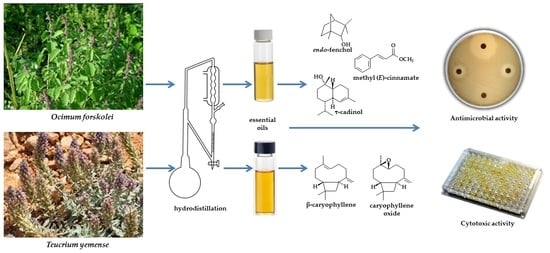
Antimicrobial, Antioxidant, and Cytotoxic Activities of Ocimum forskolei and Teucrium yemense (Lamiaceae) Essential Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Gas Chromatography-Mass Spectrometry (GC-MS)
2.3. Radical Scavenging Assay
2.4. Antimicrobial Assays
2.5. Cytotoxicity Assays
3. Results and Discussion
3.1. Essential Oil Compositions
3.1.1. Ocimum forskolei
3.1.2. Teucrium yemense
3.2. Biological Activities
3.2.1. Free Radical Scavenging
3.2.2. Antimicrobial Activity
3.2.3. Cytotoxic Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wood, J.R.I. A Handbook of the Yemen Flora; Royal Botanic Gardens: Kew, UK, 1997. [Google Scholar]
- Mabberley, D.J. Mabberley’s Plant-Book, 3rd ed.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Ryding, O. Notes on the Sweet Basil and Its Wild Relatives (Lamiaceae). Econ. Bot. 1994, 48, 65–67. [Google Scholar] [CrossRef]
- Hall, M.; Al-Khulaidi, A.W.; Miller, A.G.; Scholte, P.; Al-Qadasi, A.H. Arabia’s last forests under threat: Plant biodiversity and conservation in the valley forest of Jabal Bura (Yemen). Edinb. J. Bot. 2008, 65, 113–135. [Google Scholar] [CrossRef]
- Hammer, K.; Gebauer, J.; Al Khanjari, S.; Buerkert, A. Oman at the cross-roads of inter-regional exchange of cultivated plants. Genet. Resour. Crop Evol. 2009, 56, 547–560. [Google Scholar] [CrossRef]
- Sakkir, S.; Kabshawi, M.; Mehairbi, M. Medicinal plants diversity and their conservation status in the United Arab Emirates (UAE). J. Med. Plants Res. 2012, 6, 1304–1322. [Google Scholar]
- Al-Fatimi, M.; Wurster, M.; Schröder, G.; Lindequist, U. Antioxidant, antimicrobial and cytotoxic activities of selected medicinal plants from Yemen. J. Ethnopharmacol. 2007, 111, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Dekker, T.; Ignell, R.; Ghebru, M.; Glinwood, R.; Hopkins, R. Identification of mosquito repellent odours from Ocimum forskolei. Parasites Vectors 2011, 4. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, N.Q.M.; Wang, H.X.; Ma, C.; Lou, Z.; Bashari, M.; Thabit, R. Antimicrobial and antioxidant activities of the essential oils of some aromatic medicinal plants (Pulicaria inuloides-Asteraceae and Ocimum forskolei-Lamiaceae). Trop. J. Pharmaceut. Res. 2014, 13, 1287–1293. [Google Scholar] [CrossRef]
- Onifade, A.K. Effect of essential oils from five Ocimum sp. on the pathogenicity of Pretylenchus brachyurus (Godfrey) in tomato. Agric. J. 2007, 2, 185–191. [Google Scholar]
- Fatope, M.O.; Marwah, R.G.; Al Hadhrami, N.M.; Onifade, A.K.; Williams, J.R. Identification of the chemotypes of Ocimum forskolei and Ocimum basilicum by NMR spectroscopy. Chem. Biodivers. 2008, 5, 2457–2463. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.A.A.; Wurster, M.; Arnold, N.; Lindequist, U.; Wessjohann, L. Chemical composition of the essential oil of Teucrium yemense Deflers. Rec. Nat. Prod. 2008, 2, 25–32. [Google Scholar]
