Gabapentin-Associated Movement Disorders: A Literature Review
Abstract
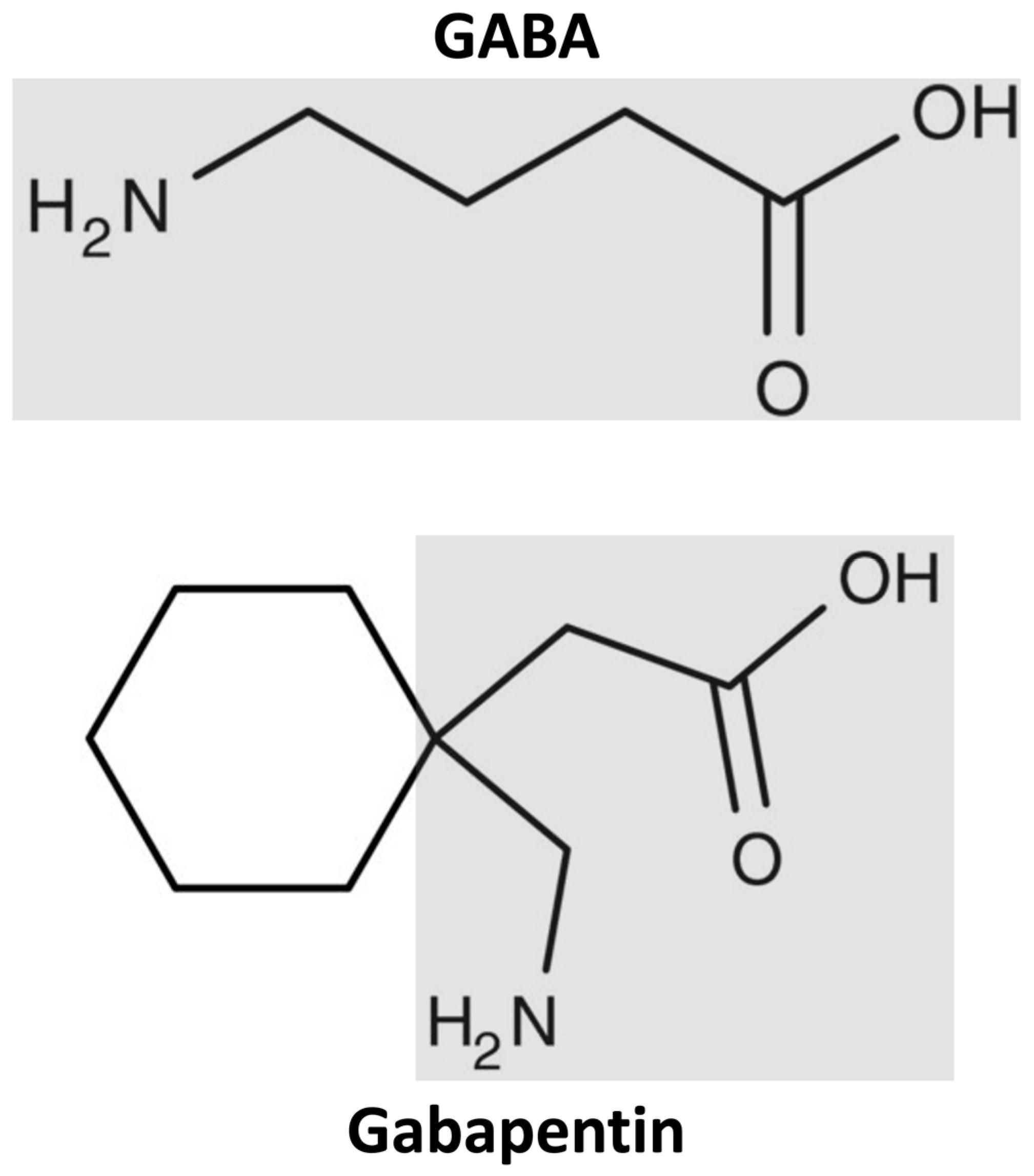
1. Introduction
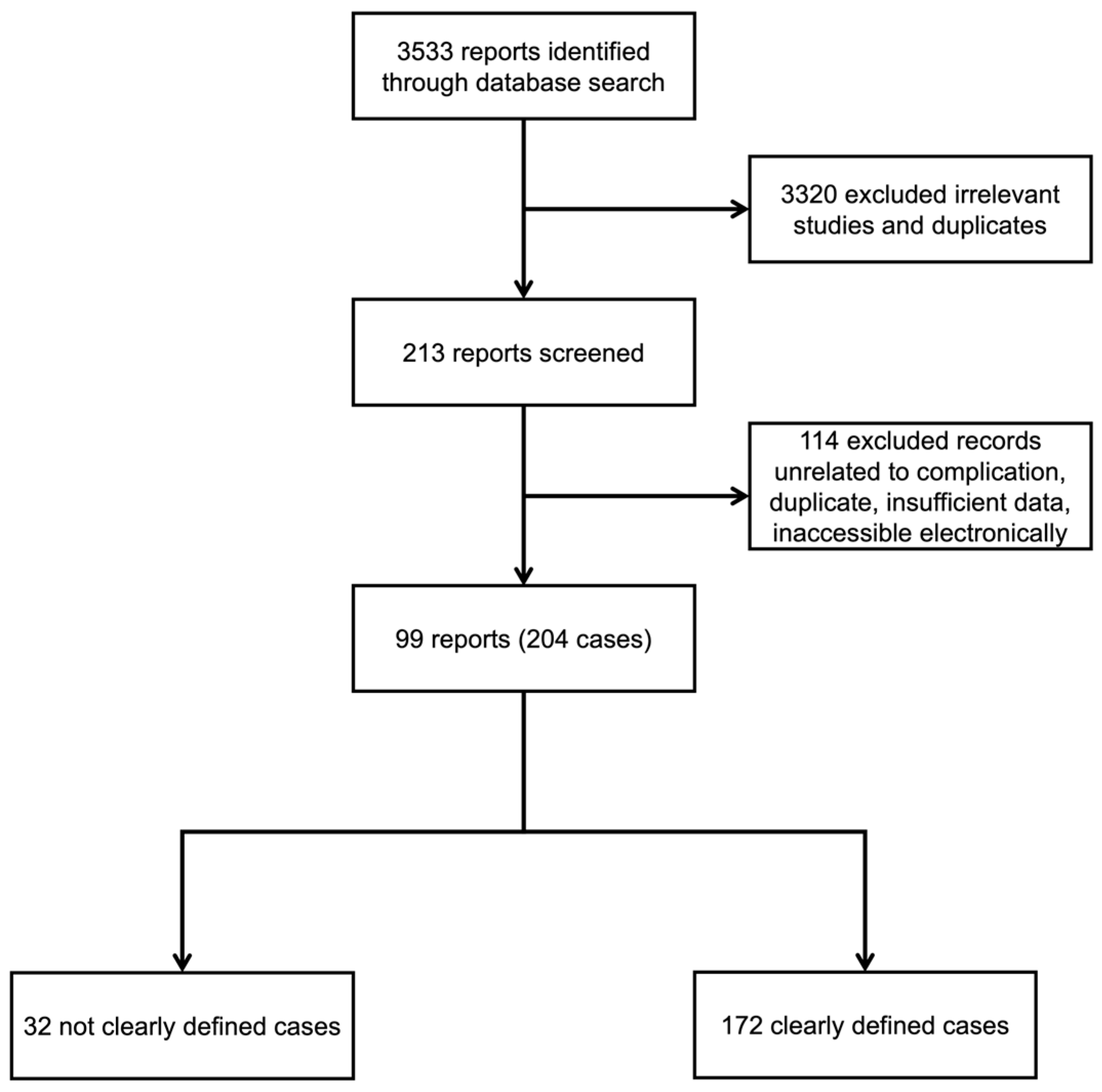
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Statistical Analysis
2.5. Definitions
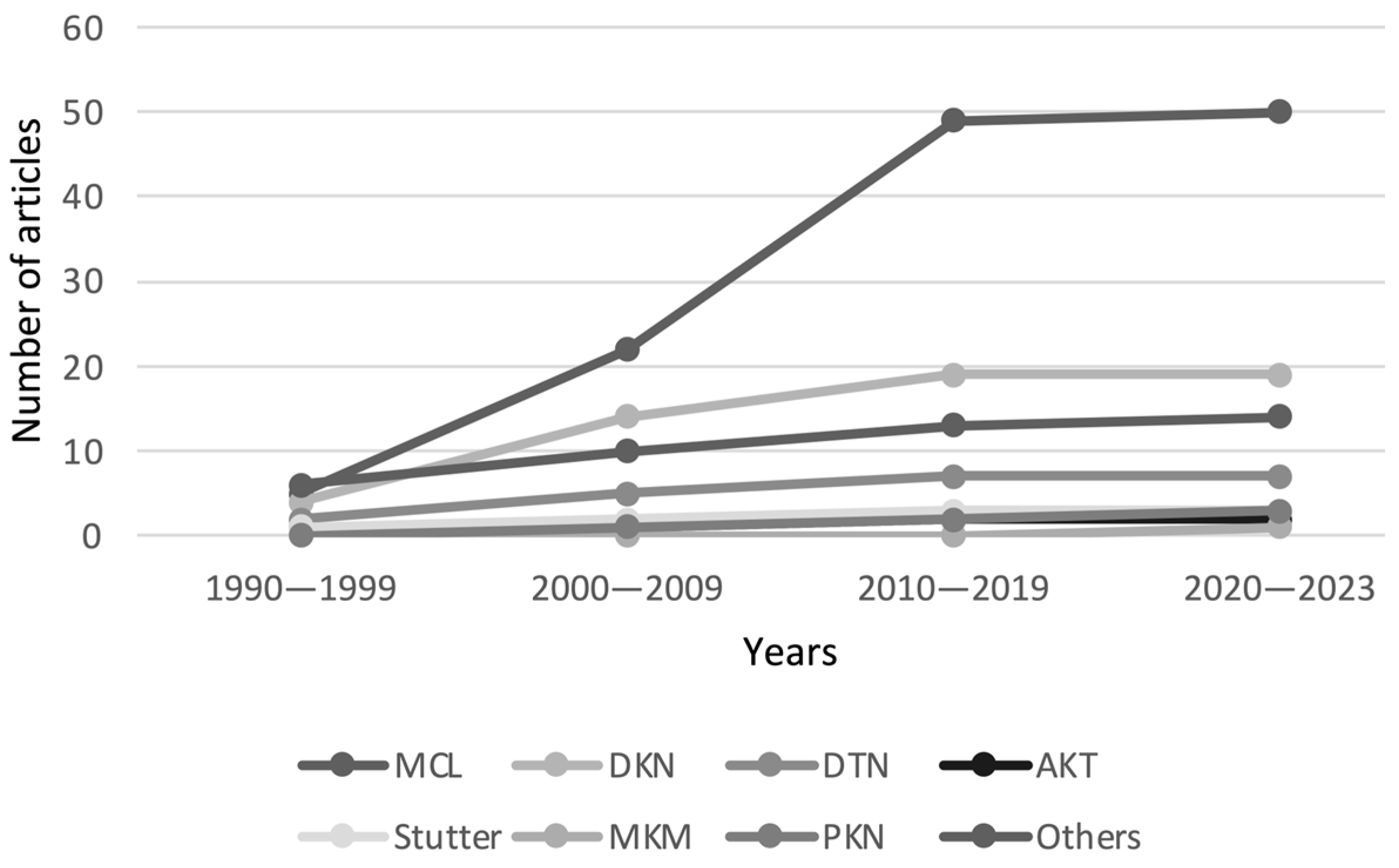
3. Results
4. Discussion
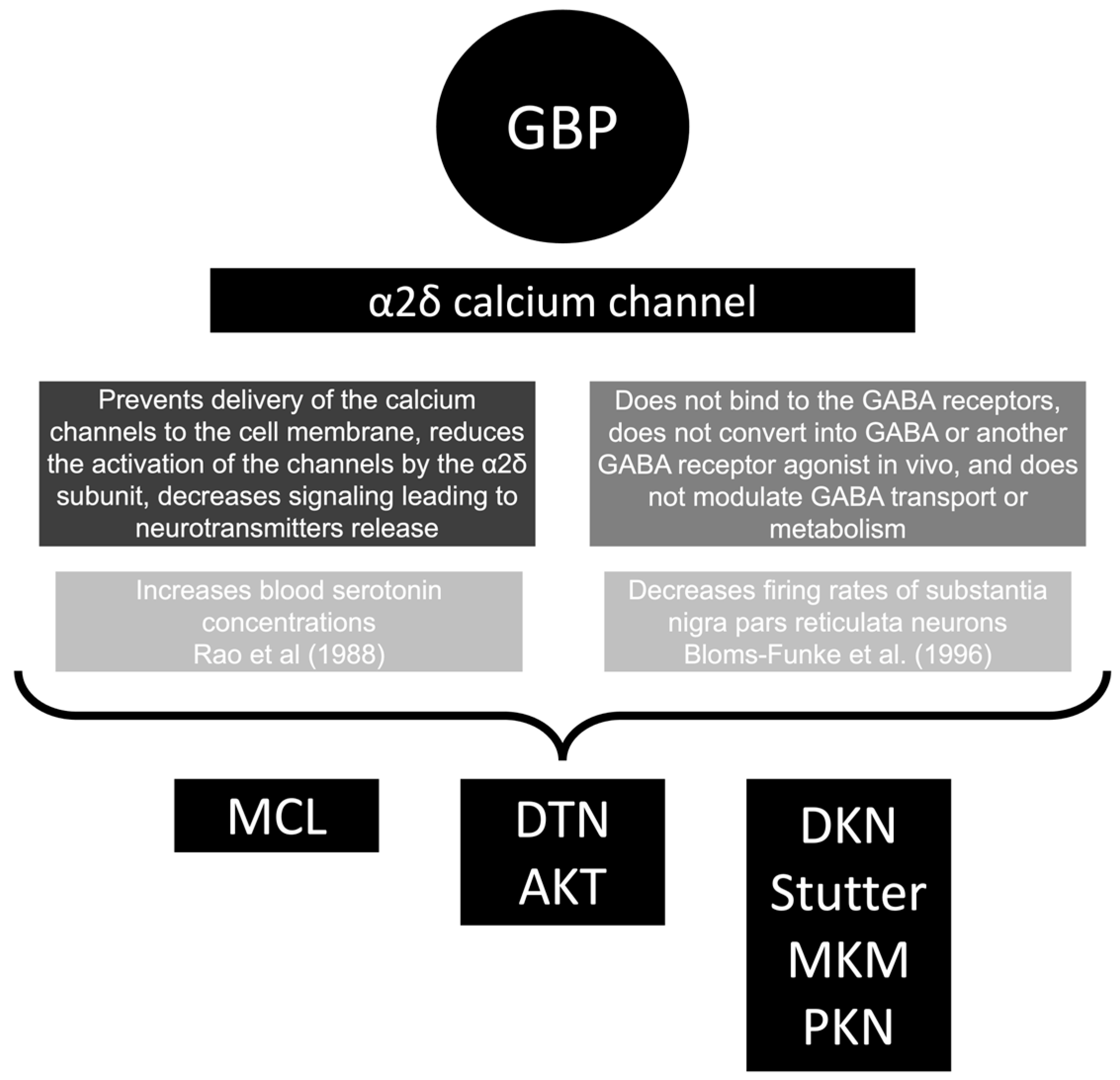
4.1. General
4.2. Myoclonus
4.3. Dyskinesia
4.4. Dystonia
4.5. Akathisia
4.6. Stuttering
4.7. Myokymia and Parkinsonism
4.8. Gabapentin and Pregabalin-Associated Movement Disorders
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarlo, G.L.; Holton, K.F. Brain Concentrations of Glutamate and GABA in Human Epilepsy: A Review. Seizure 2021, 91, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.S.; Li, J.J. The Art of Drug Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 1-118-67846-X. [Google Scholar]
- Su, T.Z.; Lunney, E.; Campbell, G.; Oxender, D.L. Transport of Gabapentin, a Gamma-Amino Acid Drug, by System l Alpha-Amino Acid Transporters: A Comparative Study in Astrocytes, Synaptosomes, and CHO Cells. J. Neurochem. 1995, 64, 2125–2131. [Google Scholar] [CrossRef]
- Bartoszyk, G.D.; Meyerson, N.; Reimann, W.; Satzinger, G.; von Hodenberg, A. Gabapentin. In Current Problems in Epilepsy: New Anticonvulsant Drugs; Meldrum, B.S., Porter, R.J., Eds.; John Libbey & Company: London, UK, 1986; pp. 147–164. [Google Scholar]
- Crawford, P.; Ghadiali, E.; Lane, R.; Blumhardt, L.; Chadwick, D. Gabapentin as an Antiepileptic Drug in Man. J. Neurol. Neurosurg. Psychiatry 1987, 50, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Group, U.G.S. The Long-Term Safety and Efficacy of Gabapentin (Neurontin®) as Add-on Therapy in Drug-Resistant Partial Epilepsy. Epilepsy Res. 1994, 18, 67–73. [Google Scholar]
- Williams, C.D.; Al-Jammali, Z.; Herink, M.C. Gabapentinoids for Pain: A Review of Published Comparative Effectiveness Trials and Data Submitted to the FDA for Approval. Drugs 2023, 83, 37–53. [Google Scholar] [CrossRef]
- Rissardo, J.P.; Caprara, A.L.F. Gabapentin-Associated Urinary Incontinence: A Case Verified by Rechallenge. Clin. Neuropharmacol. 2019, 42, 91–93. [Google Scholar] [CrossRef]
- Serpell, M.G.; Group, N.P.S. Gabapentin in Neuropathic Pain Syndromes: A Randomised, Double-Blind, Placebo-Controlled Trial. Pain 2002, 99, 557–566. [Google Scholar] [CrossRef]
- Tran-Van-Minh, A.; Dolphin, A.C. The A2δ Ligand Gabapentin Inhibits the Rab11-Dependent Recycling of the Calcium Channel Subunit A2δ-2. J. Neurosci. 2010, 30, 12856–12867. [Google Scholar] [CrossRef]
- Yu, J.; Wang, D.-S.; Bonin, R.P.; Penna, A.; Alavian-Ghavanini, A.; Zurek, A.A.; Rauw, G.; Baker, G.B.; Orser, B.A. Gabapentin Increases Expression of δ Subunit-Containing GABAA Receptors. eBioMedicine 2019, 42, 203–213. [Google Scholar] [CrossRef]
- Morris III, G.L. Efficacy and Tolerability of Gabapentin in Clinical Practice. Clin. Ther. 1995, 17, 891–900. [Google Scholar] [CrossRef]
- De Vries, E.; Schoonvelde, M.; Schumacher, G. No Longer Lost in Translation: Evidence That Google Translate Works for Comparative Bag-of-Words Text Applications. Political Anal. 2018, 26, 417–430. [Google Scholar] [CrossRef]
- Jankovic, J.; Hallett, M.; Okun, M.S.; Comella, C.L.; Fahn, S. Principles and Practice of Movement Disorders E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021; ISBN 0-323-31598-4. [Google Scholar]
- Ma, F. Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5). In Encyclopedia of Gerontology and Population Aging; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1414–1425. [Google Scholar]
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.A.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A Method for Estimating the Probability of Adverse Drug Reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245. [Google Scholar] [CrossRef]
- Rissardo, J.P.; Caprara, A.L.F. Fluoroquinolone-Associated Movement Disorder: A Literature Review. Medicines 2023, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Asconapé, J. Gabapentin-Associated Myoclonus. Neurology 1995, 45, A249–A250. [Google Scholar] [CrossRef] [PubMed]
- Reeves, A.L.; So, E.L.; Sharbrough, F.W.; Krahn, L.E. Movement Disorders Associated with the Use of Gabapentin. Epilepsia 1996, 37, 988–990. [Google Scholar] [CrossRef] [PubMed]
