Assessing the Role of Artificial Intelligence (AI) in Clinical Oncology: Utility of Machine Learning in Radiotherapy Target Volume Delineation
Abstract
1. Overview of Evolution and Advancement in Radiotherapy
2. Radiotherapy Planning, Target Volume Delineation and Quality Assurance
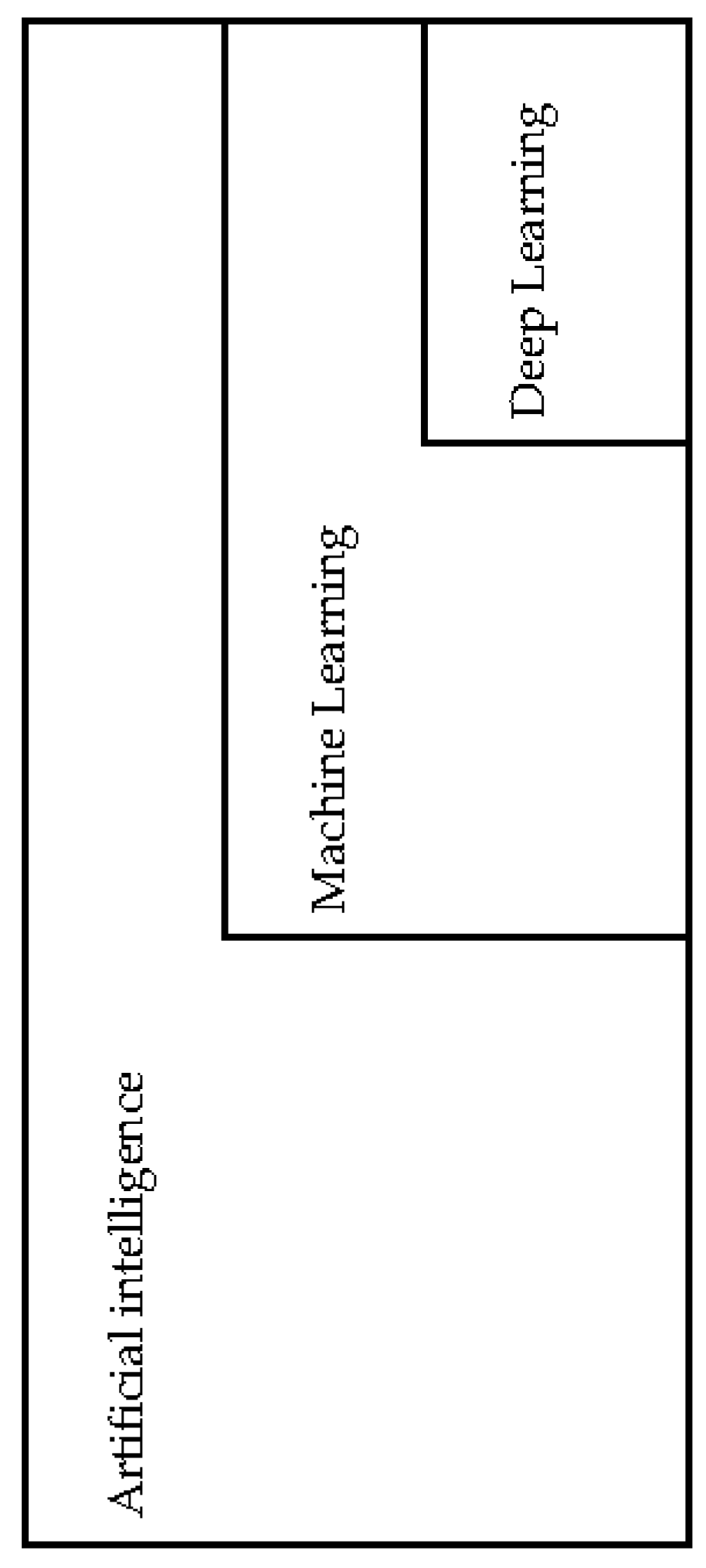
3. Artificial Intelligence, Machine Learning and Deep Learning
4. Application of Machine Learning in Radiotherapy Target Delineation
5. Application of Machine Learning in Radiotherapy Delivery and Image Guided Radiotherapy
6. Opportunity and Challenges to Application of Machine Learning Approach to Oncology and Radiotherapy
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- NHS England. Modernising Radiotherapy Services in England—Developing Proposals for Future Service Models. 28 October 2016. Available online: https://www.engage.england.nhs.uk/survey/264ceb37/supporting_documents/rtdiscussionguide.pdf (accessed on 10 November 2018).
