Composition and Biological Activities of Murraya paniculata (L.) Jack Essential Oil from Nepal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Gas Chromatographic–Mass Spectral Analysis
2.3. Antimicrobial Screening
2.4. Nematicidal Assay
2.5. Brine Shrimp Lethality Assay
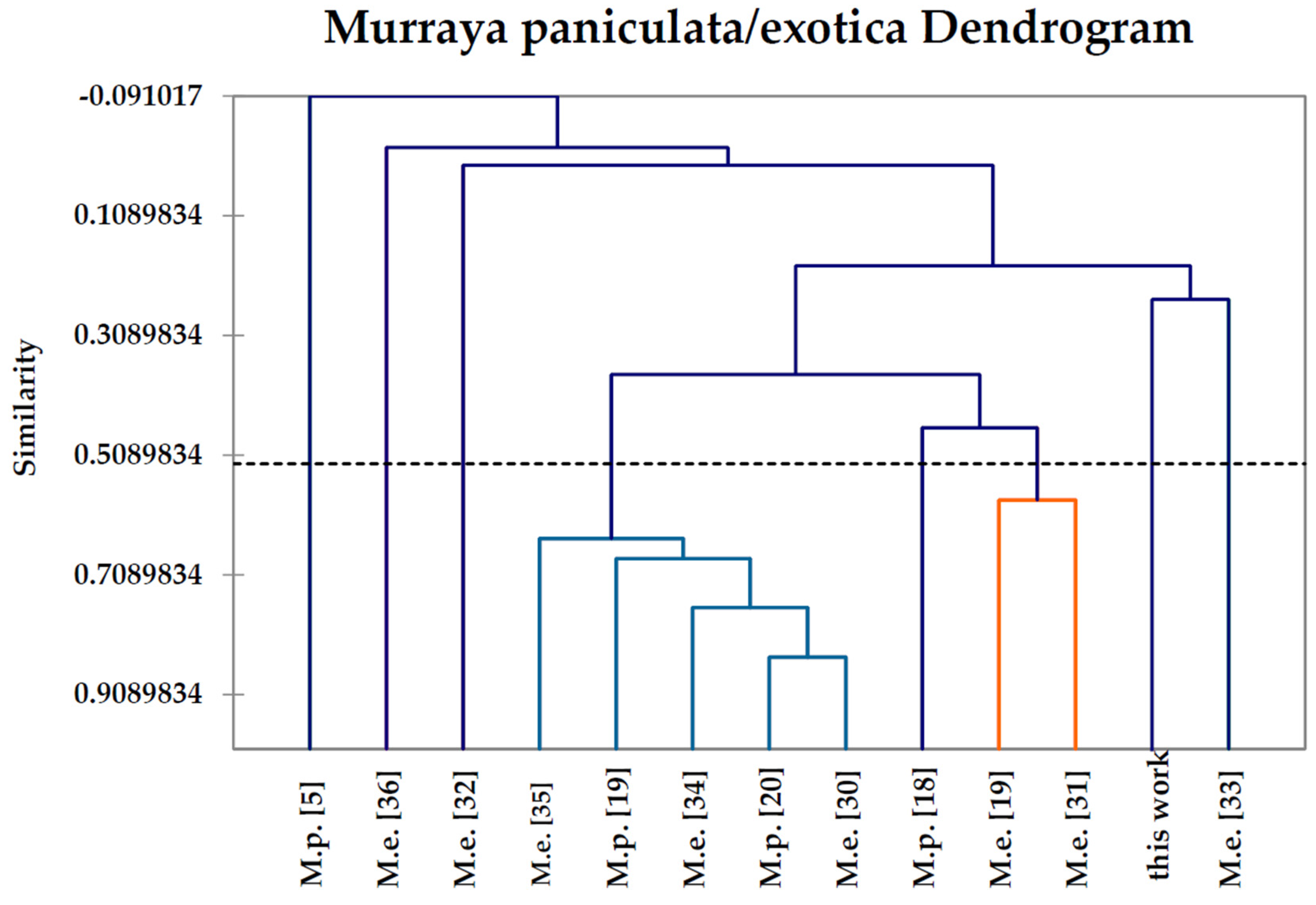
2.6. Hierarchical Cluster Analysis
3. Results and Discussion
4. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| GC-MS | Gas chromatography–mass spectrometry |
| RI | Retention indices |
| ATCC | American type culture collection |
| DMSO | Dimethylsulfoxide |
| LC50 | Median lethal concentration |
References
- Seidemann, J. World Spice Plants: Economic Usage, Botany, Taxonomy; Springer: Berlin, Germany, 2005. [Google Scholar]
- Quattrocchi, U. CRC World Dictionary of Medicinal and Poisonous Plants: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology; CRC Press: Boca Raton, FL, USA, 2012; p. 1747. [Google Scholar]
- Sambamurty, A.V.S.S. Taxonomy of Angiosperms; I.K. International Pvt. Ltd.: New Delhi, India, 2005. [Google Scholar]
- Schmelzer, G.H.; Gurib-Fakim, A. Plant Resources of Tropical Africa 11(2). Medicinal Plants 2; PROTA Foundation: Wageningen, The Netherlands, 2013. [Google Scholar]
- Olawore, N.O.; Ogunwande, I.A.; Ekundayo, O.; Adeleke, K.A. Chemical composition of the leaf and fruit essential oils of Murraya paniculata (L.) Jack. (Syn. Murraya exotica Linn.). Flavour Fragr. J. 2005, 20, 54–56. [Google Scholar] [CrossRef]
- Ng, M.K.; Abdulhadi-Noaman, Y.; Cheah, Y.K.; Yeap, S.K.; Alitheen, N.B. Bioactivity studies and chemical constituents of Murraya paniculata (Linn) Jack. Int. Food Res. J. 2012, 19, 1307–1312. [Google Scholar]
- Sharker, S.M.; Shahid, I.J.; Hasanuzzaman, M. Antinociceptive and bioactivity of leaves of Murraya paniculata (L.) Jack, Rutaceae. Braz. J. Pharmacog. 2009, 19, 746–748. [Google Scholar]
- Rahman, M.A.; Hasanuzzaman, M.; Uddin, N.; Shahid, I.Z. Antidiarrhoeal and anti-inflammatory activities of Murraya paniculata (L.) Jack. Pharmacologyonline 2010, 3, 768–776. [Google Scholar]
- Sundaram, M.; Sivakumar; Karthikeyan; Bhuvaneshwar; Aishwarya; Thirumalai; Pennarasi. Studies on in vitro antibacterial, antifungal property and antioxidant potency of Murraya paniculata. Pak. J. Nutr. 2011, 10, 925–928. [Google Scholar]
- Narkhede, M.B.; Ajmire, P.V.; Wagh, A.E. Evaluation of antinociceptive and anti-inflammatory activity of ethanol extract of Murraya paniculata leaves in experimental rodents. Int. J. Pharm. Pharmaceut. Sci. 2012, 4, 247–251. [Google Scholar]
