Chemotaxonomic Characterization and in-Vitro Antimicrobial and Cytotoxic Activities of the Leaf Essential Oil of Curcuma longa Grown in Southern Nigeria
Abstract
:1. Introduction
2. Experimental Section
2.1. Plant Material
2.2. Gas Chromatographic–Mass Spectral Analysis
2.3. Antibacterial Screening
2.4. Antifungal Screening
2.5. Cell Culture
2.6. Cytotoxicity Screening
2.7. Hierarchical Cluster Analysis
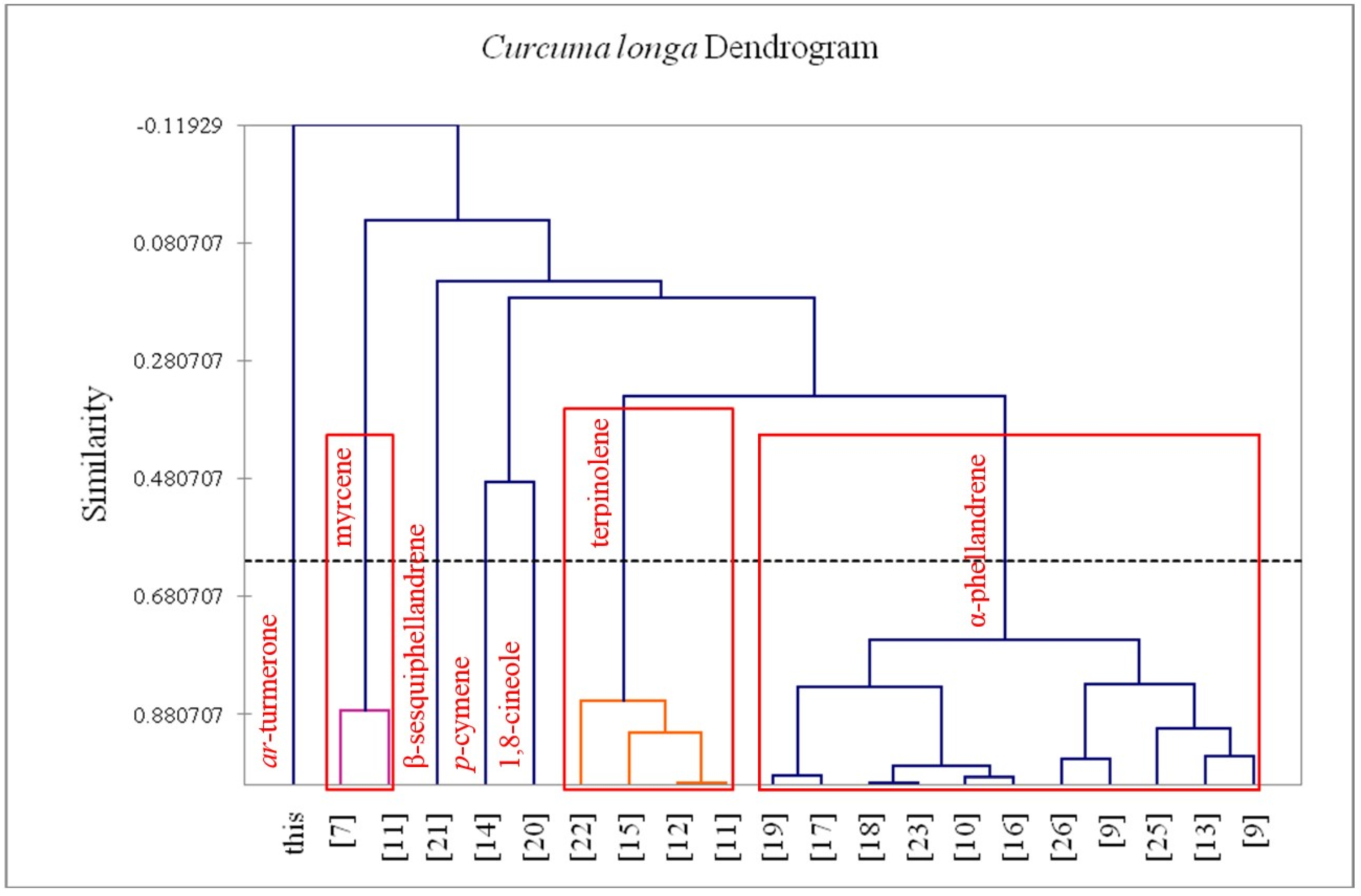
3. Results and Discussion
| RI a | Compound | % | RI a | Compound | % |
|---|---|---|---|---|---|
| 977 | β-Pinene | 0.1 | 1524 | β-Sesquiphellandrene | 0.9 |
| 1023 | p-Cymene | 1.6 | 1633 | β-Acorenol | 1.0 |
| 1029 | 1,8-Cineole | 1.6 | 1666 | ar-Turmerone | 63.4 |
| 1452 | α-Humulene | 0.2 | 1669 | β-Turmerone | 12.6 |
| 1483 | ar-Curcumene | 2.0 | 1705 | α-Turmerone | 13.7 |
| 1487 | β-Ionone | 0.6 | 1770 | (E)-α-Atlantone | 1.0 |
| 1509 | β-Bisabolene | 0.3 | - | Total Identified | 100 |
| Antimicrobial Activity (MIC, μg/mL) | ||||||
| Sample | B. cereus | S. aureus | E. coli | P. aeruginosa | C. albicans | A. niger |
| C. longa leaf EO | 78 | 78 | 312 | 625 | 312 | 19.5 |
| Positive control | 1.22 a | 0.61 a | 2.44 a | 1.22a | 0.61b | 0.61 b |
| Cytotoxic Activity | ||||||
| Sample | Hs578T c | PC-3 d | ||||
| C. longa leaf EO | 98.86 ± 0.63 | 97.94 ± 2.05 | ||||
| Tingenone | 100 | 100 | ||||
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chattopadhyay, I.; Biswas, K.; Bandyopadhyay, U.; Banerjee, R.K. Turmeric and curcumin: Biological actions and medicinal applications. Curr. Sci. 2004, 87, 44–53. [Google Scholar]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar] [PubMed]
- Mehra, A.; Bajpai, O.; Joshi, H. Diversity, utilization and sacred values of ethno-medicinal plants of Kumaun Himalaya. Trop. Plant Res. 2014, 1, 80–86. [Google Scholar]
- Thapa, S. Medico-ethnobotany of Magar community in Salija VDC of Parbat district, central Nepal. Our Nat. 2012, 10, 176–190. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, J.; Fang, H. Studies on Chinese Curcuma. III. Comparison of the volatile oil and phenolic constituents from the rhizome and tuber of Curcuma longa. Zhong Yao Tong Bao 1983, 8, 27–29. (In Chinese) [Google Scholar] [PubMed]
- Garg, S.N.; Bansal, R.P.; Gupta, M.M.; Kumar, S. Variation in the rhizome essential oil and curcumin contents and oil quality in the land races of turmeric Curcuma longa of north Indian plains. Flavour Fragr. J. 1999, 14, 315–318. [Google Scholar] [CrossRef]
- Bansal, R.P.; Bahl, J.R.; Garg, S.N.; Naqvi, A.A.; Sushil, K.; Kumar, S. Differential chemical composition of the essential oils of the shoot organs, rhizomes and rhizoids in the turmeric Curcuma longa grown in Indo-Gangetic plains. Pharm. Biol. 2002, 40, 384–389. [Google Scholar] [CrossRef]
