Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review
Abstract
:1. Introduction
2. Definition of Essential Oils
- Flowers, of course, including: orange, pink, lavender, and the (clove) flower bud or (ylang-ylang) bracts,
- Leaves, most often, including: eucalyptus, mint, thyme, bay leaf, savory, sage, pine needles, and tree underground organs, e.g., roots (vetiver),
- Rhizomes (ginger, sweet flag),
- Seeds (carvi, coriander),
- Fruits, including: fennel, anise, Citrus epicarps,
- Wood and bark, including: cinnamon, sandalwood, rosewood.
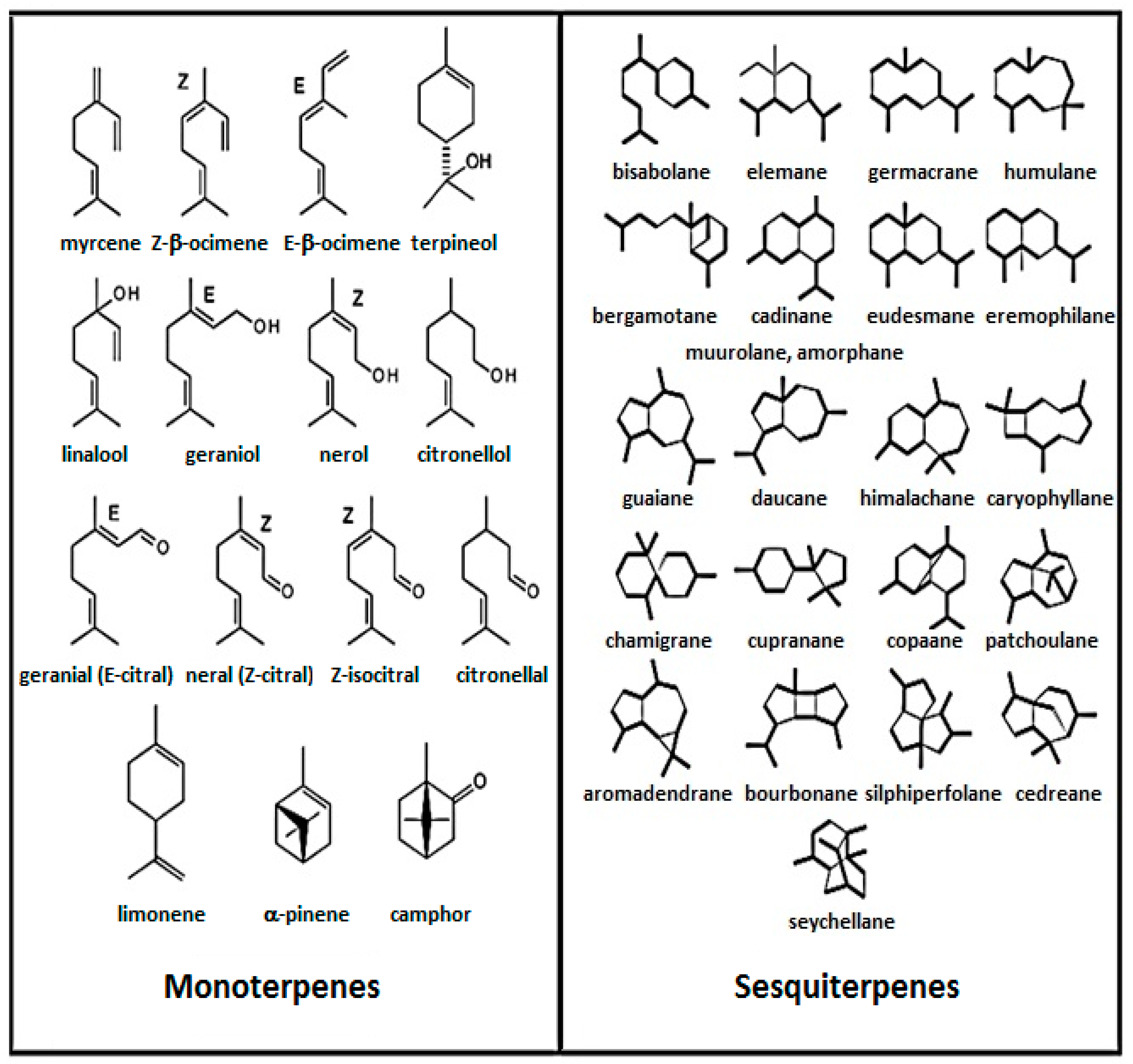
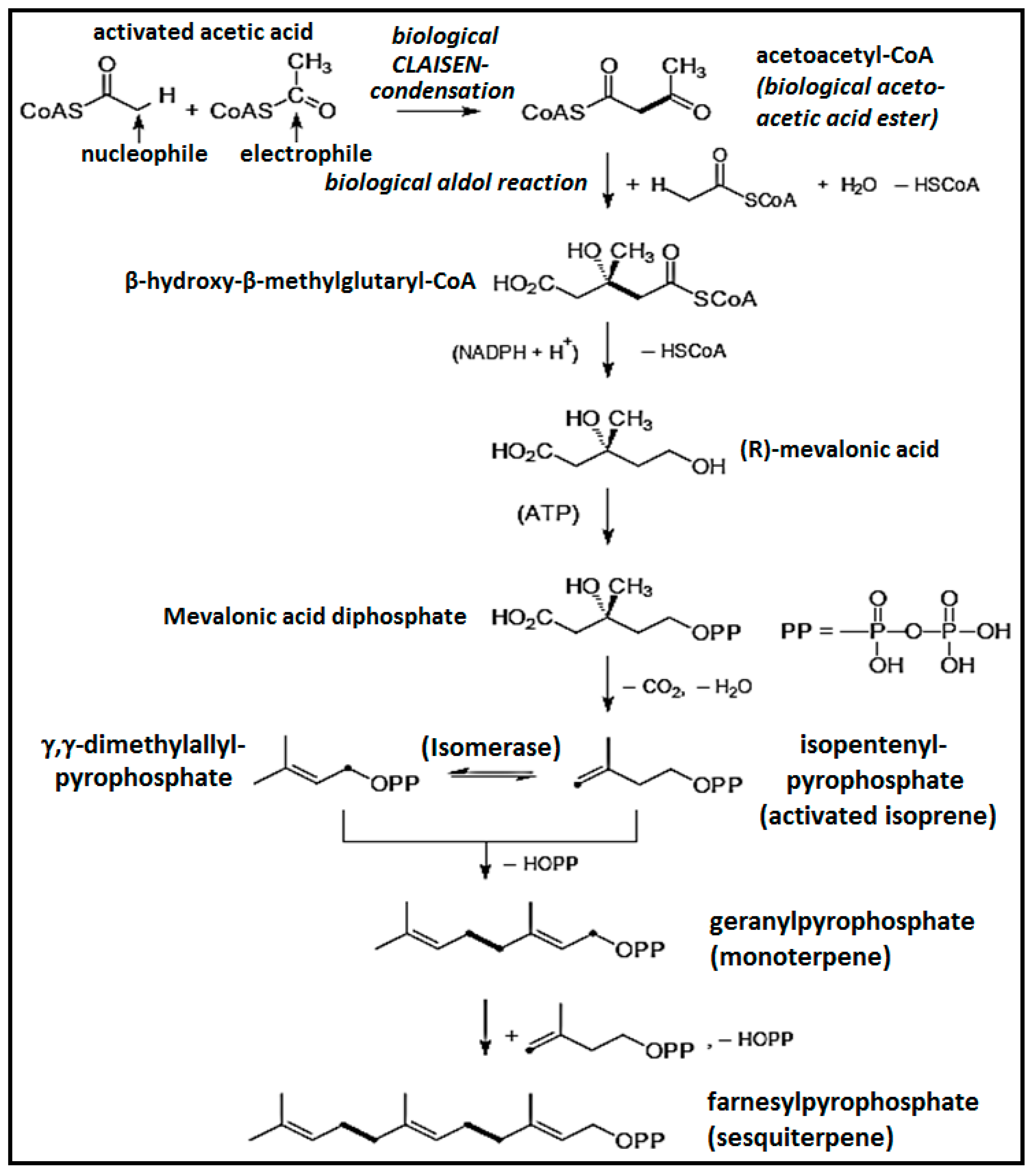
3. Chemistry of Essential Oils
- Intrinsic factors related to the plant, and interaction with the environment (soil type and climate, etc.) and the maturity of the plant concerned, even at harvest time during the day,
- Extrinsic factors related to the extraction method and the environment.