- Sawangjaroen, N.; Phongpaichit, S.; Subhadhirasakul, S.; Visutthi, M.; Srisuwan, N.; Thammapalerd, N. The anti-amoebic activity of some medicinal plants used by AIDS patients in southern Thailand. Parasitol. Res. 2006, 98, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Pangnakorn, U.; Poonpaiboonpipattana, T. Allelopathic potential of orange jessamine (Murraya paniculata L.) against weeds. J. Agric. Sci. Technol. 2013, 3, 790–796. [Google Scholar]
- Aziz, S.S.S.A.; Sukari, M.A.; Rahmani, M.; Kitajima, M.; Aimi, N.; Ahpandi, N.J. Coumarins from Murraya paniculata (Rutaceae). Malay. J. Anal. Sci. 2010, 14, 1–5. [Google Scholar]
- Saeed, S.; Shah, S.; Mehmood, R.; Malik, A. Paniculacin, a new coumarin derivative from Murraya paniculata. J. Asian Nat. Prod. Res. 2011, 13, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Firman, K. Highly oxygenated flavonoids from Murraya paniculata. Phytochemistry 1996, 42, 1207–1210. [Google Scholar] [CrossRef]
- Sukari, M.A.; Azziz, S.S.S.; Rahmani, M.; Ali, A.M.; Aimi, N.; Kitajima, M. Polysubstituted flavonoids from the leaves of Murraya paniculata (Rutaceae). Nat. Prod. Sci. 2003, 9, 56–59. [Google Scholar]
- Zhang, Y.; Li, J.; Zhou, S.X.; Tu, P.F. Polymethoxylated flavonoids from the leaves of Murraya paniculata. Chin. Pharmaceut. J. 2010, 45, 1139–1141. [Google Scholar]
- Chowdhury, J.U.; Bhuiyan, M.N.I.; Yusuf, M. Chemical composition of the leaf essential oils of Murraya koenigii (L.) Spreng and Murraya paniculata (L.) Jack. Bangladesh J. Pharmacol. 2008, 3, 59–63. [Google Scholar] [CrossRef]
- Lv, H.N.; Guo, X.Y.; Tu, P.F.; Jiang, Y. Comparative analysis of the essential oil composition of Murraya paniculata and M. exotica. Nat. Prod. Commun. 2013, 8, 1473–1475. [Google Scholar] [PubMed]
- Rodriguez, E.J.; Ramis-Ramos, G.; Vander Heyden, Y.; Simó-Alfonso, E.F.; Lerma-García, M.J.; Saucedo-Hernández, Y.; Monteagudo, U.; Morales, Y.; Holgado, B.; Herrero-Martínez, J.M. Chemical composition, antioxidant properties and antimicrobial activity of the essential oil of Murraya paniculata leaves from the mountains of central Cuba. Nat. Prod. Commun. 2012, 7, 1527–1530. [Google Scholar] [PubMed]
- Boira, H.; Blanquer, A. Environmental factors affecting chemical variability of essential oils in Thymus piperella L. Biochem. Systemat. Ecol. 1997, 26, 811–822. [Google Scholar] [CrossRef]
- Tropicos.org. Missouri Botanical Garden. Available online: http://www.tropicos.org/Name/28100030 (accessed on 8 January 2016).