- Cancer Research UK. Achieving a World-Class Radiotherapy Service Across the UK. July 2009. Available online: https://www.cancerresearchuk.org/sites/default/files/policy-achieving-a-world-class-radiotherapy-service-across-the-uk.pdf (accessed on 10 November 2018).
- Williams, M.V.; Drinkwater, K.J. Geographical variation in radiotherapy services across the UK in 2007 and the effect of deprivation. Clin. Oncol. 2009, 21, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.K.; Poortmans, P.; Chalmers, A.J.; Faivre-Finn, C.; Hall, E.; Huddart, R.A.; Lievens, Y.; Sebag-Montefiore, D.; Coles, C.E. Practice-changing radiation therapy trials for the treatment of cancer: Where are we 150 years after the birth of Marie Curie? Br. J. Cancer 2018, 119, 389–407. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Radiologists. Radiotherapy Dose-Fractionation. June 2006. Available online: https://www.rcr.ac.uk/publication/radiotherapy-dose-fractionation-second-edition (accessed on 10 November 2018).
- Moore, G.E. Cramming more components onto integrated circuits, Reprinted from Electronics, volume 38, number 8, April 19, 1965, pp. 114 ff. IEEE Solid-Stat. Circ. Soc. Newsl. 2006, 11, 33–35. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Teoh, M.; Clark, C.H.; Wood, K.; Whitaker, S.; Nisbet, A. Volumetric modulated arc therapy: A review of current literature and clinical use in practice. Br. J. Radiol. 2011, 84, 967–996. [Google Scholar] [CrossRef]
- Murray, L.; Ramasamy, S.; Lilley, J.; Snee, M.; Clarke, K.; Musunuru, H.B.; Needham, A.; Turner, R.; Sangha, V.; Flatley, M.; et al. Stereotactic Ablative Radiotherapy (SABR) in Patients with Medically Inoperable Peripheral Early Stage Lung Cancer: Outcomes for the First UK SABR Cohort. Clin. Oncol. 2016, 28, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.; Franks, K.; Hanna, G.G. A systematic review of outcomes following stereotactic ablative radiotherapy in the treatment of early-stage primary lung cancer. Br. J. Radiol. 2017, 90, 20160732. [Google Scholar] [CrossRef]
- O’Beirn, M.; Benghiat, H.; Meade, S.; Heyes, G.; Sawlani, V.; Kong, A.; Hartley, A.; Sanghera, P. The Expanding Role of Radiosurgery for Brain Metastases. Medicines 2018, 5, 90. [Google Scholar] [CrossRef]
- Crellin, A. The Road Map for National Health Service Proton Beam Therapy. Clin. Oncol. 2018, 30, 277–279. [Google Scholar] [CrossRef]
- Tree, A.C.; Huddart, R.; Choudhury, A. Magnetic Resonance-guided Radiotherapy—Can We Justify More Expensive Technology? Clin. Oncol. 2018, 30, 677–679. [Google Scholar] [CrossRef]
- Harrington, K.; Hall, E.; Hawkins, M.; Henry, A.; MacKay, R.; Maughan, T.; McDonald, A.; Nutting, C.; Oelfke, U.; Sebag-Montefiore, D.; et al. Introducing the Cancer Research UK Advanced Radiotherapy Technologies Network (ART-NET). Clin. Oncol. 2017, 29, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Burnet, N.G.; Thomas, S.J.; Burton, K.E.; Jefferies, S.J. Defining the tumour and target volumes for radiotherapy. Cancer Imaging 2004, 4, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Marks, L.B.; Yorke, E.D.; Jackson, A.; Ten Haken, R.K.; Constine, L.S.; Eisbruch, A.; Bentzen, S.M.; Nam, J.; Deasy, J.O. Use of normal tissue complication probability models in the clinic. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76 (Suppl. 3), S10–S19. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Social Care. Ionising Radiation (Medical Exposure) Regulations 2017. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/720282/guidance-to-the-ionising-radiation-medical-exposure-regulations-2017.pdf (accessed on 10 November 2018).