- Scheyer, R.D. Gabapentin Related Myoclonus. Epilepsia 1996, 37, 203. [Google Scholar]
- Wilson, E.A.; Sills, G.J.; Forrest, G.; Brodie, M.J. High Dose Gabapentin in Refractory Partial Epilepsy: Clinical Observations in 50 Patients. Epilepsy Res. 1998, 29, 161–166. [Google Scholar] [CrossRef]
- Asconapé, J.; Diedrich, A.; DellaBadia, J. Myoclonus Associated with the Use of Gabapentin. Epilepsia 2000, 41, 479–481. [Google Scholar] [CrossRef]
- Jacob, P.C.; Chand, R.P.; Omeima, E.-S. Asterixis Induced by Gabapentin. Clin. Neuropharmacol. 2000, 23, 53. [Google Scholar] [CrossRef]
- Huppertz, H.-J.; Feuerstein, T.J.; Schulze-Bonhage, A. Myoclonus in Epilepsy Patients with Anticonvulsive Add-on Therapy with Pregabalin. Epilepsia 2001, 42, 790–792. [Google Scholar] [CrossRef]
- Scullin, P.; Sheahan, P.; Kelly, S. Myoclonic Jerks Associated with Gabapentin. Palliat. Med. 2003, 17, 717. [Google Scholar] [CrossRef]
- Sechi, G.; Murgia, B.; Sau, G.; Peddone, L.; Tirotto, A.; Barrocu, M.; Rosati, G. Asterixis and Toxic Encephalopathy Induced by Gabapentin. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 195–199. [Google Scholar] [CrossRef]
- Babiy, M.; Stubblefield, M.D.; Herklotz, M.; Hand, M. Asterixis Related to Gabapentin as a Cause of Falls. Am. J. Phys. Med. Rehabil. 2005, 84, 136–140. [Google Scholar] [CrossRef]
- Bookwalter, T.; Gitlin, M. Gabapentin-Induced Neurologic Toxicities. Pharmacotherapy 2005, 25, 1817–1819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Glenn, D.G.; Bell, W.L.; O’Donovan, C.A. Gabapentin-Induced Myoclonus in End-Stage Renal Disease. Epilepsia 2005, 46, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Brefel-Courbon, C.; Gardette, V.; Ory, F.; Montastruc, J.L. Drug-Induced Myoclonus: A French Pharmacovigilance Database Study. Neurophysiol. Clin. 2006, 36, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Han, S.W.; Kim, S.Y.; Chu, M.K.; Yu, K.H.; Kim, Y.J.; Lee, B.C.; Kim, J. Gabapentin-Induced Myoclonus in a Patient with End-Stage Renal Disease. J. Korean Neurol. Assoc. 2006, 24, 495–497. [Google Scholar]
- Holtkamp, M.; Halle, A.; Meierkord, H.; Masuhr, F. Gabapentin-Induced Severe Myoclonus in a Patient with Impaired Renal Function. J. Neurol. 2006, 253, 382–383. [Google Scholar] [CrossRef]
- Striano, P.; Coppola, A.; Madia, F.; Pezzella, M.; Ciampa, C.; Zara, F.; Striano, S. Life-Threatening Status Epilepticus Following Gabapentin Administration in a Patient with Benign Adult Familial Myoclonic Epilepsy. Epilepsia 2007, 48, 1995–1998. [Google Scholar] [CrossRef]
- Cho, K.-T.; Hong, S.-K. Myoclonus Induced by the Use of Gabapentin. J. Korean Neurosurg. Soc. 2008, 43, 237–238. [Google Scholar] [CrossRef][Green Version]
- Ege, F.; Koçak, Y.; Titiz, A.P.; Oztürk, S.M.; Oztürk, S.; Ozbakir, S. Gabapentin-Induced Myoclonus: Case Report. Mov. Disord. 2008, 23, 1947–1948. [Google Scholar] [CrossRef]
- Pierce, D.A.; Holt, S.R.; Reeves-Daniel, A. A Probable Case of Gabapentin-Related Reversible Hearing Loss in a Patient with Acute Renal Failure. Clin. Ther. 2008, 30, 1681–1684. [Google Scholar] [CrossRef]
- Healy, D.G.; Ingle, G.T.; Brown, P. Pregabalin- and Gabapentin-Associated Myoclonus in a Patient with Chronic Renal Failure. Mov. Disord. 2009, 24, 2028–2029. [Google Scholar] [CrossRef]
- Koide, Y.; Ikeda, H.; Inoue, Y. Development or worsening of myoclonus associated with gabapentin therapy. Rinsho Shinkeigaku 2009, 49, 342–347. [Google Scholar] [CrossRef]
- Onuigbo, M.A.C.; Nye, D.; Iloanya, P.C. Drug-Induced Encephalopathy Secondary to Non Renal Dosing of Common Medications in Two Dialysis Patients. Adv. Perit. Dial. 2009, 25, 89–91. [Google Scholar] [PubMed]
- Chau, V.; Prasad, S.; Stewart, D.; Heckman, G. Creutzfeldt–Jakob Disease-like Syndrome Induced by Gabapentin Toxicity. Ageing Res. 2010, 1, e3. [Google Scholar] [CrossRef]
- Honma, H.; Chihara, S.; Yamada, R. Two Cases of Myoclonus Following Administration of Gabapentin for Neuropathic Pain in the End Stage of Malignancy. Palliat. Care Res. 2010, 5, 308–313. [Google Scholar] [CrossRef][Green Version]
- Zand, L.; McKian, K.P.; Qian, Q. Gabapentin Toxicity in Patients with Chronic Kidney Disease: A Preventable Cause of Morbidity. Am. J. Med. 2010, 123, 367–373. [Google Scholar] [CrossRef]
- Choe, E.Y.; Lee, B.-W.; Park, K.H.; Seok, H.; Kim, D.; Kang, E.S.; Cha, B.S.; Lee, H.C. A Case of Gabapentin-Induced Myoclonus in a Type 2 Diabetic Patient with End-Stage Renal Disease. J. Korean Diabetes 2011, 12, 171–173. [Google Scholar] [CrossRef][Green Version]