4. Biological Activities of Essential Oils
4.1. Antibacterial Activity
4.2. Antioxidant Activity
4.3. Anti-Inflammatory Activity
4.4. Cancer Chemoprotective Activity
4.5. Cytotoxicity
4.6. Allelopathic Activity
4.7. Repellent and Insecticidal Activity
5. Conclusions
Acknowledgments

Author Contributions
Conflicts of Interest
References
- Guenther, E. The Essential Oils; D. Van Nostrand Company Inc.: New York, NY, USA, 1948; p. 427. [Google Scholar]
- Association Française de Normalisation (AFNOR). Huiles Essentielles, Tome 2, Monographies Relatives Aux Huiles Essentielles, 6th ed.; AFNOR, Association Française de Normalisation: Paris, France, 2000. [Google Scholar]
- Carette Delacour, A.S. La Lavande et son Huile Essentielle. Ph.D. Thesis, Université Lille 2, Lille, France, 2000. [Google Scholar]
- Sell, C.S. The Chemistry of Fragrance. From Perfumer to Consumer, 2nd ed.; The Royal Society of Chemistry: Cambridge, UK, 2006; p. 329. [Google Scholar]
- Vainstein, A.; Lewinsohn, E.; Pichersky, E.; Weiss, D. Floral Fragrance. New Inroads into an Old Commodity. Plant Physiol. 2001, 27, 1383–1389. [Google Scholar] [CrossRef]
- Pophof, B.; Stange, G.; Abrell, L. Volatile Organic Compounds as Signals in a Plant—Herbivore System: Electrophysiological Responses in Olfactory Sensilla of the Moth Cactoblastis cactorum. Chem. Senses 2005, 30, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Modzelewska, A.; Sur, S.; Kumar, K.S.; Khan, S.R. Sesquiterpenes: Natural products that decrease cancer growth. Curr. Med. Chem. Anti-Cancer Agents 2005, 54, 477–499. [Google Scholar] [CrossRef]
- Litchenthaler, H.K. The 1-deoxy-d-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. The biosynthesis of C5-C-25 terpenoid components. Nat. Prod. Rep. 2002, 19, 181–222. [Google Scholar] [CrossRef] [PubMed]
- Marotti, M.; Piccaglia, R.; Giovanelli, E. Effects of variety and ontogenic stage on the essential oil composition and biological activity of fennel (Foeniculum vulgare Mill.). J. Essent. Oil Res. 1994, 6, 57–62. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Sherazi, S.T.H.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Hussain, A.I.; Sherazi, S.T.H.; Bhanger, M.I. Changes in composition and antioxidant and antimicrobial activities of essential oil of fennel (Foeniculum vulgare Mill.) fruit at different stages of maturity. J. Herbs Spices Med. Plants 2009, 15, 1–16. [Google Scholar] [CrossRef]
- Cocking, T.T.; Middleton, G. Improved method for the estimation of the essential oil content of drugs. Q. J. Pharm. Pharmacol. 1935, 8, 435–442. [Google Scholar]
- Nickerson, G.; Likens, S. Gas chromatographic evidence for the occurrence of hop oil components in beer. J. Chromatogr. 1996, 21, 1–5. [Google Scholar] [CrossRef]
- Shelef, L.A. Antimicrobial effects of spices. J. Food Saf. 1983, 6, 29–44. [Google Scholar] [CrossRef]
- Nychas, G.J.E. Natural antimicrobials from plants. In New Methods of Food Preservation, 1st ed.; Gould, G.W., Ed.; Blackie Academic & Professional: London, UK, 1995; pp. 58–89. [Google Scholar]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.; Nychas, G.J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef]
- Sikkema, J.; de Bont, J.A.M.; Poolman, B. Interactions of cyclic hydrocarbons with biological membranes. J. Biol. Chem. 1994, 269, 8022–8028. [Google Scholar] [PubMed]
- Gustafson, J.E.; Liew, Y.C.; Chew, S.; Markham, J.L.; Bell, H.C.; Wyllie, S.G.; Warmington, J.R. Effects of tea tree oil on Escherichia coli. Lett. Appl. Microbiol. 1998, 26, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Bell, H.C.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. The mode of antimicrobial action of essential oil of Melaleuca alternifola (tea tree oil). J. Appl. Microbiol. 2000, 88, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Riley, T.V. Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J. Appl. Bacteriol. 1995, 78, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Bennink, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Denyer, S.P.; Hugo, W.B. Biocide-induced damage to the bacterial cytoplasmic membrane. In Mechanisms of Action of Chemical Biocides, the Society for Applied Bacteriology, Technical Series No 27; Denyer, S.P., Hugo, W.B., Eds.; Oxford Blackwell Scientific Publication: Oxford, UK, 1991; pp. 171–188. [Google Scholar]
