Abstract
Background: Previous research has demonstrated the effectiveness of mindfulness interventions in improving glycemic control. By enhancing attention control, emotion regulation, and self-awareness, mindfulness shows promise in managing the lifestyle factors associated with cardiovascular disease risk. However, the impact of mindfulness on glycemic control in people with diabetes remains unclear. This overview aims to summarize the current evidence of the impact of mindfulness interventions on glycemic control in people with diabetes and propose suggestions for future research. Methods: The author searched electronic databases (PubMed/MEDLINE, Embase, and Cochrane Library) to identify relevant systematic reviews and meta-analyses. The current evidence regarding the effects of mindfulness on glycemic control in people with diabetes was summarized. Results: This review evaluated a total of five systematic reviews and meta-analyses of randomized controlled trials (RCTs). Mindfulness interventions show potential for improving glycemic control as measured by hemoglobin A1c (HbA1c) levels, as well as reducing stress, depression, and anxiety in people with diabetes. Four out of five systematic reviews and meta-analyses reported a significant reduction in HbA1c levels by approximately 0.3%. However, the available studies lacked adequate description of key characteristics of study subjects, such as body mass index, medication, and disease conditions, which are essential for assessing the impact of mindfulness on glycemic control. Moreover, there was significant heterogeneity in the intervention methods employed across the included RCTs. Conclusions: Mindfulness interventions are effective in improving glycemic control in people with type 2 diabetes. However, the overall quality of the reviewed studies raises uncertainty regarding the effectiveness of mindfulness as a treatment for people with diabetes. Further research is necessary to elucidate the biological effects of mindfulness on physiological, neurological, and endocrinological functions in humans.
1. Introduction
The prevalence of diabetes is increasing worldwide, leading to elevated risks of cardiovascular diseases (CVD) and mortality, which pose significant health challenges. The global population living with diabetes was estimated to be 529 million individuals in 2021, and the global prevalence of diabetes adjusted for age was approximately 6% [1]. It is essential for people with diabetes to engage in lifestyle modifications, including a healthy diet and regular exercise, to improve glycemic control and reduce the risks of CVD and mortality. However, maintaining a healthy lifestyle is not easy for people with diabetes due to the impact of psychological and environmental factors on their motivation for managing the condition [2]. People with diabetes need to focus on their treatment and increase their awareness of health.
Mindfulness is a state of intentional and non-judgmental attention to one’s present moment experience. It involves bringing one’s awareness to the present moment, including thoughts, feelings, bodily sensations, and the surrounding environment, with an attitude of curiosity, openness, and acceptance [3]. Mindfulness is often cultivated through various practices such as meditation, where individuals focus on the breath, bodily sensations, or a particular thought or phrase. Mindfulness is often researched for its potential benefits on mental health, well-being, stress reduction, and cognitive function [4]. Therapeutic approaches such as mindfulness-based stress reduction (MBSR) or mindfulness-based cognitive therapy (MBCT) often incorporate mindfulness practices. These interventions aim to help individuals cultivate abilities to effectively navigate their thoughts, emotions, and overall psychological well-being [5]. Furthermore, previous studies have shown that mindfulness interventions are effective in improving chronic pain, clinical symptoms related to irritable bowel syndrome, as well as insulin resistance and glycemic control in people with diabetes [3].
Mindfulness improves attention control, emotion regulation, and self-awareness, enhancing the management of lifestyles related to CVD risk factors, such as diet, physical activity, smoking, and medication adherence [6,7]. Diabetes is one of the lifestyle-related diseases that requires appropriate self-management; thus, mindfulness interventions should have an impact on treating diabetes. Indeed, there is a number of randomized controlled trials (RCTs) and systematic reviews assessing the effectiveness of mindfulness interventions on glycemic control in people with diabetes. The most recent RCT reported that a mindfulness intervention integrated into a diabetes self-management education and support program improved diabetes distress as well as hemoglobin A1c (HbA1c) levels in veterans with type 2 and type 1 diabetes [8].
Diabetes is a multifaceted and chronic disease that necessitates demanding self-management by patients. This includes organizing their diet and physical activity according to the guidance of healthcare professionals, monitoring blood glucose levels, and maintaining medication adherence. Diabetic complications, including CVD, are a major cause of morbidity and mortality among those with diabetes. Additionally, the considerable burdens of impaired functioning and self-management responsibilities can produce significant emotional distress [9]. Therefore, the beneficial effects of mindfulness on disease distress may help diabetes patients live happy lives no different from healthy individuals, which is the ultimate goal in the management of diabetes.
On the other hand, the underlying mechanism of the effects of mindfulness on physical health has not yet been clarified. Creswell et al. [3] argued that its mechanism of action consists of three pathways: the biological pathway, the health behavior pathway, and the psychological pathway. Among these, the biological pathway is vital in investigating the effect of mindfulness on glucose metabolism. The main biological effect of mindfulness is attributed to controlling the stress regulatory regions of the prefrontal cortex (the regulatory pathway) and the stress alarm system of the brain (the reactivity pathway). Previous studies have reported that mindfulness interventions are associated with a reduction in interleukin (IL)-6 levels in stressful individuals and can also modulate the autonomic nervous system (ANS) and the hypothalamic-pituitary-adrenal (HPA) axis in response to external stress [3].
