Histological Alterations in Hashimoto’s Disease: A Case-Series Ultrastructural Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Electron Microscopy
2.3. Quantitative Morphometric Data and Statistics
3. Results
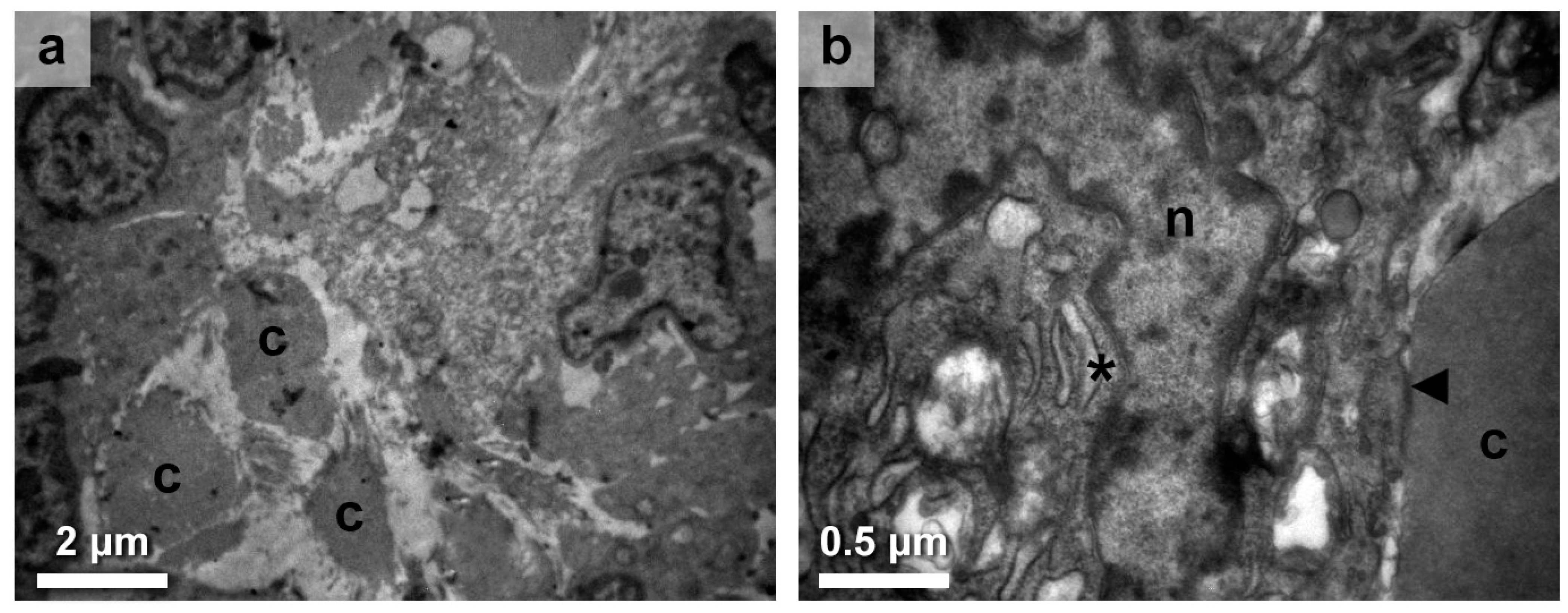
3.1. Immune Response in the Thyroid Gland Interstitium
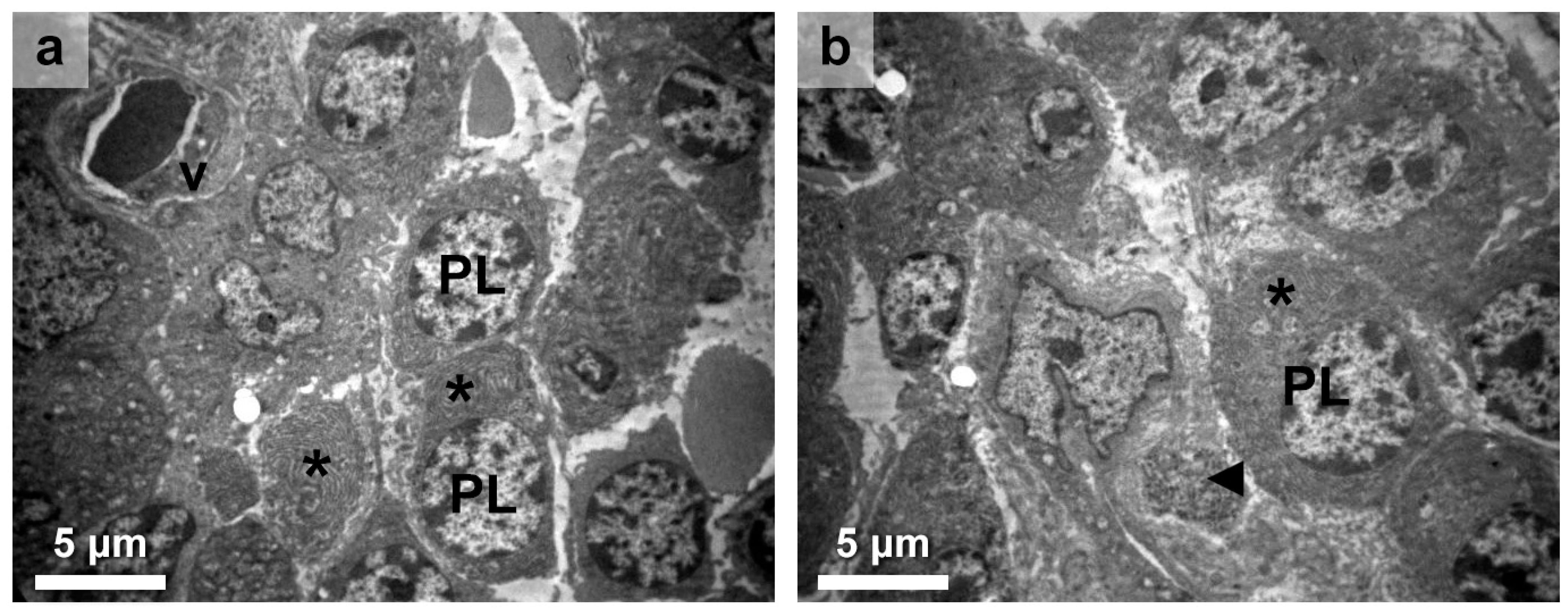
3.2. Thyroid Follicular Cell Alterations
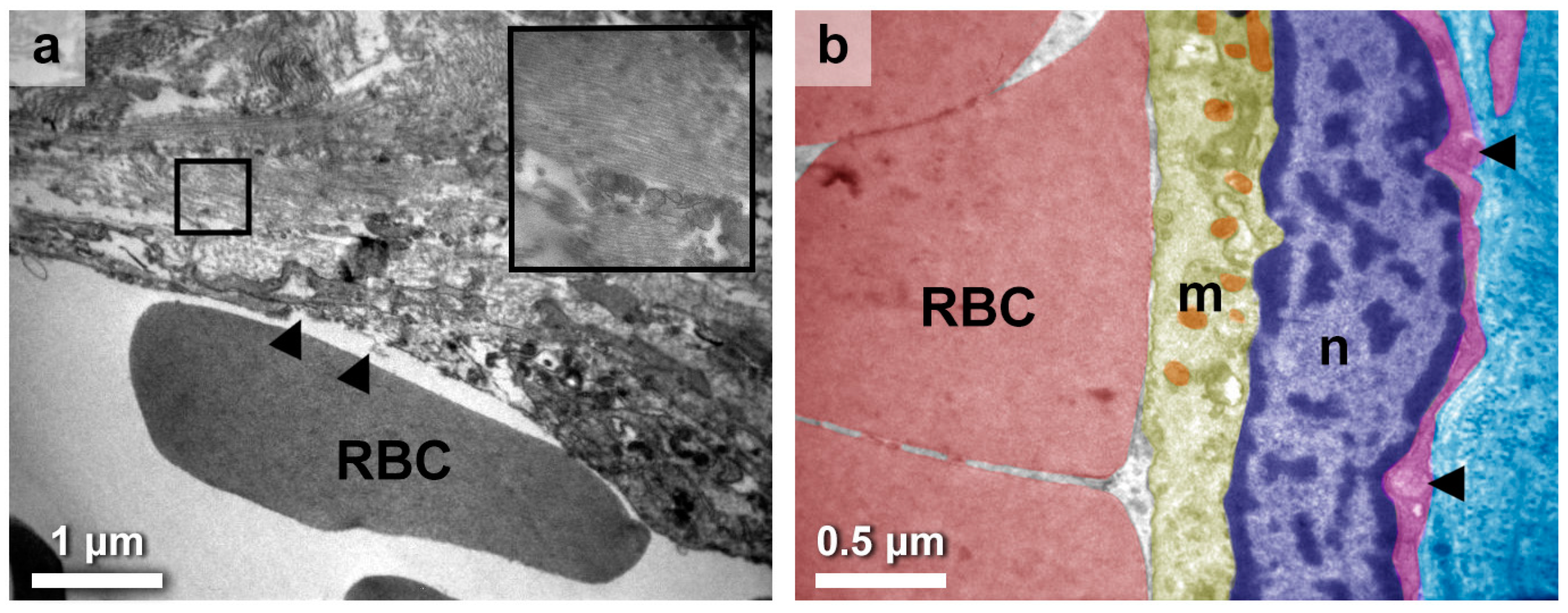
3.3. Eclectic Endothelial Perturbations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hashimoto, H. Zur Kenntniss der lymphomatösen Veränderung der Schilddrüse (Struma lymphomatosa). Arch. Klin. Chir. 1912, 97, 219–248. [Google Scholar]
- Chandanwale, S.S.; Nair, R.; Gambhir, A.; Kaur, S.; Pandey, A.; Shetty, A.; Naragude, P. Cytomorphological Spectrum of Thyroiditis: A Review of 110 Cases. J. Thyroid Res. 2018, 2018, 5246516. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. Iodine Deficiency. Endocr. Rev. 2009, 30, 376–408. [Google Scholar] [CrossRef]
- Vanderpump, M.P.; Tunbridge, W.M.; French, J.M.; Appleton, D.; Bates, D.; Clark, F.; Evans, J.G.; Hasan, D.M.; Rodgers, H.; Tunbridge, F. The Incidence of Thyroid Disorders in the Community: A Twenty-Year Follow-up of the Whickham Survey. Clin. Endocrinol. 1995, 43, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Rossi, R.T.; Bonaffini, O.; Scisca, C.; Altavilla, G.; Calbo, L.; Rosanò, A.; Sindoni, A.; Trimarchi, F.; Benvenga, S. Increased Annual Frequency of Hashimoto’s Thyroiditis between Years 1988 and 2007 at a Cytological Unit of Sicily. Ann. Endocrinol. 2010, 71, 525–534. [Google Scholar] [CrossRef]
- Cheserek, M.J.; Wu, G.-R.; Ntazinda, A.; Shi, Y.-H.; Shen, L.-Y.; Le, G.-W. Association Between Thyroid Hormones, Lipids and Oxidative Stress Markers in Subclinical Hypothyroidism. J. Med. Biochem. 2015, 34, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Mincer, D.L.; Jialal, I. Hashimoto Thyroiditis, 2022 Jun 21. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Holcomb, S.S. Detecting thyroid disease. Nursing 2005, 35 (Suppl. S10), 4–8. [Google Scholar] [CrossRef]
