. Technol. Adv. Mater. 2006, 8, 19–24. [Google Scholar] [CrossRef]
- Hutchison, J.E. Greener nanoscience: A proactive approach to advancing applications and reducing implications of nanotechnology. ACS Nano 2008, 2, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.W.; James, J.T.; McCluskey, R.; Arepalli, S.; Hunter, R.L. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit. Rev. Toxicol. 2006, 36, 189–217. [Google Scholar] [CrossRef]
- Schulte, P.A.; McKernan, L.T.; Heidel, D.S.; Okun, A.H. Occupational safety and health, green chemistry, and sustainability: A review of areas of convergence. Environ. Health 2013, 12, 1186–1476. [Google Scholar] [CrossRef] [PubMed]
- Eastlake, A.; Hodson, L.; Geraci, C.L.; Crawford, C.A. Critical evaluation of material safety data sheets (MSDS) for engineered nanomaterials. J. Chem. Health Saf. 2012, 53, S108–S112. [Google Scholar]
- Carnovale, C.; Bryant, G.; Shukla, R.; Bansal, V. Size, shape and surface chemistry of nano-gold dictate its cellular intraction, uptake and toxicity. Prog. Mater. Sci. 2016, 83, 152–190. [Google Scholar] [CrossRef]
- Khan, A.K.; Rashid, R.; Murtaza, G.; Zahra, A. Gold Nanoparticles: Synthesis and Applications in Drug Delivery. Trop. J. Pharm. Res. 2014, 13, 1169–1177. [Google Scholar] [CrossRef]
- Manuel, C.; Toni, U.; Allan, P.; Katrin, L. Challenges in Determining the Size Distribution of Nanoparticles in Consumer Products by Asymmetric Flow Field-Flow Fractionation Coupled to Inductively Coupled Plasma-Mass Spectrometry: The Example of Al2O3, TiO2, and SiO2 Nanoparticles in Toothpaste. Separations 2018, 5, 56. [Google Scholar]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W. Heavy metal removal from water/wastewater by nano-sized metal oxides: A review. J. Hazard. Mater. 2012, 211, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Centi, M.; Perathoner, S. Carbon nanotubes for sustainable energy applications. ChemSusChem 2011, 4, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Li, C.M. Nanostructured catalysts in fuel cells. J. Mater. Chem. 2011, 21, 4027–4036. [Google Scholar] [CrossRef]
- Cardellina, J.H. Challenges and opportunities confronting the botanical dietary supplement industry. J. Nat. Prod. 2000, 65, 1073–1084. [Google Scholar] [CrossRef]
- Williamson, E.M. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Samaligy, M.S.; Afif, N.N.; Mahmoud, E.A. Increasing bioavailability of silymarin using a buccal liposomal delivery system: Preparation and experimental design investigation. Int. J. Pharm. 2006, 308, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, L.; Jia, A.; Chang, X.; Xue, H. Preparation and characterization of solid lipid nanoparticles loaded traditional Chinese medicine. Int. J. Biol. Macromol. 2006, 38, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chang, X.; Du, D.; Liu, W.; Yang, X. Podophyllotoxin-loaded solid lipid nanoparticles for epidermal targeting. J. Control. Release 2006, 110, 296–306. [Google Scholar] [CrossRef]
- Lawrencea, M.J.; Reesb, G.D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Deliv. Rev. 2000, 45, 89–121. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, Y.; Zhu, S.; Feng, F.; Zheng, X. Characterization and antimicrobial activity of a pharmaceutical microemulsion. Int. J. Pharm. 2010, 395, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as Curecumin: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lin, J.H.; Chou, S.C.; Chang, S.; Chung, C.C. Berberine-loaded targeted nanoparticles as specific Helicobacter pylori eradication therapy: In vitro and in vivo study. Nanomedicine 2015, 10, 157–171. [Google Scholar] [CrossRef]
- Bhatt, R.L.; Vries, P.; Tulinsky, J.; Bellamy, G.; Baker, B.; Singer, J.W. Synthesis and in vivo antitumor activity of poly(L-glutamic acid) conjugates of 20(S)-camptothecin. J. Med. Chem. 2003, 46, 190–193. [Google Scholar] [CrossRef]
