Modes of Action of Herbal Medicines and Plant Secondary Metabolites
Abstract
:1. Introduction
Why do Plants Produce so many Bioactive Metabolites?—A Lesson from Evolutionary Pharmacology
2. General Modes of Action of Secondary Metabolites
| Plant Species | Substance (Class) | Mode of Action | Properties/Applications |
|---|---|---|---|
| Aconitum napellus | aconitine (A) | activates Na+ channels | analgesic |
| Atropa belladonna | l-hyoscyamine (A) | antagonist of mAChR | parasympathomimetic |
| Camptotheca acuminate | camptothecin (A) | inhibitor of DNA topoisomerase | tumour therapy |
| Cannabis sativa | tetrahydrocannabinol (T) | activates THC receptor | analgesic |
| Catharanthus roseus | dimeric Vinca alkaloids (A) | inhibit microtubule assembly | tumor therapy |
| Chondrodendron tomentosum | tubocurarine (A) | inhibits nAChR | muscle relaxant |
| Cinchona pubescens | quinidine (A) | inhibits Na+ channels | antiarrhythmic |
| Coffea arabica | caffeine (A) | inhibits phosphodiesterase and adenosine receptors | stimulant |
| Colchicum autumnale | colchicine (A) | inhibits microtubule assembly | gout treatment |
| Cytisus scoparius | sparteine (A) | inhibits Na+ channels | antiarrhythmic |
| Digitalis lanata | digitoxin, digoxin (T) | inhibits Na+,K+-ATPase | heart insufficiency |
| Erythroxylum coca | cocaine (A) | inhibits Na+ channels and reuptake of noradrenaline and dopamine | analgesic; stimulant |
| Galanthus woronowii | galanthamine (A) | inhibits AChE | Alzheimer treatment |
| Lycopodium clavatum | huperzine A (A) | inhibits AChE | Alzheimer treatment |
| Papaver somniferum | morphine (A) | agonist of endorphine receptors | analgesic, hallucinogen |
| Physostigma venenosum | physostigmine (A) | inhibits AChE | Alzheimer treatment |
| Pilocarpus joborandi | pilocarpine (A) | agonist of mAChR | glaucoma treatment |
| Psychotria ipecacuanha | emetine (A) | protein biosynthesis inhibitor | treatment of amebae infections; emetic |
| Rauvolfia reserpina | reserpine (A) | inhibits the uptake of noradrenalin into postsynaptic vesicles | hypertonia treatment |
| Sanguinaria canadensis | sanguinarine (A) | DNA intercalator | antibacterial, antiviral |
| Strophantus gratus | ouabain (T) | inhibits Na+, K+-ATPase | heart insufficiency |
| Taxus brevifolia | paclitaxel (taxol) (A) | inhibits microtubule disassembly | tumour therapy |
| Medicinal Plant/Drug | Phenolics * | Terpenoids * | Saponins * | Polysaccharides * | Covalent Interactions ** |