- Coucil of Europe. European Pharmacopoeia, 3rd ed.; Council of Europe Press: Strasbourg, France, 1997. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- Mohamad, H.; Abas, F.; Permana, D.; Lajis, N.H.; Ali, A.M.; Sukari, M.A.; Hin, T.Y.Y.; Kikuzaki, H.; Nakatani, N. DPPH free radical ccavenger components from the fruits of Alpinia rafflesiana Wall. Ex. Bak. (Zingiberaceae). Z. Naturforsch. C 2004, 59, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.A.A.; Sharopov, F.S.; Alhaj, M.; Hill, G.M.; Porzel, A.; Arnold, N.; Setzer, W.N.; Schmidt, J.; Wessjohann, L. Chemical composition and biological activity of essential oil from Pulicaria undulata from Yemen. Nat. Prod. Commun. 2012, 7, 257–260. [Google Scholar] [PubMed]
- Setzer, M.C.; Setzer, W.N.; Jackes, B.R.; Gentry, G.A.; Moriarity, D.M. The medicinal value of tropical rainforest plants from Paluma, North Queensland, Australia. Pharmaceut. Biol. 2001, 39, 67–78. [Google Scholar] [CrossRef]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar] [PubMed]
- Johansson, S.; Lindholm, P.; Gullbo, J.; Larsson, R.; Bohlin, L.; Claeson, P. Cytotoxicity of digitoxin and related cardiac glycosides in human tumor cells. Anti-Cancer Drugs 2001, 12, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar]
- Setzer, W.N.; Setzer, M.C.; Hopper, A.L.; Moriarity, D.M.; Lehrman, G.K.; Niekamp, K.L.; Morcomb, S.M.; Bates, R.B.; McClure, K.J.; Stessman, C.C.; et al. The cytotoxic activity of a Salacia liana species from Monteverde, Costa Rica, is due to a high concentration of tingenone. Planta Med. 1998, 64, 583. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Fornasiero, C.M.; Isetta, A.M. MTT Colorimetric assay for testing macrophage cytotoxic activity in vitro. J. Immunol. Meth. 1990, 131, 165–172. [Google Scholar] [CrossRef]
- Wood, J.R.I. The genus Ocimum (Labiatae) in Forsskal’s Flora Aegyptiaco-Arabica. Kew Bull. 1983, 37, 597–603. [Google Scholar] [CrossRef]
- Bezić, N.; Vuko, E.; Dunkić, V.; Ruščić, M.; Blažević, I.; Burčul, F. Antiphytoviral activity of sesquiterpene-rich essential oils from four Croatian Teucrium species. Molecules 2011, 16, 8119–8129. [Google Scholar] [CrossRef] [PubMed]
- Kremer, D.; Košir, I.J.; Kosalec, I.; Končić, M.Z.; Potočnik, T.; Čerenak, A.; Bezić, N.; Srečec, S.; Dunkić, V. Investigation of chemical compounds, antioxidant and antimicrobial properties of Teucrium arduini L. (Lamiaceae). Curr. Drug Targets 2013, 14, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, M.A.; Pérez, I.; Boira, H. Essential oil analysis of Teucrium libanitis and T. turredanum by GC and GC-MS. Flavour Fragr. J. 2003, 18, 497–501. [Google Scholar] [CrossRef]