- Chen, A.M.; Chin, R.; Beron, P.; Yoshizaki, T.; Mikaeilian, A.G.; Cao, M. Inadequate target volume delineation and local-regional recurrence after intensity-modulated radiotherapy for human papillomavirus-positive oropharynx cancer. Radiother. Oncol. 2017, 123, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Fong, C.T.; Boon, C.S.; Tiffany, M.; Roques, T.; Brammer, C.; Foran, B.; Prestwich, R.; Chan, A.K.; Howard, H.; Sanghera, P.; et al. UK contouring variation in nasopharyngeal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96 (Suppl. 2), E393. [Google Scholar] [CrossRef]
- Pettit, L.; Hartley, A.; Bowden, S.J.; Mehanna, H.; Glaholm, J.; Cashmore, J.; Sanghera, P. Variation in volume definition between UK head and neck oncologists treating oropharyngeal carcinoma. Clin. Oncol. 2011, 23, 654–655. [Google Scholar] [CrossRef] [PubMed]
- The Royal College of Radiologists. Radiotherapy Target Volume Definition and Peer Review—RCR Guidance. August 2017. Available online: https://www.rcr.ac.uk/system/files/publication/field_publication_files/bfco172_peer_review_outlining.pdf (accessed on 10 November 2018).
- Hague, C.; Beasley, W.; Dixon, L.; Gaito, S.; Garcez, K.; Green, A.; Lee, L.W.; Maranzano, M.; McPartlin, A.; Mistry, H.; et al. Use of a novel atlas for muscles of mastication to reduce inter observer variability in head and neck radiotherapy contouring. Radiother. Oncol. 2018. [Google Scholar] [CrossRef] [PubMed]
- The Royal College of Radiologists. Towards Safer Radiotherapy. 2008. Available online: https://www.rcr.ac.uk/system/files/publication/field_publication_files/Towards_saferRT_final.pdf (accessed on 10 November 2018).
- Gwynne, S.; Gilson, D.; Dickson, J.; McAleer, S.; Radhakrishna, G. Evaluating Target Volume Delineation in the Era of Precision Radiotherapy: FRCR, Revalidation and Beyond. Clin. Oncol. 2017, 29, 436–438. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.; Noblet, V.; Mazzara, C.; Lallement, A. Survey on deep learning for radiotherapy. Comput. Biol. Med. 2018, 98, 126–146. [Google Scholar] [CrossRef] [PubMed]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Bibault, J.E.; Giraud, P.; Burgun, A. Big Data and machine learning in radiation oncology: State of the art and future prospects. Cancer Lett. 2016, 382, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, Y.; Honda, K.; Osaka, Y.; Hara, T.; Umaki, T.; Tsuchida, A.; Aoki, T.; Hirohashi, S.; Yamada, T. Possible prediction of chemoradiosensitivity of esophageal cancer by serum protein profiling. Clin. Cancer Res. 2005, 11, 8042–8047. [Google Scholar] [CrossRef] [PubMed]
- Klement, R.J.; Allgäuer, M.; Appold, S.; Dieckmann, K.; Ernst, I.; Ganswindt, U.; Holy, R.; Nestle, U.; Nevinny-Stickel, M.; Semrau, S.; et al. Support vector machine-based prediction of local tumor control after stereotactic body radiation therapy for early-stage non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Gulliford, S.L.; Webb, S.; Rowbottom, C.G.; Corne, D.W.; Dearnaley, D.P. Use of artificial neural networks to predict biological outcomes for patients receiving radical radiotherapy of the prostate. Radiother. Oncol. 2004, 71, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.D.; Wernicke, A.G.; Nori, D.; Chao, K.C.; Chang, J.; Parashar, B. Predicting Radiation Therapy Outcome for Head and Neck Cancer Patients Using Artificial Neural Network (ANN). Int. J. Radiat. Oncl. Biol. Phys. 2014, 90, S852. [Google Scholar] [CrossRef]
- Nikolov, S.; Blackwell, S.; Mendes, R.; De Fauw, J.; Meyer, C.; Hughes, C.; Askham, H.; Romera-Paredes, B.; Karthikesalingam, A.; Chu, C.; et al. Deep learning to achieve clinically applicable segmentation of head and neck anatomy for radiotherapy. arXiv, 2018; arXiv:1809.04430. [Google Scholar]