- Verma, S.; Rana, T.S.; Ranade, S.A. Genetic variation and clustering in Murraya paniculata complex as revealed by single primer amplification reaction methods. Curr. Sci. 2009, 96, 1210–1216. [Google Scholar]
- Monzote, L.; Nance, M.R.; Garcia, M.; Scull, R.; Setzer, W.N. Comparative chemical, cytotoxicity and antileishmanial properties of essential oils from Chenopodium ambrosioides. Nat. Prod. Commun. 2011, 6, 281–286. [Google Scholar] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- Setzer, M.C.; Setzer, W.N.; Jackes, B.R.; Gentry, G.A.; Moriarity, D.M. The medicinal value of tropical rainforest plants from Paluma, North Queensland, Australia. Pharmaceut. Biol. 2001, 39, 67–78. [Google Scholar] [CrossRef]
- Park, I.K.; Kim, J.; Lee, S.G.; Shin, S.C. Nematicidal activity of plant essential oils and components from ajowan (Trachyspermum ammi), allspice (Pimenta dioica) and litsea (Litsea cubeba) essential oils against pine wood nematode (Bursaphelenchus xylophilus). J. Nematol. 2007, 39, 257–279. [Google Scholar]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent end points. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- McLaughlin, J.L. Bench-top bioassays for the discovery of bioactive compounds in higher plants. Brenesia 1990, 34, 1–14. [Google Scholar]
- Huang, Y.S.; Wang, Y.; Luo, X.P.; Yuan, K. Composition, antimicrobial and antioxidant activities of the essential oil of Murraya exotica from Hainan of China. Asian J. Chem. 2013, 25, 5055–5058. [Google Scholar]
- Li, W.Q.; Jiang, C.H.; Chu, S.S.; Zuo, M.X.; Liu, Z.L. Chemical composition and toxicity against Sitophilus zeamais and Tribolium castaneum of the essential oil of Murraya exotica aerial parts. Molecules 2010, 15, 5831–5839. [Google Scholar] [CrossRef] [PubMed]
- El-Sakhawy, F.S.; El-Tantawy, M.E.; Ross, S.A.; El-Sohly, M.A. Composition and antimicrobial activity of the essential oil of Murraya exotica L. Flavour Fragr. J. 1998, 13, 59–62. [Google Scholar] [CrossRef]
- Raina, V.K.; Verma, S.C.; Dhawan, S.; Khan, M.; Ramesh, S.; Singh, S.C.; Yadav, A.; Srivastava, S.K. Essential oil composition of Murraya exotica from the plains of northern India. Flavour Fragr. J. 2006, 21, 140–142. [Google Scholar] [CrossRef]
- Pino, J.A.; Marbot, R.; Fuentes, V. Aromatic plants from western Cuba. VI. Composition of the leaf oils of Murraya exotica L., Amyris balsamifera L., Severinia buxifolia (Poir.) Ten. and Triphasia trifolia (Burm. f.) P. Wilson. J. Essent. Oil Res. 2006, 18, 24–28. [Google Scholar] [CrossRef]