|---|---|---|---|---|---|
| Actaea (syn. Cimicifuga) racemosa | ++ | ++ | |||
| Aesculus hippocastanum | ++ | ++ | |||
| Allium sativum | + | ++ | |||
| Althaea officinalis | + | ++ | |||
| Andrographis paniculata | + | ++ | |||
| Arctostaphylos uva-ursi | ++ | ++ | |||
| Arnica montana | ++ | ++ | + | + | + |
| Boswellia sacra | ++ | ++ | + | ||
| Calendula officinalis | ++ | ++ | ++ | + | |
| Centella asiatica | + | ++ | |||
| Cistus creticus | ++ | + | |||
| Crataegus monogyna | ++ | + | |||
| Curcuma longa | ++ | ++ | + | ||
| Cynara cardunculus | ++ | ++ | + | ||
| Echinacea purpurea | ++ | ++ | |||
| Eleutherococcus senticosus | ++ | ++ | ++ | + | |
| Eucalyptus globulus | + | ++ | |||
| Filipendula ulmaria | ++ | + | |||
| Gentiana lutea | ++ | ++ | + | ||
| Ginkgo biloba | ++ | ++ | |||
| Glycyrrhiza glabra | ++ | ++ | |||
| Harpagophytum procumbens | ++ | ++ | ++ | ||
| Hypericum perforatum | ++ | ++ | |||
| Matricaria chamomilla | ++ | ++ | + | + | |
| Mentha piperita | + | ++ | |||
| Orthosiphon aristatus | ++ | ++ | + | ||
| Panax ginseng | + | + | ++ | ||
| Pelargonium sidoides | ++ | ++ | |||
| Plantago lanceolata | ++ | ++ | + | ++ | + |
| Potentilla erecta | ++ | ++ | |||
| Quercus robur | ++ | + | |||
| Rhemannia glutinosa | ++ | + | ++ | ||
| Rosmarinus officinalis | ++ | ++ | + | ||
| Salix alba | ++ | ||||
| Silybum marianum | ++ | ||||
| Urtica dioica | ++ | + | |||
| Vaccinium macrocarpon | ++ | ||||
| Verbascum phlomoides | ++ | + | ++ | + | |
| Vitex agnus-castus | ++ | ++ | ++ | ||
| Zingiber officinale | ++ |
2.1. How Secondary Metabolites Used in Phytotherapy can Mediate Biological Activities?
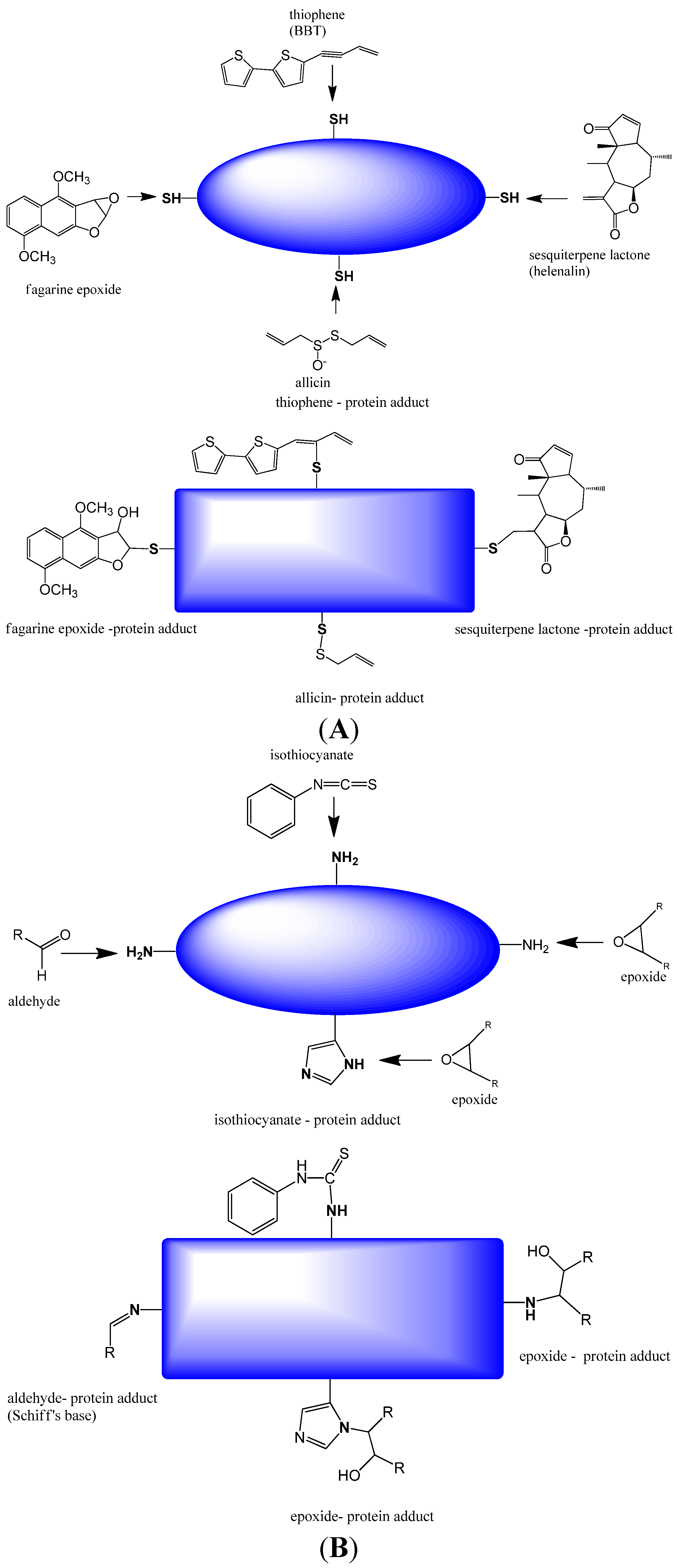
2.1.1. Covalent Modification of Proteins and DNA Bases
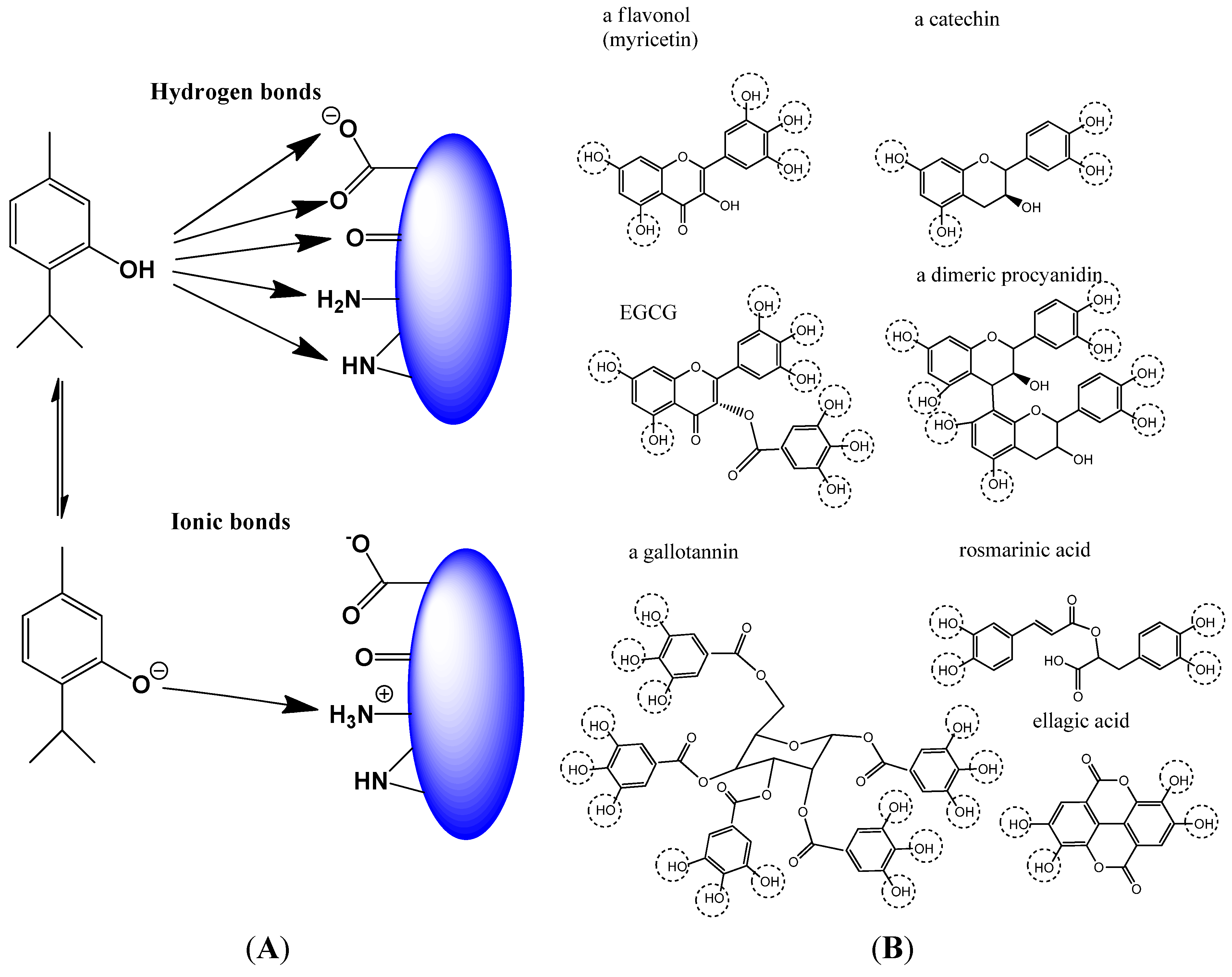
2.1.2. Non-Covalent Modification of Proteins
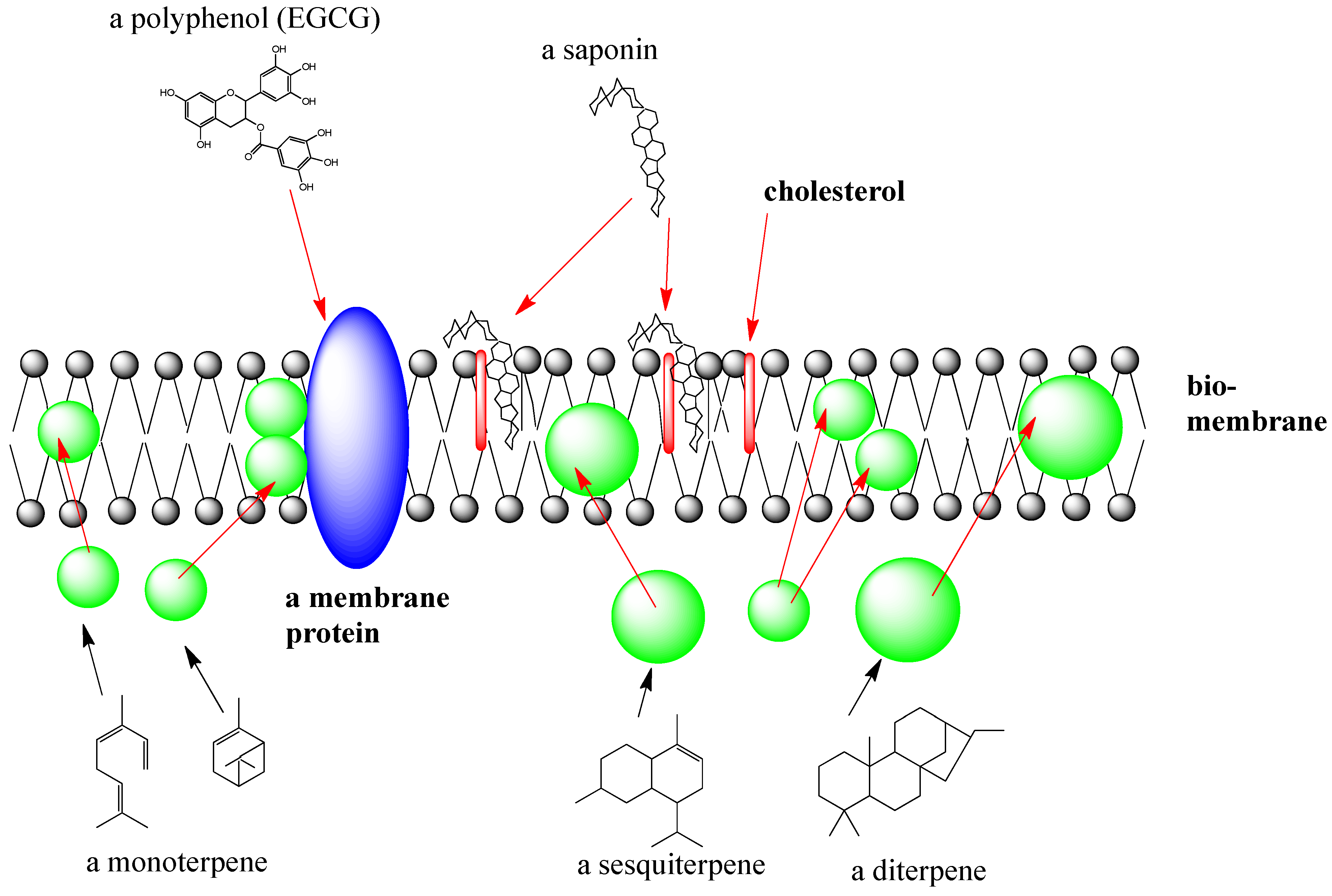
2.1.3. Interactions of SM with Biomembranes
2.1.4. Interactions of SM with Nucleic Acids
2.1.5. SM with Antioxidant Properties
3. Which Secondary Metabolites Occur in Plants and how do They Function?
| Class | Numbers of Structures | Toxic or Repellent for Herbivores | Antimicrobial Activity | Attraction of Pollinators or Fruit Dispersers |
|---|---|---|---|---|
| With nitrogen | ||||
| Alkaloids | 27,000 | ++++ | ++ | − |
| Non-protein amino acids (NPAA) | 700 | ++++ | +++ | − |
| Cyanogenic Glucosides/HCN | 60 | ++++ | + | − |
| Mustard oils (Glucosinolates) | 150 | ++++ | ++++ | +/− |
| Amines | 100 | +++ | + | +++ |
| Lectins, Peptides, AMPs | 2000 | +++ | +++ | − |
| Without nitrogen | ||||
| Terpenes | ||||
| Monoterpenes (including Iridoid glucosides) | 3000 | ++ | +++ | +++ |
| Sesquiterpenes | 5000 | +++ | +++ | ++ |
| Diterpenes | 2500 | +++ | +++ | − |
| Triterpenes, Steroids, Saponins (including cardiac glycosides) | 5000 | +++ | +++ | − |