- Kawicka, A.; Regulska-Ilow, B.; Regulska-Ilow, B. Metabolic Disorders and Nutritional Status in Autoimmune Thyroid Diseases. Postep. Hig. Med. Dosw. 2015, 69, 80–90. [Google Scholar] [CrossRef]
- Matsuta, M. Immunohistochemical and electron microscopic studies on Hashimoto’s thyroiditis. Acta Pathol. Jpn. 1982, 32, 41–56. [Google Scholar] [CrossRef]
- Shamsuddin, A.K.M.; Lane, R.A. Ultrastructural Pathology in Hashimoto’s Thyroiditis. Hum. Pathol. 1981, 12, 561–573. [Google Scholar] [CrossRef]
- Nève, P. The Ultrastructure of Thyroid in Chronic Autoimmune Thyroiditis. Virchows Arch. A 1969, 346, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Reidbord, H.E.; Fisher, E.R. Ultrastructural Features of Subacute Granulomatous Thyroiditis and Hashimoto’s Disease. Am. J. Clin. Pathol. 1973, 59, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Ben-Skowronek, I.; Szewczyk, L.; Ciechanek, R.; Korobowicz, E. Interactions of Lymphocytes, Thyrocytes and Fibroblasts in Hashimoto’s Thyroiditis: An Immunohistochemical and Ultrastructural Study. Horm. Res. Paediatr. 2011, 76, 335–342. [Google Scholar] [CrossRef]
- Irvine, W.J.; Muir, A.R. An electron microscopic study of Hashimoto thyroiditis. Q. J. Exp. Physiol. Cogn. Med. Sci. 1963, 48, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Nève, P. Ultrastructure of the Thyroid in a Case of Hashimoto Goitre. (Struma Lymphomatosa). Pathol. Eur. 1966, 1, 234–245. [Google Scholar]
- Harris, M. The Cellular Infiltrate in Hashimoto’s Disease and Focal Lymphocytic Thyroiditis. J. Clin. Path 1969, 22, 326–333. [Google Scholar] [CrossRef]
- Kalderon, A.E.; Bogaars, H.A.; Diamond, I. Ultrastructural Alterations of the Follicular Basement Membrane in Hashimoto’s Thyroiditis. Rep. Eight Cases Basement Deposits. Am. J. Med. 1973, 55, 485–491. [Google Scholar] [CrossRef]
- Ketelbant-Balasse, P.; Nève, P. Ultrastructural Study of the Thyroid in Adult Hypothyroidism. Virchows Arch. A Path. Anat. Histol. 1974, 362, 195–205. [Google Scholar] [CrossRef]