- Djabou, N.; Muselli, A.; Allali, H.; Dib, M.E.A.; Tabti, B.; Varesi, L.; Costa, J. Chemical and genetic diversity of two Mediterranean subspecies of Teucrium polium L. Phytochemistry 2012, 83, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Hachicha, S.F.; Skanji, T.; Barrek, S.; Zarrouk, H.; Ghrabi, Z.G. Chemical composition of Teucrium alopecurus essential oil from Tunisia. J. Essent. Oil Res. 2007, 19, 413–415. [Google Scholar] [CrossRef]
- Baher, Z.F.; Mirza, M. Volatile constituents of Teucrium flavum L. from Iran. J. Essent. Oil Res. 2003, 15, 106–107. [Google Scholar] [CrossRef]
- Ricci, D.; Fraternale, D.; Giamperi, L.; Bucchini, A.; Epifano, F.; Burini, G.; Curini, M. Chemical composition, antimicrobial and antioxidant activity of the essential oil of Teucrium marum (Lamiaceae). J. Ethnopharmacol. 2005, 98, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Aburjai, T.; Hudaib, M.; Cavrini, V. Composition of the essential oil from Jordanian germander (Teucrium polium L.). J. Essent. Oil Bear. Plants 2006, 18, 97–99. [Google Scholar] [CrossRef]
- Vukovic, N.; Milosevic, T.; Sukdolak, S.; Solujic, S. Antimicrobial activities of essential oil and methanol extract of Teucrium montanum. Evid. Based Complement. Altern. Med. 2007, 4 (Suppl. 1), 17–20. [Google Scholar] [CrossRef] [PubMed]
- Antunes, T.; Sevinate-Pinto, I.; Barroso, J.G.; Cavaleiro, C.; Salgueiro, L.R. Micromorphology of trichomes and composition of essential oil of Teucrium capitatum. Flavour Fragr. J. 2004, 19, 336–340. [Google Scholar] [CrossRef]
- De Martino, L.; Formisano, C.; Mancini, E.; de Feo, V.; Piozzi, F.; Rigano, D.; Senatore, F. Chemical composition and phytotoxic effects of essential oils from four Teucrium species. Nat. Prod. Commun. 2010, 5, 1969–1976. [Google Scholar] [PubMed]
- Hachicha, S.F.; Skanji, T.; Barrek, S.; Ghrabi, Z.G.; Zarrouk, H. Composition of the essential oil of Teucrium ramosissimum Desf. (Lamiaceae) from Tunisia. Flavour Fragr. J. 2007, 22, 101–104. [Google Scholar] [CrossRef]
- Shah, S.M.M.; Ullah, F.; Shah, S.M.H.; Zahoor, M.; Sadiq, A. Analysis of chemical constituents and antinociceptive potential of essential oil of Teucrium stocksianum Bioss collected from the north west of Pakistan. BMC Complement. Altern. Med. 2012, 12, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Amiri, H. Antioxidant activity of the essential oil and methanolic extract of Teucrium orientale (L.) subsp. taylori (Boiss.) Rech. F. Iran. J. Pharm. Res. 2010, 9, 417–423. [Google Scholar]
- Menichini, F.; Conforti, F.; Rigano, D.; Formisano, C.; Piozzi, F.; Senatore, F. Phytochemical composition, anti-inflammatory and antitumour activities of four Teucrium essential oils from Greece. Food Chem. 2009, 115, 679–686. [Google Scholar] [CrossRef]
- Dunkić, V.; Bezić, N.; Vuko, E. Antiphytoviral activity of essential oil from endemic species Teucrium arduini. Nat. Prod. Commun. 2011, 6, 1385–1388. [Google Scholar] [PubMed]
- Kabouche, A.; Kabouche, Z.; Ghannadi, A.; Sajjadi, S.E. Analysis of the essential oil of Teucrium polium ssp. aurasiacum from Algeria. J. Essent. Oil Res. 2007, 19, 44–46. [Google Scholar] [CrossRef]