- You, C.; Zhang, W.; Guo, S.; Wang, C.; Yang, K.; Liang, J.; Wang, Y.; Geng, Z.; Du, S.; Deng, Z. Chemical composition of essential oils extracted from six Murraya species and their repellent activity against Tribolium castaneum. Ind. Crops Prod. 2015, 76, 681–687. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Chandrasekaran, M.; Raj, G.A.; Jayaraman, M.; Venkatesalu, V. Identification of chemical constituents and larvicidal activity of essential oil from Murraya exotica L. (Rutaceae) against Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus (Diptera: Culicidae). Parasitol. Res. 2015, 114, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L.; Villanueva, H.E.; Palazzo, M.C.; Wright, B.S.; Setzer, W.N. Seasonal variation and bioactivity in the leaf oil of Liriodendron tulipifera growing in Huntsville, Alabama. Nat. Prod. Commun. 2009, 4, 839–843. [Google Scholar] [PubMed]
- Hudaib, M.; Speroni, E.; Di Pietra, A.M.; Cavrini, V. GC/MS evaluation of thyme (Thymus vulgaris L.) oil composition and variations during the vegetative cycle. J. Pharmaceut. Biomed. Anal. 2002, 29, 691–700. [Google Scholar] [CrossRef]
- Telci, I.; Devirtas, I.; Bayram, E.; Arabaci, O.; Kacar, O. Environmental variation on aroma components of pulegone/piperitone rich spearmint (Mentha spicata L.). Ind. Crops Prod. 2010, 32, 588–592. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Coroneo, V.; Dessi, S.; Cabras, P. Chemical composition, seasonal variability, and antifungal activity of Lavandula stoechas L. ssp. stoechas essential oils from stem/leaves and flowers. J. Agric. Food Chem. 2006, 54, 4364–4370. [Google Scholar] [CrossRef] [PubMed]
- Amzallag, G.N.; Larkov, O.; Ben Hur, M.; Dudai, N. Soil microvariations as a source of variability in the wild: The case of secondary metabolism in Origanum dayi Post. J. Chem. Ecol. 2005, 31, 1235–1254. [Google Scholar] [CrossRef] [PubMed]
- Sáez, F. Volatile oil variability in Thymus serpylloides ssp. gadorensis growing wild in southeastern Spain. Biochem. System. Ecol. 2001, 29, 189–198. [Google Scholar]
- Satyal, P.; Crouch, R.A.; Monzote, L.; Cos, P.; Ali, N.A.A.; Alhaj, M.A.; Setzer, W.N. The chemical diversity of Lantana camara: Analyses of essential oil samples from Cuba, Nepal, and Yemen. Chem. Biodivers. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Satyal, P.; Paudel, P.; Lamichhane, B.; Setzer, W.N. Leaf essential oil composition and bioactivity of Psidium guajava from Kathmandu, Nepal. Am. J. Essent. Oils Nat. Prod. 2016, in press. [Google Scholar]