- Akbar, A.; Kuanar, A.; Sandeep, I.S.; Kar, B.; Singh, S.; Mohanty, S.; Patnaik, J.; Nayak, S. GC-MS analysis of essential oil of some high drug yielding genotypes of turmeric (Curcuma longa L.). Int. J. Pharm. Pharmaceut. Sci. 2015, 7, 35–40. [Google Scholar]
- Awasthi, P.K.; Dixit, S.C. Chemical composition of Curcuma longa leaves and rhizome oil from the plains of northern India. J. Young Pharm. 2009, 1, 312–316. [Google Scholar]
- Behura, S.; Sahoo, S.; Srivastava, V.K. Major constituents in leaf essential oils of Curcuma longa L. and Curcuma aromatica Salisb. Curr. Sci. 2002, 83, 1312–1313. [Google Scholar]
- Behura, S.; Srivastava, V.K. Essential oils of leaves of Curcuma species. J. Essent. Oil Res. 2004, 16, 109–110. [Google Scholar] [CrossRef]
- Chane-Ming, J.; Vera, R.; Chalchat, J.C.; Cabassu, P. Chemical composition of essential oils from rhizomes, leaves and flowers of Curcuma longa L. from Reunion Island. J. Essent. Oil Res. 2002, 14, 249–251. [Google Scholar] [CrossRef]
- Dūng, N.X.; Tuyêt, N.T.B.; Leclercq, P.A. Constituents of the leaf oil of Curcuma domestica L. from Vietnam. J. Essent. Oil Res. 1995, 7, 701–703. [Google Scholar] [CrossRef]
- Garg, S.N.; Mengi, N.; Patra, N.K.; Charles, R.; Kumar, S. Chemical examination of the leaf essential oil of Curcuma longa L. from the north Indian plains. Flav. Fragr. J. 2002, 17, 103–104. [Google Scholar] [CrossRef]
- Kiran Babu, G.D.; Shanmugam, V.; Ravindranath, S.D.; Joshi, V.P. Comparison of chemical composition and antifungal activity of Curcuma longa L. leaf oils produced by different water distillation techniques. Flav. Fragr. J. 2007, 22, 191–196. [Google Scholar] [CrossRef]
- Kuanar, A.; Mohanty, S.; Panda, M.K.; Nayak, S. Essential oils from leaves of micropropagated turmeric. Curr. Sci. 2009, 96, 1166–1167. [Google Scholar]
- Leela, N.K.; Tava, A.; Shafi, P.M.; John, S.P.; Chempakam, B. Chemical composition of essential oils of turmeric (Curcuma longa L.). Acta Pharm. 2002, 52, 137–141. [Google Scholar]
- McCarron, M.; Mills, A.J.; Whittaker, D.; Sunny, T.P.; Verghese, J. Comparison of the monoterpenes derived from green leaves and fresh rhizomes of Curcuma longa L. from India. Flav. Fragr. J. 1995, 10, 355–357. [Google Scholar] [CrossRef]
- Oguntimein, B.O.; Weyerstahl, P.; Marschall-Weyerstahl, H. Essential oil of Curcuma longa L. leaves. Flav. Fragr. J. 1990, 5, 89–90. [Google Scholar] [CrossRef]
- Parveen, Z.; Nawaz, S.; Siddique, S.; Shahzad, K. Composition and antimicrobial activity of the essential oil from leaves of Curcuma longa L. Kasur variety. Indian J. Pharmaceut. Sci. 2013, 75, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Priya, R.; Prathapan, A.; Raghu, K.G.; Menon, A.N. Chemical composition and in vitro antioxidative potential of essential oil isolated from Curcuma longa L. leaves. Asian Pac. J. Trop. Biomed. 2012, 2, S695–S699. [Google Scholar] [CrossRef]