| Tetraterpenes | 500 | + | + | +++ |
| Phenols | ||||
| Phenylpropanoids, coumarins, lignans | 2000 | +++ | +++ | ++ |
| Flavonoids, anthocyanins, tannins | 4000 | +++ | +++ | ++ |
| Polyketides (Anthraquinones) | 800 | ++++ | +++ | − |
| Others | ||||
| Polyacetylenes | 1500 | ++++ | ++++ | − |
| Carbohydrates, organic acids | 600 | + | ++ | + |
3.1. Nitrogen-Free Secondary Metabolites
3.1.1. Terpenes
Monoterpenes
Iridoid Glucosides
Sesquiterpenes and Sesquiterpenes Lactones
Diterpenes
Triterpenes and Steroids
Saponins
Cardiac Glycosides (CG)
Tetraterpenes
Polyterpenes
3.1.2. Phenolics
Phenylpropanoids
Coumarins and Furanocoumarins
Lignans and Lignin
Flavonoids and Anthocyanins
Catechins and Tannins
3.1.3. Quinones
Quinones and Naphthoquinones
Anthraquinones and other Polyketides
3.1.4. Polyacetylenes, Polyenes and Alkamides
3.1.5. Carbohydrates
3.1.6. Organic Acids
Ranunculin and Tuliposide
3.2. Nitrogen-Containing Secondary Metabolites
3.2.1. Alkaloids (Including Amines)
Amaryllidaceae Alkaloids
Bufotenin, Tryptamines and Tyramines
Colchicine
Diterpene Alkaloids
Ergot Alkaloids (EA)
Indole Alkaloids (including Monoterpene Indole Alkaloids)
Isoquinoline Alkaloids (including Protoberberine, Aporphine, and Morphinane Alkaloids)
Phenylpropylamines
Piperidine Alkaloids
Purine Alkaloids
Pyrrolidine Alkaloids
Pyrrolizidine Alkaloids (PA)
Quinolizidine Alkaloids (QA)
Quinoline Alkaloids (including Acridone Alkaloids)
Steroid Alkaloids
Tropane Alkaloids (TA)
3.2.2. Non-Protein Amino Acids (NPAAs)
3.2.3. Cyanogenic Glucosides (CG) and HCN
3.2.4. Glucosinolates and Mustard Oils
3.2.5. Lectins and Peptides
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bellamy, D.; Pfister, A. World Medicine—Plants, Patients and People; Blackwell Publishers: Oxford, UK, 1992. [Google Scholar]
- Hänsel, R.; Sticher, O.; Steinegger, E. Pharmakognosie—Phytopharmazie, 8th ed.; Springer Verlag: Heidelberg, Germany, 2007. [Google Scholar]
- Mutschler, E.; Geisslinger, G.; Kroemer, H.K.; Schäfer-Korting, M. Arzneimittelwirkungen—Lehrbuch der Pharmakologie und Toxikologie, 9th ed.; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 2008. [Google Scholar]
- Schmeller, T.; Wink, M. Utilization of alkaloids in modern medicine. In Alkaloids: Biochemistry, Ecology and Medicinal Applications; Roberts, M.F., Wink, M., Eds.; Plenum: New York, NY, USA, 2008; pp. 435–459. [Google Scholar]
- Bruneton, J. Phytothérapie—Les Données de L‘évaluation; Lavoisier: Paris, France, 2002. [Google Scholar]
- Chevallier, A. Encyclopedia of Medicinal Plants; Dorling Kindersley: London, UK, 2001. [Google Scholar]
- Duke, J.A. Handbook of Medicinal Herbs, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Saller, R.; Reichling, J.; Hellenbrecht, D. Phytotherapie—Klinische, Pharmakologische und Pharmazeutische Grundlagen; Karl F. Haug Verlag: Heidelberg, Germany, 1995. [Google Scholar]