- Fonseca, C.; Simões, S.; Gaspar, R. Paclitaxel-loaded PLGA nanoparticles: Preparation, physicochemical characterization and in vitro anti-tumoral activity. J. Control. Release 2002, 4, 273–286. [Google Scholar] [CrossRef]
- Harikumar, P.S.; Aravind, A. Antibacterial Activity of Copper Nanoparticles and Copper Nanocomposites against Escherichia Coli Bacteria. Int. J. Sci. 2016, 2, 84–90. [Google Scholar] [CrossRef]
- Tran, Q.H.; Van Quy, N.; Le, A.T. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. 2013, 4, 1–21. [Google Scholar] [CrossRef]
- Vijay Kumar, P.P.N.; Pammi, S.V.N.; Kollu, P.; Satyanarayana, K.V.; Shameem, V.U. Green synthesis and characterization of silver nanoparticles using Boerhaaviadiffusa plant extract and their anti-bacterial activity. Ind. Crops Prod. 2014, 52, 562–566. [Google Scholar] [CrossRef]
- Dhuper, S.; Panda, D.; Nayak, P.L. Green synthesis and characterization of zero valent iron nanoparticles from the leaf extract of Mangiferaindica. Nano Trends J. Nanotechnol. Appl. 2012, 13, 16–22. [Google Scholar]
- Kalishwaralal, K.; Deepak, V.; Pandian, S.R.K.; Kottaisamy, M.; BarathManiKanth, S.; Kartikeyan, B.; Gurunathan, S. Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloids Surf. B Biointerfaces 2010, 77, 257–262. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Park, H.H. Antifungal activity of silver nanoparticles synthesized using turnip leaf extract (Brassica rapa L.) against wood rotting pathogens. Eur. J. Plant Pathol. 2014, 140, 185–192. [Google Scholar] [CrossRef]
- Mondal, S.N.; Roy, R.A.; Laskar, I.; Sk, S.; Basu, D. Mandal Biogenic synthesis of Ag, Au and bimetallic Au/Ag alloy nanoparticles using aqueous extract of mahogany (Swieteniamahogani JACQ) leaves. Colloids Surf. B Biointerfaces 2011, 82, 497–504. [Google Scholar] [CrossRef]
- Sharma, R.; Rana, V. Effect of carboxymethylation on rheological and drug release characteristics of Terminaliacatappa gum. Carbohydr. Polym. 2017, 175, 728–738. [Google Scholar] [CrossRef]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef]
- Brewer, E.; Coleman, J.; Lowman, A. Emerging technologies of polymeric nanoparticles in cancer drug delivery. J. Nanomater. 2011, 2011, 1–10. [Google Scholar] [CrossRef]
- Lim, Z.Z.J.; Li, J.E.J.; Yung, L.Y.L.; Bay, B.H. Gold nanoparticles in cancer therapy. Acta Pharmacol. Sin. 2011, 32, 983–990. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Kundu, S.; Ghosh, S.K.; Mandal, M.; Pal, T. Silver and gold nanocluster catalyzed reduction of methylene blue by arsine in micellar medium. Mater. Sci. 2002, 25, 577–579. [Google Scholar] [CrossRef]
- Justin, T.; Seil, J.T.; Webster, T.J. Antimicrobial applications of nanotechnology: Methods and literature. Int. J. Nanomed. 2012, 7, 2767–2781. [Google Scholar]
- Cao, Y.W.; Jin, R.; Mirkin, C.A. DNA-modified core–shell Ag/Au nanoparticles. J. Am. Chem. Soc. 2001, 123, 7961–7962. [Google Scholar] [CrossRef]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Alanazi, F.K.; Radwan, A.A.; Alsarra, I.A. Biopharmaceutical applications of nanogold. Saudi Pharm. J. 2010, 18, 179–193. [Google Scholar] [CrossRef]
- Tedesco, S.; Doyle, H.; Blasco, J.; Redmond, G.; Sheehan, D. Oxidative stress and toxicity of gold nanoparticles in Mytilusedulis. Aquat. Toxicol. 2010, 100, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Nalawade, P.; Mukherjee, T.; Kapoor, S. High-yield synthesis of multispiked gold nanoparticles: Characterization and catalytic reactions. Colloid Surf. A Physicochem. Eng. Asp. 2012, 396, 336–340. [Google Scholar] [CrossRef]
- Kareem, A.M.; Ismail, S.; Kundan, K. Potential Biotechnological Strategies for the Cleanup of Heavy Metals and Metalloids. Plant Sci. 2016, 7, 303. [Google Scholar]
- Hemen, S. Metal Hyperaccumulation in Plants: A Review Focusing on Phytoremediation Technology. J. Environ. Sci. Technol. 2011, 4, 118–138. [Google Scholar]