- Gosselin, S.J.; Capen, C.C.; Martin, S.L. Histologic and Ultrastructural Evaluation of Thyroid Lesions Associated with Hypothyroidism in Dogs. Vet. Pathol. 1981, 18, 299–309. [Google Scholar] [CrossRef]
- Knecht, H.; Hedinger, C.E. Ultrastructural Findings in Hashimoto’s Thyroiditis and Focal Lymphocytic Thyroiditis with Reference to Giant Cell Formation. Histopathology 1982, 6, 511–538. [Google Scholar] [CrossRef]
- Nesland, J.M.; Sobrinho-Simões, M.A.; Holm, R.; Sambade, M.C.; Johannessen, J.V. Hürthle-Cell Lesions of the Thyroid: A Combined Study Using Transmission Electron Microscopy, Scanning Electron Microscopy, and Immunocytochemistry. Ultrastruct. Pathol. 1985, 8, 269–290. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-C.; Lai, S.-M.; Wen, C.-Y.; Hsiao, Y.-L. Three-Dimensional Cytomorphology in Fine Needle Aspiration Biopsy of Subacute Thyroiditis. Acta Cytol. 2004, 48, 155–160. [Google Scholar] [CrossRef]
- Masini-Repiso, A.M.; Bonaterra, M.; Spitale, L.; Di Fulvio, M.; Bonino, M.I.; Coleoni, A.H.; Orgnero-Gaisán, E. Ultrastructural Localization of Thyroid Peroxidase, Hydrogen Peroxide-Generating Sites, and Monoamine Oxidase in Benign and Malignant Thyroid Diseases. Hum. Pathol. 2004, 35, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Yagi, Y. Electron microscopic and immunohistochemical studies on Hashimoto’s thyroiditis. Pathol. Jpn. 1981, 31, 611–622. [Google Scholar]
- Williams, D.E.; Le, S.N.; Hoke, D.E.; Chandler, P.G.; Gora, M.; Godlewska, M.; Banga, J.P.; Buckle, A.M. Structural Studies of Thyroid Peroxidase Show the Monomer Interacting with Autoantibodies in Thyroid Autoimmune Disease. Endocrinology 2020, 161, bqaa016. [Google Scholar] [CrossRef]
- Abbasalizad Farhangi, M. The Correlation between Inflammatory and Metabolic Parameters with Thyroid Function in Patients with Hashimoto’s Thyroiditis: The Potential Role of Interleukin 23 (Il-23) and Vascular Endothelial Growth Factor (Vegf)—1. Acta Endocrinol. 2018, 14, 163–168. [Google Scholar] [CrossRef]