The author acknowledges that yoga, qigong, and tai chi differ from mindfulness interventions in this review because these mind-body exercises involve a stronger exercise aspect compared to MBSR or MBCT. However, the mechanisms of action of mind-body therapies, such as yoga, qigong, and tai chi, are highly suggestive in considering the effects of mindfulness interventions in people with diabetes. Slow breathing and meditation reduce sympathetic nerve activity and enhance parasympathetic nerve activity, balancing the ANS and leading to stable glycemic control [10]. Furthermore, yoga practice may improve cardiovascular health by producing adiponectin and endothelial nitric oxide, and have an anti-inflammatory effect on IL-6 and tumor necrosis factor (TNF)-α [11]. Mindfulness interventions induce epigenetic modifications related to glucose metabolism and inflammation, including DNA methylation [12]. For example, García-Campayo et al. [13] performed a genome-wide screening of DNA methylation in peripheral blood leukocytes and found that there were 64 differentially methylated regions corresponding to 43 genes related to glucose and lipid metabolism in individuals who usually practice mindfulness meditation. They also identified TNF and nuclear factor kappa light chain enhancer of activated B cells (NF-κB) signaling as vital regulators of genes related to mindfulness interventions. The growing evidence suggests that mindfulness benefits physical health, including glycemic control, by modulating the ANS, HPA axis, and pro-inflammatory cytokines.
This review aims to summarize the current evidence of the impact of mindfulness interventions on glycemic control in people with diabetes and propose suggestions for future research.
2. Methods
This overview adhered to the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Supplementary file). The protocol for this overview was not registered in a database such as PROSPERO before conducting the literature search. The author searched the literature on mindfulness using MEDLINE/PubMed, Embase, and Cochrane Library from their inception to May 2023. The search terms for the overview were “mindfulness” OR “meditation” AND “diabetes” AND “systematic review”. The eligibility criteria were as follows: (1) Systematic review with meta-analysis of RCTs; (2) Subjects are people with type 2 and/or type 1 diabetes; (3) Interventions are mindfulness or mindfulness-based meditation; (4) Study outcomes include parameters related to glycemic control.
The initial search yielded 203 systematic reviews. Of these, 76 articles were excluded because their subjects were not diabetes patients. Additionally, 112 articles related to diet, exercise, and drug therapy were excluded. Fifteen articles were assessed for eligibility, and eight articles were excluded because they were qualitative analyses. The titles and abstracts of the screened articles were reviewed to determine their relevance. Additionally, three full-text articles that did not meet the eligibility criteria were excluded. A total of five systematic reviews were included.
The author utilized the Joanna Briggs Institute data extraction guide [14] to extract information from the included systematic reviews and meta-analyses. The methodological quality of the meta-analysis was assessed using A Measurement Tool to Assess Systematic Reviews version 2 (AMSTAR 2) [15].
Figure 1 depicts the identification, inclusion, and exclusion procedures of this overview.
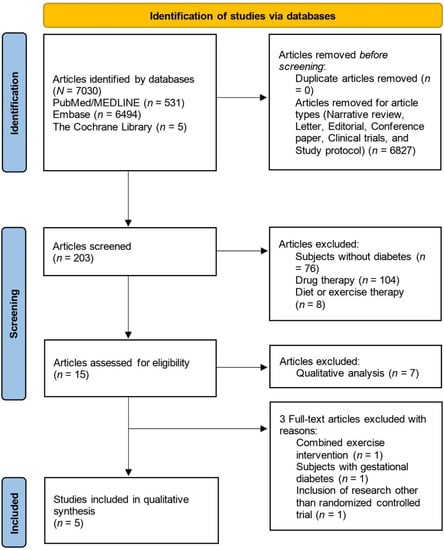
Figure 1.
Flow diagram of the study identification, inclusion, and exclusion.
3. Results
3.1. Effects of Mindfulness Interventions on Glycemic Control
The primary outcomes of the previous studies assessing the effects of mindfulness interventions on health in people with diabetes were mostly psychological outcomes. Ni et al. [16] assessed the effectiveness of MBSR and MBCT on depression, quality of life, and HbA1c levels in people with type 2 or type 1 diabetes. The authors included nine RCTs (11 articles) with 741 subjects in the meta-analysis [17,18,19,20,21,22,23,24,25,26,27]. This systematic review reported that MBSR or MBCT significantly reduced HbA1c levels (mean difference (MD) = −0.28%; 95% confidence interval (CI) −0.47 to −0.09, p = 0.004) with no heterogeneity between the included studies (I2 = 0%). However, three RCTs conducted in China [24,25,26,27,28] and one RCT conducted in Canada [23] did not perform intention-to-treat analysis. The authors also did not disclose conflicts of interest and funding sources in their paper.
The same research group reported beneficial effects of mindfulness interventions on glycemic control and psychological outcomes in people with diabetes in another academic journal [28]. This systematic review included eight RCTs with 841 subjects (11 articles) [17,18,19,20,21,22,23,29,30,31,32]. The differences between the authors’ previous and present systematic review were as follows. First, the authors excluded studies published in Chinese. Second, the authors included studies using mindfulness-based eating awareness training as a mindfulness intervention in addition to MBSR and MBCT. The pooled analysis showed that mindfulness interventions significantly reduced HbA1c levels (MD = −0.25%; 95% CI, −0.43 to −0.07, p = 0.006) with no heterogeneity between the included studies (I2 = 0%). Subgroup analysis also showed that subjects with better glycemic control at baseline (HbA1c levels < 8.0%) had greater reductions in HbA1c levels (−0.26%) compared to those with poor glycemic control (HbA1c levels ≥ 8.0%) (−0.22%, non-significant within-group change). The authors evaluated the risk of bias using the Cochrane risk-of-bias tool; however, other quality assessments were not performed to improve the certainty of the evidence.