- Kampf, C.; Benecke, R.; Roesche, J. Provocation of Myoclonic Seizures and Generalized Electroencephalographic Epileptic Patterns in a Patient with Renal Failure and Gabapentin Intoxication. Am. J. Case Rep. 2012, 19, 159–163. [Google Scholar]
- Prieto-Pérez, L.; Montastruc, J.; García-Ruiz, P.J. Myoclonus secondary to gabapentin in a patient with chronic renal failure. Rev. Neurol. 2011, 52, 512. [Google Scholar] [PubMed]
- Guddati, A.K.; Zafar, Z.; Cheng, J.T.; Mohan, S. Treatment of Gabapentin-Induced Myoclonus with Continuous Renal Replacement Therapy. Indian J. Nephrol. 2012, 22, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Torregrosa-de Juan, E.; Olagüe-Díaz, P.; Royo-Maicas, P.; Fernández-Nájera, E.; García-Maset, R. Acute renal failure due to gabapentin. A case report and literature. Nefrologia 2012, 32, 130–131. [Google Scholar] [CrossRef]
- Atalay, H.; Solak, Y.; Biyik, Z.; Gaipov, A.; Guney, F.; Turk, S. Cross-over, Open-Label Trial of the Effects of Gabapentin versus Pregabalin on Painful Peripheral Neuropathy and Health-Related Quality of Life in Haemodialysis Patients. Clin. Drug Investig. 2013, 33, 401–408. [Google Scholar] [CrossRef]
- Kagnoff, M.; Shahed, J.J. Improvement in Cortical Myoclonus in a Patient with VIM Deep Brain Stimulation for Essential Tremor (P07.202). Neurology 2013, 80, P07.202. [Google Scholar]
- Wahba, M.; Waln, O. Asterixis Related to Gabapentin Intake: A Case Report and Review. Postgrad. Med. 2013, 125, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, T.; Gorodetsky, R. Recreational Abuse of Gabapentin and Pregabalin Are Under-Recognized Causes of Hospitalization Related to These Prescription Drugs. Clin. Toxicol. 2013, 51, 316. [Google Scholar]
- Kaufman, K.R.; Parikh, A.; Chan, L.; Bridgeman, M.; Shah, M. Myoclonus in Renal Failure: Two Cases of Gabapentin Toxicity. Epilepsy Behav. Case Rep. 2014, 2, 8–10. [Google Scholar] [CrossRef]
- Shea, Y.-F.; Mok, M.M.-Y.; Chang, R.S.-K. Gabapentin-Induced Myoclonus in an Elderly with End-Stage Renal Failure. J. Formos. Med. Assoc. 2014, 113, 660–661. [Google Scholar] [CrossRef]
- Clark, S.L.; Rabinstein, A.A.; Hocker, S.E. Disabling Asterixis Induced By Gabapentin. J. Med. Cases 2015, 6, 285–286. [Google Scholar] [CrossRef][Green Version]
- Guner, S.I.; Pamukçuoglu, M.; Sucak, G. A Patient with Multiple Myeloma Who Developed Severe Myoclonus after Stem Cell Transplantation. J. Leuk. 2015, 3, 4. [Google Scholar] [CrossRef]
- Ozmenoglu, M.; Gazioglu, S.; Cakmak, V.; Horozoglu, H. Gabapentin-Induced Myoclonus: A Case Report. J. Neurol. Sci. 2015, 357, e282–e283. [Google Scholar] [CrossRef]
- Schnitzer, O.; Gober, J.F.; Dalal, K. Poster 396 Gabapentin-Induced Myoclonus in Spinal Cord Injury. PM&R 2016, 8, S290–S291. [Google Scholar] [CrossRef]
- Ahmad, A.; Thrush, K. Gabapentin Induced Myoclonus in Elderly Patients-More Common than You Think! J. Am. Geriatr. Soc. 2017, 65, 152. [Google Scholar]
- Khan, M.; Khalid, S.; Marwat, A.; Aldergash, S.; Joshi, M.; Malhotra, V. Myoclonus in a Patient with Acute Kidney Injury: A Rare Presentation of Gabapentin Toxicity. Am. J. Case Rep. 2017, 5, 232–233. [Google Scholar] [CrossRef][Green Version]
- Kim, J.B.; Jung, J.-M.; Park, M.-H.; Lee, E.J.; Kwon, D.-Y. Negative Myoclonus Induced by Gabapentin and Pregabalin: A Case Series and Systematic Literature Review. J. Neurol. Sci. 2017, 382, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C. Myoclonus from Gabapentin Toxicity in the Setting Ofacute Kidney Injury. J. Gen. Intern. Med. 2017, 32, S545. [Google Scholar]
- Perez, A.; Williams, B. Severe Gabapentin Toxicity After Acute Kidney Injury in Hospitalized Patient with Acute Pain (P6.020). Neurology 2018, 90, P6.020. [Google Scholar]
- Desai, A.; Kherallah, Y.; Szabo, C.; Marawar, R. Gabapentin or Pregabalin Induced Myoclonus: A Case Series and Literature Review. J. Clin. Neurosci. 2019, 61, 225–234. [Google Scholar] [CrossRef]
- Hui, C.-H.; Leung, J.K.-C.; Chang, R.S.-K.; Shea, Y.-F. Reversible Dysphagia Due to Gabapentin-Induced Jaw Myoclonus. Chin. Med. J. 2019, 132, 1485–1486. [Google Scholar] [CrossRef]
- Medsafe Gathering Knowledge from Adverse Reaction Reports: March 2019. Prescr. Update 2019, 40, 22–23.