- Farag, R.S.; Daw, Z.Y.; Hewedi, F.M.; El-Baroty, G.S.A. Antimicrobial activity of some Egyptian spice essential oils. J. Food Prot. 1989, 52, 665–667. [Google Scholar]
- Cosentino, S.; Tuberoso, C.I.G.; Pisano, B.; Satta, M.; Mascia, V.; Arzedi, E.; Palmas, F. In vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett. Appl. Microbiol. 2002, 29, 130–135. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.M. Chemical preservatives and natural antimicrobial compounds. In Food Microbiology: Fundamentals and Frontiers; Doyle, M.P., Beuchat, L.R., Montville, T.J., Eds.; ASM Press: Washington, DC, USA, 1997; pp. 520–556. [Google Scholar]
- Knobloch, K.; Weigand, H.; Weis, N.; Schwarm, H.M.; Vigenschow, H. Action of terpenoids on energy metabolism. In Progress in Essential Oil Research: 16th International Symposium on Essential Oils; Brunke, E.J., Ed.; De Walter de Gruyter: Berlin, Germany, 1986; pp. 429–445. [Google Scholar]
- Pauli, A. Antimicrobial properties of essential oil constituents. Int. J. Aromather. 2001, 11, 126–133. [Google Scholar] [CrossRef]
- Fabian, D.; Sabol, M.; Domaracké, K.; Bujnékovâ, D. Essential oils, their antimicrobial activity against Escherichia coli and effect on intestinal cell viability. Toxicol. in Vitro 2006, 20, 1435–1445. [Google Scholar] [PubMed]
- Marino, M.; Bersani, C.; Comi, G. Antimicrobial activity of the essential oils of Thymus vulgaris L. measured using a bioimpedometric method. J. Food Prot. 1999, 62, 1017–1023. [Google Scholar] [PubMed]
- Senatore, F.; Napolitano, F.; Ozcan, M. Composition and antibacterial activity of the essential oil from Crithmum maritimum L. (Apiaceae) growing wild in Turkey. Flav. Frag. J. 2000, 15, 186–189. [Google Scholar] [CrossRef]
- Canillac, N.; Mourey, A. Antibacterial activity of the essential oil of Picea excelsa on Listeria, Staphylococcus aureus and coliform bacteria. Food Microbiol. 2001, 18, 261–268. [Google Scholar] [CrossRef]
- Cimanga, K.; Kambu, K.; Tona, L.; Apers, S.; de Bruyne, T.; Hermans, N.; Totté, J.; Pieters, L.; Vlietinck, A.J. Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. J. Ethnopharmacol. 2002, 79, 213–220. [Google Scholar] [CrossRef]
- Delaquis, P.J.; Stanich, K.; Girard, B.; Mazza, G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int. J. Food Microbiol. 2002, 74, 101–109. [Google Scholar] [CrossRef]
- Ratledge, C.; Wilkinson, S.G. An overview of microbial lipids. In Microbial Lipids; Ratledge, C., Wilkinson, S.G., Eds.; Academic Press Limited: London, UK, 1988; Volume 1, pp. 3–22. [Google Scholar]
- Davidson, P.M.; Parish, M.E. Methods for testing the efficacy of food antimicrobials. Food Technol. 1989, 43, 148–155. [Google Scholar]
- Gill, A.O.; Delaquis, P.; Russo, P.; Holley, R.A. Evaluation of antilisterial action of cilantro oil on vacuum packed ham. Int. J. Food Microbiol. 2002, 73, 83–92. [Google Scholar] [CrossRef]
- Reichling, J.; Schnitzler, P.; Suschke, U.; Saller, R. Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotocic properties-an overview. Forsch. Komplement. 2009, 16, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Edris, A.E. Pharmaceutical and Therapeutic Potentials of Essential Oils and Their Individual Volatile Constituents. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Braga, P.C.; dal Sasso, M.; Culici, M.; Gasastri, L.; Marceca, M.X.; Guffanti, E.E. Antioxidant potential of thymol determined by chemiluminescence inhibition in human neutrophils and cell-free systems. Pharmacology 2006, 76, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Viljoen, A.M. A review of the application and pharmacological properties of α-Bisabolol and α-Bisabolol-rich oils. J. Am. Oil Chem. Soc. 2010, 87, 1–7. [Google Scholar] [CrossRef]
- Maruyama, N.; Sekimoto, N.; Ishibashi, H. Suppression of neutrophil accumulation in mice by cutaneous application of geranium essential oil. J. Inflamm. 2005, 2, 1–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koh, K.J.; Pearce, A.L.; Marshman, G.; Finlay-Jones, J.J.; Hart, P.H. Tea tree oil reduces histamine-induced skin inflammation. Br. J. Dermatol. 2002, 147, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Caldefie-Chézet, F.; Guerry, M.; Chalchat, J.C.; Fusillier, C.; Vasson, M.P.; Guillot, J. Antiinflammatory effects of Malaleuca alternifolia essential oil on human polymorphonuciear neutrophils and monocytes. Free Radic. Res. 2004, 38, 805–811. [Google Scholar]