- Raina, V.K.; Srivastava, S.K.; Jain, N.; Ahmad, A.; Syamasundar, K.V.; Aggarwal, K.K. Essential oil composition of Curcuma longa L. cv. Roma from the plains of northern India. Flavour Fragr. J. 2002, 17, 99–102. [Google Scholar] [CrossRef]
- Raina, V.K.; Srivastava, S.K.; Syamsundar, K.V. Rhizome and leaf oil composition of Curcuma longa from the lower Himalayan region of northern India. J. Essent. Oil Res. 2005, 17, 556–559. [Google Scholar] [CrossRef]
- Sharma, R.K.; Mishra, B.P.; Sharma, T.C.; Bordloi, A.K.; Pathak, M.G.; Leclercq, P.A. Essential oil of Curcuma longa L. from Bhutan. J. Essent. Oil Res. 1997, 9, 589–592. [Google Scholar] [CrossRef]
- Sindhu, S.; Chempakam, B.; Leela, N.K.; Bhai, R.S. Chemoprevention by essential oil of turmeric leaves (Curcuma longa L.) on the growth of Aspergillus flavus and aflatoxin production. Food Chem. Toxicol. 2011, 49, 1188–1192. [Google Scholar] [CrossRef] [PubMed]
- Zaibunnisa, A.H.; Norashikin, S.; Mamot, S.; Osman, H. An experimental design approach for the extraction of volatile compounds from turmeric leaves (Curcuma domestica) using pressurised liquid extraction (PLE). LWT-Food Sci. Technol. 2009, 42, 233–238. [Google Scholar] [CrossRef]
- Rhyu, H.Y. Gas chromatographic characterization of sages of various geographic origins. J. Food Sci. 1979, 44, 758–762. [Google Scholar] [CrossRef]
- Piccaglia, R.; Marotti, M.; Galletti, G.C. Characterization of essential oil from a Satureja montana L. chemotype grown in northern Italy. J. Essent. Oil Res. 1991, 3, 147–152. [Google Scholar] [CrossRef]
- Shu, C.K.; Lawrence, B.M. Reasons for the variation in composition of some commercial essential oils. In Spices, Flavor Chemistry and Antioxidant Properties; ACS Symposium Series 660; Risch, S.J., Ho, C.T., Eds.; American Chemical Society: Washington, DC, USA, 1997; pp. 138–159. [Google Scholar]
- Mohagheghzaded, A.; Ardakani, M.S.; Ghannadi, A. Linalol-rich essential oil of Zataria multiflora Boiss. (Lamiaceae). Flavour Frag. J. 2000, 15, 119–122. [Google Scholar]
- British Pharmacopoeia Commission. British Pharmacopoeia; H.M. Stationery Office, Pharmaceutical Press: London, UK, 1980. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Sahm, D.H.; Washington, J.A. Antibacterial susceptibility tests: Dilution methods. In Manual of Clinical Microbiology, 5th ed.; Balows, A., Hausler, W.J., Herrmann, K.L., Isenberg, H.D., Shamody, H.J., Eds.; American Society for Microbiology: Washington, DC, USA, 1991. [Google Scholar]