- Yeddi, A.; Adam, O.; Khalid, M.; Benjaram, S.; Abu-Heija, A.; Abdallah, M.A.; Shah, P. Myoclonus and Altered Mental Status Induced by Single Dose of Gabapentin in a Patient With End-Stage Renal Disease: A Case Report and Literature Review. Am. J. Ther. 2019, 26, e768–e770. [Google Scholar] [CrossRef]
- Latief, M.; Bhat, M.; Wani, M.; Shafi, O.; Goud, L.; Abbas, F.; Wani, M. Gabapentin Toxicity and Role of Dialysis: Case Series and Literature Review. J. Ren. Hepatic Disord. 2021, 6, 7–9. [Google Scholar] [CrossRef]
- Zadikoff, C.; Munhoz, R.P.; Asante, A.N.; Politzer, N.; Wennberg, R.; Carlen, P.; Lang, A. Movement Disorders in Patients Taking Anticonvulsants. J. Neurol. Neurosurg. Psychiatry 2007, 78, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Paez, T.; Montastruc, F.; Rousseau, V.; Chebane, L.; Lapeyre-Mestre, M.; Renoux, C.; Montastruc, J.-L. Parkinsonism Associated with Gabapentinoid Drugs: A Pharmacoepidemiologic Study. Mov. Disord. 2020, 35, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Ri, K.; Fukasawa, T.; Yoshida, S.; Takeuchi, M.; Kawakami, K. Risk of Parkinsonism and Related Movement Disorders with Gabapentinoids or Tramadol: A Case-Crossover Study. Pharmacotherapy 2023, 43, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Buetefisch, C.M.; Gutierrez, A.; Gutmann, L. Choreoathetotic Movements: A Possible Side Effect of Gabapentin. Neurology 1996, 46, 851–852. [Google Scholar] [PubMed]
- Millichap, J.G. Choreoathetosis with Gabapentin. Pediatr. Neurol. Briefs 1996, 10, 29. [Google Scholar] [CrossRef]
- Chudnow, R.S.; Dewey, R.B.J.; Lawson, C.R. Choreoathetosis as a Side Effect of Gabapentin Therapy in Severely Neurologically Impaired Patients. Arch. Neurol. 1997, 54, 910–912. [Google Scholar]
- Millichap, J.G. Gabapentin-Induced Choreoathetosis. Pediatr. Neurol. Briefs 1997, 11, 66–67. [Google Scholar] [CrossRef][Green Version]
- Norton, J.W.; Quarles, E. Gabapentin-Related Dyskinesia. J. Clin. Psychopharmacol. 2001, 21, 623–624. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.H.; Wang, T.Y.; Li, T.Y.; Chang, C.C.; Chang, S.T. Choreoathetosis as Side Effect of Gabapentin Therapy in a Patient with Spontaneous Spinal Epidural Hematoma: A Case Report. Taiwan J. Rehabil. Med. 2007, 35, 179–185. [Google Scholar]
- Raju, P.M.; Walker, R.W.; Lee, M.A. Dyskinesia Induced by Gabapentin in Idiopathic Parkinson’s Disease. Mov. Disord. 2007, 22, 288–289. [Google Scholar] [CrossRef]
- Lai, M.-H.; Wang, T.-Y.; Chang, C.-C.; Tsai, K.-C.; Chang, S.-T. Hemichorea Associated with Gabapentin Therapy with Hypoperfusion in Contralateral Basal Ganglion—A Case of a Paraplegic Patient with Neuropathic Pain. J. Clin. Pharm. Ther. 2008, 33, 83–86. [Google Scholar] [CrossRef]
- Shin, Y.-K.; Hong, S.-C.; Ihn, Y.K.; Jeong, J.-H.; Han, J.-H.; Lee, S.-P. A Case of a Patient with Both Chorea and Restless Legs Syndrome. J. Korean Med. Sci. 2008, 23, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Twardowschy, C.A.; Teive, H.A.G.; Fernandes, A.F.; Búrigo, I.P.; Lange, M.; Werneck, L.C. Chorea Due to Gabapentin Monotherapy in a Not Encephalopatic Patient. Arq. Neuropsiquiatr. 2008, 66, 107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zesiewicz, T.A.; Shimberg, W.R.; Hauser, R.A.; Robinson, W.; Wilson, M.-C.; Sullivan, K.L. Chorea as a Side Effect of Gabapentin (Neurontin) in a Patient with Complex Regional Pain Syndrome Type 1. Clin. Rheumatol. 2008, 27, 389–390. [Google Scholar] [CrossRef]
- Attupurath, R.; Aziz, R.; Wollman, D.; Muralee, S.; Tampi, R.R. Chorea Associated with Gabapentin Use in an Elderly Man. Am. J. Geriatr. Pharmacother. 2009, 7, 220–224. [Google Scholar] [CrossRef]
- Bonakis, A.; Papageorgiou, S.G.; Potagas, C.; Karahalios, G.; Kalfakis, N. A Case of Refractory Secondary Paroxysmal Kinesigenic Dyskinesia with High Sensitivity to Phenytoin Monotherapy. Park. Relat. Disord. 2009, 15, 68–70. [Google Scholar] [CrossRef]
- Erol, C.; Ozben, S.; Ozer, F.; Cetin, S.; Tiras, R. Bilateral Ballism Induced by Gabapentin in Idiopatic Parkinson’s Disease. Clin. Neurol. Neurosurg. 2009, 111, 397. [Google Scholar] [CrossRef]
- Aksoy, D.; Çevik, B.; Solmaz, V.; Kurt, S.G.; Sümbül, O. A Dyskinesia Case Induced by Pramipexole, Pregabalin and Gabapentin After Cardiopulmonary Resuscitation. Turk. J. Neurol. 2013, 19, 148–150. [Google Scholar]
- Souzdalnitski, D.; Chang, A.K.; Guirguis, M. Chorea in a Chronic Pain Patient Using Gabapentin. Ochsner J. 2014, 14, 276–278. [Google Scholar] [PubMed]
- VanHook, C.J.; Laredo, R.; Beer, M.; Tangel, D. Gabapentin Toxicitypresenting as Choreoathetosis. Hosp. Med. 2017, 12, 769. [Google Scholar]
- Rahil, A.; Abdulqader, M.; Suliman, A. Gabapentin-Induced Hemichorea in a Young Female: A Case Report and Literature Review. Libyan J. Med. Sci. 2018, 2, 152–154. [Google Scholar]
- Hampton, Z.; Shahrestani, N.; Little, A. Gabapentin-Induced Facial Myoclonus in the Setting of Acute on Chronic Kidney Disease. Cureus 2019, 11, e4758. [Google Scholar] [CrossRef]
- Childers, M.K.; Holland, D. Psychomotor Agitation Following Gabapentin Use in Brain Injury. Brain Inj. 1997, 11, 537–540. [Google Scholar] [CrossRef]
- See, S.; Hendriks, E.; Hsiung, L. Akathisia Induced by Gabapentin Withdrawal. Ann. Pharmacother. 2011, 45, e31. [Google Scholar] [CrossRef]
- Nissani, M.; Sanchez, E.A. Stuttering Caused by Gabapentin. Ann. Intern. Med. 1997, 126, 410. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, Y.J.; Baik, H.J.; Kim, J.H. Stuttering during Management of Postherpetic Neuralgia A Case Report. Korean J. Pain 2004, 17, 275–278. [Google Scholar] [CrossRef]
- Zeldin, E.; Byrd, M.; Barfield, W.; Darr, E. Gabapentin Treatment Resulting in New-Onset Stuttering: A Case Report and Literature Review. J. Orthop. Rehabil. Res. 2019, 1, 3–4. [Google Scholar] [CrossRef]
- Bernal, M.; Arcocha, J.; Peralta, P.; Valdizán, J.R. Dystonic movements: A possible secondary effect of gabapentin. Rev. Neurol. 1999, 28, 1215. [Google Scholar] [PubMed]
- Palomeras, E.; Sanz, P.; Cano, A.; Fossas, P. Dystonia in a Patient Treated with Propranolol and Gabapentin. Arch. Neurol. 2000, 57, 570–571. [Google Scholar] [CrossRef] [PubMed]
- Pina, M.A.; Modrego, P.J. Dystonia Induced by Gabapentin. Ann. Pharmacother. 2005, 39, 380–382. [Google Scholar] [CrossRef]
- Allford, M.A. Prolonged Myotonia and Dystonia after General Anaesthesia in a Patient Taking Gabapentin. Br. J. Anaesth. 2007, 99, 218–220. [Google Scholar] [CrossRef][Green Version]
- Lal, N. Drug-Induced Secondary Dystonia: Clinical Presentation and Management. Indian J. Med. Spec. 2011, 2, 147–149. [Google Scholar] [CrossRef]
- Rohman, L.; Hebron, A. Acute Dystonic Reaction Caused by Gabapentin. J. Emerg. Med. 2014, 46, e89. [Google Scholar] [CrossRef]
- Brown, A.; Esechie, A.; Gogia, B.; Shanina, E. Gabapentin-Induced Myokymia: A Case Report. Clin. Neuropharmacol. 2021, 44, 75–76. [Google Scholar] [CrossRef]
- Bergey, G.K.; Morris, H.H.; Rosenfeld, W.; Blume, W.T.; Penovich, P.E.; Morrell, M.J.; Leiderman, D.B.; Crockatt, J.G.; LaMoreaux, L.; Garofalo, E.; et al. Gabapentin Monotherapy: I. An 8-Day, Double-Blind, Dose-Controlled, Multicenter Study in Hospitalized Patients with Refractory Complex Partial or Secondarily Generalized Seizures. The US Gabapentin Study Group 88/89. Neurology 1997, 49, 739–745. [Google Scholar] [CrossRef]
- Steinhoff, B.J.; Herrendorf, G.; Bittermann, H.J.; Kurth, C. Isolated Ataxia as an Idiosyncratic Side-Effect under Gabapentin. Seizure 1997, 6, 503–504. [Google Scholar] [CrossRef]
- Mayer, T.; Schütte, W.; Wolf, P.; Elger, C.E. Gabapentin Add-on Treatment: How Many Patients Become Seizure-Free? An Open-Label Multicenter Study. Acta Neurol. Scand. 1999, 99, 1–7. [Google Scholar] [CrossRef]
- McLean, M.J.; Morrell, M.J.; Willmore, L.J.; Privitera, M.D.; Faught, R.E.; Holmes, G.L.; Magnus-Miller, L.; Bernstein, P.; Rose-Legatt, A. Safety and Tolerability of Gabapentin as Adjunctive Therapy in a Large, Multicenter Study. Epilepsia 1999, 40, 965–972. [Google Scholar] [CrossRef]
- Perugi, G.; Toni, C.; Ruffolo, G.; Sartini, S.; Simonini, E.; Akiskal, H. Clinical Experience Using Adjunctive Gabapentin in Treatment-Resistant Bipolar Mixed States. Pharmacopsychiatry 1999, 32, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Dallocchio, C.; Buffa, C.; Mazzarello, P.; Chiroli, S. Gabapentin vs. Amitriptyline in Painful Diabetic Neuropathy: An Open-Label Pilot Study. J. Pain Symptom Manag. 2000, 20, 280–285. [Google Scholar] [CrossRef]
- Mellegers, M.A.; Furlan, A.D.; Mailis, A. Gabapentin for Neuropathic Pain: Systematic Review of Controlled and Uncontrolled Literature. Clin. J. Pain 2001, 17, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Wilton, L.V.; Shakir, S. A Postmarketing Surveillance Study of Gabapentin as Add-on Therapy for 3100 Patients in England. Epilepsia 2002, 43, 983–992. [Google Scholar] [CrossRef]
- Klein-Schwartz, W.; Shepherd, J.G.; Gorman, S.; Dahl, B. Characterization of Gabapentin Overdose Using a Poison Center Case Series. J. Toxicol. Clin. Toxicol. 2003, 41, 11–15. [Google Scholar] [CrossRef]
- Karagoz, A.; Guney, I.; Yazici, R.; Arslan, S.; Altintepe, L. Gabapentin Toxicity in Patients with Chronic Kidney Disease. Nephrol. Dial. Transplant. Turk. 2013, 22, 297–299. [Google Scholar] [CrossRef]
- Pal, G.; Lin, M.M.; Laureno, R. Asterixis: A Study of 103 Patients. Metab. Brain Dis. 2014, 29, 813–824. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Park, M.H. Regardless of Impaired Renal Function, Negative Myoclonus Can Be Induced by Gabapetin and Pregabalin. Mov. Disord. 2013, 34, S597. [Google Scholar]
- Ghayur, M.N. Potential Adverse Consequences of Combination Therapy with Gabapentin and Pregabalin. Case Rep. Med. 2021, 2021, 5559981. [Google Scholar] [CrossRef] [PubMed]
- ClinCalc. The Top 300 of 2020. Available online: https://clincalc.com/DrugStats/Top300Drugs.aspx (accessed on 23 June 2023).