- Caldefie-Chézet, F.; Fusillier, C.; Jarde, T.; Laroye, H.; Damez, M.; Vasson, M.P. Potential antiinflammatory effects of Malaleuca alternifolia essential oil on human peripheral blood leukocytes. Phytother. Res. 2006, 20, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Brand, C.; Carson, C.F.; Riley, T.V.; Prager, R.H.; Finlay-Jones, J.J. Terpinen-4-ol, the main component of the essential oil of Malaleuca altemifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm. Res. 2000, 49, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Sharififar, F.; Mirtajadini, M.; Azampour, M.J.; Zamani, E. Essential Oil and Methanolic Extract of Zataria multiflora Boiss with Anticholinesterase Effect. Pak. J. Biol. Sci. 2012, 15, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Manjamalai, A.; Jiflin, G.J.; Grace, V.M. Study on the effect of essential oil of Wedelia chinensis (Osbeck) against microbes and inflammation. Asian J. Pharm. Clin. Res. 2012, 5, 155–163. [Google Scholar]
- Yoon, H.S.; Moon, S.C.; Kim, N.D.; Park, B.S.; Jeong, M.H.; Yoo, Y.H. Genistein induces apoptosis of RPE-J cells by opening mitochondrial PTP. Biochem. Biophys. Res. Commun. 2000, 276, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Pyun, M.S.; Shin, S. Antifungal effects of the volatile oils from Asiium plants against Trichophyton species and synergism of the oils wih ketoconazole. Phytomedicine 2006, 13, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.A. A historical perspective on garlic and cancer. Recent advances on the nutritional effects associated with the use of garlic as a supplement. J. Nutr. 2001, 131, 1027–1031. [Google Scholar]
- Milner, J.A. Preclinical perspectives on garlic and cancer. Signifiance of garlic and its constituents in cancer and cardiovascular disease. J. Nutr. 2006, 136, 827–831. [Google Scholar]
- Wu, C.C.; Sheen, L.Y.; Chen, H.W.; Kuo, W.W.; Tsai, S.J.; Lii, C.K. Differential effects of garlic oil and its three major organosulfur components on the hepatic detoxification system in rats. J. Agric. Food Chem. 2002, 50, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Tijerina, M.T.; Tobola, A.S. Preferential overexpression of a class MU glutathione S-transferase subunit in mouse liver by myristicin. Biochem. Biophys. Res. Commun. 1997, 236, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Kenny, P.; Lam, L. Inhibition of benzo[α]-pyrene-induced tumorigenesis by myristicin, a volatile aroma constituent of parsley leaf oil. Carcinogenesis 1992, 13, 1921–1923. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Kim, J.H.; Jung, J.W.; Choi, J.W.; Han, E.S.; Lee, S.H.; Ko, K.H.; Ryu, J.H. Myristicin induced neurotoxicity in human neuroblastoma MSK-N-SH cells. Toxicol. Lett. 2005, 157, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Carnesecchi, S.; Bras-Gonçalves, R.; Bradaia, A.; Zeisel, M.; Gossé, F.; Poupon, M.F.; Raul, F. Geraniol, a component of plant essential oils, modulates DNA synthesis and potentiates 5-fluorourcil efficacy on human colon tumor xenografts. Cancer Lett. 2004, 215, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Carnesecchi, S.; Langley, K.; Exinger, F.; Gossé, F.; Raul, F. Geraniol, a component of plant essential oils, sensitizes human colonie cancer cells to 5-fluorouracil treatment. J. Pharm. Exp. Ther. 2002, 301, 625–630. [Google Scholar] [CrossRef]
- Legault, J.; Dahl, W.; Debiton, E.; Pichette, A.; Madelmont, J.C. Antitumor activity of balsam fir oil: Production of reactive oxygen species induced by α-Humulene as possible mechanism of action. Planta Med. 2003, 69, 402–407. [Google Scholar] [PubMed]
- Uedo, N.; Tatsuta, M.; Lishi, H.; Baba, M.; Sakai, N.; Yano, H.; Otani, T. Inhibition by Dlimonene of gastric carcinogenesis induced by N methyl N’ nitro N-nitrosoguanimidine in wistar rats. Cancer Lett. 1999, 137, 131–136. [Google Scholar] [CrossRef]