- Yu, J.; Dabing, Z.; Deok-Chun, Y. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 7–18. [Google Scholar]
- Niederberger, M.; Garnweitner, G. Reduction of Metal Ions in Polymer Matrices as a Condensation Method of Nanocomposite Synthesis. Eur. J. 2006, 12, 7282. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, S.; Azamal, H.; Rifaqat, A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar]
- Palaniselvam, K.; Mashitah, M.; Yusoff, G.; Natanamurugaraj, G. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—An updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar]
- Barbara, D.; Phil, S.; Günter, S.; Rothen, R. In vitro approaches to assess the hazard of nanomaterials. Nano Impact 2017, 8, 99–116. [Google Scholar]
- Peter, L.; Jutta, T.; Christian, R.; Albert, B. Nanomaterials: Certain aspects of application, risk assessment and risk communication. Arch. Toxicol. 2018, 92, 121–141. [Google Scholar]
- Becker, H.; Herzberg, F.; Schulte, A.; Kolossa-Gehring, M. The carcinogenic potential of nanomaterials, their release from products and options for regulating them. Int. J. Hyg. Environ. Health 2011, 214, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Ivo, I.; Veruscka, A.; Walter, R.; Laura, L.H.; Mark, D. Opportunities and challenges of nanotechnology in the green economy. Environ. Health 2014, 13, 78. [Google Scholar]
| Formulation | Active Ingredients | Biological Activity | Method of Preparation | References |
|---|---|---|---|---|
| Curcuminoids solid lipid nanoparticles | Curcuminoids | Anticancer and antioxidant | Micro-emulsion technique | [8] |
| Glycyrrhizic acid loaded nanoparticles | Glycyrrhizin acid | Antihypertensive and anti-inflammatory | Rotary-evaporated film ultrasonication method | [9] |
| Nanoparticles of cuscuta chinensis | Flavonoids and lignans | Hepatoprotective and antioxidant effects | Nanosuspension method | [10] |
| Artemisinin nanocapsules | Artemisinin | Anticancer | Self-assembly procedure | [11] |
| Berberine-loaded nanoparticles | Berberine | Anticancer | Ionic gelation method | [12] |
| CPTencapsulated nanoparticles | Camptothecin | Anticancer | Dialysis method | [13] |
| Taxel-loaded nanoparticles | Taxel | Anticancer | Emulsion solvent evaporation | [14] |
| Plant | Nanoparticle | Size (nm) | Shape | Reference |
|---|---|---|---|---|
| Aloe vera | Au & Ag | 50 to 350 | Spherical, triangular | [18] |
| Aloe vera | In2O3 | 5 to 50 | Spherical | [19] |
| Citrullus colocynthis | Ag | 31 | Spherical | [20] |
| Curcuma longa | Pd | 10 to 15 | Spherical | [21] |
| Diopyros kaki | Pt | 15 to 19 | Crystalline | [22] |
| Eucalyptus macrocarpa | Au | 20 to 100 | Spherical, triangular, hexagonal | [23] |
| Mangifera indica | Ag | 20 | Spherical, triangular, hexagonal | [24] |
| Rhododendron dauricum | Ag | 25 to 40 | Spherical | [25] |
| Psidium guajava | Au | 25 to 30 | Spherical | [26] |
| Pyrus sp. (Pear fruit extract) | Au | 200 to 500 | Triangular, hexagonal | [27] |
| Terminalia catappa | Au | 10 to 35 | Spherical | [28] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, A.; Gautam, S.P.; Bansal, K.K.; Prabhakar, N.; Rosenholm, J.M. Green Nanotechnology: Advancement in Phytoformulation Research. Medicines 2019, 6, 39. https://doi.org/10.3390/medicines6010039
Verma A, Gautam SP, Bansal KK, Prabhakar N, Rosenholm JM. Green Nanotechnology: Advancement in Phytoformulation Research. Medicines. 2019; 6(1):39. https://doi.org/10.3390/medicines6010039
Chicago/Turabian StyleVerma, Ajay, Surya P. Gautam, Kuldeep K. Bansal, Neeraj Prabhakar, and Jessica M. Rosenholm. 2019. "Green Nanotechnology: Advancement in Phytoformulation Research" Medicines 6, no. 1: 39. https://doi.org/10.3390/medicines6010039
APA StyleVerma, A., Gautam, S. P., Bansal, K. K., Prabhakar, N., & Rosenholm, J. M. (2019). Green Nanotechnology: Advancement in Phytoformulation Research. Medicines, 6(1), 39. https://doi.org/10.3390/medicines6010039