- Shorvon, S.D. Drug Treatment of Epilepsy in the Century of the ILAE: The Second 50 Years, 1959–2009. Epilepsia 2009, 50 (Suppl. S3), 93–130. [Google Scholar] [CrossRef]
- Walton, S.M.; Schumock, G.T.; Lee, K.-V.; Alexander, G.C.; Meltzer, D.; Stafford, R.S. Prioritizing Future Research on Off-Label Prescribing: Results of a Quantitative Evaluation. Pharmacotherapy 2008, 28, 1443–1452. [Google Scholar] [CrossRef]
- Peckham, A.M.; Ananickal, M.J.; Sclar, D.A. Gabapentin Use, Abuse, and the US Opioid Epidemic: The Case for Reclassification as a Controlled Substance and the Need for Pharmacovigilance. Risk Manag. Healthc. Policy 2018, 11, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.L.; Clarenbach, P.; Vahlensieck, M.; Krätzschmar, S. Gabapentin Augments Whole Blood Serotonin in Healthy Young Men. J. Neural Transm. 1988, 73, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Bloms-Funke, P.; Löscher, W. The Anticonvulsant Gabapentin Decreases Firing Rates of Substantia Nigra Pars Reticulata Neurons. Eur. J. Pharmacol. 1996, 316, 211–218. [Google Scholar] [CrossRef]
- Rissardo, J.P.; Caprara, A.L.F. The Link Between Amitriptyline and Movement Disorders: Clinical Profile and Outcome. Ann. Acad. Med. Singap. 2020, 49, 236–251. [Google Scholar] [CrossRef]
- Rissardo, J.P.; Caprara, A.L.F.; Durante, Í.; Rauber, A. Lithium-Associated Movement Disorder: A Literature Review. Brain Circ. 2022, 8, 76–86. [Google Scholar] [CrossRef]
- Caviness, J.N.; Brown, P. Myoclonus: Current Concepts and Recent Advances. Lancet Neurol. 2004, 3, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Charlier, B.; Coglianese, A.; De Rosa, F.; de Grazia, U.; Operto, F.F.; Coppola, G.; Filippelli, A.; Dal Piaz, F.; Izzo, V. The Effect of Plasma Protein Binding on the Therapeutic Monitoring of Antiseizure Medications. Pharmaceutics 2021, 13, 1208. [Google Scholar] [CrossRef]
- Blum, R.A.; Comstock, T.J.; Sica, D.A.; Schultz, R.W.; Keller, E.; Reetze, P.; Bockbrader, H.; Tuerck, D.; Busch, J.A.; Reece, P.A. Pharmacokinetics of Gabapentin in Subjects with Various Degrees of Renal Function. Clin. Pharmacol. Ther. 1994, 56, 154–159. [Google Scholar] [CrossRef]
- Rissardo, J.P.; Caprara, A.L.F. Buspirone-Associated Movement Disorder: A Literature Review. Prague Med. Rep. 2020, 121, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Klawans, H.L.J.; Goetz, C.; Weiner, W.J. 5-Hydroxytryptophan-Induced Myoclonus in Guinea Pigs and the Possible Role of Serotonin in Infantile Myoclonus. Neurology 1973, 23, 1234–1240. [Google Scholar] [CrossRef]
- Atkin, T.; Comai, S.; Gobbi, G. Drugs for Insomnia beyond Benzodiazepines: Pharmacology, Clinical Applications, and Discovery. Pharmacol. Rev. 2018, 70, 197–245. [Google Scholar] [CrossRef] [PubMed]
- Rissardo, J.P.; Caprara, A.L.F. Phenytoin-Associated Movement Disorder: A Literature Review. Tzu Chi Med. J. 2022, 34, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Rissardo, J.P.; Caprara, A.L.F.; Durante, Í. Valproate-Associated Movement Disorder: A Literature Review. Prague Med. Rep. 2021, 122, 140–180. [Google Scholar] [CrossRef]
- Rissardo, J.P.; Caprara, A.L.F. Topiramate-Associated Movement Disorder: Case Series and Literature Review. Clin. Neuropharmacol. 2020, 43, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Middleton, O. Suicide by Gabapentin Overdose. J. Forensic Sci. 2011, 56, 1373–1375. [Google Scholar] [CrossRef] [PubMed]
- McCann, U.D.; Penetar, D.M.; Belenky, G. Acute Dystonic Reaction in Normal Humans Caused by Catecholamine Depletion. Clin. Neuropharmacol. 1990, 13, 565–568. [Google Scholar] [CrossRef]
- Smit, M.; Bartels, A.L.; van Faassen, M.; Kuiper, A.; Niezen-Koning, K.E.; Kema, I.P.; Dierckx, R.A.; de Koning, T.J.; Tijssen, M.A. Serotonergic Perturbations in Dystonia Disorders-a Systematic Review. Neurosci. Biobehav. Rev. 2016, 65, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Rissardo, J.P.; Caprara, A.L.F. Mirtazapine-Associated Movement Disorders: A Literature Review. Tzu Chi Med. J. 2020, 32, 318–330. [Google Scholar] [CrossRef]
- Sullivan, M.A.; Wilbur, R. Gabapentin Pharmacotherapy for Antipsychotic-Induced Akathisia: Single-Patient Experiment and Case Report. Ther. Adv. Psychopharmacol. 2014, 4, 100–102. [Google Scholar] [CrossRef]
- Pfeffer, G.; Chouinard, G.; Margolese, H.C. Gabapentin in the Treatment of Antipsychotic-Induced Akathisia in Schizophrenia. Int. Clin. Psychopharmacol. 2005, 20, 179–181. [Google Scholar] [CrossRef]
- Perez, H.R.; Stoeckle, J.H. Stuttering: Clinical and Research Update. Can. Fam. Physician 2016, 62, 479–484. [Google Scholar]
- Reimann, W. Inhibition by GABA, Baclofen and Gabapentin of Dopamine Release from Rabbit Caudate Nucleus: Are There Common or Different Sites of Action? Eur. J. Pharmacol. 1983, 94, 341–344. [Google Scholar] [CrossRef]
- Rissardo, J.P.; Caprara, A.L.F. Pregabalin-Associated Movement Disorders: A Literature Review. Brain Circ. 2020, 6, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Maguire, G.A.; Bird, A.A. Gabapentin for Treating Acquired Neurogenic Stuttering. Ann. Clin. Psychiatry 2012, 24, 240. [Google Scholar]
- Rissardo, J.P.; Caprara, A.L.F. Cinnarizine- and Flunarizine-Associated Movement Disorder: A Literature Review. Egypt. J. Neurol. Psychiatry Neurosurg. 2020, 56, 61. [Google Scholar] [CrossRef]
- Deokule, S.; Burdon, M.; Matthews, T. Superior Oblique Myokymia Improved with Gabapentin. J. Neuroophthalmol. 2004, 24, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Serrao, M.; Cardinali, P.; Rossi, P.; Parisi, L.; Tramutoli, R.; Pierelli, F. A Case of Myokymia-Cramp Syndrome Successfully Treated with Gabapentin. Acta Neurol. Scand. 1998, 98, 458–460. [Google Scholar] [CrossRef]
| Category | Search Terms | Results |
|---|---|---|
| Akathisia | (“gabapentin”[MeSH Terms] OR “gabapentin”[All Fields] OR “gabapentine”[All Fields] OR “gabapentin s”[All Fields]) AND (“akathisias”[All Fields] OR “psychomotor agitation”[MeSH Terms] OR (“psychomotor”[All Fields] AND “agitation”[All Fields]) OR “psychomotor agitation”[All Fields] OR “akathisia”[All Fields]) | 30 |
| Ataxia | (“gabapentin”[MeSH Terms] OR “gabapentin”[All Fields] OR “gabapentine”[All Fields] OR “gabapentin s”[All Fields]) AND (“ataxia”[MeSH Terms] OR “ataxia”[All Fields] OR “ataxias”[All Fields]) | 132 |
| Ballism | (“gabapentin”[MeSH Terms] OR “gabapentin”[All Fields] OR “gabapentine”[All Fields] OR “gabapentin s”[All Fields]) AND (“dyskinesias”[MeSH Terms] OR “dyskinesias”[All Fields] OR “ballism”[All Fields]) | 193 |
| Bradykinesia | (“gabapentin”[MeSH Terms] OR “gabapentin”[All Fields] OR “gabapentine”[All Fields] OR “gabapentin s”[All Fields]) AND (“hypokinesia”[MeSH Terms] OR “hypokinesia”[All Fields] OR “bradykinesia”[All Fields]) | 4 |
| Chorea | (“gabapentin”[MeSH Terms] OR “gabapentin”[All Fields] OR “gabapentine”[All Fields] OR “gabapentin s”[All Fields]) AND (“chorea”[MeSH Terms] OR “chorea”[All Fields] OR “choreas”[All Fields]) | 26 |
| Dyskinesia | (“gabapentin”[MeSH Terms] OR “gabapentin”[All Fields] OR “gabapentine”[All Fields] OR “gabapentin s”[All Fields]) AND (“dyskinesiae”[All Fields] OR “dyskinesias”[MeSH Terms] OR “dyskinesias”[All Fields] OR “dyskinesia”[All Fields]) | 208 |
| Dystonia | (“gabapentin”[MeSH Terms] OR “gabapentin”[All Fields] OR “gabapentine”[All Fields] OR “gabapentin s”[All Fields]) AND (“dystonia”[MeSH Terms] OR “dystonia”[All Fields] OR “dystonias”[All Fields] OR “dystonic disorders”[MeSH Terms] OR (“dystonic”[All Fields] AND “disorders”[All Fields]) OR “dystonic disorders”[All Fields]) | 49 |
| Extrapyramidal | (“gabapentin”[MeSH Terms] OR “gabapentin”[All Fields] OR “gabapentine”[All Fields] OR “gabapentin s”[All Fields]) AND “extrapyramidal”[All Fields] | 6 |
| Hyperkinetic | (“gabapentin”[MeSH Terms] OR “gabapentin”[All Fields] OR “gabapentine”[All Fields] OR “gabapentin s”[All Fields]) AND (“hyperkinetic”[All Fields] OR “hyperkinetics”[All Fields]) | 5 |
| Hypokinetic | (“gabapentin”[MeSH Terms] OR “gabapentin”[All Fields] OR “gabapentine”[All Fields] OR “gabapentin s”[All Fields]) AND (“hypokinesia”[MeSH Terms] OR “hypokinesia”[All Fields] OR “hypokinetic”[All Fields]) | 1 |
| Movement disorder | (“gabapentin”[MeSH Terms] OR “gabapentin”[All Fields] OR “gabapentine”[All Fields] OR “gabapentin s”[All Fields]) AND (“movement disorders”[MeSH Terms] OR (“movement”[All Fields] AND “disorders”[All Fields]) OR “movement disorders”[All Fields] OR (“movement”[All Fields] AND “disorder”[All Fields]) OR “movement disorder”[All Fields]) | 224 |
| Myoclonus | (“gabapentin”[MeSH Terms] OR “gabapentin”[All Fields] OR “gabapentine”[All Fields] OR “gabapentin s”[All Fields]) AND (“myoclonus”[MeSH Terms] OR “myoclonus”[All Fields]) | 80 |
| Myokymia | (“gabapentin”[MeSH Terms] OR “gabapentin”[All Fields] OR “gabapentine”[All Fields] OR “gabapentin s”[All Fields]) AND (“myokymia”[MeSH Terms] OR “myokymia”[All Fields] OR “myokymias”[All Fields]) | 12 |
| Parkinsonism | (“gabapentin”[MeSH Terms] OR “gabapentin”[All Fields] OR “gabapentine”[All Fields] OR “gabapentin s”[All Fields]) AND (“parkinson disease”[MeSH Terms] OR (“parkinson”[All Fields] AND “disease”[All Fields]) OR “parkinson disease”[All Fields] OR “parkinsons”[All Fields] OR “parkinson”[All Fields] OR “parkinson s”[All Fields] OR “parkinsonian disorders”[MeSH Terms] OR (“parkinsonian”[All Fields] AND “disorders”[All Fields]) OR “parkinsonian disorders”[All Fields] OR “parkinsonism”[All Fields] OR “parkinsonisms”[All Fields] OR “parkinsons s”[All Fields]) | 82 |