- Hackett, A.J.; Smith, H.S.; Springer, E.L.; Owens, R.B.; Nelson-Rees, W.A.; Riggs, J.L.; Gardner, M.B. Two syngeneic cell lines from human breast tissues: The aneuploid mammary epithelial (Hs578T) and the diploid myoepithelial (Hs578Bst) cell lines. J. Natl. Cancer Inst. 1977, 58, 1795–1806. [Google Scholar] [PubMed]
- Kaighn, M.E.; Narayan, K.S.; Ohnuki, Y.; Lechner, J.F.; Jones, L. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Investig. Urol. 1979, 17, 16–23. [Google Scholar]
- Setzer, W.N.; Setzer, M.C.; Hopper, A.L.; Moriarity, D.M.; Lehrman, G.K.; Niekamp, K.L.; Morcomb, S.M.; Bates, R.B.; McClure, K.J.; Stessman, C.C.; et al. The cytotoxic activity of a Salacia liana species from Monteverde, Costa Rica, is due to a high concentration of tingenone. Planta Med. 1998, 64, 583. [Google Scholar] [CrossRef] [PubMed]
- CellTiter 96®AQueous One Solution Cell Proliferation Assay. Available online: https://cn.promega.com/resources/protocols/technical-bulletins/0/celltiter-96-aqueous-one-solution-cell-proliferation-assay-system-protocol/ (accessed on 15 December 2015).
- Lee, H.S. Antimicrobial properties of turmeric (Curcuma longa L.) rhizome-derived ar-turmerone and curcumin. Food Sci. Biotechnol. 2006, 15, 559–563. [Google Scholar]
- Negi, P.S.; Jayaprakasha, G.K.; Rao, L.J.M.; Sakariah, K.K. Antibacterial activity of turmeric oil: A byproduct from curcumin manufacture. J. Agric. Food Chem. 1999, 47, 4297–4300. [Google Scholar] [CrossRef] [PubMed]
- Norajit, K.; Laohakunjit, N.; Kerdchoechuen, O. Antibacterial effect of five Zingiberaceae essential oils. Molecules 2007, 12, 2047–2060. [Google Scholar] [CrossRef] [PubMed]
- Giang, P.M.; Huong, V.N.; Son, P.T. Antimicrobial activity of sesquiterpene constituents from some Curcuma species of Vietnam. Vietnamese J. Chem. 2000, 38, 91–94. [Google Scholar]
- Alzoreky, N.S.; Nakahara, K. Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int. J. Food Microbiol. 2003, 80, 223–230. [Google Scholar] [CrossRef]
- Jankasem, M.; Wuthi-udomlert, M.; Gritsanapan, W. Antidermatophytic properties of ar-turmerone, turmeric oil, and Curcuma longa preparations. ISRN Dermatol. 2013, 2013, 250597. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.D.; Mossini, S.A.G.; Ferreira, F.M.D.; Arrotéia, C.C.; da Costa, C.L.; Nakamura, C.V.; Junior, M.M. The inhibitory effects of Curcuma longa L. essential oil and curcumin on Aspergillus flavus Link growth and morphology. Sci. World J. 2013, 2013, 343804. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, O.D.; Jham, G.N.; Barcelos, R.C.; Mendonça, F.A.; Ghiviriga, I. Isolation and identification of the principal fungitoxic component of turmeric essential oil. J. Essent. Oil Res. 2007, 19, 387–391. [Google Scholar] [CrossRef]