- Viglietto, G.; Maglione, D.; Rambaldi, M.; Cerutti, J.; Romano, A.; Trapasso, F.; Fedele, M.; Ippolito, P.; Chiappetta, G.; Botti, G.; et al. Upregulation of Vascular Endothelial Growth Factor (VEGF) and Downregulation of Placenta Growth Factor (P1GF) Associated with Malignancy in Human Thyroid Tumors and Cell Lines. Oncogene 1995, 11, 1569–1579. [Google Scholar]
- Schwaighofer, B.; Kurtaran, A.; Hubsch, P.; Fruhwald, F.; Barton, P.; Trattnig, S. Colour-Coded Doppler Sonography in Thyroid Gland Diagnosis: Preliminary Results. Rofo 1988, 149, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Ralls, P.W.; Mayekawa, D.S.; Lee, K.P.; Colletti, P.M.; Radin, D.R.; Boswell, W.D.; Halls, J.M. Color-Flow Doppler Sonography in Graves Disease: “Thyroid Inferno”. Am. J. Roentgenol. 1988, 150, 781–784. [Google Scholar] [CrossRef]
- Wollman, S.H.; Herveg, J.P.; Zeligs, J.D.; Ericson, L.E. Blood Capillary Enlargement during the Development of Thyroid Hyperplasia in the Rat. Endocrinology 1978, 103, 2306–2314. [Google Scholar] [CrossRef]
- Iitaka, M.; Miura, S.; Yamanaka, K.; Kawasaki, S.; Kitahama, S.; Kawakami, Y.; Kakinuma, S.; Oosuga, I.; Wada, S.; Katayama, S. Increased Serum Vascular Endothelial Growth Factor Levels and Intrathyroidal Vascular Area in Patients with Graves’ Disease and Hashimoto’s Thyroiditis. J. Clin. Endocrinol. Metab. 1998, 83, 3908–3912. [Google Scholar] [CrossRef][Green Version]
- Fu, X.; Guo, L.; Zhang, H.; Ran, W.; Fu, P.; Li, Z.; Chen, W.; Jiang, L.; Wang, J.; Jia, J. “Focal Thyroid Inferno” on Color Doppler Ultrasonography: A Specific Feature of Focal Hashimoto’s Thyroiditis. Eur. J. Radiol. 2012, 81, 3319–3325. [Google Scholar] [CrossRef]
- Jebreel, A.; England, J.; Bedford, K.; Murphy, J.; Karsai, L.; Atkin, S. Vascular Endothelial Growth Factor (VEGF), VEGF Receptors Expression and Microvascular Density in Benign and Malignant Thyroid Diseases. Int. J. Exp. Pathol. 2007, 88, 271–277. [Google Scholar] [CrossRef]