| Restless legs syndrome | (“gabapentin”[MeSH Terms] OR “gabapentin”[All Fields] OR “gabapentine”[All Fields] OR “gabapentin s”[All Fields]) AND (“restless legs syndrome”[MeSH Terms] OR (“restless”[All Fields] AND “legs”[All Fields] AND “syndrome”[All Fields]) OR “restless legs syndrome”[All Fields]) | 203 |
| Restlessness | (“gabapentin”[MeSH Terms] OR “gabapentin”[All Fields] OR “gabapentine”[All Fields] OR “gabapentin s”[All Fields]) AND (“psychomotor agitation”[MeSH Terms] OR (“psychomotor”[All Fields] AND “agitation”[All Fields]) OR “psychomotor agitation”[All Fields] OR “restlessness”[All Fields] OR “restless”[All Fields]) | 240 |
| Stuttering | (“gabapentin”[MeSH Terms] OR “gabapentin”[All Fields] OR “gabapentine”[All Fields] OR “gabapentin s”[All Fields]) AND (“stammerers”[All Fields] OR “stammers”[All Fields] OR “stutterer”[All Fields] OR “stutterer s”[All Fields] OR “stutterers”[All Fields] OR “stuttering”[MeSH Terms] OR “stuttering”[All Fields] OR “stammer”[All Fields] OR “stammering”[All Fields] OR “stutter”[All Fields] OR “stuttered”[All Fields] OR “stutters”[All Fields] OR “stutterings”[All Fields]) | 5 |
| Tics | (“gabapentin”[MeSH Terms] OR “gabapentin”[All Fields] OR “gabapentine”[All Fields] OR “gabapentin s”[All Fields]) AND (“tics”[MeSH Terms] OR “tics”[All Fields]) | 0 |
| Tremor | (“gabapentin”[MeSH Terms] OR “gabapentin”[All Fields] OR “gabapentine”[All Fields] OR “gabapentin s”[All Fields]) AND (“tremor”[MeSH Terms] OR “tremor”[All Fields] OR “tremors”[All Fields] OR “tremoring”[All Fields] OR “tremorous”[All Fields]) | 133 |
| Reference | N | Age (Years); Sex | GBP-Indication | GBP-Dose (mg/day) | MD Onset a | MD Recovery b | Follow-Up | CT, MRI; EEG, EMG |
|---|---|---|---|---|---|---|---|---|
| Myoclonus | ||||||||
| Asconape et al. (1995) [18] | 1 | NA | NA | NA | NA | NA | NA | NA |
| Reeves et al. (1996) [19] | 1 | 23; F | Complex partial seizures | 900 | 2 weeks and 4 weeks | 4 days | CR | NA |
| Scheyer et al. (1996) [20] | 8 | NA | 5 focal epilepsy, 2 generalized epilepsy, 1 focal-generalized epilepsy | 2170 (mean) | NA | NA | CR | NA; EEG available in 4 patients only—normal |
| Wilson et al. (1998) * [21] | 2 | NA | Refractory partial epilepsy | >3600 mg/day | NA | NA | NA | NA |
| Asconape et al. (2000) * [22] | 13 | 29.2 (mean); 9F, 4M | Refractory epilepsy | 2000 (mean) | 8 weeks (mean) | 2–12 months (range) | 2 CR | NA; EEG performed in 3 patients showed no correlation with MCL |
| Jacob et al. (2000) [23] | 1 | 60; F | Postherpetic neuralgia | First 2 days: 300 mg; other days: 900 mg a day | 4 days | 3 days | CR | Cranial CT scan: normal; NA |
| Huppertz et al. (2001) [24] | 4 | 33.25 (mean); 2F, 2M | Simple and complex partial seizures | 312.5 (mean) | NA | NA | CR | NA; EEG and EMG: normal |
| Scullin et al. (2003) [25] | 2 | 50.5 (mean); 1F, 1M | Neuropathic pain | 1500 (mean) | NA | NA | CR | NA |
| Sechi et al. (2004) [26] | 2 | 76.5 (mean); 2F | Simple partial seizure, DM polineuropathy | 900 to 3600 | Weeks–2 months | 11 days (mean) | CR | Brain MRI: unspecific; EEG: normal; EMG: asterixis |
| Babiy et al. (2005) [27] | 1 | 74; F | Neuropathic pain | 600 | Several months | NA | CR | NA; EMG: asterixis |
| Bookwalter et al. (2005) [28] | 1 | 75; M | Postherpetic neuralgia | 3200 | 1 day | 1 day | CR | NA |
| Zhang et al. (2005) [29] | 3 | 52.6 (mean); 1F, 2M | Muscle spasm, chronic paresthesia, arm pain | 1033 (mean) | 4 months | 4–15 days | CR | NA; EEG: normal |
| Brefel-Courbon et al. (2006) * [30] | 26 | NA | NA | NA | NA | NA | NA | NA |
| Han et al. (2006) [31] | 1 | 57; F | Legs paresthesia | 900 | 2 days | 3 days | CR | NA; EEG: abnormal |
| Holtkamp et al. (2006) [32] | 1 | 66; M | Painful sensorimotor neuropathy | 900 | 2 years | 1 day | CR | NA |
| Striano et al. (2007) [33] | 1 | 57; M | ET | 900 | 3 days | 1 day | CR | Brain MRI: normal; EEG: diffuse theta slowing and multifocal spikes |
| Cho et al. (2008) [34] | 1 | 69; F | Neuropathic pain | 900 | 1 week | 2 days | CR | NA; EEG: normal |
| Ege et al. (2008) [35] | 1 | 87; M | Neuropathic pain | 600 | single-dose | 20 h | CR | Brain MRI: periventricular ischemia and cortical atrophy; EEG: normal |
| Pierce et al. (2008) [36] | 1 | 46; F | DM peripheral neuropathy | 900 | 4 years | 2 days | CR | NA |
| Healy et al. (2009) [37] | 1 | 47; M | Neuropathic pain | 900 | NA | NA | CR | NA |
| Koide et al. (2009) [38] | 3 | NA | NA | 600–1800 | 2 weeks (mean) | NA | CR | NA |
| Onuigbo et al. (2009) [39] | 1 | NA | NA | NA | NA | 3 days | CR | NA |
| Chau et al. (2010) [40] | 1 | 78; M | Trigeminal neuralgia | 100 | 3 months | 2 days | CR | Cranial CT scan: normal; EEG showed diffuse background slowing with larger amplitude delta discharges, which at times appeared triphasic and were interpreted as consistent with periodic sharp wave complexes |
| Honma et al. (2010) [41] | 2 | NA; 1F, 1M | Neuropathic pain | 200–400 | 1 day–4 months | 1–2 days | CR | NA |
| Zand et al. (2010) [42] | 8 | NA | NA | NA | NA | NA | NA | NA |
| Choe et al. (2011) [43] | 1 | 69; M | NA | 1200 | 2 days | 1 week | CR | NA |
| Kampf et al. (2011) [44] | 1 | 40; F | Chronic pain syndrome | 3200 | Months | NA | CR | Brain MRI: EEG: slowed background activity with generalized spike-wave-complexes up to 7 s with a density of 0–4/min normal |
| Prieto-Pérez et al. (2011) [45] | 1 | 77; M | Restless legs syndrome | NA | 2 weeks | 3 days | CR | Brain MRI: chronic stroke; NA |
| Guddati et al. (2012) [46] | 1 | 57; M | Neuropathic pain | 900 | NA | 3 days | CR | NA |
| Torregrosa-Juan et al. (2012) [47] | 1 | 49; NA | Lumbosacral pain | 1800 | 2 days | NA | NA | Brain MRI: normal; NA |
| Atalay et al. (2013) * [48] | 1 | NA | NA | NA | NA | NA | NA | NA |
| Kagnoff et al. (2013) [49] | 1 | 85; M | NA | 1200 | NA | NA | NA | NA |
| Wahba et al. (2013) [50] | 1 | 68; M | Neuropathic pain | 300 | 2 days | 3 days | CR | NA; EMG: irregular MCL on isometric muscle contraction manifesting as brief interruption of muscle tone for 50 to 200 msec |
| Wiegand et al. (2013) [51] | 4 | 31.5 (mean); 2F, 2M | Recreational abuse | NA | NA | NA | NA | NA |
| Kaufman et al. (2013) [52] | 2 | 66.5 (mean); 1F, 1M | Neuropathic pain | 600–900 | 3 days–months | days | CR | NA |
| Shea et al. (2014) [53] | 1 | 79; F | Postherpetic neuralgia | 300 | 10 h | 5 days | CR | NA |
| Clark et al. (2015) [54] | 1 | 80; F | Postherpetic neuralgia | 900 | 4 days | 2 days | CR | NA |
| Guner et al. (2015) [55] | 1 | 65; M | Neuropathic pain | 1200 | 1 week | 2 days | CR | NA |
| Ozmenoglu et al. (2015) [56] | 1 | 68; M | Neuropathic pain | 1800 | 2 weeks | 2 days | CR | NA |
| Schnitzer et al. (2016) [57] | 1 | 69; F | Neuropathic pain | 3200 | Days | Days | CR | NA |
| Ahmad et al. (2017) [58] | 3 | 68 (mean); 3M | NA | 900 | NA | NA | CR | NA |
| Khan et al. (2017) [59] | 1 | 55; F | Lumbosacral pain | 1500 | NA | 1 day | CR | Cranial CT scan: normal; EEG: generalized mild–moderate slowing and generalized discharges with triphasic morphology |
| Kim et al. (2017) [60] | 12 | 62.58 (mean); 4F, 8M | NA | 400 (mean) | 3.33 (mean) | NA | CR | NA |
| Zheng et al. (2017) [61] | 1 | 74; F | Neuropathic pain | 900 | NA | NA | CR | NA |
| Perez et al. (2018) [62] | 1 | 47; F | Neuropathic pain | 2400 | NA | 3 days | CR | Cranial CT scan: normal; EEG: generalized, slow waveforms consistent with diffuse encephalopathy |
| Desai et al. (2019) [63] | 7 | 53 (mean); 4F, 3M | Neuropathic pain | NA | NA | NA | CR | NA; Pt 1: Background slow wave activity. MCL captured on EEG. Pt 4: Periodic discharges of triphasic morphology with background slowing. MCL captured on EEG |
| Hui et al. (2019) [64] | 1 | 89; F | Postherpetic neuralgia | 300 | 2 months | 2 days | CR | Cranial CT scan: normal; EEG: abnormal |
| Medsafe et al. (2019) * [65] | 1 | 35; M | NA | NA | NA | NA | CR | NA |
| Yeddi et al. (2019) [66] | 1 | 62; F | Painful leg muscle spasms | 200 | 2 days | 3 days | CR | Cranial CT scan: normal; EEG: normal |
| Latief et al. (2021) [67] | 1 | 64; M | Neuropathic pain | 300 | 1 week | NA | NA | NA |
| Parkinsonism | ||||||||
| Zadikoff et al. (2007) * [68] | 1 | 61; F | Seizure | NA | NA | NA | NA | NA |
| Pacheco-Paez et al. (2020) * [69] | NA | NA | NA | NA | NA | NA | NA | NA |
| Ri et al. (2023) * [70] | NA | NA | NA | NA | NA | NA | NA | NA |
| Dyskinesia | ||||||||
| Buetefisch et al. (1996) [71] | 1 | NA | NA | NA | NA | NA | NA | NA |
| Millichap et al. (1996) [72] | 1 | 37; M | Intractable seizure | NA | 5 days | 2 days | NA | NA |
| Chudnow et al. (1997) [73] | 2 | NA | Intractable seizure | 1200–1800 | NA | NA | CR | NA |
| Millichap et al. (1997) [74] | 2 | 41.5 (mean); NA | Intractable seizure | 1200–1800 | 14 days | NA | No | |
| Norton et al. (2001) [75] | 2 | 50.5 (mean); 2M | Anxiety disorder | 1050 (mean) | 3 days | 2 days | CR | Brain MRI: normal |
| Lai et al. (2007) [76] | 1 | NA | Neuropathic pain | 1200 | NA | 5 days | CR | NA |
| Raju et al. (2007) [77] | 1 | 75; M | Neuropathic pain | 600 | 2 weeks | 3 days | CR | NA |
| Lai et al. (2008) [78] | 1 | 41; M | Neuropathic pain | 1200 | 4 days | 1 week | CR | NA |
| Shin et al. (2008) [79] | 1 | 44; M | Peripheral neuroapthy, and RLS | 900 | NA | NA | NA | NA |
| Twardowschy et al. (2008) [80] | 1 | 68; F | Neuropathic pain | NA | 30 days | 1 week | CR | Brain MRI: unspecific |
| Zesiewicz et al. (2008) [81] | 1 | 46; F | Complex regional pain syndrome type 1 | 2100 | 4 weeks | 10 days | CR | Brain MRI: normal |
| Attupurath et al. (2009) [82] | 1 | 75; M | Anxiety disorder | 900 | >1 month | 2 days | CR | Brain MRI: normal; EEG: normal |