- Setzer, M.C.; Moriarity, D.M.; Lawton, R.O.; Setzer, W.N.; Gentry, G.A.; Haber, W.A. Phytomedicinal potential of tropical cloudforest plants from Monteverde, Costa Rica. Rev. Biol. Trop. 2003, 51, 647–674. [Google Scholar] [PubMed]
- Setzer, M.C.; Werka, J.S.; Irvine, A.K.; Jackes, B.R.; Setzer, W.N. Biological activity of rainforest plant extracts from far north Queensland, Australia. In Biologically Active Natural Products for the 21st Century; Williams, L.A.D., Ed.; Research Signpost: Trivandrum, India, 2006; pp. 21–46. [Google Scholar]
- Schmidt, J.M.; Noletto, J.A.; Vogler, B.; Setzer, W.N. Abaco bush medicine: Chemical composition of the essential oils of four aromatic medicinal plants from Abaco Island, Bahamas. J. Herbs Spices Med. Plants 2007, 12, 43–65. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Wink, M.; Khalifaev, D.R.; Zhang, H.; Dosoky, N.S.; Setzer, W.N. Composition and bioactivity of the essential oil of Melissa officinalis L. growing wild in Tajikistan. Int. J. Trad. Nat. Med. 2013, 2, 86–96. [Google Scholar]
- Owolabi, M.S.; Lawal, O.A.; Dosoky, N.S.; Satyal, P.; Setzer, W.N. Chemical composition, antimicrobial, and cytotoxic assessment of Mitracarpus scaber Zucc. (Rubiaceae) essential oil from southwestern Nigeria. Am. J. Essent. Oil Nat. Prod. 2013, 1, 4–6. [Google Scholar]
- Woods, K.E.; Chhetri, B.K.; Jones, C.D.; Goel, N. Bioactivities and compositions of Betula nigra essential oils. J. Med. Active Plants 2013, 2, 1–9. [Google Scholar]
- Satyal, P.; Paudel, P.; Raut, J.; Deo, A.; Dosoky, N.S.; Setzer, W.N. Volatile constituents of Pinus roxburghii from Nepal. Phcog. Res. 2013, 5, 43–48. [Google Scholar] [PubMed]
- Satyal, P.; Paudel, P.; Poudel, A.; Dosoky, N.S.; Pokharel, K.K.; Setzer, W.N. Bioactivities and compositional analyses of Cinnamomum essential oils from Nepal: C. camphora, C. tamala, and C. glaucescens. Nat. Prod. Commun. 2013, 8, 1777–1784. [Google Scholar] [PubMed]
- Essien, E.E.; Newby, J.S.; Walker, T.M.; Setzer, W.N.; Ekundayo, O. Chemotaxonomic characterization and in-vitro antimicrobial and cytotoxic activities of Curcuma longa grown in southern Nigeria. Medicines 2015, 2, 340–349. [Google Scholar] [CrossRef]
- Essien, E.E.; Newby, J.S.; Walker, T.M.; Setzer, W.N.; Ekundayo, O. Characterization and antimicrobial activity of volatile constituents from fresh fruits of Alchornea cordifolia and Canthium subcordatum. Medicines 2016, 3, 1. [Google Scholar] [CrossRef]