Ngan et al. [33] examined the effects of MBSR, MBCT, and acceptance-based interventions, which are a form of cognitive behavioral therapy (CBT) that aims to alleviate psychological distress and discomfort by enhancing skills related to acceptance, on diabetes distress and HbA1c levels in people with type 2 diabetes. A total of nine RCTs with 801 subjects were included in the meta-analysis [20,21,31,32,34,35,36,37,38]. HbA1c levels were significantly reduced up to one month after the interventions (MD = −0.35%; 95% CI, −0.67 to −0.04, p = 0.03) with no heterogeneity between the included studies (I2 = 0%); however, this beneficial effect disappeared at three to six months after the interventions. This systematic review evaluated the methodological quality and the certainty of the evidence of the included studies using the Cochrane risk-of-bias tool for randomized trials ver.2 (RoB 2.0) [39] and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria [40], respectively, leading to the rigorous assessment of the quality of RCTs. However, the author could not identify the protocol of this systematic review in an international prospective register of systematic reviews, such as PROSPERO and Cochrane Collaboration. Moreover, although acceptance-based interventions are related to mindfulness, emphasizing the cultivation of awareness to accept present circumstances and emotions [41], the author has unresolved questions regarding whether it is appropriate to recognize mindfulness and acceptance-based interventions in a similar manner.
Heo et al. [42] assessed the effects of meditation on self-management in people with type 2 diabetes. A total of nine RCTs (10 articles) with 698 subjects were included in the analysis [17,19,31,32,36,43,44,45,46,47]. The meta-analysis revealed that meditation, including MBSR, MBCT, and mindfulness meditation, improved HbA1c levels (effect size = −0.75; 95% CI, −1.30 to −0.21, p = 0.007). The mean difference in HbA1c levels was −0.73 ± 0.63% in the intervention group and −0.12 ± 0.28% in the control group, respectively. However, the heterogeneity between the included studies was high (I2 = 93.2%). On the other hand, there was no significant effect on fasting blood glucose levels. The effect of meditation on lifestyle (e.g., diet, exercise), blood pressure, and blood cholesterol levels was inconclusive because the quantitative analysis was not performed.
In contrast, Bersch-Ferreira et al. [48] could not find a beneficial effect of mindfulness practice on glycemic control in people with type 2 diabetes. Only four RCTs [17,30,32,36] with 322 subjects were included in their systematic reviews. Mindfulness interventions, including MBSR and mindfulness-based eating awareness training, did not reduce blood glucose levels (two RCTs; MD = −0.73 mg/dL; 95% CI, −10.49 to 9.02) and HbA1c levels (two RCTs; MD = 0.05%; 95% CI, −0.22 to 0.32). The quality of evidence as evaluated by the GRADE system was very low for blood glucose levels and low for HbA1c levels, respectively. The authors concluded that mindfulness interventions have no effect on glycemic control in type 2 diabetes patients.
Table 1 summarizes the results of these systematic reviews.

Table 1.
Systematic reviews and meta-analyses assessing the effects of mindfulness interventions on people with diabetes.
Overall, the quality of these systematic reviews and meta-analyses is low to moderate for the following reasons: (1) the review authors did not adequately describe the characteristics of study subjects, such as metabolic parameters and medication, which are essential for assessing glycemic control in people with diabetes in the included RCTs; (2) the authors did not refer to the sources of funding for the studies included in the reviews.
Table 2 presents the quality of systematic reviews and meta-analyses included in the overview based on AMSTAR 2 criteria.

Table 2.
Quality assessment of studies included in the overview using A Measurement Tool to Assess Systematic Reviews (AMSTAR) 2.
3.2. Other Effects of Mindfulness Interventions in People with Diabetes
The author briefly mentions other health effects of mindfulness interventions that were confirmed in the previous systematic reviews. Three out of four systematic reviews assessed the effects of mindfulness interventions on psychological outcomes. Ni et al. [16] showed that MBSR or MBCT reduced depression scores measured by various scales (standardized mean difference (SMD) = −0.84; 95% CI, −1.16 to −0.51, p < 0.0001) and improved the mental health composite of quality of life (MD = 7.06, 95% CI, 5.19 to 9.03, p < 0.0001). Ni et al. [28] also reported that mindfulness interventions reduced depression (SMD = −0.56; 95% CI, −0.82 to −0.30, p < 0.0001), stress (SMD = −0.53; 95% CI, −0.75 to −0.31, p < 0.0001), and diabetes-related distress (MD = −5.81; 95% CI, −10.10 to −1.52, p = 0.008). However, the effect of mindfulness interventions on anxiety was inconsistent among the included studies. Two RCTs reported a significant reduction in anxiety in the intervention group compared to the control group, while three RCTs found no significant differences between groups. Moreover, Ngan et al. [33] showed that MBCT and self-directed mindfulness practice reduced anxiety (SMD = −0.41; 95% CI, −0.66 to −0.15, p = 0.002); however, self-management was not improved by the interventions. Additionally, no significant improvements in acceptance of diabetes, understanding of diabetes, and diabetes treatment satisfaction were observed.