- Tyler, V.E. The Honest Herbal, 3rd ed.; Pharmaceutical Products Press: New York, NY, USA, 1993. [Google Scholar]
- Tyler, V.E. Herb of Choice—The Therapeutic Use of Phytomedicinals; Pharmaceutical Products Press: New York, NY, USA, 1994. [Google Scholar]
- Van Wyk, B.-E.; Wink, M. Medicinal Plants of the World; Timber Press: Portland, OR, USA, 2004. [Google Scholar]
- Van Wyk, B.-E.; Wink, C.; Wink, M. Handbuch der Arzneipflanzen, 3rd ed.; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 2015. [Google Scholar]
- Van Wyk, B.-E.; Wink, M. Phytomedicines, Herbal drugs and Poisons; Briza, Kew Publishing, Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Wagner, H.; Vollmar, A.; Bechthold, A. Pharmazeutische Biologie 2. Biogene Arzneistoffe und Grundlagen von Gentechnik und Immunologie, 7th ed.; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 2007. [Google Scholar]
- Weiß, R.F.; Fintelmann, V. Lehrbuch der Phytotherapie, 10th ed.; Hippokrates Verlag: Stuttgart, Germany, 2002. [Google Scholar]
- Wichtl, M.; Bisset, N.G. Herbal Drugs and Phytopharmaceuticals; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Wiesenauer, M. PhytoPraxis, 5th ed.; Springer: Heidelberg, Germany, 2012. [Google Scholar]
- Bown, D. The RHS Encyclopedia of Herbs and Their Uses; Dorling Kindersley: London, UK, 1995. [Google Scholar]
- Bejeuhr, G. Hagers Handbuch der Pharmazeutischen Praxis, 5th ed.; Blaschek, W., Hänsel, R., Keller, K., Reichling, J., Rimpler, G., Schneider, G., Eds.; Springer Verlag: Heidelberg, Germany, 1998; Volumes 2,3. [Google Scholar]
- Bejeuhr, G. Hagers Handbuch der Pharmazeutischen Praxis, 5th ed.; Hänsel, R., Keller, K., Rimpler, H., Schneider, G., Eds.; Springer Verlag: Heidelberg, Germany, 1992–1994; Volumes 4–6. [Google Scholar]
- Schmeller, T.; El-Shazly, A.; Wink, M. Allelochemical activities of pyrrolizidine alkaloids: Interactions with neuroreceptors and acetylcholine related enzymes. J. Chem. Ecol. 1997, 23, 399–416. [Google Scholar] [CrossRef]
- Seigler, D.S. Plant Secondary Metabolism; Kluwer Academic Publishers: Boston, MA, USA, 1995. [Google Scholar]
- Teuscher, E.; Lindequist, U. Biogene Gifte—Biologie, Chemie, Pharmakologie, Toxikologie, 3rd ed.; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 2010. [Google Scholar]
- Teuscher, E.; Melzig, M.F.; Lindequist, U. Biogene Arzneimittel. Ein Lehrbuch der Pharmazeutischen Biologie, 7th ed.; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 2012. [Google Scholar]
- Wink, M. Functions of Plant Secondary Metabolites and their Exploitation in Biotechnology. Annual Plant Reviews; Wiley-Blackwell: London, UK, 2010; Volume 39. [Google Scholar]
- Wink, M. Plant secondary metabolism: Diversity, function and its evolution. Nat. Prod. Commun. 2008, 3, 1205–1216. [Google Scholar]