- Cavalieri, E.; Mariotto, S.; Fabrizi, C.; Carcereri de Prati, A.; Gottardo, R.; Leone, S.; Berra, L.V.; Lauro, G.M.; Ciampa, A.R.; Suzuki, H. α-Bisabolol, a nontoxic natural compound, strongly induces apoptosis in glioma cells. Biochem. Biophys. Res. Commun. 2004, 315, 589–594. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, A.; Alviano, A.; Blank, A.; Alves, P.; Alviano, C.; Gattass, C. Melisa officinalis L. essential oil: Antitumoral and antioxidant activities. J. Pharm. Pharmacol. 2004, 56, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Calcabrini, A.; Stringaro, A.; Toccacieli, L.; Meschini, S.; Marra, M.; Colone, M.; Salvatore, G.; MondeIlo, F.; Arancia, G.; Molinari, A. Terpinen-4-ol, the main component of Melaieuca aitemifolia (tea tree) oil inhibits the in vitro growth of human melanoma cells. J. Investig. Dermatol. 2004, 122, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, M.; Wang, L.; Jiang, Z.; Li, W.; Li, H. Induction of apoptosis of cultured hepatocarcinoma cell by essential oil of Artemisia annua L. Sichuan Da Xue Xue Bao Yi Xue Ban 2004, 35, 337–339. [Google Scholar] [PubMed]
- Sylvestre, M.; Pichette, A.; Lavoie, S.; Longtin, A.; Legault, J. Composition and cytotoxic activity of the leaf essential oil of Comptonia peregrine L. Coulter. Phytother. Res. 2007, 6, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Kets, E.P.; Alberda, M.; Hoekstra, F.A.; Smid, E.J. Adaptation of the food-borne pathogen Bacillus cereus to carvacrol. Arch. Microbiol. 2000, 174, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Di Pasqua, R.; Hoskins, N.; Betts, G.; Mauriello, G. Changes in membrane fatty acids composition of microbial cells induced by addiction of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J. Agric. Food Chem. 2006, 54, 2745–2749. [Google Scholar] [CrossRef] [PubMed]
- Turina, A.V.; Nolan, M.V.; Zygadlo, J.A.; Perillo, M.A. Natural terpenes: Self-assembly and membrane partitioning. Biophys. Chem. 2006, 122, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Oussalah, M.; Caillet, S.; Lacroix, M. Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157:H7 and Listeria monocytogenes. J. Food Prot. 2006, 69, 1046–1055. [Google Scholar] [PubMed]
- Novgorodov, S.A.; Gudz, T.I. Permeability transition pore of the inner mitochondrial membrane can operate in two open states with different selectivities. J. Bioenerg. Biomembr. 1996, 28, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Vercesi, A.E.; Kowaltowski, A.J.; Grijalba, M.T.; Meinicke, A.R.; Castilho, R.F. The role of reactive oxygen species in mitochondrial permeability transition. Biosci. Rep. 1997, 1, 43–52. [Google Scholar] [CrossRef]
- Armstrong, J.S. Mitochondrial membrane permeabilization: The sine qua non for cell death. Bioessays 2006, 28, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Soylu, E.M.; Soylu, S.; Kurt, S. Antimicrobial activity of the essential oils of various plants against tomato late blight disease agent Phytophthora infestans. Mycopathologia 2006, 161, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Santoro, G.F.; Cardoso, M.G.; Guimaraes, L.G.; Mendonca, L.Z.; Soares, M.J. Trypanosoma cruzi: Activity of essential oils from Achillea millefolium L., Syzygiumaromaticum L. and Ocimumbasilicum L. on epimastigotes and trypomastigotes. Exp. Parasitol. 2007, 116, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Santoro, G.F.; Das Gracas Cardoso, M.; Guimaraes, L.G.; Salgado, A.P.; Menna-Barreto, R.F.; Soares, M.J. Effect of Oregano (Origanum vulgare L.) and Thyme (Thymus vulgaris L.) essential oils on Trypanosoma cruzi (Protozoa: Kinetoplastida) growth and ultrastructure. Parasitol. Res. 2007, 100, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, P.; Koch, C.; Reichling, J. Susceptibility of drugresistant clinical HSV-1 strains to essential oils of Ginger, Thyme, Hyssop and Sandalwood. Antimicrob. Agents Chemother. 2007, 51, 1859–1862. [Google Scholar] [CrossRef] [PubMed]
- Parveen, M.; Hasan, M.K.; Takahashi, J.; Murata, Y.; Kitagawa, E.; Kodama, O.; Iwahashi, H. Response of Saccharomyces cerevisiae to a monoterpene: Evaluation of antifungal potential by DNA microarray analysis. J. Antimicrob. Chemother. 2004, 54, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.J.; Na, K.J.; Choi, I.G.; Choi, K.C.; Jeung, E.B. Antibacterial and antifungal effects of essential oils from coniferous trees. Biol. Pharm. Bull. 2004, 27, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Rota, C.; Carraminana, J.J.; Burillo, J.; Herrera, A. In vitro antimicrobial activity of essential oils from aromatic plants against selected foodborne pathogens. J. Food Prot. 2004, 67, 1252–1256. [Google Scholar] [PubMed]