- Choi, G.J.; Jang, K.S.; Choi, Y.H.; Yu, J.H.; Kim, J.C. Antifungal activity of lower alkyl fatty acid esters against powdery mildews. Plant Pathol. J. 2010, 26, 360–366. [Google Scholar] [CrossRef]
- Setzer, W.N.; Park, G.; Agius, B.R.; Stokes, S.L.; Walker, T.M.; Haber, W.A. Chemical compositions and biological activities of leaf essential oils of twelve species of Piper from Monteverde, Costa Rica. Nat. Prod. Commun. 2008, 3, 1367–1374. [Google Scholar]
- Werka, J.S.; Boehme, A.K.; Setzer, W.N. Biological activities of essential oils from Monteverde, Costa Rica. Nat. Prod. Commun. 2007, 2, 1215–1219. [Google Scholar]
- Satyal, P.; Woods, K.E.; Dosoky, N.S.; Neupane, S.; Setzer, W.N. Biological activities and volatile constituents of Aegle marmelos (L.) Corrêa from Nepal. J. Med. Active Plants 2012, 1, 114–122. [Google Scholar]
| RI a | Compound | % |
|---|---|---|
| 1100 | Linalool | 1.20 |
| 1112 | Phenylethyl alcohol | 0.73 |
| 1138 | Benzeneacetonitrile | 0.14 |
| 1189 | α-Terpineol | 0.16 |
| 1254 | Geraniol | 0.19 |
| 1290 | Indole | 1.25 |
| 1325 | p-Vinylguaiacol | 0.69 |
| 1332 | Bicycloelemene | 0.12 |
| 1334 | δ-Elemene | 3.19 |
| 1336 | Methyl anthranilate | 2.05 |
| 1391 | β-Elemene | 0.38 |
| 1398 | (Z)-Jasmone | 0.59 |
| 1418 | β-Caryophyllene | 3.97 |
| 1451 | cis-Murrola-3,5-diene | 0.25 |
| 1453 | α-Humulene | 1.10 |
| 1476 | γ-Gurjunene | 1.07 |
| 1480 | Germacrene D | 3.39 |
| 1483 | α-Curcumene | 0.84 |
| 1485 | β-Selinene | 0.16 |
| 1491 | δ-Selinene | 0.52 |
| 1496 | α-Zingiberene | 2.16 |
| 1504 | Germacrene A | 0.42 |
| 1509 | (E,E)-α-Farnesene | 2.79 |
| 1514 | Cubebol | 0.39 |
| 1521 | α-Chamigrene | 0.91 |
| 1523 | β-Sesquiphellandrene | 0.71 |
| 1549 | α-Elemol | 0.45 |
| 1556 | Germacrene B | 3.62 |
| 1564 | (E)-Nerolidol | 0.43 |
| 1571 | (3Z)-Hexenyl benzoate | 0.27 |
| 1581 | trans-Sesquisabinene hydrate | 0.16 |
| 1583 | Caryophyllene oxide | 0.40 |
| 1586 | Phenylethyl tiglate | 0.27 |
| 1593 | Spathulenol isomer | 1.39 |
| 1606 | β-Nootkatol | 0.35 |
| 1614 | Zingiberenol | 0.39 |
| 1620 | Selin-6-en-4-ol | 4.01 |
| 1624 | 1,10-di-epi-Cubenol | 1.09 |
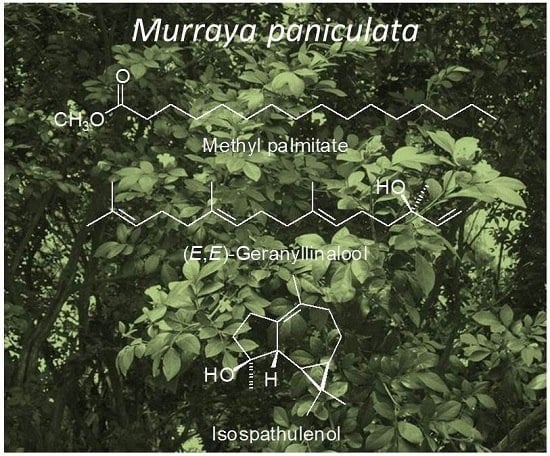
| 1628 | Isospathulenol | 9.44 |
| 1639 | τ-Cadinol | 2.02 |
| 1644 | epi-β-Muurolol | 0.34 |
| 1647 | β-Eudesmol | 0.17 |
| 1649 | τ-Muurolol | 1.01 |
| 1651 | α-Cadinol | 1.07 |
| 1663 | Intermedeol | 0.22 |
| 1680 | Germacra-4(15),5,10(14)-trien-1α-ol | 0.12 |
| 1689 | (2Z,6Z)-Farnesol | 0.22 |
| 1713 | Eudesma-4,11-dien-2-ol | 0.42 |
| 1716 | (2E,6Z)-Farnesol | 0.26 |
| 1720 | Nuciferol | 2.13 |