- Wink, M. Allelochemical properties and the raison d’être of alkaloids. Alkaloids 1993, 43, 1–118. [Google Scholar]
- Wink, M.; Schmeller, T.; Latz-Brüning, B. Modes of action of allelochemical alkaloids: Interaction with neuroreceptors, DNA and other molecular targets. J. Chem. Ecol. 1998, 24, 1881–1937. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Wink, M. Plant breeding: Importance of plant secondary metabolites for protection against pathogens and herbivores. Theor. Appl. Gen. 1988, 75, 225–233. [Google Scholar] [CrossRef]
- Mann, J. Murder, Magic and Medicine; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Mebs, D. Gifttiere; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 1992. [Google Scholar]
- Perrine, D. The Chemistry of Mind-Altering Drugs: History, Pharmacology, and Cultural Context; American Chemical Society: Washington, DC, USA, 1996. [Google Scholar]
- Rätsch, C. The Encyclopedia of Psychoactive Plants: Ethnopharmacology and its Applications; Park Street Press: South Paris, ME, USA, 2005. [Google Scholar]
- Russo, E. Handbook of Psychotropic Herbs: A Scientific Analysis of Herbal Remedies for Psychiatric Conditions; Haworth Press: Binghampton, NY, USA, 2001. [Google Scholar]
- Schulz, V.; Hänsel, R.; Tyler, V.E. Rational Phytotherapy—A Physician’s Guide to Herbal Medicine, 4th ed.; Springer: Heidelberg, Germany, 2001. [Google Scholar]
- Swerdlow, J.L. Nature’s Medicine—Plants that Heal; National Geographic: Washington, DC, USA, 2000. [Google Scholar]
- Wink, M.; van Wyk, B.-E. Mind-Altering and Poisonous Plants of the World; Timber Press: Portland, OR, USA, 2010. [Google Scholar]
- Ernst, E. The Complete German Commission E Monographs; Blumenthal, M., Ed.; American Botanical Council: Austin, TX, USA, 1998. [Google Scholar]
- European Pharmacopoeia, 8th ed.; Directorate for the Quality of Medicines and Health Care of the Council of Europe (EDQM): Strasbourg, France, 2014.
- ESCOP Monographs: The Scientific Foundations for Herbal Medicinal Products, 2nd ed.; ESCOP (Ed.) Thieme: Stuttgart, Germany, 2003.
- ESCOP Monographs Supplement. The Scientific Foundations for Herbal Medicinal Products, 2nd ed.; ESCOP (Ed.) Thieme: Stuttgart, Germany, 2009.
- Dewick, P.M. Medicinal Natural Products; Wiley: Chichester, UK, 2001. [Google Scholar]
- Duke, J.A. Database of Phytochemical Constituents of GRAS Herbs and Other Economic Plants; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Harborne, J.B.; Baxter, H. Phytochemical Dictionary—A Handbook of Bioactive Compounds from Plants; Taylor & Francis: London, UK, 1993. [Google Scholar]
- Hocking, G.M. A Dictionary of Natural Products; Plexus Publishing: Medford, UK, 1997. [Google Scholar]
- The Merck Index, 14th ed.; Merck: Rahway, NJ, USA, 2006.