4. Discussion
This review demonstrated that mindfulness interventions improved glycemic control in people with type 2 diabetes. Overall, the quality of the included systematic reviews and meta-analyses was rated as low to moderate. Two out of the five studies did not clarify the protocol of the systematic review in an international prospective register of systematic reviews. Moreover, most of the reviews did not provide adequate details about the included RCTs. None of the systematic reviews reported the sources of funding for the included RCTs. Unfortunately, the assessment of publication bias was not possible due to the limited number of included RCTs. These issues likely increased the risk of bias in the included systematic reviews and meta-analyses. There are also significant limitations regarding the interpretation of the results.
First, a standardized mindfulness intervention does not exist. MBSR and MBCT are widely used as interventions in previous studies; however, the difference between conventional CBT and mindfulness interventions remains ambiguous in terms of their definition. The study by Ngan et al. [33] included acceptance and commitment therapy, which is similar to CBT, in mindfulness interventions. On the other hand, the study by Ni et al. [26] described CBT as one of the comparators. To distinguish mindfulness interventions from psychotherapeutic approaches, including CBT, it is important to explore and clarify the differences in their impact on physiological and biochemical factors, not just psychological indicators measured using subjective methods. For instance, a meta-analysis reported that yoga practice was associated with the improvement in regulating ANS and HPA axis, indicated by decreased ambulatory blood pressure, resting heart rate, and cortisol levels [49]. Mindfulness interventions may also significantly reduce cortisol, IL-6, and TNF-α levels in depressed individuals [50]. Additionally, mindfulness meditation could probably decrease NF-kB activity and circulating C-reactive protein levels, increase T helper cell count in individuals with HIV, and increase telomerase activity [51]. Future research should explore the differences in biological impacts between mindfulness interventions and various psychotherapeutic approaches on physical functions in humans, such as cardiovascular, endocrine, immune, and central nervous system functioning. It is essential to clarify the distinctions between these interventions in terms of their effects.
Furthermore, the delivery method of mindfulness interventions was heterogeneous in previous studies. Most studies delivered the sessions in person; however, some studies used individual sessions while others used group sessions. In addition, interventionists and supervisors varied depending on the studies: certified instructors, healthcare professionals with training, psychologists, and researchers. The effectiveness of mindfulness interventions could be affected by such differences. As the review authors mentioned the limitation of their study, future research should focus on standardizing the intervention methods, including the delivery method (who and how), the number and duration of sessions, and the overall duration of interventions. This standardization should be based on a consensus derived from scientific evidence.
Third, it is imperative that researchers investigate the influence of drug therapy on study outcomes in people with diabetes. However, the systematic reviews included in this review did not report or analyze the interaction between the effects of mindfulness interventions and drugs, such as oral hypoglycemic agents. Furthermore, the systematic review should describe how the included RCTs deal with the diet and exercise of study subjects. Mindfulness interventions should affect stress-related health behaviors. Several RCTs have suggested that mindfulness interventions can alter eating behaviors, such as binge eating [52] and sweet consumption [53], and increase self-reported moderate-to-vigorous physical activity [54]. Thus, future systematic reviews and meta-analyses should take into consideration the impact of diet, exercise, and drug therapy when assessing the effect of mindfulness interventions on glycemic control in people with diabetes.
Fourth, the included systematic reviews lack some essential information on study subjects. People with diabetes often have comorbid obesity; thus, indices of obesity, such as body mass index or body weight, should be described in the systematic reviews. HbA1c levels of study subjects at baseline, which were used as an indicator of the disease condition in diabetes patients, should also be presented in summary tables. Although the study by Ni et al. [28] performed a subgroup analysis dividing the included studies into two subgroups based on baseline HbA1c levels (<8% or ≥8%), specific HbA1c levels should be shown as critical information in all the included studies. Additionally, HbA1c levels are average blood glucose levels for the last two to three months. Thus, the results of the studies with a duration of less than 12 weeks could be unreliable. Furthermore, the status of diabetic complications in study subjects is completely unknown. Uncontrolled diabetes can cause vascular damage, typically resulting in neuropathy, retinopathy, and nephropathy over time. Most people with diabetes have at least one complication, and CVD due to uncontrolled diabetes is one of the major causes of morbidity and mortality in these patients [55,56]. Such severe vascular complications significantly impair the physical function of people with diabetes. The effects of mindfulness interventions on glycemic control or other health outcomes in patients with progressed diabetic complications remains unknown based on the systematic reviews and meta-analyses included in this review. Therefore, future research should investigate whether mindfulness interventions provide health benefits to those patients.
Fifth, previous studies did not seem to investigate the effects of mindfulness interventions on other parameters related to glucose metabolism, such as insulin sensitivity. A recent study showed that adolescents who exhibit lower levels of mindfulness and experience shorter sleep durations have a higher risk of developing insulin resistance, whereas higher levels of mindfulness may serve as a protective factor [57]. Inflammation, insulin resistance, and leptin resistance appear to be key risk factors for the development of depression and anxiety, which mindfulness interventions could potentially benefit [58]. Future studies should investigate the endocrinological mechanisms that underlie the relationship between mindfulness and glucose metabolism to elucidate the impact of mindfulness interventions on diabetes.