- El-Shazly, A.M.; Hussein, K.T. Chemical analysis and biological activities of the essential oil of Teucrium leucocladum Boiss. (Lamiaceae). Biochem. Syst. Ecol. 2004, 32, 665–674. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Demirçakmak, B. Composition of the essential oils of three Teucrium species from Turkey. J. Essent. Oil Res. 1997, 9, 545–549. [Google Scholar] [CrossRef]
- Vukovic, N.; Sukdolak, S.; Solujic, S.; Mihailovic, V.; Mladenovic, M.; Stojanovic, J.; Stankovic, M.S. Chemical composition and antimicrobial activity of Teucrium arduini essential oil and cirsimarin from Montenegro. J. Med. Plants Res. 2011, 5, 1244–1250. [Google Scholar]
- Morteza-Semnani, K.; Akbarzadeh, M.; Rostami, B. The essential oil composition of Teucrium chamaedrys L. from Iran. Flavour Fragr. J. 2005, 20, 544–546. [Google Scholar] [CrossRef]
- Bagci, E.; Yazgın, A.; Hayta, S.; Cakılcıoglu, U. Composition of the essential oil of Teucrium chamaedrys L. (Lamiaceae) from Turkey. J. Med. Plants Res. 2010, 4, 2587–2589. [Google Scholar]
- Javidnia, K.; Miri, R.; Khosravi, A.R. Composition of the essential oil of Teucrium persicum from Iran. J. Essent. Oil Res. 2007, 19, 430–432. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Wink, M.; Setzer, W.N. Radical scavenging and antioxidant activities of essential oil components—An experimental and computational investigation. Nat. Prod. Commun. 2015, 10, 153–156. [Google Scholar] [PubMed]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Gilles, M.; Zhao, J.; An, M.; Agboola, S. Chemical composition and antimicrobial properties of essential oils of three Australian Eucalyptus species. Food Chem. 2010, 119, 731–737. [Google Scholar] [CrossRef]
- Su, Y.-C.; Ho, C.-L. Composition of the leaf essential oil of Phoebe formosana from Taiwan and its in vitro cytotoxic, antibacterial, and antifungal activities. Nat. Prod. Commun. 2016, 11, 845–848. [Google Scholar] [PubMed]
- Kotan, R.; Kordali, S.; Cakir, A. Screening of antibacterial activities of twenty-one oxygenated monoterpenes. Z. Naturforsch. C 2007, 62, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Marei, G.I.K.; Abdel Rasoul, M.A.; Abdelgaleil, S.A.M. Comparative Antifungal Activities and Biochemical Effects of Monoterpenes on Plant Pathogenic Fungi. Pestic. Biochem. Physiol. 2012, 103, 56–61. [Google Scholar] [CrossRef]
- Schmidt, J.M.; Noletto, J.A.; Vogler, B.; Setzer, W.N. Abaco bush medicine: Chemical composition of the essential oils of four aromatic medicinal plants from Abaco Island, Bahamas. J. Herbs Spices Med. Plants 2006, 12, 43–65. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Muroi, H.; Kubo, A. Naturally occurring antiacne agents. J. Nat. Prod. 1994, 57, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Morimitsu, Y. Cytotoxicity of green tea flavor compounds against two solid tumor cells. J. Agric. Food Chem. 1995, 43, 1626–1628. [Google Scholar] [CrossRef]