- Wink, M. Biochemistry of Plant Secondary Metabolism. Annual Plant Reviews; Wiley Blackwell: London, Great Britain, 2010. [Google Scholar]
- Lewin, L. Phantastica: A Classic Survey on the Use and Abuse of Mind-Altering Plants; Park Street Press: South Paris, ME, USA, 1998. [Google Scholar]
- Spinella, M. The Psychopharmacology of Herbal Medicine: Plant Drugs That Alter Mind, Brain, and Behavior; MIT Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Wink, M.; Schimmer, O. Molecular modes of action of defensive secondary metabolites. In Functions and Biotechnology of Plant Secondary Metabolites. Annual Plant Reviews 39; Wink, M., Ed.; Wiley-Blackwell: London, UK, 2010; pp. 21–161. [Google Scholar]
- Wink, M. Interference of alkaloids with neuroreceptors and ion channels. In Bioactive Natural Products; Atta-Ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 11, pp. 3–129. [Google Scholar]
- Wink, M. Modes of action of alkaloids. In Alkaloids: Biochemistry, Ecology and Medicinal Applications; Roberts, M.F., Wink, M., Eds.; Plenum: New York, NY, USA, 1998; pp. 301–326. [Google Scholar]
- Wink, M. Molecular modes of action of cytotoxic alkaloids—From DNA intercalation, spindle poisoning, topoisomerase inhibition to apoptosis and multiple drug resistance. In The Alkaloids; Cordell, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 64, pp. 1–48. [Google Scholar]
- Goodman, L.S.; Gilman, A.G.; Limbird, L.E.; Hardman, J.G. Goodman Gilman A. The Pharmacological Basis of Therapeutics, 10th ed.; The McGraw-Hill Co.: London, UK, 2001. [Google Scholar]
- Martindale, W. Extra Pharmacopoeia, 30th ed.; The Pharmaceutical Press: London, UK, 1993. [Google Scholar]
- Samuelsson, G. Drugs of Natural Origin; Swedish Pharmaceutical Press: Stockholm, Sweden, 1992. [Google Scholar]
- Dingermann, T.; Loew, D. Phytopharmakologie; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 2003. [Google Scholar]
- Poyala, G. Biochemical Targets of Plant Bioactive Compounds; Taylor & Francis: London, UK, 2003. [Google Scholar]
- Roth, L.; Daunderer, M.; Kormann, K. Giftpflanzen—Pflanzengifte; Ecomed Verlagsgesellschaft: Landsberg, Germany, 1994. [Google Scholar]
- Wink, M. Wie funktionieren Phytopharmaka? Wirkmechanismen der Vielstoffgemische. Z. Phytother. 2005, 26, 271–274. [Google Scholar] [CrossRef]
- Wink, M. Molecular modes of action of drugs used in phytomedicine. In Herbal Medicines: Development and Validation of Plant-derived Medicines for Human Health; Bagetta, G., Cosentino, M., Corasaniti, M.T., Sakurada, S., Eds.; Taylor & Francis: London, UK, 2012; pp. 161–172. [Google Scholar]
- Wink, M. Evolutionary advantage and molecular modes of action of multi-component mixtures used in phytomedicine. Curr. Drug Metab. 2008, 9, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Jastorf, B.; Störmann, R.; Wölcke, U. Struktur-Wirkungs-Denken in der Chemie; Universitätsverlag Aschenbeck & Isensee: Bremen, Germany, 2003. [Google Scholar]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. Herbal Medicines—A Guide for Health Care Professionals, 2nd ed.; Rittenhouse Book Distributors: King of Prussia, PA, USA, 1996. [Google Scholar]
- Ody, P. Handbook of Over-The-Counter Herbal Medicines; Kyle Cathie Limited: London, UK, 1996. [Google Scholar]
- Wink, M. Medicinal plants: Source of anti-parasitic secondary metabolites. Molecules 2012, 17, 12771–12791. [Google Scholar] [CrossRef] [PubMed]
- Aleinein, R.A.; Schäfer, H.; Wink, M. Secretory ranalexin produced in recombinant Pichia pastoris exhibits additive bactericidal activity when used in combination with polymyxin B or linezolid against multi-drug resistant bacteria. Biotechnol. J. 2014, 9, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.Y.; El-Readi, M.Z.; Eldin, E.E.M.N.; Fatani, S.H.; Wink, M. Influence of combinations of digitonin with selected phenolics, terpenoids, and alkaloids on the expression and activity of P-glycoprotein in leukemia and colon cancer cells. Phytomedicine 2013, 21, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.Y.; El-Readi, M.Z.; Wink, M. Synergism of three-drug combinations of sanguinarine and other plant secondary metabolites with digitonin and doxorubicin in multi-drug resistant cancer cells. Phytomedicine 2012, 19, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.Y.; El-Readi, M.Z.; Wink, M. Carotenoids reverse multidrug resistance by interfering with ABC-transporters in cancer cells. Phytomedicine 2012, 19, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Hamoud, R.; Sporer, F.; Reichling, J.; Wink, M. Antimicrobial activity of a traditionally used blend of essential oils (Olbas®) in comparison to its individual essential oil ingredients. Phytomedicine 2012, 19, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Hamoud, R.; Reichling, J.; Wink, M. Synergistic antibacterial activity of the alkaloid sanguinarine with EDTA and the antibiotic streptomycin against multidrug resistant bacteria. J. Pharm. Pharmacol. 2015, 67, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Hamoud, R.; Zimmermann, S.; Sporer, F.; Reichling, J.; Wink, M. Synergistic interactions in two-drug and three-drug combinations (thymol, EDTA and vancomycin) against multidrug resistant bacteria including E. coli. Phytomedicine 2014, 21, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, J.K.; Münter, S.; Wink, M.; Frischknecht, F. Synergistic and additive effects of epigallocatechin gallate and digitonin on Plasmodium sporozoite survival and motility. PLoS ONE 2010, 5, e8682. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, F.; Hamoud, R.; Sporer, F.; Tahrani, A.; Wink, M. Carlina oxide- a natural polyacetylene from Carlina acaulis (Asteraceae) with potent antitrypanosomal and antimicrobial properties. Planta Med. 2011, 77, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, F.; Wink, M. Synergistic interactions of saponins and monoterpenes in HeLa and Cos7 cells and in erythrocytes. Phytomedicine 2011, 18, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.; Weiss, J.; Wink, M. Reduction of cytotoxicity of the alkaloid emetine through P-glycoprotein in human Caco-2 cells and leukemia cell lines. Planta Med. 2006, 72, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Mulyaningsih, S.; Sporer, F.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic resistant pathogens. Phytomedicine 2010, 17, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Wink, M. Tetrandrine and fangchinoline, bisbenzylisoquinoline alkaloids from Stephania tetrandra, are can reverse multidrug resistance by inhibiting P-glycoprotein activity in multidrug resistant human cancer cells. Phytomedicine 2014, 21, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Wink, M.; Ashour, M.; El-Readi, M.Z. Secondary metabolites inhibiting ABC transporters and reversing resistance of cancer cells and fungi to cytotoxic and antimicrobial agents. Front. Microbiol. 2012, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- El-Readi, M.Z.; Eid, S.Y.; Ashour, M.L.; Tahrani, A.; Wink, M. Modulation of multidrug resistance in cancer cells by chelidonine and Chelidonium majus alkaloids. Phytomedicine 2013, 20, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Pakalapati, G.; Wink, M.; Li, L.; Gretz, N.; Koch, E. Influence of Red Clover (Trifolium pratense) isoflavones on gene and protein expression profiles in liver of ovariectomized rats. Phytomedicine 2009, 16, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Holtrup, F.; Bauer, A.; Fellenberg, K.; Hilger, R.A.; Wink, M.; Hoheisel, J.D. Microarray analysis of nemorosone-induced cytotoxic effects on pancreatic cancer cells reveals activation of the unfolded protein response (UPR). Br. J. Pharmacol. 2011, 162, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, N.; Sudji, I.R.; Wink, M.; Tanaka, M. Mechanistic Investigation of Interactions between the Steroidal Saponin Digitonin and Cell Membrane Models. J. Phys. Chem. B 2014, 118, 14632–14639. [Google Scholar] [CrossRef] [PubMed]
- El-Shazly, A.; Wink, M. Structures, distribution, and biological properties of pyrrolizidine alkaloids of the Boraginaceae. Diversity 2014, 6, 188–282. [Google Scholar] [CrossRef]
- Schmeller, T.; Latz-Brüning, B.; Wink, M. Biochemical activities of berberine, palmatine and sanguinarine mediating chemical defence against microorganisms and herbivores. Phytochemistry 1997, 44, 257–266. [Google Scholar] [CrossRef]
- Abbas, S.; Wink, M. Epigallocatechin gallate (EGCG) from green tea (Camellia sinensis) increases lifespan and stress resistance in Caenorhabditis elegans. Planta Med.ic 2009, 75, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Wink, M. Epigallocatechin gallate inhibits beta-amyloid oligomerization in Caenorhabditis elegans and affects the daf-2/insulin-like signaling pathway. Phytomedicine 2010, 17, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Joubert, E.; van Wyk, B.E.; Wink, M. Ameliorative effect of aspalathin from rooibos (Aspalathus linearis) on acute oxidative stress in Caenorhabditis elegans. Phytomedicine 2013, 20, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Müller, D.; Richling, E.; Wink, M. Anthocyanin-rich purple wheat prolongs the life span of Caenorhabditis elegans probably by activating the DAF-16/FOXO transcription factor. J. Agric. Food Chem. 2013, 61, 3047–3053. [Google Scholar] [CrossRef] [PubMed]
- Link, P.; Fu, Y.; Wetterauer, B.; Wink, M. Extracts of Glycyrrhiza uralensis and isoliquiritigenin, one of its compounds, counteract amyloid-beta toxicity in Caenorhabditis elegans. Planta Med. 2015, 81, 357–362. [Google Scholar] [PubMed]
- Rezaizadehnajafi, L.; Wink, M. EPs7630® from Pelargonium sidoides increases stress resistance in Caenorhabditis elegans and enhances life span probably via the DAF-16/FOXO pathway. Phytomedicine 2014, 21, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wink, M. Natural lignans from Arctium lappa as antiaging agents in Caenorhabditis elegans. Phytochemistry 2015, 117, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Woldemichael, G.M.; Wink, M. Identification of biological activities of triterpenoid saponins from Chenopodium quinoa. J. Agric. Food Chem. 2001, 49, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Herrmann, F.; Tahrani, A.; Wink, M. Cytotoxic activity of secondary metabolites from Artemisia annua L. towards cancer cells in comparison to its designated active constituent artemisinin. Phytomedicine 2011, 18, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Kuljanabhagavad, T.; Wink, M. Biological activities and chemistry of saponins from Chenopodium quinoa Willd. Phytochem. Rev. 2009, 8, 473–490. [Google Scholar] [CrossRef]