- Sharp, G.; Fritscher, K.D.; Pekar, V.; Peroni, M.; Shusharina, N.; Veeraraghavan, H.; Yang, J. Vision 20/20: Perspectives on automated image segmentation for radiotherapy. Med. Phys. 2014, 41, 050902. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J. Reflections on the current status of commercial automated segmentation systems in clinical practice. J. Med. Radiat. Sci. 2014, 61, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Leech, M. Use of auto-segmentation in the delineation of target volumes and organs at risk in head and neck. Acta Oncol. 2016, 55, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.H.; Warfield, S.K.; Bharatha, A.; Tempany, C.M.; Kaus, M.R.; Haker, S.J.; Wells, W.M., III; Jolesz, F.A.; Kikinis, R. Statistical validation of image segmentation quality based on a spatial overlap index. Acad. Radiol. 2004, 11, 178–189. [Google Scholar] [CrossRef]
- Li, Q.; Xu, Y.; Chen, Z.; Liu, D.; Feng, S.-T.; Law, M.; Ye, Y.; Huang, B. Tumor Segmentation in Contrast-Enhanced Magnetic Resonance Imaging for Nasopharyngeal Carcinoma: Deep Learning with Convolutional Neural Network. Biomed. Res. Int. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Cardenas, C.E.; McCarroll, R.E.; Court, L.E.; Elgohari, B.A.; Elhalawani, H.; Fuller, C.D.; Kamal, M.J.; Meheissen, M.A.M.; Mohamed, A.S.R.; Rao, A.; et al. Deep Learning Algorithm for Auto-Delineation of High-Risk Oropharyngeal Clinical Target Volumes with Built-In Dice Similarity Coefficient Parameter Optimization Function. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 468–478. [Google Scholar] [CrossRef] [PubMed]
- McCarroll, R.; Yang, J.; Cardenas, C.; Balter, P.; Burger, H.; Dalvie, S.; Kisling, K.; Mejia, M.; Naidoo, K.; Nelson, C.; et al. Machine learning for the prediction of physician edits to clinical auto-countous in head and neck: Tu-fg-605-09. Med. Phys. 2017, 44, 3160. [Google Scholar]
- Speight, R.; Karakaya, E.; Prestwich, R.; Sen, M.; Lindsay, R.; Harding, R.; Sykes, J. Evaluation of atlas based auto-segmentation for head and neck target volume delineation in adaptive/replan IMRT. J. Phys. Conf. Ser. 2014, 489, 012060. [Google Scholar] [CrossRef]
- Martin, S.; Rodrigues, G.; Patil, N.; Bauman, G.; D’Souza, D.; Sexton, T.; Palma, D.; Louie, A.V.; Khalvati, F.; Tizhoosh, H.R.; et al. A multiphase validation of atlas-based automatic and semiautomatic segmentation strategies for prostate MRI. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Lustberg, T.; van Soest, J.; Gooding, M.; Peressutti, D.; Aljabar, P.; van der Stoep, J.; van Elmpt, W.; Dekker, A. Clinical evaluation of atlas and deep learning based automatic contouring for lung cancer. Radiother. Oncol. 2018, 126, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.R.; Dowling, J.A.; Pogson, E.M.; Metcalfe, P.; Holloway, L. Atlas-based segmentation technique incorporating inter-observer delineation uncertainty for whole breast. J. Phys. Conf. Ser. 2016, 777, 012002. [Google Scholar] [CrossRef]
- Silver, D.; Hubert, T.; Schrittwieser, J.; Antonoglou, I.; Lai, M.; Guez, A.; Lanctot, M.; Sifre, L.; Kumaran, D.; Graepel, T.; et al. Mastering Chess and Shogi by Self-Play with a General Reinforcement Learning Algorithm. arXiv, 2017; arXiv:1712.01815. [Google Scholar]
- Health Education England, National Health Service. The Topol Review. Preparing the Healthcare Workforce to Deliver the Digital Future: Interim Report June 2018—A Call for Evidence. June 2018. Available online: https://www.hee.nhs.uk/sites/default/files/documents/Topol%20Review%20interim%20report_0.pdf (accessed on 10 November 2018).