- Wright, B.S.; Bansal, A.; Moriarity, D.M.; Takaku, S.; Setzer, W.N. Cytotoxic leaf essential oils from Neotropical Lauraceae: Synergistic effects of essential oil components. Nat. Prod. Commun. 2007, 2, 1241–1244. [Google Scholar]
- Da Silva, S.L.; Figueiredo, P.M.; Yano, T. Cytotoxic evaluation of essential oil from Zanthoxylum rhoifolium Lam. leaves. Acta Amazon. 2007, 37, 281–286. [Google Scholar] [CrossRef]
- Su, Y.-C.; Hsu, K.-P.; Ho, C.-L. Composition, in vitro cytotoxicity, and anti-mildew activities of the leaf essential oil of Machilus thunbergii from Taiwan. Nat. Prod. Commun. 2015, 10, 2013–2016. [Google Scholar] [PubMed]
- Sylvestre, M.; Pichette, A.; Lavoie, S.; Longtin, A.; Legault, J. Composition and cytotoxic activity of the leaf essential oil of Comptonia peregrina (L.) Coulter. Phytother. Res. 2007, 21, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Legault, J.; Dahl, W.; Debiton, E.; Pichette, A.; Madelmont, J.-C. Antitumor activity of balsam fir oil: Production of reactive oxygen species induced by α-humulene as possible mechanism of action. Planta Med. 2003, 69, 402–407. [Google Scholar] [PubMed]
- Jun, N.J.; Mosaddik, A.; Moon, J.Y.; Jang, K.C.; Lee, D.S.; Ahn, K.S.; Cho, S.K. Cytotoxic activity of β-caryophyllene oxide isolated from Jeju guava (Psidium cattleianum Sabine) leaf. Rec. Nat. Prod. 2011, 5, 242–246. [Google Scholar]
- He, K.; Zeng, L.; Shi, G.; Zhao, G.X.; Kozlowski, J.F.; McLaughlin, J.L. Bioactive compounds from Taiwania cryptomerioides. J. Nat. Prod. 1997, 60, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Legault, J.; Pichette, A. Potentiating effect of β-caryophyllene on anticancer activity of α-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2007, 59, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
| RIcalc a | RIlit b | Compound | % Composition | ||
|---|---|---|---|---|---|
| EOOF | EOTY-d | EOTY-t | |||
| 936 | 930 | α-Thujene | --- | tr c | tr |
| 942 | 939 | α-Pinene | 0.5 | 2.3 | 6.6 |
| 954 | 954 | Camphene | 0.3 | --- | 0.1 |
| 959 | 960 | Thuja-2,4(10)-diene | --- | --- | tr |
| 976 | 975 | Sabinene | tr | 0.5 | tr |
| 979 | 979 | β-Pinene | tr | 1.1 | 3.1 |
| 982 | 979 | 1-Octen-3-ol | tr | --- | --- |
| 992 | 990 | Myrcene | 0.4 | --- | 0.2 |
| 1004 | 1002 | α-Phellandrene | tr | --- | --- |
| 1009 | 1005 | (3Z)-Hexenyl acetate | tr | --- | --- |
| 1025 | 1024 | p-Cymene | 0.1 | tr | tr |
| 1028 | 1029 | Limonene | 2.5 | 0.5 | 1.2 |
| 1031 | 1031 | 1,8-Cineole | 0.3 | --- | --- |
| 1067 | 1070 | cis-Sabinene hydrate | 0.3 | --- | --- |
| 1073 | 1068 | 1-Octanol | tr | --- | --- |
| 1088 | 1086 | Fenchone | 12.2 | 0.3 | --- |
| 1094 | 1092 | 6,7-Epoxymyrcene | 0.1 | --- | --- |
| 1100 | 1096 | Linalool | 5.7 | 0.2 | 0.1 |