- Baik, K.U.; Jung, S.H.; Ahn, B.Z. Recognition of pharmacophore of ar-turmerone for its anticancer activity. Arch. Pharmacal Res. 1993, 16, 254–256. [Google Scholar] [CrossRef]
- Schmidt, E.; Ryabchenko, B.; Wanner, J.; Jäger, W.; Jirovetz, L. Cytotoxic active constituents of essential oils of Curcuma longa and Curcuma zanthorrhiza. Nat. Prod. Commun. 2015, 10, 139–141. [Google Scholar] [PubMed]
- Ji, M.; Choi, J.; Lee, J.; Lee, Y. Induction of apoptosis by ar-turmerone on various cell lines. Int. J. Mol. Med. 2004, 14, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Paek, S.H.; Kim, G.J.; Jeong, H.S.; Yum, S.K. Ar-turmerone and β-atlantone induce internucleosomal DNA fragmentation associated with programmed cell death in human myeloid leukemia HL-60 cells. Arch. Pharmacal Res. 1996, 19, 91–94. [Google Scholar] [CrossRef]
- Aratanechemuge, Y.; Komiya, T.; Moteki, H.; Katsuzaki, H.; Imai, K.; Hibasami, H. Selective induction of apoptosis by ar-turmerone isolated from turmeric (Curcuma longa L) in two human leukemia cell lines, but not in human stomach cancer cell line. Int. J. Mol. Med. 2002, 9, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.B.; Wu, L.C.; Hsieh, Y.C.; Wu, C.H.; Chan, Y.J.; Chang, L.H.; Chang, C.M.J.; Hsu, S.L.; Teng, C.L.; Wu, C.C. Supercritical carbon dioxide extraction of aromatic turmerone from Curcuma longa Linn. induces apoptosis through reactive oxygen species-triggered intrinsic and extrinsic pathways in human hepatocellular carcinoma HepG2 cells. J. Agric. Food Chem. 2012, 60, 9620–9630. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Essien, E.E.; Newby, J.S.; Walker, T.M.; Setzer, W.N.; Ekundayo, O. Chemotaxonomic Characterization and in-Vitro Antimicrobial and Cytotoxic Activities of the Leaf Essential Oil of Curcuma longa Grown in Southern Nigeria. Medicines 2015, 2, 340-349. https://doi.org/10.3390/medicines2040340
Essien EE, Newby JS, Walker TM, Setzer WN, Ekundayo O. Chemotaxonomic Characterization and in-Vitro Antimicrobial and Cytotoxic Activities of the Leaf Essential Oil of Curcuma longa Grown in Southern Nigeria. Medicines. 2015; 2(4):340-349. https://doi.org/10.3390/medicines2040340
Chicago/Turabian StyleEssien, Emmanuel E., Jennifer Schmidt Newby, Tameka M. Walker, William N. Setzer, and Olusegun Ekundayo. 2015. "Chemotaxonomic Characterization and in-Vitro Antimicrobial and Cytotoxic Activities of the Leaf Essential Oil of Curcuma longa Grown in Southern Nigeria" Medicines 2, no. 4: 340-349. https://doi.org/10.3390/medicines2040340
APA StyleEssien, E. E., Newby, J. S., Walker, T. M., Setzer, W. N., & Ekundayo, O. (2015). Chemotaxonomic Characterization and in-Vitro Antimicrobial and Cytotoxic Activities of the Leaf Essential Oil of Curcuma longa Grown in Southern Nigeria. Medicines, 2(4), 340-349. https://doi.org/10.3390/medicines2040340