- Okamoto, M.; Watanabe, M.; Inoue, N.; Ogawa, K.; Hidaka, Y.; Iwatani, Y. Gene Polymorphisms of VEGF and VEGFR2 Are Associated with the Severity of Hashimoto’s Disease and the Intractability of Graves’ Disease, Respectively. Endocr. J. 2020, 67, 545–559. [Google Scholar] [CrossRef]
- Micaily, I.; Johnson, J.; Argiris, A. An Update on Angiogenesis Targeting in Head and Neck Squamous Cell Carcinoma. Cancers Head Neck 2020, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Gu, L.; Liu, Z.; Li, J.; Yao, M.; Fang, C. Correlation between Vascular Endothelial Growth Factor Pathway and Immune Microenvironment in Head and Neck Squamous Cell Carcinoma. BMC Cancer 2021, 21, 836. [Google Scholar] [CrossRef]
- Ma, Y.; He, J.; Shen, N.; Guo, R. Expression of NIS, VEGF-A and Thyroid Autoantibody in Papillary Thyroid Carcinoma with or without Hashimoto’s Disease. ORL 2019, 81, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Soh, E.Y.; Sobhi, S.A.; Wong, M.G.; Meng, Y.G.; Siperstein, A.E.; Clark, O.H.; Duh, Q.Y. Thyroid-Stimulating Hormone Promotes the Secretion of Vascular Endothelial Growth Factor in Thyroid Cancer Cell Lines. Surgery 1996, 120, 944–947. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Yamazaki, K.; Shizume, K.; Kanaji, Y.; Obara, T.; Ohsumi, K.; Demura, H.; Yamaguchi, S.; Shibuya, M. Stimulation by Thyroid-Stimulating Hormone and Graves’ Immunoglobulin G of Vascular Endothelial Growth Factor MRNA Expression in Human Thyroid Follicles in Vitro and Fit MRNA Expression in the Rat Thyroid In Vivo. J. Clin. Investig. 1995, 96, 1295–1302. [Google Scholar] [CrossRef]
- Viglietto, G.; Romano, A.; Manzo, G.; Chiappetta, G.; Paoletti, I.; Califano, D.; Galati, M.G.; Mauriello, V.; Bruni, P.; Lago, C.T.; et al. Upregulation of the Angiogenic Factors PlGF, VEGF and Their Receptors (Flt-1, Flk-1/KDR) by TSH in Cultured Thyrocytes and in the Thyroid Gland of Thiouracil-Fed Rats Suggest a TSH-Dependent Paracrine Mechanism for Goiter Hypervascularization. Oncogene 1997, 15, 2687–2698. [Google Scholar] [CrossRef]