| 1113 | 1116 | endo-Fenchol | 31.1 | 0.1 | --- |
| 1122 | 1122 | trans-Pinene hydrate | 0.1 | --- | --- |
| 1125 | 1127 | Chrysanthenone | --- | tr | --- |
| 1126 | 1126 | α-Campholenal | --- | tr | 0.1 |
| 1137 | 1140 | Nopinone | --- | tr | --- |
| 1138 | 1139 | trans-Pinocarveol | --- | 0.4 | 0.2 |
| 1141 | 1141 | cis-Verbenol | --- | tr | 0.1 |
| 1144 | 1144 | trans-Verbenol | --- | 1.0 | 0.3 |
| 1145 | 1146 | Camphor | 6.2 | --- | --- |
| 1157 | 1159 | Sabina ketone | --- | tr | --- |
| 1162 | 1164 | Pinocarvone | --- | --- | tr |
| 1165 | 1169 | Borneol | 1.0 | --- | tr |
| 1167 | 1169 | p-Mentha-1,5-dien-8-ol | --- | --- | 0.1 |
| 1177 | 1177 | Terpinen-4-ol | 0.2 | 0.2 | tr |
| 1183 | 1182 | p-Methylacetophenone | tr | tr | --- |
| 1185 | 1182 | p-Cymen-8-ol | 0.2 | 0.1 | 0.1 |
| 1190 | 1188 | α-Terpineol | 0.8 | 0.1 | 0.1 |
| 1195 | 1195 | Myrtenal | --- | 0.4 | 0.2 |
| 1196 | 1195 | Myrtenol | --- | --- | tr |
| 1198 | 1196 | Estragole (=Methyl chavicol) | 0.2 | --- | --- |
| 1207 | 1205 | Verbenone | --- | 0.6 | 0.2 |
| 1217 | 1216 | trans-Carveol | --- | tr | 0.1 |
| 1219 | 1220 | endo-Fenchyl acetate | 2.8 | --- | --- |
| 1237 | 1241 | Cuminaldehyde | --- | tr | --- |
| 1241 | 1243 | Carvone | --- | tr | tr |
| 1243 | 1244 | Carvacrol methyl ether | --- | --- | tr |
| 1252 | 1252 | Geraniol | tr | --- | --- |
| 1285 | 1288 | Bornyl acetate | 0.1 | 0.4 | 0.3 |
| 1299 | 1298 | trans-Pinocarvyl acetate | --- | tr | --- |
| 1302 | 1299 | Carvacrol | tr | --- | 0.2 |
| 1305 | 1299 | Methyl (Z)-cinnamate | 0.9 | --- | --- |
| 1324 | 1326 | Myrtenyl acetate | --- | 1.0 | --- |
| 1334 | 1338 | δ-Elemene | --- | --- | 0.1 |
| 1349 | 1348 | α-Cubebene | 0.1 | tr | 0.1 |
| 1375 | 1376 | α-Copaene | 0.1 | tr | 0.6 |
| 1385 | 1378 | Methyl (E)-cinnamate | 5.1 | tr | --- |
| 1387 | 1388 | β-Bourbonene | --- | 0.3 | 1.0 |
| 1390 | 1388 | β-Cubebene | 0.2 | --- | 0.2 |
| 1393 | 1390 | β-Elemene | 0.3 | 0.9 | 0.3 |
| 1406 | 1403 | Methyl eugenol | tr | --- | --- |
| 1408 | 1408 | (Z)-Caryophyllene | --- | tr | 0.1 |
| 1409 | 1409 | α-Gurjunene | --- | --- | 0.2 |
| 1416 | 1412 | α-cis-Bergamotene | tr | --- | --- |
| 1419 | 1419 | (E)-Caryophyllene | 1.1 | 11.2 | 19.1 |
| 1429 | 1432 | β-Copaene | --- | --- | 0.2 |
| 1437 | 1434 | α-trans-Bergamotene | 3.1 | 0.1 | tr |
| 1439 | 1439 | α-Guaiene | 0.4 | tr | tr |
| 1444 | 1441 | Aromadendrene | --- | --- | tr |
| 1454 | 1454 | α-Humulene | 0.2 | 4.0 | 6.4 |
| 1459 | 1456 | (E)-β-Farnesene | 0.1 | 0.1 | 0.1 |
| 1461 | 1454 | Alloaromadendrene | --- | tr | 2.2 |
| 1464 | 1466 | cis-Muurola-4(14),5-diene | 0.5 | --- | 0.1 |
| 1467 | 1467 | Ethyl (E)-cinnamate | --- | tr | --- |
| 1474 | 1479 | trans-Cadina-1(6),4-diene | --- | --- | 0.2 |
| 1476 | 1477 | γ-Gurjunene | --- | tr | --- |
| 1478 | 1479 | γ-Muurolene | --- | --- | 0.4 |
| 1482 | 1485 | Germacrene-D | 0.8 | 0.1 | 0.4 |
| 1484 | 1479 d | γ-Selinene | --- | 5.5 | 0.4 |