Finally, the systematic reviews in this review did not evaluate the publication bias since the number of included studies in the meta-analyses was less than 10. The Cochrane Handbook for Systematic Reviews of Interventions suggests a minimum of 10 studies per examined covariate in meta-analysis [59]. Therefore, a future systematic review should include at least 10 high-quality RCTs to obtain rigorous evidence on whether mindfulness interventions have specific health benefits in people with diabetes.
The RCTs included in previous systematic reviews exhibited significant heterogeneity attributed to differences in the characteristics of study subjects, study duration/follow-up period, sample size, and outcomes. Therefore, high-quality RCTs with larger sample sizes and longer durations/follow-up periods are warranted.
Although it might be beyond the scope of this overview, future research should focus on the gut-brain interaction and epigenetics. The gut-brain axis demonstrates bidirectional communication between the central nervous system and gastrointestinal function, including the ANS function, HPA axis, neuroendocrine system, immune system, and gut microbiota [60]. The bidirectional microbiota-gut-brain axis connects stress and depression in both directions: stress influences the microbiota through the efferent brain-gut axis, and the gut microbiota, in turn, affects the central nervous system through the afferent gut-brain axis. Thus, mindfulness interventions could reduce stress, modulate the gut microbiota, and regulate endocrinological and immune functions, benefiting physical health [61]. On the other hand, the gut microbiota has been associated with the development of obesity, metabolic syndrome, and type 2 diabetes due to its impact on reduced glucose tolerance and insulin sensitivity, and gut microbiota dysbiosis is associated with inflammation and the progression of diabetic complications in people with type 2 diabetes [62,63]. The gut microbiota may be the key to elucidate the mechanisms of action of mindfulness interventions in glycemic control. Recently, mindfulness practice has also been shown to have a beneficial impact on epigenetic changes by regulating the HPA axis, serotonergic, metabolic, immunological, and neurological pathways [64]. Future clinical studies are required to explore these biological factors in addition to glycemic control in people with diabetes.
This overview also had some limitations. First, it was conducted by a single author, which may introduce biases in the literature search, eligibility screening, and data extraction processes. Second, quantitative analysis of the results from previous systematic reviews and meta-analyses was not conducted. To ensure higher-quality conclusions, it will be necessary to incorporate both qualitative and quantitative analysis in future studies.
5. Conclusions
Previous systematic reviews and meta-analyses have shown that mindfulness interventions are effective in improving glycemic control in people with type 2 diabetes. The reduction in HbA1c levels is approximately 0.3%, which somewhat limits the clinical significance in managing people with type 2 diabetes. Nevertheless, mindfulness interventions could effectively complement diet and exercise therapy for people with type 2 diabetes. On the other hand, mindfulness interventions had no impact on fasting blood glucose levels. Mindfulness interventions could potentially be beneficial in reducing stress, depression, and anxiety in people with diabetes. However, it should be noted that the quality of the evidence supporting this claim was not high. These health benefits are partly due to the regulation of ANS function and the HPA axis, as well as behavioral changes facilitated by mindfulness. However, the effects of mindfulness interventions on cardiovascular, endocrine, and central nervous system function are not fully clarified. The beneficial effect of mindfulness interventions on glycemic control in people with diabetes is inconclusive due to the suboptimal quality of previous systematic reviews and meta-analyses. Mindfulness could be a feasible and supportive practice for people with diabetes who also suffer from stress, depression, and anxiety; however, more rigorous scientific evidence will be needed in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medicines10090053/s1, PRISM Checklist [65].
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Lakerveld, J.; Palmeira, A.L.; van Duinkerken, E.; Whitelock, V.; Peyrot, M.; Nouwen, A. Motivation: Key to a healthy lifestyle in people with diabetes? Current and emerging knowledge and applications. Diabet. Med. 2020, 37, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Creswell, J.D.; Lindsay, E.K.; Villalba, D.K.; Chin, B. Mindfulness training and physical health: Mechanisms and outcomes. Psychosom. Med. 2019, 81, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lee, E.K.P.; Mak, E.C.W.; Ho, C.Y.; Wong, S.Y.S. Mindfulness-based interventions: An overall review. Br. Med. Bull. 2021, 138, 41–57. [Google Scholar] [CrossRef]