- Balzan, S.; Del Carratore, R.; Nicolini, G.; Beffy, P.; Lubrano, V.; Forini, F.; Iervasi, G. Proangiogenic Effect of TSH in Human Microvascular Endothelial Cells through Its Membrane Receptor. J. Clin. Endocrinol. Metab. 2012, 97, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Vural, P.; Degirmencioglu, S.; Erden, S.; Gelincik, A. The Relationship between Transforming Growth Factor-Beta1, Vascular Endothelial Growth Factor, Nitric Oxide and Hashimoto’s Thyroiditis. Int. Immunopharmacol. 2009, 9, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Caturegli, P.; De Remigis, A.; Rose, N.R. Hashimoto Thyroiditis: Clinical and Diagnostic Criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef]
- Ciccone, M.M.; De Pergola, G.; Porcelli, M.T.; Scicchitano, P.; Caldarola, P.; Iacoviello, M.; Pietro, G.; Giorgino, F.; Favale, S. Increased Carotid IMT in Overweight and Obese Women Affected by Hashimoto’s Thyroiditis: An Adiposity and Autoimmune Linkage? BMC Cardiovasc. Disord. 2010, 10, 22. [Google Scholar] [CrossRef]
- Taddei, S.; Caraccio, N.; Virdis, A.; Dardano, A.; Versari, D.; Ghiadoni, L.; Ferrannini, E.; Salvetti, A.; Monzani, F. Low-Grade Systemic Inflammation Causes Endothelial Dysfunction in Patients with Hashimoto’s Thyroiditis. J. Clin. Endocrinol. Metab. 2006, 91, 5076–5082. [Google Scholar] [CrossRef] [PubMed]
- Haapala, A.M.; Hyoty, H.; Parkkonen, P.; Mustonen, J.; Soppi, E. Antibody Reactivity Against Thyroid Peroxidase and Myeloperoxidase in Autoimmune Thyroiditis and Systemic Vasculitis. Scand. J. Immunol. 1997, 46, 78–85. [Google Scholar] [CrossRef]
- Lionaki, S.; Hogan, S.L.; Falk, R.J.; Joy, M.S.; Chin, H.; Jennette, C.E.; Jennette, J.C.; Nachman, P.H. Association between Thyroid Disease and Its Treatment with ANCA Small-Vessel Vasculitis: A Case–Control Study. Nephrol. Dial. Transplant. 2007, 22, 3508–3515. [Google Scholar] [CrossRef]
- Kermani, T.A.; Cuthbertson, D.; Carette, S.; Khalidi, N.A.; Koening, C.L.; Langford, C.A.; McAlear, C.A.; Monach, P.A.; Moreland, L.; Pagnoux, C.; et al. Hypothyroidism in Vasculitis. Rheumatology 2022, 61, 2942–2950. [Google Scholar] [CrossRef]