| 1487 | 1490 | β-Selinene | 1.2 | 0.3 | 2.5 |
| 1495 | 1494 | epi-Cubebol | --- | --- | 0.9 |
| 1495 | 1496 | Valencene | --- | 3.7 | --- |
| 1496 | 1492 | δ-Selinene | 0.8 | --- | --- |
| 1497 | 1500 | Bicyclogermacrene | --- | --- | 0.8 |
| 1500 | 1496 | Viridiflorene | 0.1 | --- | --- |
| 1502 | 1500 | α-Muurolene | --- | tr | 1.0 |
| 1506 | 1509 | Germacrene A | --- | --- | 0.1 |
| 1507 | 1509 | α-Bulnesene | 0.9 | --- | --- |
| 1510 | 1505 | β-Bisabolene | --- | tr | 0.1 |
| 1516 | 1513 | γ-Cadinene | 2.9 | --- | 2.7 |
| 1521 | 1522 | 7-epi-α-Selinene | 0.1 | 20.1 | 1.3 |
| 1523 | 1522 | trans-Calamenene | 0.2 | --- | --- |
| 1525 | 1523 | δ-Cadinene | 0.2 | 0.4 | 6.5 |
| 1532 | 1535 | 10-epi-Cubebol | 0.1 | --- | --- |
| 1534 | 1534 | trans-Cadina-1,4-diene | --- | --- | 0.1 |
| 1537 | 1538 | α-Cadinene | tr | --- | 0.2 |
| 1547 | 1544 | cis-Sesquisabinene hydrate | --- | 3.4 | 0.9 |
| 1553 | --- | Unidentified | --- | 1.2 | --- |
| 1557 | 1561 | cis-Muurol-5-en-4α-ol | 0.1 | --- | --- |
| 1560 | 1563 | (E)-Nerolidol | --- | tr | --- |
| 1565 | 1565 | β-Calacorene | --- | tr | --- |
| 1566 | --- | Unidentified | --- | 0.5 | --- |
| 1578 | 1575 | Germacrene D-4-ol | --- | --- | 3.1 |
| 1584 | 1583 | Caryophyllene oxide | 0.2 | 20.1 | 4.3 |
| 1604 | 1602 | Ledol | --- | 3.6 | 0.5 |
| 1610 | 1608 | Humulene epoxide II | --- | --- | 0.9 |
| 1616 | 1619 | 1,10-di-epi-Cubenol | 1.6 | --- | 0.1 |
| 1619 | 1623 | 10-epi-γ-Eudesmol | --- | 0.6 | 0.3 |
| 1629 | 1628 | 1-epi-Cubenol | --- | 0.6 | 0.6 |
| 1637 | 1640 | Caryophylla-4(12),8(13)-dien-5-ol | --- | 0.7 | 0.4 |
| 1643 | 1640 | τ-Cadinol | 12.2 | 2.0 | 5.7 |
| 1646 | 1642 | τ-Muurolol | --- | 0.6 | 4.9 |
| 1647 | 1646 | α-Muurolol (= Torreyol) | --- | --- | 0.7 |
| 1648 | 1649 | Methyl (Z)-jasmonate | 0.2 | --- | --- |
| 1653 | 1650 | β-Eudesmol | 0.1 | 0.9 | 0.3 |
| 1657 | 1654 | α-Cadinol | 0.4 | 2.0 | 9.5 |
| 1660 | 1663 1666 1661 | 7-epi-α-Eudesmol + Intermediol + cis-Calamenen-10-ol | --- | 3.3 | --- |
| 1668 | 1669 | trans-Calamenen-10-ol | --- | 0.6 | --- |
| 1672 | 1671 | epi-β-Bisabolol | --- | --- | 0.3 |
| 1672 | 1669 | 14-Hydroxy-9-epi-(E)-caryophyllene | --- | 0.8 | --- |
| 1674 | 1676 | Cadalene | --- | 0.4 | --- |
| 1685 | 1685 | α-Bisabolol | 0.1 | --- | --- |
| 1687 | 1689 | cis-14-nor-Muurol-5-en-4-one | 0.3 | --- | --- |
| 1690 | 1689 | Shyobunol | --- | --- | 4.6 |
| 1704 | 1702 | 10-Norcalamenen-10-one | --- | 0.2 | --- |
| 1738 | 1740 | Oplopanone | --- | 0.3 | tr |
| 1773 | 1775 | epi-Cyclocolorenone | --- | 0.8 | --- |
| 1803 | 1806 | Nootkatone | --- | 0.6 | --- |
| Total Identified | 100 | 91.7 | 98.6 | ||
| Major Sesquiterpenoid | Teucrium Species | % | Refs. |
|---|---|---|---|
| (E)-Caryophyllene | T. chamaedrys | 47.6 | [24] |
| T. polium | 52.0 | [24] | |
| T. arduini | 35.2 | [25] | |
| T. turredanum | 15.6–32.6 | [26] | |