| 1733 | Oplopanone | 0.20 |
| 1745 | Curcumen-12-ol | 2.33 |
| 1761 | Benzyl benzoate | 4.20 |
| 1771 | Isospathulenol isomer | 0.93 |
| 1852 | Phenylethyl octanoate | 2.39 |
| 1865 | Benzyl salicylate | 0.29 |
| 1919 | Methyl palmitate | 11.05 |
| 1953 | Phenylethyl salicylate | 0.13 |
| 1957 | Palmitic acid | 0.80 |
| 1996 | Ethyl palmitate | 0.39 |
| 2031 | (E,E)-Geranyl linalool | 5.29 |
| 2094 | Methyl linoleate | 0.73 |
| 2100 | Methyl linolenate | 1.30 |
| 2110 | Phytol | 2.11 |
| 2123 | Methyl stearate | 0.60 |
| 2130 | Osthole | 2.58 |
| 2232 | Isogeigerin | 0.24 |
| 2238 | Suberosin epoxide | 0.82 |
| 2268 | 7-Methoxy-6-(3′-metylbuta-1′,3′-dienyl)coumarin | 0.47 |
| 2270 | Muurialongin | 0.59 |
| 2407 | Minimicrolin isovalerate | 0.45 |
| 2416 | Paniculol | tr b |
| 2504 | Octyl palmitate | 0.59 |
| 2705 | Octyl stearate | 0.21 |
| 2711 | Murpaniculol senecioate | tr |
| 2828 | Squalene | 0.25 |
| Compounds Identified | 76 (91.5%) | |
| Compound | M.p. a | M.p. | M.p. | M.p. | M.p. | M.e. b | M.e. | M.e. | M.e. | M.e. | M.e. | M.e. | M.e. |
| This | [18] | [20] | [5] | [19] | [19] | [33] | [34] | [35] | [32] | [31] | [30] | [36] | |
| α-pinene | 0 | 0 | 0 | tr | 0 | 0 | 0 | tr | 0 | 62.5 | 13.2 | 0.3 | 0 |
| Methyl salicylate | 0 | 0 | 0 | 22.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| β-Cyclocitral | 0 | 0 | 0 | 22.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| δ-Elemene | 3.2 | 3.6 | 0.4 | 0 | 3.3 | 3.4 | 5.1 | 0.2 | 0 | 0.4 | 0 | 0 | 0 |
| α-Cubebene | 0 | 3.0 | 2.2 | 7.9 | 0.9 | 0.1 | 0 | 1.0 | 6.9 | 0 | 0 | 0.4 | 0 |
| α-Copaene | 0 | 2.3 | 3.8 | 0.2 | 0 | 0.1 | 0.5 | 4.4 | 1.4 | 0.4 | 1.7 | 0.2 | 0 |
| β-Cubebene | 0 | 0 | 5.3 | 5.8 | 1.6 | 0 | 1.6 | 10.5 | 0 | 1.6 | 0 | 0 | 0 |
| β-Elemene | 0.4 | 8.9 | 0 | 0 | 0.1 | 2.0 | 0 | 0 | 0 | 0 | 0.9 | 0.1 | 7.6 |
| β-Caryophyllene | 4.0 | 11.8 | 29.8 | 0 | 23.3 | 11.7 | 9.7 | 24.1 | 20.3 | 5.2 | 4.5 | 45.5 | 7.1 |
| Cedrene | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 15.1 | 0 |
| α-(E)-Bergamotene | 0 | 0 | 0 | 0 | 9.3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| β-Humulene | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 40.6 |
| (E)-β-Farnesene | 0 | 0 | 0 | 0 | 2.6 | 0 | 1.5 | 0 | 2.4 | 0 | 0 | 0 | 0 |
| α-Humulene | 1.1 | 3.1 | 5.3 | tr | 0 | 3.0 | 0.6 | 5.8 | 0 | 0.8 | 7.3 | 0.3 | t |
| Alloaromadendrene | 0 | 0 | 0 | 0 | 0.1 | 1.9 | 0 | 0 | 5.9 | 0 | 0.2 | 0 | 0 |
| GermacreneD | 3.4 | 7.0 | 4.2 | 0 | 0.5 | 2.4 | 2.6 | 11.9 | 0 | 2.1 | 0.8 | 0 | 0 |