- Si, W.; Gong, J.; Tsao, R.; Zhou, T.; Yu, H.; Poppe, C.; Johnson, R.; Du, Z. Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J. Appl. Microbiol. 2006, 100, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Sonboli, A.; Babakhani, B.; Mehrabian, A.R. Antimicrobial activity of six constituents of essential oil from Salvia. Z. Naturforschung 2006, 61, 160–164. [Google Scholar] [CrossRef]
- Bruni, R.; Medici, A.; Andreotti, E.; Fantin, C.; Muzzoli, M.; Dehesa, M. Chemical composition and biological activities of Isphingo essential oil, a traditional Ecuadorian spice from Ocotea quixos (Lam.) Kosterm. (Lauraceae) flower calices. Food Chem. 2003, 85, 415–421. [Google Scholar] [CrossRef]
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in food. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- Basile, A.; Senatore, F.; Gargano, R.; Sorbo, S.; Del Pezzo, M.; Lavitola, A.; Ritieni, A.; Bruno, M.; Spatuzzi, D.; Rigano, D.; et al. Antibacterial and antioxidant activities in Sideritis italica (Miller) Greuter et Burdet essential oils. J. Ethnopharmacol. 2006, 107, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Duschatzky, C.B.; Possetto, M.L.; Talarico, L.B.; Garcia, C.C.; Michis, F.; Almeida, N.V.; de Lampasona, M.P.; Schuff, C.; Damonte, E.B. Evaluation of chemical and antiviral properties of essential oils from South American plants. Antivir. Chem. Chemother. 2005, 16, 247–251. [Google Scholar] [CrossRef] [PubMed]
- El Hadri, A.; Gómez Del Río, M.A.; Sanz, J.; González Coloma, A.; Idaomar, M.; Ribas Ozonas, B.; Benedí González, J.; Sánchez Reus, M.I. Cytotoxic activity of α-humulene and transcaryophyllene from Salvia officinalis in animal and human tumor cells. An. Real Acad. Nac. Farm. 2010, 76, 343–356. [Google Scholar]
- Zeytinoglu, H.; Incesu, Z.; Baser, K.H. Inhibition of DNA synthesis by carvacrol in mouse myoblast cells bearing a human NRAS oncogene. Phytomedicine 2003, 10, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Asekun, O.T.; Adeniyi, B.A. Antimicrobial and cytotoxic activities of the fruit essential oil of Xylopia aethiopica from Nigeria. Fitoterapia 2004, 75, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.S.; Lee, S.Y.; Jang, C.G. Involvement of 5-HT1A and GABAA receptors in the anxiolytic-like effects of Cinnamomum cassia in mice. Pharmacol. Biochem. Behav. 2007, 87, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Ravizza, R.; Gariboldi, M.B.; Molteni, R.; Monti, E. Linalool, a plant-derived monoterpene alcohol, reverses doxorubicin resistance in human breast adenocarcinoma cells. Oncol. Rep. 2008, 20, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Kilani, S.; Abdelwahed, A.; Ben Ammar, R. Chemical Composition of the Essential Oil of Juniperus phoenicea L. from Algeria. J. Essent. Oil. 2008, 20, 695–700. [Google Scholar]
- Moon, T.; Wilkinson, J.M.; Cavanagh, H.M. Antiparasitic activity of two Lavandula essential oils against Giardia duodenalis, Trichomonas vaginalis and Hexamitainflata. Parasitol. Res. 2006, 99, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Priestley, C.M.; Burgess, I.F.; Williamson, F.M. Lethality of essential oil constituents towards the human louse, Pediculus humanus, and its eggs. Fitoterapia 2006, 77, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Rim, I.S.; Jee, C.H. Acaricidal effects of herb essential oils against Dermatophagoides farinae and D. pteronyssinus (Acari: Pyroglyphidae) and qualitative analysis of a herb Mentha pulegium (pennyroyal). Korean J. Parasitol. 2006, 44, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Macías, F.A.; Chinchilla, N.; Varela, R.M.; Molinillo, J.M. Bioactive steroids from Oryza sativa L. Steroids 2006, 71, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.P.; Kaur, S.; Mittal, S.; Batish, D.R.; Kohli, R.K. Essential oil of Artemisia scoparia inhibits plant growth by generating reactive oxygen species and causing oxidative damage. J. Chem. Ecol. 2009, 35, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Tellez, M.R.; Kobaisy, M.; Duke, S.O.; Schrader, K.K.; Dayan, F.E.; Romagni, J. Terpenoid based defense in plants and other organisms. In Lipid Technology; Kuo, T.M., Gardner, H.W., Eds.; Marcel Dekker: New York, NY, USA, 2002; p. 354. [Google Scholar]