| Bonakis et al. (2009) [83] | 1 | 42; M | Paroxysmal kinesigenic DKN | 900 | 2 days | NA | NA | NA |
| Erol et al. (2009) [84] | 1 | 83; F | Neuropathic pain | NA | 5 days | 4 weeks | CR | Cranial CT scan: normal |
| Aksoy et al. (2013) [85] | 1 | 70; M | Restless legs syndrome | 900 | NA | NA | NA | Brain MRI: normal |
| Souzdalnitski et al. (2014) [86] | 1 | 70; F | Lumbosacral pain | 900 | 3 months | Months | CR | Brain MRI: unspecific |
| VanHook et al. (2017) [87] | 1 | 75; M | Lumbosacral pain | 1200 | one year | 2 days | CR | Brain MRI: normal |
| Rahil et al. (2018) [88] | 1 | 39; F | Upper limb pain | 300 | 2 days | 1 week | CR | Brain MRI: normal |
| Hampton et al. (2019) [89] | 1 | 82; M | NA | 1200 | NA | 1 day | CR | Cranial CT scan: normal; EEG: normal |
| Akathisia | ||||||||
| Childers et al. (1997) [90] | 2 (CR) | NA | Neuropathic pain | 900 | 1 week | 2 days | NA | NA |
| See et al. (2011) [91] | 1 | 76; F | Neuropathic pain | 3600 | NA | NA | NA | NA |
| Stuttering | ||||||||
| Nissani et al. (1997) [92] | 1 | 58; F | Intractable seizure | NA | NA | 4 days | CR | NA |
| Choi et al. (2004) [93] | 1 | 66; M | Postherpetic neuralgia | NA | NA | NA | CR | NA |
| Zeldin et al. (2019) [94] | 1 | 62; M | Lumbosacral pain | 900 | 2 days | Weeks | CR | Cranial CT scan: normal |
| Dystonia | ||||||||
| Reeves et al. (1996) [19] | 1 | 24; M | Complex partial seizures | 1800 | 2 months | <1 h | CR | NA; EEG: normal |
| Bernal et al. (1999) [95] | 1 | 10; M | Behavioral problems | 900 | 16 months | NA | No | NA; EEG normal |
| Palomeras et al. (2000) [96] | 1 | 68; M | ET | GBP 900 mg/day + propranolol 80 mg/day | 2 days when adding propranolol to GBP therapy | 20 days | CR | NA |
| Pina et al. (2005) [97] | 1 | 72; F | ET | 2100 | NA | Weeks | CR | Cranial CT scan: normal; EEG normal |
| Allford et al. (2007) [98] | 1 | 55; F | Neuropathic pain | 1800 | NA | 2 days | CR | Brain MRI; EEG normal: |
| Lal et al. (2011) [99] | 1 | NA | NA | NA | NA | NA | NA | NA |
| Rohman et al. (2014) [100] | 1 | 26; M | NA | 1600 | Single-dose | 1 day | CR | NA |
| Myokymia | ||||||||
| Brown et al. (2021) [101] | 1 | 69; M | Neuropathic pain | 9600 | NA | 3 days | CR | Brain MRI; EEG: normal |
| Others not clearly defined | ||||||||
| The US Gabapentin Study Group (1994) * [6] | 15% developed tremor | NA | NA | NA | NA | NA | NA | NA |
| Bergey et al. (1997) * [102] | 4 | NA | NA | NA | NA | NA | NA | NA |
| Steinhoff et al. (1997) [103] | 2 | 26 (mean); 1F, 1M | Drug-resistant epilepsy | 300 (mean) | 6 days (mean) | 1 day (mean) | CR | Case 1—NA, case 2—MRI revealed cerebellar atrophy mainly of the vermis; Case 1—NA; case 2—frontal lobe origin |
| Mayer et al. (1999) * [104] | NA | 37.6 (mean); NA | Seizure | ≤2400 | 43 days | NA | NA | NA |
| McLean et al. (1999) * [105] | NA | NA | Seizure | A total of 2.2% developed with less than 1800 mg/day. A total of 0.6% developed with 1800–2400 and 2400–3600 mg/day. | NA | NA | NA | NA |
| Perugi et al. (1999) * [106] | 1 | NA | NA | NA | NA | NA | NA | NA |
| Dallocchio et al. (2000) * [107] | 1 | 78; F | DM neuropathy | 1600 | NA | NA | NA | NA |
| Mellegers et al. (2001) * [108] | 19 | NA | NA | NA | NA | NA | NA | NA |
| Wilton et al. (2002) * [109] | NA | NA | NA | NA | NA | NA | NA | NA |
| Klein-Schwartz et al. (2003) [110] | 1 | 42; NA | Intoxication | 1200 | NA | NA | NA | NA |
| Karagoz et al. (2013) [111] | 3 | 71 (mean); 2F, 1M | Neuropathic pain | 1200 | NA | NA | CR | NA |
| Pal et al. (2014) * [112] | NA | NA | NA | NA | NA | NA | NA | NA |
| Kwon et al. (2019) [113] | NA | NA | NA | NA | NA | NA | NA | NA |
| Ghayur et al. (2021) [114] | 1 | 57; F | Neuropathic pain | 300 | 3 months | NA | NA | NA |
| Reference | CH and CM |
|---|---|
| Myoclonus | |
| Reeves et al. (1996) [19] | CH: She reported muscle twitches. These occurred once a minute and involved arm, leg, and neck muscles. The twitches were painless and were not of sufficient magnitude to cause the limb or head to move. After one week, the MCL disappeared, and GBP continued. After four weeks, the MCL reappeared. GBP was discontinued with complete recovery within four days. |
| Scheyer et al. (1996) [20] | CM: discontinue GBP and recovery from movement disorder. |
| Wilson et al. (1998) [21] | Refractory partial epilepsy (all the 50 patients in the study). |
| Asconape et al. (2000) [22] | Three patients were tapered off GBP, and the MCL completely resolved (two cases) or returned to baseline frequency (one case). In eight cases, in which GBP was continued at the same dose, six showed no change in the MCL, whereas two had a gradual spontaneous improvement, but not complete resolution. One patient had a decline in the frequency of MCL after an increase in dosage. Finally, one patient had a decrease in dosage but no change in frequency. The period during which patients had MCL ranged from 2 to 12 months. |
| Jacob et al. (2000) [23] | Severe postherpetic neuralgia that did not respond to amitryptiline or carbamazepine, and these were discontinued 1 month before admission. A week later, the drug was reintroduced at 300 mg/d without any recurrence of asterixis and she reported 50% pain relief. |
| Huppertz et al. (2001) [24] | In one individual MCL decreased with reduction of GBP. In the other three, MCL was unchanged with a GBP increase. |
| Scullin et al. (2003) [25] | Discontinuation of GBP. |
| Sechi et al. (2004) [26] | GBP-dose reduced. |
| Babiy et al. (2005) [27] | GBP was discontinued. |
| Bookwalter et al. (2005) [28] | GBP-dose reduced. |
| Zhang et al. (2005) [29] | GBP was discontinued. |
| Han et al. (2006) [31] | GBP was discontinued. |
| Holtkamp et al. (2006) [32] | The patient had a worsening of renal function. The MCL was observed. GBP was discontinued. |
| Striano et al. (2007) [33] | GBP was discontinued. |
| Cho et al. (2008) [34] | GBP was discontinued. |
| Ege et al. (2008) [35] | GBP was discontinued. |
| Pierce et al. (2008) [36] | The patient presented with hearing loss, MCL, and confusion. GBP levels were high. GBP was discontinued, and a hemodyalisis was performed. |
| Healy et al. (2009) [37] | The patient had MCL with GBP and pregabalin. But, MCL was not observed with carbamazepine or amitriptyline. |
| Koide et al. (2009) [38] | Discontinuation of GBP or clonazepam add-on resulted in cessation of MCL with no serious sequela. |
| Onuigbo et al. (2009) [39] | Following prompt drug discontinuation and continued daily hemodialysis or peritoneal dialysis respectively, both patients were discharged home, in normal clinical condition, after 3 days. |
| Chau et al. (2010) [40] | GBP was discontinued. After two days, MCL improved. After two months, EEG was normal. |
| Honma et al. (2010) [41] | GBP was discontinued. |
| Choe et al. (2011) [43] | After increasing hemodialysis to three times a week and discontinuing GBP, MCL spontaneously resolved. |
| Kampf et al. (2011) [44] | She had MCL with morphine and GBP. |
| Prieto-Pérez et al. (2011) [45] | GBP was discontinued. |
| Guddati et al. (2012) [46] | GBP level 6.3 mcg/mL, managed with continuous venovenous hemodiafiltration. |
| Torregrosa-Juan et al. (2012) [47] | GBP was discontinued, hemodialysis was performed. Encephalopathy improved progressively. |
| Kagnoff et al. (2013) [49] | The movement disorder was sudden multi-focal jerking limb movements that were worse with action, multiple metabolic abnormalities, and a history of high-dose GBP exposure. |
| Wahba et al. (2013) [50] | GBP was discontinued. |
| Kaufman et al. (2013) [52] | In one, GBP was mantained, and in the other, GBP was discontinued. Hemodialysis was performed in both cases. |
| Shea et al. (2014) [53] | Clonazepam was prescribed. GBP-dose was maintaned. |
| Clark et al. (2015) [54] | GBP-dose was reduced 100 mg 3×/d, but there was no improvement of MCL. GBP was discontinued, and within two days the MCL improved. |
| Guner et al. (2015) [55] | GBP was discontinued. |
| Ozmenoglu et al. (2015) [56] | GBP was discontinued. |
| Schnitzer et al. (2016) [57] | GBP was discontinued. |
| Ahmad et al. (2017) [58] | GBP-dose was reduced 100 mg 3×/d, and the symptoms completely improved. |
| Khan et al. (2017) [59] | GBP was discontinued. |
| Kim et al. (2017) [60] | GBP was discontinued. |
| Zheng et al. (2017) [61] | Patient was in use of GBP. She had an abdominal surgery, which led to the worsening of renal function. GBP toxicity was observed. GBP was discontinued. The abnormal movement improved. |
| Perez et al. (2018) [62] | Patient had worsening of renal function due to contrast-induced nephropathy leading to GBP toxicity. GBP was discontinued, and hemodialysis was performed. |
| Desai et al. (2019) [63] | One patient received lorazepam. All patient GBP was discontinued. |
| Hui et al. (2019) [64] | GBP was discontinued. |
| Medsafe et al. (2019) [65] | GBP was discontinued. |
| Yeddi et al. (2019) [66] | Hemodialysis was performed. No description about the GBP-dose is provided. |
| Latief et al. (2021) [67] | Hemodialysis was performed. No description about the GBP-dose is provided. |
| Parkinsonism | |
| Zadikoff et al. (2007) [68] | The patient was in use of valproic acid and GBP. Both antiepiletics can cause PKN. |
| Dyskinesia | |
| Millichap et al. (1996) [72] | Severe mental retardation and seizures. CM: Diphenhydramine 25 mg IV. Full recovery with GBP discontinuation. |
| Chudnow et al. (1997) [73] | CM: Both patients experienced resolution of abnormal movements on discontinuation of the therapy. One patient developed recurrent choreiform movements after drug rechallenge. |