- The Royal College of Radiologists. RCR Position Statement on Artificial Intelligence. July 2018. Available online: https://www.rcr.ac.uk/posts/rcr-position-statement-artificial-intelligence (accessed on 10 November 2018).
- Kortesniemi, M.; Tsapaki, V.; Trianni, A.; Russo, P.; Maas, A.; Källman, H.E.; Brambilla, M.; Damilakis, J. The European Federation of Organisations for Medical Physics (EFOMP) White Paper: Big data and deep learning in medical imaging and in relation tomedical physics profession. Phys Med. 2018, 16, S1120–S1797. [Google Scholar] [CrossRef] [PubMed]
- Kourou, K.; Exarchos, T.P.; Exarchos, K.P.; Karamouzis, M.V.; Fotiadis, D.I. Machine learning applications in cancer prognosis and prediction. Comput. Struct. Biotechnol. J. 2014, 13, 8–17. [Google Scholar] [CrossRef]
- Kim, W.; Kim, K.S.; Lee, J.E.; Noh, D.Y.; Kim, S.W.; Jung, Y.S.; Park, M.Y.; Park, R.W. Development of novel breast cancer recurrence prediction model using support vector machine. J. Breast Cancer 2012, 15, 230–238. [Google Scholar] [CrossRef]
- Exarchos, K.P.; Goletsis, Y.; Fotiadis, D.I. Multiparametric decision support system for the prediction of oral cancer reoccurrence. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 1127–1134. [Google Scholar] [CrossRef]
- Tseng, C.-J.; Lu, C.-J.; Chang, C.-C.; Chen, G.-D. Application of machine learning to predict the recurrence-proneness for cervical cancer. Neural Comput. Appl. 2014, 24, 1311–1316. [Google Scholar] [CrossRef]
- Dos Reis, F.J.; Wishart, G.C.; Dicks, E.M.; Greenberg, D.; Rashbass, J.; Schmidt, M.K.; van den Broek, A.J.; Ellis, I.O.; Green, A.; Rakha, E.; et al. An updated PREDICT breast cancer prognostication and treatment benefit prediction model with independent validation. Breast Cancer Res. 2017, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Delen, D.; Walker, G.; Kadam, A. Predicting breast cancer survivability: A comparison of three data mining methods. Artif. Intell. Med. 2005, 34, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Ke, W.C.; Chiu, H.W. Risk classification of cancer survival using ANN with gene expression data from multiple laboratories. Comput. Biol. Med. 2014, 48, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Guidi, G.; Maffei, N.; Meduri, B.; D’Angelo, E.; Mistretta, G.M.; Ceroni, P.; Ciarmatori, A.; Bernabei, A.; Maggi, S.; Cardinali, M.; et al. A machine learning tool for re-planning and adaptive RT: A multicenter cohort investigation. Phys. Med. 2016, 32, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Valdes, G.; Scheuermann, R.; Hung, C.Y.; Olszanski, A.; Bellerive, M.; Solberg, T.D. A mathematical framework for virtual IMRT QA using machine learning. Med. Phys. 2016, 43, 4323–4334. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, C.; Wu, Q.J.; Yuan, L.; Liu, J.; Hood, R.; Yin, F.F.; Adamson, J. Utilizing knowledge from prior plans in the evaluation of quality assurance. Phys. Med. Biol. 2015, 60, 4873–4891. [Google Scholar] [CrossRef]
- Nguyen, A.; Yosinski, J.; Clune, J. Deep Neural Networks are Easily Fooled: High Confidence Predictions for Unrecognizable Images. In Proceedings of the IEEE Computer Vision and Pattern Recognition (CVPR ’15), Boston, MA, USA, 7–12 June 2015. [Google Scholar]
- Valentini, V.; Boldrini, L.; Damiani, A.; Muren, L.P. Recommendations on how to establish evidence from auto-segmentation software in radiotherapy. Radiother. Oncol. 2014, 112, 317–320. [Google Scholar] [CrossRef]