- Alsubaie, M.; Abbott, R.; Dunn, B.; Dickens, C.; Keil, T.F.; Henley, W.; Kuyken, W. Mechanisms of action in mindfulness-based cognitive therapy (MBCT) and mindfulness-based stress reduction (MBSR) in people with physical and/or psychological conditions: A systematic review. Clin. Psychol. Rev. 2017, 55, 74–91. [Google Scholar] [CrossRef]
- Loucks, E.B.; Schuman-Olivier, Z.; Britton, W.B.; Fresco, D.M.; Desbordes, G.; Brewer, J.A.; Fulwiler, C. Mindfulness and cardiovascular disease risk: State of the evidence, plausible mechanisms, and theoretical framework. Curr. Cardiol. Rep. 2015, 17, 112. [Google Scholar] [CrossRef]
- Tang, Y.Y.; Hölzel, B.K.; Posner, M.I. The neuroscience of mindfulness meditation. Nat. Rev. Neurosci. 2015, 16, 213–225. [Google Scholar] [CrossRef]
- DiNardo, M.M.; Greco, C.; Phares, A.D.; Beyer, N.M.; Youk, A.O.; Obrosky, D.S.; Morone, N.E.; Owen, J.E.; Saba, S.K.; Suss, S.J.; et al. Effects of an integrated mindfulness intervention for veterans with diabetes distress: A randomized controlled trial. BMJ Open Diabetes Res. Care 2022, 10, e002631. [Google Scholar] [CrossRef]
- Baek, R.N.; Tanenbaum, M.L.; Gonzalez, J.S. Diabetes burden and diabetes distress: The buffering effect of social support. Ann. Behav. Med. 2014, 48, 145–155. [Google Scholar] [CrossRef]
- Yadav, A.; Kaushik, R.M.; Kaushik, R. Effects of Diaphragmatic Breathing and Systematic Relaxation on Depression, Anxiety, Stress, and Glycemic Control in Type 2 Diabetes Mellitus. Int. J. Yoga Ther. 2021, 31, Article_13. [Google Scholar] [CrossRef]
- Kurian, J.; Mohanthy, S.; Nanjumdaiah, R.M. Mechanism of action of yoga on prevention and management of type 2 diabetes mellitus: Narrative review. J. Bodyw. Mov. Ther. 2022, 29, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Koh, E.; Sung, M.K.; Kang, H. Changes induced by mind-body intervention including epigenetic marks and its effects on diabetes. Int. J. Mol. Sci. 2021, 22, 1317. [Google Scholar] [CrossRef]
- García-Campayo, J.; Puebla-Guedea, M.; Labarga, A.; Urdánoz, A.; Roldán, M.; Pulido, L.; de Morentin, X.M.; Perdones-Montero, Á.; Montero-Marín, J.; Mendioroz, M. Epigenetic response to mindfulness in peripheral blood leukocytes involves genes linked to common human diseases. Mindfulness 2018, 9, 1146–1159. [Google Scholar] [CrossRef]
- Aromataris, E.; Fernandez, R.; Godfrey, C.M.; Holly, C.; Khalil, H.; Tungpunkom, P. Summarizing systematic reviews: Methodological development, conduct and reporting of an umbrella review approach. Int. J. Evid. Based Healthc. 2015, 13, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Ma, L.; Li, J. Effects of mindfulness-based stress reduction and mindfulness-based cognitive therapy in people with diabetes: A systematic review and meta-analysis. J. Nurs. Scholarsh. 2020, 52, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Kopf, S.; Kircher, C.; Faude-Lang, V.; Djuric, Z.; Augstein, F.; Friederich, H.C.; Kieser, M.; Bierhaus, A.; Humpert, P.M.; et al. Sustained effects of a mindfulness-based stress-reduction intervention in type 2 diabetic patients: Design and first results of a randomized controlled trial (the Heidelberger Diabetes and Stress-study). Diabetes Care 2012, 35, 945–947. [Google Scholar] [CrossRef] [PubMed]
- van Son, J.; Nyklícek, I.; Pop, V.J.; Blonk, M.C.; Erdtsieck, R.J.; Spooren, P.F.; Toorians, A.W.; Pouwer, F. The effects of a mindfulness-based intervention on emotional distress, quality of life, and HbA(1c) in outpatients with diabetes (DiaMind): A randomized controlled trial. Diabetes Care 2013, 36, 823–830. [Google Scholar] [CrossRef]
- Kopf, S.; Oikonomou, D.; Hartmann, M.; Feier, F.; Faude-Lang, V.; Morcos, M.; Häring, H.U.; Herzog, W.; Bierhaus, A.; Humpert, P.M.; et al. Effects of stress reduction on cardiovascular risk factors in type 2 diabetes patients with early kidney disease—Results of a randomized controlled trial (HEIDIS). Exp. Clin. Endocrinol. Diabetes 2014, 122, 341–349. [Google Scholar] [CrossRef]
- van Son, J.; Nyklíček, I.; Pop, V.J.; Blonk, M.C.; Erdtsieck, R.J.; Pouwer, F. Mindfulness-based cognitive therapy for people with diabetes and emotional problems: Long-term follow-up findings from the DiaMind randomized controlled trial. J. Psychosom. Res. 2014, 77, 81–84. [Google Scholar] [CrossRef]
- Tovote, K.A.; Fleer, J.; Snippe, E.; Peeters, A.C.; Emmelkamp, P.M.; Sanderman, R.; Links, T.P.; Schroevers, M.J. Individual mindfulness-based cognitive therapy and cognitive behavior therapy for treating depressive symptoms in patients with diabetes: Results of a randomized controlled trial. Diabetes Care 2014, 37, 2427–2434. [Google Scholar] [CrossRef] [PubMed]
- Schroevers, M.J.; Tovote, K.A.; Keers, J.C.; Links, T.P.; Sanderman, R.; Fleer, J. Individual mindfulness-based cognitive therapy for people with diabetes: A pilot randomized controlled trial. Mindfulness 2015, 6, 99–110. [Google Scholar] [CrossRef]