- Su, S.; Cheng, X.; Wink, M. Cytotoxicity of arctigenin and matairesinol against the T-cell lymphoma cell line CCRF-CEM. J. Pharm. Pharmacol. 2015, 67, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Cheng, X.; Wink, M. Natural lignans from Arctium lappa modulate P-glycoprotein efflux function in multidrug resistant cancer cells. Phytomedicine 2015, 22, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.F.; Wink, M. Alkaloids. Biochemistry, Ecology and Medicinal Applications; Plenum Press: New York, NY, USA, 1998. [Google Scholar]
- Körper, S.; Wink, M.; Fink, R.A. Differential effects of alkaloids on sodium currents of isolated single skeletal muscle fibres. FEBS Lett. 1998, 436, 251–255. [Google Scholar] [CrossRef]
- Schmeller, T.; Sauerwein, M.; Sporer, F.; Müller, W.E.; Wink, M. Binding of quinolizidine alkaloids to nicotinic and muscarinic receptors. J. Nat. Prod. 1994, 57, 1316–1319. [Google Scholar] [CrossRef] [PubMed]
- Schmeller, T.; Sporer, F.; Sauerwein, M.; Wink, M. Binding of tropane alkaloids to nicotinic and muscarinic receptors. Pharmazie 1995, 50, 493–495. [Google Scholar] [PubMed]
- Walstab, J.; Wohlfarth, C.; Hovius, R.; Schmitteckert, S.; Röth, R.; Lasitschka, L.; Wink, M.; Bönisch, H.; Niesler, B. Natural compounds boldine and menthol are antagonists of human 5-HT3 receptors: Implications for treating functional gastrointestinal disorders. Neurogastroenterol. Motil. 2014, 26, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.; Herzer, K.; Wenger, T.; Herr, I.; Wink, M. The alkaloid emetine as a promising agent for the induction and enhancement of drug-induced apoptosis in leukaemia cells. Oncol. Rep. 2007, 14, 737–744. [Google Scholar]
- Noureini, S.K.; Wink, M. Dose-dependent cytotoxic effects of boldine in HepG-2 cells—Telomerase inhibition and apoptosis induction. Molecules 2015, 20, 3730–3743. [Google Scholar] [CrossRef] [PubMed]
- Noureini, S.K.; Wink, M. Antiproliferative effect of the isoquinoline alkaloid papaverine in hepatocarcinoma HepG2 cells—Inhibition of telomerase and induction of senescence. Molecules 2014, 19, 11846–11859. [Google Scholar] [CrossRef] [PubMed]
- Noureini, S.K.; Wink, M. Transcriptional down-regulation of hTERT and induction of senescence in HepG2 cells by the benzophenanthridine alkaloid chelidonine. World J. Gastroenterol. 2009, 7, 3603–3610. [Google Scholar] [CrossRef]
- Rosenkranz, V.; Wink, M. Induction of apoptosis by alkaloids in human promyelotic HL-60 cells. Z. Naturforschung J. Biosci. 2007, 62, 458–466. [Google Scholar]
- Rosenkranz, V.; Wink, M. Alkaloids induce programmed cell death in bloodstream forms of Trypanosomes (Trypanosoma b. brucei). Molecules 2008, 13, 2462–2473. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.; Wink, M. Characteristics of apoptosis induction by the alkaloid emetine in human tumor cell lines. Planta Med. 2007, 73, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wink, M. The beta-carboline alkaloid harmine inhibits telomerase activity of MCF-7 cells by down-regulating hTERT mRNA expression accompanied by an accelerated senescent phenotype. PeerJ 2013, 1, e174. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Fu, Y.; Zu, Y.; Schwarz, G.; Newman, D.; Wink, M. Molecular target-guided tumor therapy with natural products derived from Traditional Chinese Medicine. Curr. Med. Chem. 2007, 14, 2024–2032. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Wink, M. Chemical-biology of natural products from medicinal plants for cancer therapy. In Alternative and Complementary Therapies for Cancer; Alaoui-Jamali, M., Ed.; Springer: New York, NY, USA.
- Fu, Y.; Li, S.; Zu, Y.; Yang, G.; Yang, Z.; Luo, M.; Jiang, S.; Wink, M.; Efferth, T. Medicinal chemistry of paclitaxel and its analogues. Curr. Med. Chem. 2009, 16, 3966–3985. [Google Scholar] [CrossRef] [PubMed]
- El-Readi, Z.M.; Hamdan, D.; Nawal, M.; Farrag, A.; El-Shazly, A.; Wink, M. Inhibition of p-glycoprotein by limonin and other secondary metabolites from Citrus species in human colon and leukemia cell lines. Eur. J. Pharmacol. 2010, 626, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lei, Y.; Ma, Y.; Wink, M. Piperine, a piperidine alkaloid from Piper nigrum re-sensitizes P-gp, MRP1 and BCRP dependent multidrug resistant cancer cells. Phytomedicine 2011, 19, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wink, M. The beta-carboline alkaloid harmine inhibits BCRP and can reverse resistance to the anticancer drugs mitoxanthrone and camptothecin. Phytother. Res. 2010, 24, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wink, M. Lobeline, a piperidine alkaloid from Lobelia can reverse P-gp dependent multidrug resistance in tumor cells. Phytomedicine 2008, 15, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Schäfer, H.; Reichling, J.; Wink, M. Antibacterial properties of the antimicrobial peptide Ib-AMP4 from Impatiens balsamina produced in E. coli. Biotechnol. J. 2013, 8, 1213–1220. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wink, M. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines 2015, 2, 251-286. https://doi.org/10.3390/medicines2030251
Wink M. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines. 2015; 2(3):251-286. https://doi.org/10.3390/medicines2030251
Chicago/Turabian StyleWink, Michael. 2015. "Modes of Action of Herbal Medicines and Plant Secondary Metabolites" Medicines 2, no. 3: 251-286. https://doi.org/10.3390/medicines2030251
APA StyleWink, M. (2015). Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines, 2(3), 251-286. https://doi.org/10.3390/medicines2030251