| Millichap et al. (1997) [74] | CM: Case 1 also receiving valproic acid, intermittent choreoathetosis occurred for many weeks after GBP was discontinued. In case 2, receiving phenytoin, a GBP rechallenge caused recurrence of choreoathetosis in 7 days but to a lesser degree; the reduced severity of movements was related to a reduction in dosage of phenytoin. |
| Norton et al. (2001) [75] | GBP was discontinued. |
| Lai et al. (2007) [76] | The patient had spontaneous spinal epidural hematoma that caused neuropathic pain. GBP was started due to the neuropathic pain. |
| Raju et al. (2007) [77] | GBP was discontinued. |
| Lai et al. (2008) [78] | GBP was discontinued. |
| Shin et al. (2008) [79] | Patient was in use of GBP, clonazepam, oral iron supplements, citalopram, and haloperidol, when he had worsening of chorea. |
| Twardowschy et al. (2008) [80] | GBP was discontinued. |
| Zesiewicz et al. (2008) [81] | GBP was discontinued. |
| Attupurath et al. (2009) [82] | GBP was discontinued. |
| Bonakis et al. (2009) [83] | The patient had paroxysmal kinesigenic dyskinesia, which worsened with GBP therapy. |
| Erol et al. (2009) [84] | GBP was discontinued. |
| Souzdalnitski et al. (2014) [86] | GBP was discontinued. Chorea improved within 2 weeks. Orofacial DKN improved within months of GBP withdrawal. |
| VanHook et al. (2017) [87] | GBP was discontinued. |
| Rahil et al. (2018) [88] | GBP was discontinued. |
| Hampton et al. (2019) [89] | GBP was discontinued. |
| Akathisia | |
| Childers et al. (1997) [90] | GBP was discontinued, and the abnormal movements improved within 48 h. |
| Stuttering | |
| Nissani et al. (1997) [92] | CM–GBP was discontinued and stuttering was gone after 4 days of discontinuation. |
| Choi et al. (2004) [93] | GBP was discontinued. |
| Zeldin et al. (2019) [94] | GBP was discontinued. |
| Dystonia | |
| Reeves et al. (1996) [19] | One minute after the patient received 2 mg intravenous lorazepam, all abnormal movements ceased. GBP was discontinued, and the movements did not recur. |
| Bernal et al. (1999) [95] | GBP discontinuation. Abnormal movements in the upper right limb persisted. |
| Palomeras et al. (2000) [96] | The authors described that by reducing propranolol dose to 40 mg/day, the DTN disappeared immediately. |
| Pina et al. (2005) [97] | GBP was discontinued. |
| Allford et al. (2007) [98] | In the first surgery, she had myoclonic movements involving mainly the upper limbs. The episode persisted for 3 h and was not relieved by benztropine. In a second surgery, she had axial DTN. Therapy with long-acting benzodiazepines was ineffective. |
| Rohman et al. (2014) [100] | Treated with an intravenous dose of procyclidine 10 mg. |
| Myokymia | |
| Brown et al. (2021) [101] | GBP holiday was started. After restarting on a lower dose of GBP with multimodal pain control, the patient continued to improve with diminution of his MCL, tremor, and gait instability. |
| MD | MCL | DKN | DTN | AKT | Stutter | MKM | PKN | Others | General Data | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cases (%) | 135 (66.17%) | 22 (10.78%) | 7 (3.43%) | 3 (1.47%) | 3 (1.47%) | 1 (0.49%) | 1 (0.49%) | 32 (15.68%) | 204 (100%) | |
| Continent (%) | Africa | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Australia | 1 (0.74%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.49%) | |
| Asia | 24 (17.77%) | 4 (18.18%) | 1 (14.28%) | 0 (0%) | 1 (33.33%) | 0 (0%) | 0 (0%) | 0 (0%) | 30 (14.70%) | |
| Europe | 46 (34.07%) | 4 (18.18%) | 5 (71.42%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 26 (81.25%) | 81 (39.13%) | |
| N. America | 64 (47.40%) | 13 (59.09%) | 1 (14.28%) | 3 (100%) | 2 (66.66%) | 1 (100%) | 1 (100%) | 6 (18.75%) | 91 (44.60%) | |
| S. America | 0 (0%) | 1 (4.54%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.49%) | |
| Sex (%) | Female | 42 (31.11%) | 5 (22.72%) | 2 (28.57%) | 1 (33.33%) | 1 (33.33%) | 0 (0%) | 1 (100%) | 52 (25.49%) | |
| Male | 42 (31.11%) | 11 (50%) | 4 (57.14%) | 0 (0%) | 2 (66.66%) | 1 (100%) | 0 (0%) | 60 (29.41%) | ||
| Unknown | 51 (37.77%) | 6 (27.27%) | 1 (14.28%) | 2 (66.66%) | 0 (0%) | 0 (0%) | 0 (0%) | 92 (45.09%) | ||
| Age (year) | Rg | 23–89 | 73–83 | 10–72 | 76 | 58–66 | 69 | 61 | 10–89 (Md: 57) | |
| Mn | 53.98 | 57.2 | 42.5 | 76 | 62 | 69 | 61 | 54.54 (SD: 17.79) | ||
| GBP-dose (Mn mg) | 1277.75 | 1133.33 | 1516.66 | 1800 | 900 | 9600 | NA | 1324.66 (SD: 1117.66; Rg: 100–9600; Md: 1033) | ||
| MD onset | Range | 1 h–4 years | 2 days–1 years | 1 day–16 months | 1 week | 2 days | NA | NA | 1 h–4 years | |
| Mean | 4.01 weeks | 2.51 weeks | 2.80 weeks | 1 week | 2 days | NA | NA | 4.58 weeks (Sd: 8.08 Md: 1) | ||
| MD recovery | Range | 1 day–12 months | 1 day–months | 1 day–weeks | 2 days | 4 days–weeks | 3 days | NA | 1 day–12 months | |
| Mean | 3.66 days | 7.85 days | 9 days | 2 days | 4 days | 3 days | NA | 4.17 days (Sd: 4.87 Md: 2.5) | ||
| Follow-up—% CR (number of reports) | 79.24% (42/53) | 88.23% (15/17) | 83.33% (5/6) | NA (0/0) | 100% (3/3) | 100% (1/1) | NA | 82.5% (66/80) | ||
| MD | Incidence | N Cases | Population | Note | Reference |
|---|---|---|---|---|---|
| MCL | 1.09% | 8 | 729 | Assessment of GBP dosage and risk of toxicity in patients with chronic kidney disease. | Zand et al. [42] |
| MCL | 1.85% | 3 | 162 | NA | Koide et al. [38] |
| MCL | 2.50% | 1 | 40 | Cross-over, open-label trial of the effects of GBP on peripheral neuropathy. | Atalay et al. [48] |
| ATX | 2.60% | 58 | 2216 | Open-label multicenter study, which investigates how many patients become seizure free after GBP use. | McLean et al. [105] |
| ATX | 3.60% | 4 | 110 | Open-label multicenter study, which investigates how many patients become seizure free after GBP use. | Mayer et al. [104] |
| MCL | 4% | 2 | 50 | Efficacy of high-dose GBP in refractory partial epilepsy. | Wilson et al. [21] |
| ATX | 4% | 1 | 25 | Open-label pilot study on GBP vs. amitriptyline in diabetic neuropathy. | Dallochio et al. [107] |
| MCL | 4.22% | 3 | 71 | Patients with end-stage renal disease. | Zhang et al. [29] |
| ATX | 4.76% | 1 | 21 | Adjunctive GBP therapy efficacy in bipolar disorder type I. | Perugi et al. [106] |
| Tremor | 4.87% | 4 | 82 | Efficacy and safety of GBP administered as monotherapy in refractory complex partial or secondarily generalized seizures. | Bergey et al. [102] |
| Choreoathetosis | 7.14% | 2 | 28 | NA | Millichap et al. [74] |
| ATX | 7.42% | 19 | 256 | NA | Mellegers et al. [108] |
| ATX | 12.19% | 10 | 82 | Efficacy and safety of GBP administered as monotherapy in refractory complex partial or secondarily generalized seizures. | Bergey et al. [102] |
| MCL | 12.50% | 13 | 104 | NA | Asconape et al. [22] |
| ATX | 14.16% | 34 | 240 | Safety of GBP as add-on therapy in patients with refractory partial seizures. | U.S. Gabapentin Study Group [6] |
| Tremor | 15% | 36 | 240 | Safety of GBP as add-on therapy in patients with refractory partial seizures. | U.S. Gabapentin Study Group [6] |
| Asterix | 15.58% | 12 | 77 | NA | Kim et al. [60] |
| MCL | 21.05% | 4 | 19 | NA | Huppertz et al. [24] |
| MD | Gabapentin General Data | Pregabalin General Data a | |
|---|---|---|---|
| Cases (%) | 204 (100%) | 305 (100%) | |
| Movement disorders | Akathisia | 3 (1.47%) | 1 (0.32%) |
| Ataxia | NA | 184 (60.32%) | |
| Dyskinesia | 22 (10.78%) | 1 (0.32%) | |
| Dystonia | 7 (3.43%) | 1 (0.32%) | |
| Myoclonus | 135 (66.17%) | 39 (12.78%) | |
| Myokymia | 1 (0.49%) | 0 (0%) | |
| Parkinsonism | 1 (0.49%) | 8 (2.62%) | |
| Restless legs syndrome | 0 (0%) | 1 (0.32%) | |
| Stuttering | 3 (1.47%) | 0 (0%) | |
| Tremors | NA | 61 (20%) | |
| Others | 32 (15.68%) | 9 (2.95%) | |
| Continent (%) | Africa | 0 (0%) | 0 (0%) |
| Australia | 1 (0.49%) | 0 (0%) | |
| Asia | 30 (14.70%) | 40 (13.11%) | |
| Europe | 81 (39.13%) | 68 (22.29%) | |
| N. America | 91 (44.60%) | 196 (64.26%) | |
| S. America | 1 (0.49%) | 1 (0.32%) | |
| Sex (%) | Female | 52 (25.49%) | 21 (6.88%) |
| Male | 60 (29.41%) | 25 (8.19%) | |
| Unknown | 92 (45.09%) | 259 (84.91%) | |
| Age (year) | Rg | 10–89 (Md: 57) | 23–94 (Md: 66.5) |
| Mn | 54.54 (SD: 17.79) | 62.89 (SD: 18.12) | |
| Dose (Mn mg) | 1324.66 (SD: 1117.66; Rg: 100–9600; Md: 1033) | 238 (SD: 136.95; Rg: 50–600; Md: 150) | |
| MD onset | Range | 1 h–4 years | 1 day–9 months |
| Mean | 4.58 weeks (Sd: 8.08; Md: 1) | 9.48 days (Sd: 16.78; Md: 3) | |
| MD recovery | Range | 1 day–12 months | 1 day–6 months |
| Mean | 4.17 days (Sd: 4.87; Md: 2.5) | 12.17 days (Sd: 22.13; Md: 2.5) | |
| Follow-up—% CR (number of reports) | 82.5% (66/80) | 100% (18/18) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rissardo, J.P.; Medeiros Araujo de Matos, U.; Fornari Caprara, A.L. Gabapentin-Associated Movement Disorders: A Literature Review. Medicines 2023, 10, 52. https://doi.org/10.3390/medicines10090052
Rissardo JP, Medeiros Araujo de Matos U, Fornari Caprara AL. Gabapentin-Associated Movement Disorders: A Literature Review. Medicines. 2023; 10(9):52. https://doi.org/10.3390/medicines10090052
Chicago/Turabian StyleRissardo, Jamir Pitton, Ursula Medeiros Araujo de Matos, and Ana Letícia Fornari Caprara. 2023. "Gabapentin-Associated Movement Disorders: A Literature Review" Medicines 10, no. 9: 52. https://doi.org/10.3390/medicines10090052
APA StyleRissardo, J. P., Medeiros Araujo de Matos, U., & Fornari Caprara, A. L. (2023). Gabapentin-Associated Movement Disorders: A Literature Review. Medicines, 10(9), 52. https://doi.org/10.3390/medicines10090052