- Angelini, L.G.; Carpanese, G.; Cioni, P.L.; Morelli, I.; Macchia, M.; Flamini, G. Essential oils from Mediterranean Lamiaceae as weed germination inhibitors. J. Agric. Food Chem. 2003, 51, 6158–6164. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Moraes, M.L.L.; Rezende, M.O.O.; Souza-Filho, A.P.S. Potencial alelopático e identificação de compostos secundários em extratos de calopogônio (Calopogonium mucunoides) utilizando eletroforese capilar. Eclética Quím. 2011, 36, 51–68. [Google Scholar] [CrossRef]
- Dudai, N.; Poljakoff-Mayber, A.; Mayer, A.M.; Putievsky, E.; Lerne, H.R. Essential oils as allelochemicals and their potential use as bioherbicides. J. Chem. Ecol. 1999, 25, 1079–1089. [Google Scholar] [CrossRef]
- De Oliveira, C.M.; das Graças Cardoso, M.; Ionta, M.; Gomes Soares, M.; de Andrade Santiago, J.; Ferreira Da Silva, G.A.; Teixeira, M.L.; de Carvalho Selvati Rezende, D.A.; Vieira de Souza, R.; Isac Soares, L.; et al. Chemical Characterization and in vitro Antitumor Activity of the Essential Oils from the Leaves and Flowers of Callistemon viminalis. Am. J. Plant Sci. 2015, 6, 2664–2671. [Google Scholar] [CrossRef]
- Saad, L.M.M.G.; Abdelgaleil, S.A.M. Allelopathic Potential of Essential Oils Isolated from Aromatic Plants on Silybum marianum. Glob. Adv. Res. J. Agric. Sci. 2014, 3, 289–297. [Google Scholar]
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother. Res. 2010, 24, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Kotan, R.; Kordali, S.; Cakir, A.; Kesdek, M.; Kaya, Y.; Kilic, H. Antimicrobial and insecticidal activities of essential oil isolated from Turkish Salvia hydrangea DC. Ex. Benth. Biochem. Syst. Ecol. 2008, 36, 360–368. [Google Scholar] [CrossRef]
- De Almeida, R.; Fernando, L.; Fernando, F.; Mancini, E.; de Feo, V. Phytotoxic Activities of Mediterranean Essential Oils. Molecules 2010, 15, 4309–4323. [Google Scholar] [CrossRef] [PubMed]
- Vokou, D.; Douvli, P.; Blionis, G.J.; Halley, J.M. Effects of monoterpenoids, acting alone or in pairs, on seed germination and subsequent seedling growth. J. Chem. Ecol. 2003, 29, 2281–2301. [Google Scholar] [CrossRef] [PubMed]
- Bouajaj, S.; Romane, A.; Benyamna, A.; Amri, I.; Hanana, M.; Hamrouni, L.; Romdhane, M. Essential oil composition, phytotoxic and antifungal activities of Ruta chalepensis L. leaves from High Atlas Mountains (Morocco). Natl. Prod. Res. 2014, 28, 1910–1914. [Google Scholar] [CrossRef] [PubMed]
- Tabana, A.; Saharkhiza, M.J.; Hadian, J. Allelopathic potential of essential oils from four Satureja spp. Biol. Agric. Hortic. 2013, 29, 244–255. [Google Scholar] [CrossRef]
- Paluch, G.E.; Zhu, J.; Bartholomay, L.; Coats, R.J. Amyris and Siam-wood Essential Oils: Insect Activity of Sesquiterpenes. Pestic. Househ. Struct. Resid. Pest Manag. 2011, 1015, 5–18. [Google Scholar]
- Ahmed, S.M.; Eapen, M. Vapour toxicity and repellency of some essential oils to insect pests. Indian Perfum. 1986, 30, 273–278. [Google Scholar]
- Mateeva, A.; Karov, S. Studies on the insecticidal effect of some essential oils. Naushni Tr. Vissha Selskostop. Inst. Vasil Kolar. Plodiv. 1983, 28, 129–139. [Google Scholar]
- Hamraoui, A.; Regnault-Roger, C. Oviposition and larval growth of Acanthoscelides obtectus Say (Col., Bruchidae) in regard to host and non-host plants from leguminosae family. J. Appl. Entomol. 1995, 119, 195–196. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Hamraoui, A. Fumigant toxic activity and reproductive inhibition induced by Monoterpenes upon Acanthoscelides obtectus Say (Coleoptera), bruchid of kidney bean (Phaseolus vulgaris). J. Stored Prod. Res. 1995, 31, 291–299. [Google Scholar] [CrossRef]
- Maia, M.F.; Moore, S.J. Plant-based insect repellents: A review of their efficacy, development and testing. Malar. J. 2011, 10 (Suppl. 1). [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. https://doi.org/10.3390/medicines3040025
Dhifi W, Bellili S, Jazi S, Bahloul N, Mnif W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines. 2016; 3(4):25. https://doi.org/10.3390/medicines3040025
Chicago/Turabian StyleDhifi, Wissal, Sana Bellili, Sabrine Jazi, Nada Bahloul, and Wissem Mnif. 2016. "Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review" Medicines 3, no. 4: 25. https://doi.org/10.3390/medicines3040025
APA StyleDhifi, W., Bellili, S., Jazi, S., Bahloul, N., & Mnif, W. (2016). Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines, 3(4), 25. https://doi.org/10.3390/medicines3040025