- Nathan, H.J.; Poulin, P.; Wozny, D.; Taljaard, M.; Smyth, C.; Gilron, I.; Sorisky, A.; Lochnan, H.; Shergill, Y. Randomized trial of the effect of mindfulness-based stress reduction on pain-related disability, pain intensity, health-related quality of life, and A1C in patients with painful diabetic peripheral neuropathy. Clin. Diabetes 2017, 35, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yang, J.; Zhao, X.; Kang, S.; Ge, Y.; Qin, L.; Zhang, Y. Effects of mindfulness training on diabetes-related distress and quality of life in patients with type 2 diabetes mellitus. Chin. J. Mod. Nurs. 2018, 24, 186–190. [Google Scholar]
- Teng, L.; Zhao, Q. Effect of mindfulness-base therapy on diabetes distress and quality of life in patients with type 2 diabetes. China J. Health Psychol. 2018, 26, 197–200. [Google Scholar]
- Xiang, Y.; Luo, W.; Lu, G. Effects of mindfulness-based stress reduction in patients with type 2 diabetes mellitus. Prev. Med. 2019, 31, 1068–1069, 1073. [Google Scholar]
- Ellis, D.A.; Carcone, A.I.; Slatcher, R.; Naar-King, S.; Hains, A.; Graham, A.; Sibinga, E. Efficacy of mindfulness-based stress reduction in emerging adults with poorly controlled, type 1 diabetes: A pilot randomized controlled trial. Pediatr. Diabetes 2019, 20, 226–234. [Google Scholar] [CrossRef]
- Ni, Y.X.; Ma, L.; Li, J.P. Effects of mindfulness-based intervention on glycemic control and psychological outcomes in people with diabetes: A systematic review and meta-analysis. J. Diabetes Investig. 2021, 12, 1092–1103. [Google Scholar] [CrossRef]
- Miller, C.K.; Kristeller, J.L.; Headings, A.; Nagaraja, H. Comparison of a mindful eating intervention to a diabetes self-management intervention among adults with type 2 diabetes: A randomized controlled trial. Health Educ. Behav. 2014, 41, 145–154. [Google Scholar] [CrossRef]
- Miller, C.K.; Kristeller, J.L.; Headings, A.; Nagaraja, H.; Miser, W.F. Comparative effectiveness of a mindful eating intervention to a diabetes self-management intervention among adults with type 2 diabetes: A pilot study. J. Acad. Nutr. Diet. 2012, 112, 1835–1842. [Google Scholar] [CrossRef]
- Pearson, S.; Wills, K.; Woods, M.; Warnecke, E. Effects of mindfulness on psychological distress and HbA1c in people with diabetes. Mindfulness 2018, 9, 1615–1626. [Google Scholar] [CrossRef]
- Jung, H.Y.; Lee, H.; Park, J. Comparison of the effects of Korean mindfulness-based stress reduction, walking, and patient education in diabetes mellitus. Nurs. Health Sci. 2015, 17, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Ngan, H.Y.; Chong, Y.Y.; Chien, W.T. Effects of mindfulness- and acceptance-based interventions on diabetes distress and glycaemic level in people with type 2 diabetes: Systematic review and meta-analysis. Diabet. Med. 2021, 38, e14525. [Google Scholar] [CrossRef] [PubMed]
- Gregg, J.A.; Callaghan, G.M.; Hayes, S.C.; Glenn-Lawson, J.L. Improving diabetes self-management through acceptance, mindfulness, and values: A randomized controlled trial. J. Consult. Clin. Psychol. 2007, 75, 336–343. [Google Scholar] [CrossRef]
- Shayeghian, Z.; Hassanabadi, H.; Aguilar-Vafaie, M.E.; Amiri, P.; Besharat, M.A. A randomized controlled trial of acceptance and commitment therapy for type 2 diabetes management: The moderating role of coping styles. PLoS ONE 2016, 11, e0166599. [Google Scholar] [CrossRef]
- Armani Kian, A.; Vahdani, B.; Noorbala, A.A.; Nejatisafa, A.; Arbabi, M.; Zenoozian, S.; Nakhjavani, M. The impact of mindfulness-based stress reduction on emotional wellbeing and glycemic control of patients with type 2 diabetes mellitus. J. Diabetes Res. 2018, 2018, 1986820. [Google Scholar] [CrossRef]
- Maghsoudi, Z.; Razavi, Z.; Razavi, M.; Javadi, M. Efficacy of acceptance and commitment therapy for emotional distress in the elderly with type 2 diabetes: A randomized controlled trial. Diabetes Metab. Syndr. Obes. 2019, 12, 2137–2143. [Google Scholar] [CrossRef]
- Whitehead, L.C.; Crowe, M.T.; Carter, J.D.; Maskill, V.R.; Carlyle, D.; Bugge, C.; Frampton, C.M.A. A nurse-led education and cognitive behaviour therapy-based intervention among adults with uncontrolled type 2 diabetes: A randomised controlled trial. J. Eval. Clin. Pract. 2017, 23, 821–829. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Brozek, J.L.; Canelo-Aybar, C.; Akl, E.A.; Bowen, J.M.; Bucher, J.; Chiu, W.A.; Cronin, M.; Djulbegovic, B.; Falavigna, M.; Guyatt, G.H.; et al. GRADE Working Group GRADE Guidelines 30: The GRADE approach to assessing the certainty of modeled evidence-An overview in the context of health decision-making. J. Clin. Epidemiol. 2021, 129, 138–150. [Google Scholar] [CrossRef]
- Forman, E.M.; Butryn, M.L. A new look at the science of weight control: How acceptance and commitment strategies can address the challenge of self-regulation. Appetite 2015, 84, 171–180. [Google Scholar] [CrossRef]