| Publication | Cancer Site | Machine Learning Method | Target Volume Delineation | Radiotherapy Planning Modality | Number of Patients | Validation | Outcome and Important Features |
|---|---|---|---|---|---|---|---|
| Nikolov S [32] | Head and neck | Deep Learning | OAR | CT | 663 | Compared against manual contours by senior radiographers adjudicated by senior consultant clinical oncologist | 19 out of 21 OAR surface DSC scores less than 5% deviation when compared to clinician manual contours. Did not achieved target for brainstem and right lens |
| Li Q [37] | Head and neck | Deep Learning | Tumour | MRI | 29 | Compared against manual contours by consultant clinical oncologists | Mean DSC 0.89. Good agreement when compared to manual contours |
| Cardenas CE [38] | Head and neck | Deep Learning | High risk CTV | CT | 52 | Compared against manual contours by clinicians | Median DSC 0.81. Good agreement when compared to manual contours by clinicians with only minor or no change |
| McCarroll R [39] | Head and neck | Machine Learning | OAR | CT | 128 | Compared against manual contours by consultant clinical oncologist | Mean DSC 0.78. Once validated was used in clinical setting and prospectively tested with accuracy of 63%. 50% of auto-contours were used without changes |
| Speight R [40] | Head and neck | Machine Learning | CTV | CT | 15 | Auto-contours edited by clinicians compared against manual contours by clinician | Edited CTV DSC 0.87. Mean clinician time saved by 112 min per plan when compared to manual contours |
| Martin S [41] | Prostate | Machine Learning | Tumour | MRI | 15 | Compared against manual contours by 5 clinicians of varying experience | 3 phases of trial. Mean DSC 0.89. Good agreement with clinician contours requiring minimal changes. Time saved in all cases |
| Lustberg T [42] | Lung | Deep Learning | OAR | CT | 20 | Compared against manual contours by a single radiotherapy technician | Median DSC 0.57 and median time saved by 79%. Saved time in lung and spinal cord contouring but not for left lung and oesophagus |
| Bell LR [43] | Breast | Machine Learning | Tumour | CT | 28 | Compared against manual contours by 8 clinicians | DSC more than 0.70. Good agreement with clinician manual contours. Coverage agreement poorest towards heart border structures |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boon, I.S.; Au Yong, T.P.T.; Boon, C.S. Assessing the Role of Artificial Intelligence (AI) in Clinical Oncology: Utility of Machine Learning in Radiotherapy Target Volume Delineation. Medicines 2018, 5, 131. https://doi.org/10.3390/medicines5040131
Boon IS, Au Yong TPT, Boon CS. Assessing the Role of Artificial Intelligence (AI) in Clinical Oncology: Utility of Machine Learning in Radiotherapy Target Volume Delineation. Medicines. 2018; 5(4):131. https://doi.org/10.3390/medicines5040131
Chicago/Turabian StyleBoon, Ian S., Tracy P. T. Au Yong, and Cheng S. Boon. 2018. "Assessing the Role of Artificial Intelligence (AI) in Clinical Oncology: Utility of Machine Learning in Radiotherapy Target Volume Delineation" Medicines 5, no. 4: 131. https://doi.org/10.3390/medicines5040131
APA StyleBoon, I. S., Au Yong, T. P. T., & Boon, C. S. (2018). Assessing the Role of Artificial Intelligence (AI) in Clinical Oncology: Utility of Machine Learning in Radiotherapy Target Volume Delineation. Medicines, 5(4), 131. https://doi.org/10.3390/medicines5040131