| GermacreneB | 0 | 0 | 0 | 0 | 0 | 0 | 3.5 | 0 | 0 | 1.9 | 0 | 0 | 0.9 |
| Bicyclogermacrene | 0 | 0 | 5.6 | 0 | 1.9 | 4.1 | 0 | 11.8 | 9.6 | 0 | 7.1 | 0 | 0 |
| α-Zingiberene | 2.2 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 12.7 | 0 | 0 | 0 | 0 |
| trans-β-Guaiene | 0 | 0 | 0 | 0 | 0 | 13.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| γ-Cadinene | 0 | 0 | 2.2 | 0 | 0 | 0 | 0 | tr | 2.1 | 1.1 | 0 | 0 | 0 |
| Cubebol | 0.4 | 0 | 0 | 6.8 | 0 | 0 | 0 | 3.1 | 0 | 0 | 0 | 0 | 0 |
| δ-Cadinene | 0 | 0 | 5.6 | tr | 2.2 | 1.2 | 0 | 4.4 | 8.0 | 0.5 | 4.4 | 0 | 0 |
| α-Elemol | 0.5 | 0 | 0 | 0 | 0.2 | 1.7 | 0.2 | 1.3 | 0 | 0 | 0 | 0 | 0.1 |
| (E)-Nerolidol | 0.4 | 0 | 0 | 11.7 | 4.6 | 0.4 | 27.8 | 1.5 | 2.7 | 0 | 0 | 0 | 0 |
| Spathulenol | 0 | 10.2 | 5.1 | 3.6 | 16.1 | 25.6 | 0.9 | 1.0 | 6.3 | 0.5 | 17.7 | 4.4 | 0.1 |
| Caryophyllene oxide | 0.4 | 16.6 | 6.3 | tr | 2.8 | tr | 0.1 | 0.8 | 4.0 | 0.5 | 8.6 | 1.3 | tr |
| Viridiflorol | 0 | 2.2 | 5.7 | 0 | 0 | 0 | 0.6 | 0.4 | 0 | 0 | 0 | 0 | 0 |
| 1,10-di-epi-Cubenol | 1.1 | 2.4 | 1.9 | 0 | 0 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1-epi-Cubenol | 0 | 0 | 2.5 | 1.2 | 0.4 | 0.1 | 0 | 1.1 | 0 | 0 | 0 | 0 | 0 |
| τ-Cadinol | 2.0 | 0 | 4.3 | 3.2 | 0 | 0 | 2.2 | 0 | 0 | 0.6 | 3.6 | 0 | 0 |
| β-Eudesmol | 0.2 | 0 | 0 | 0 | 2.9 | 2.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| α-Cadinol | 1.1 | 0 | 1.7 | 0 | 0 | 0 | 0.2 | 1.4 | 0.6 | 0.3 | 0 | 0 | 0 |
| Benzyl benzoate | 4.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 24.0 |
| Methyl palmitate | 11.1 | 0 | 0 | 0 | tr | tr | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dosoky, N.S.; Satyal, P.; Gautam, T.P.; Setzer, W.N. Composition and Biological Activities of Murraya paniculata (L.) Jack Essential Oil from Nepal. Medicines 2016, 3, 7. https://doi.org/10.3390/medicines3010007
Dosoky NS, Satyal P, Gautam TP, Setzer WN. Composition and Biological Activities of Murraya paniculata (L.) Jack Essential Oil from Nepal. Medicines. 2016; 3(1):7. https://doi.org/10.3390/medicines3010007
Chicago/Turabian StyleDosoky, Noura S., Prabodh Satyal, Tilak P. Gautam, and William N. Setzer. 2016. "Composition and Biological Activities of Murraya paniculata (L.) Jack Essential Oil from Nepal" Medicines 3, no. 1: 7. https://doi.org/10.3390/medicines3010007
APA StyleDosoky, N. S., Satyal, P., Gautam, T. P., & Setzer, W. N. (2016). Composition and Biological Activities of Murraya paniculata (L.) Jack Essential Oil from Nepal. Medicines, 3(1), 7. https://doi.org/10.3390/medicines3010007