- Stamatelopoulos, K.S.; Kyrkou, K.; Chrysochoou, E.; Karga, H.; Chatzidou, S.; Georgiopoulos, G.; Georgiou, S.; Xiromeritis, K.; Papamichael, C.M.; Alevizaki, M. Arterial Stiffness but Not Intima-Media Thickness Is Increased in Euthyroid Patients with Hashimoto’s Thyroiditis: The Effect of Menopausal Status. Thyroid 2009, 19, 857–862. [Google Scholar] [CrossRef]
- Hu, Y.; Yao, Z.; Wang, G. The Relationship between the Impairment of Endothelial Function and Thyroid Antibodies in Hashimoto’s Thyroiditis Patients with Euthyroidism. Horm. Metab. Res. 2020, 52, 642–646. [Google Scholar] [CrossRef]
- François, B. Angiogenesis: Insights from a Systematic Overview; Santulli, G., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013; ISBN 978-1-62618-114-4. [Google Scholar]
- Li, Y.; Zhou, G.; Ozaki, T.; Nishihara, E.; Matsuzuka, F.; Bai, Y.; Liu, Z.; Taniguchi, E.; Miyauchi, A.; Kakudo, K. Distinct Histopathological Features of Hashimoto’s Thyroiditis with Respect to IgG4-Related Disease. Mod. Pathol. 2012, 25, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avramidou, E.; Gkantaras, A.; Dermitzakis, I.; Sapalidis, K.; Manthou, M.E.; Theotokis, P. Histological Alterations in Hashimoto’s Disease: A Case-Series Ultrastructural Study. Medicines 2023, 10, 51. https://doi.org/10.3390/medicines10090051
Avramidou E, Gkantaras A, Dermitzakis I, Sapalidis K, Manthou ME, Theotokis P. Histological Alterations in Hashimoto’s Disease: A Case-Series Ultrastructural Study. Medicines. 2023; 10(9):51. https://doi.org/10.3390/medicines10090051
Chicago/Turabian StyleAvramidou, Eleni, Antonios Gkantaras, Iasonas Dermitzakis, Konstantinos Sapalidis, Maria Eleni Manthou, and Paschalis Theotokis. 2023. "Histological Alterations in Hashimoto’s Disease: A Case-Series Ultrastructural Study" Medicines 10, no. 9: 51. https://doi.org/10.3390/medicines10090051
APA StyleAvramidou, E., Gkantaras, A., Dermitzakis, I., Sapalidis, K., Manthou, M. E., & Theotokis, P. (2023). Histological Alterations in Hashimoto’s Disease: A Case-Series Ultrastructural Study. Medicines, 10(9), 51. https://doi.org/10.3390/medicines10090051