- Heo, S.; Kang, J.; Umeakunne, E.; Lee, S.; Bertulfo, T.F.; Barbé, T.; Kim, J.; Black, V.; An, M.; Randolph, J. Effects of meditation intervention on self-management in adult patients with type 2 diabetes: A systematic literature review and meta-analysis. J. Cardiovasc. Nurs. 2023; Epub ahead of print. [Google Scholar] [CrossRef]
- Chen, S.M.; Lin, H.S.; Atherton, J.J.; MacIsaac, R.J.; Wu, C.J. Effect of a mindfulness programme for long-term care residents with type 2 diabetes: A cluster randomised controlled trial measuring outcomes of glycaemic control, relocation stress and depression. Int. J. Older People Nurs. 2020, 15, e12312. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, H.; Ge, L.; Valimaki, M.; Wiley, J.; Whittemore, R. Effectiveness of a nurse-led mindfulness stress-reduction intervention on diabetes distress, diabetes self-management, and HbA1c levels among people with type 2 diabetes: A pilot randomized controlled trial. Res. Nurs. Health 2022, 45, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.S.; Ahadi, M.; Hatami, M.; Khalatbari, J. Effectiveness of mindfulness-based therapy on resilience, psychological well-being, and blood sugar levels in patients with type II diabetes. Razavi Int. J. Med. 2021, 9, 74–80. [Google Scholar]
- Ravari, O.N.; Mousavi, S.Z.; Babak, A. Evaluation of the effects of 12 weeks mindfulness-based stress reduction on glycemic control and mental health indices in women with diabetes mellitus type 2. Adv. Biomed. Res. 2020, 9, 61. [Google Scholar]
- Xiao, L. Blood sugar reduction of type 2 diabetic patients through a mindfulness intervention program. NeuroQuantology 2018, 16, 57–62. [Google Scholar] [CrossRef]
- Bersch-Ferreira, Â.C.; Weber, B.; da Silva, J.G.S.T.; Pagano, R.; Figueiro, M.F.; da Silva, L.R.; de Souza Mota, L.G.; Suzumura, E.A.; Torreglosa, C.R.; de Sousa Lara, E.; et al. Mindfulness practice for glycemic control: Could it be a new strategy for an old problem? A systematic review and meta-analysis. Curr. Diabetes Rev. 2021, 17, 65–80. [Google Scholar] [CrossRef]
- Pascoe, M.C.; Thompson, D.R.; Ski, C.F. Yoga, mindfulness-based stress reduction and stress-related physiological measures: A meta-analysis. Psychoneuroendocrinology 2017, 86, 152–168. [Google Scholar] [CrossRef]
- Marinovic, D.A.; Hunter, R.L. Examining the interrelationships between mindfulness-based interventions, depression, inflammation, and cancer survival. CA Cancer J. Clin. 2022, 72, 490–502. [Google Scholar] [CrossRef]
- Black, D.S.; Slavich, G.M. Mindfulness meditation and the immune system: A systematic review of randomized controlled trials. Ann. N. Y. Acad. Sci. 2016, 1373, 13–24. [Google Scholar] [CrossRef]
- O’Reilly, G.A.; Cook, L.; Spruijt-Metz, D.; Black, D.S. Mindfulness-based interventions for obesity-related eating behaviours: A literature review. Obes. Rev. 2014, 15, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Mason, A.E.; Epel, E.S.; Kristeller, J.; Moran, P.J.; Dallman, M.; Lustig, R.H.; Acree, M.; Bacchetti, P.; Laraia, B.A.; Hecht, F.M.; et al. Effects of a mindfulness-based intervention on mindful eating, sweets consumption, and fasting glucose levels in obese adults: Data from the SHINE randomized controlled trial. J. Behav. Med. 2016, 39, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Sala, M.; Geary, B.; Baldwin, A.S. A mindfulness-based physical activity intervention: A randomized pilot study. Psychosom. Med. 2021, 83, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Avogaro, A.; Fadini, G.P. Microvascular complications in diabetes: A growing concern for cardiologists. Int. J. Cardiol. 2019, 291, 29–35. [Google Scholar] [CrossRef]
- Clark, E.L.M.; Gulley, L.D.; Prince, M.A.; Casamassima, M.; Sanchez, N.; Jimenez, V.; Johnson, S.A.; Miller, R.L.; Conte, I.; Kaar, J.L.; et al. The role of mindfulness in associations among depression symptoms, sleep duration, and insulin resistance in adolescents. J. Behav. Med. 2021, 44, 694–703. [Google Scholar] [CrossRef]
- Fulton, S.; Décarie-Spain, L.; Fioramonti, X.; Guiard, B.; Nakajima, S. The menace of obesity to depression and anxiety prevalence. Trends Endocrinol. Metab. 2022, 33, 18–35. [Google Scholar] [CrossRef]
- Higgins, J.P.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A. Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Chichester, UK, 2019; Chapter 8; pp. 205–228. [Google Scholar]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Tan, H.E. The microbiota-gut-brain axis in stress and depression. Front. Neurosci. 2023, 17, 1151478. [Google Scholar] [CrossRef]
- Iatcu, C.O.; Steen, A.; Covasa, M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients 2021, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sun, B.; Yu, D.; Zhu, C. Gut Microbiota: An Important Player in Type 2 Diabetes Mellitus. Front. Cell Infect. Microbiol. 2022, 12, 834485. [Google Scholar] [CrossRef] [PubMed]
- Househam, A.M. Effects of stress and mindfulness on epigenetics. In Vitamins and Hormones; Litwack, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 122, pp. 283–306. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.F.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).