| T. scorodonia ssp. baeticum | 33.8 | [27] | |
| α-Humulene | T. alopecurus | 12.3 | [28] |
| T. flavum | 8.4 | [29] | |
| T. marum | 7.2 | [30] | |
| T. polium | 4.3 | [31] | |
| T. scorodonia ssp. baeticum | 9.1 | [27] | |
| T. turredanum | 4.7–10.1 | [26] | |
| Germacrene D a | T. sandrasicum | 27.9 | [42] |
| T. arduini | 17.0–18.7 | [25,43] | |
| T. chamaedrys | 16.5–32.1 | [24,44,45] | |
| T. scorodonia ssp. baeticum | 22.2 | [27] | |
| δ-Cadinene | T. montanum | 17.2 | [32] |
| T. capitatum | 3.0–9.8 | [33] | |
| T. maghrebinum | 13.5 | [34] | |
| T. ramosissimum | 20.0 | [35] | |
| T. stocksianum | 12.9 | [36] | |
| Caryophyllene oxide | T. orientale ssp. taylori | 15.6 | [37] |
| T. montbretti | 12.7 | [38] | |
| T. arduini | 14.6 | [39] | |
| α-Cadinol | T. polium ssp. aurasiacum | 46.8 | [40] |
| T. polium ssp. capitatum | 4.5 | [38] | |
| T. ramosissimum | 9.9 | [35] | |
| T. leucocladum | 9.3 | [41] | |
| T. capitatum | 1.6–9.8 | [33] | |
| τ-Cadinol | T. capitatum | 1.6–24.1 | [33] |
| T. montanum | 5.5 | [42] | |
| T. leucocladum | 5.5 | [41] |
| Organism | EOOF a | EOTY-t b | Positive Control |
|---|---|---|---|
| S. aureus | 4300 | 156 | 0.61 e |
| MRSA | 8600 | nt c | <10.0 f |
| B. cereus | nt | 156 | 1.22 e |
| B. subtilis | 4300 | nt | <10.0 e |
| E. coli | na d | 313 | 1.22 e |
| P. aeruginosa | na | 1250 | 2.44 e |
| C. albicans | 8600 | 1250 | 0.61 g |
| A. niger | nt | 313 | 0.61 g |
| B. cinerea | nt | 313 | <19.5 g |
| Cell Line | EOOF | EOTY-d | EOTY-t |
|---|---|---|---|
| HT-29 | na a | 43.7 ± 7.1 | nt b |
| MCF-7 | nt | nt | 24.4 ± 1.8 |
| MDA-MB-231 | nt | nt | 59.9 ± 4.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, N.A.A.; Chhetri, B.K.; Dosoky, N.S.; Shari, K.; Al-Fahad, A.J.A.; Wessjohann, L.; Setzer, W.N. Antimicrobial, Antioxidant, and Cytotoxic Activities of Ocimum forskolei and Teucrium yemense (Lamiaceae) Essential Oils. Medicines 2017, 4, 17. https://doi.org/10.3390/medicines4020017
Ali NAA, Chhetri BK, Dosoky NS, Shari K, Al-Fahad AJA, Wessjohann L, Setzer WN. Antimicrobial, Antioxidant, and Cytotoxic Activities of Ocimum forskolei and Teucrium yemense (Lamiaceae) Essential Oils. Medicines. 2017; 4(2):17. https://doi.org/10.3390/medicines4020017
Chicago/Turabian StyleAli, Nasser A. Awadh, Bhuwan K. Chhetri, Noura S. Dosoky, Khola Shari, Ahmed J. A. Al-Fahad, Ludger Wessjohann, and William N. Setzer. 2017. "Antimicrobial, Antioxidant, and Cytotoxic Activities of Ocimum forskolei and Teucrium yemense (Lamiaceae) Essential Oils" Medicines 4, no. 2: 17. https://doi.org/10.3390/medicines4020017
APA StyleAli, N. A. A., Chhetri, B. K., Dosoky, N. S., Shari, K., Al-Fahad, A. J. A., Wessjohann, L., & Setzer, W. N. (2017). Antimicrobial, Antioxidant, and Cytotoxic Activities of Ocimum forskolei and Teucrium yemense (Lamiaceae) Essential Oils. Medicines, 4(2), 17. https://doi.org/10.3390/medicines4020017