Bridging the Gap between Basic Research and Clinical Practice: The Growing Role of Translational Neurorehabilitation
Abstract
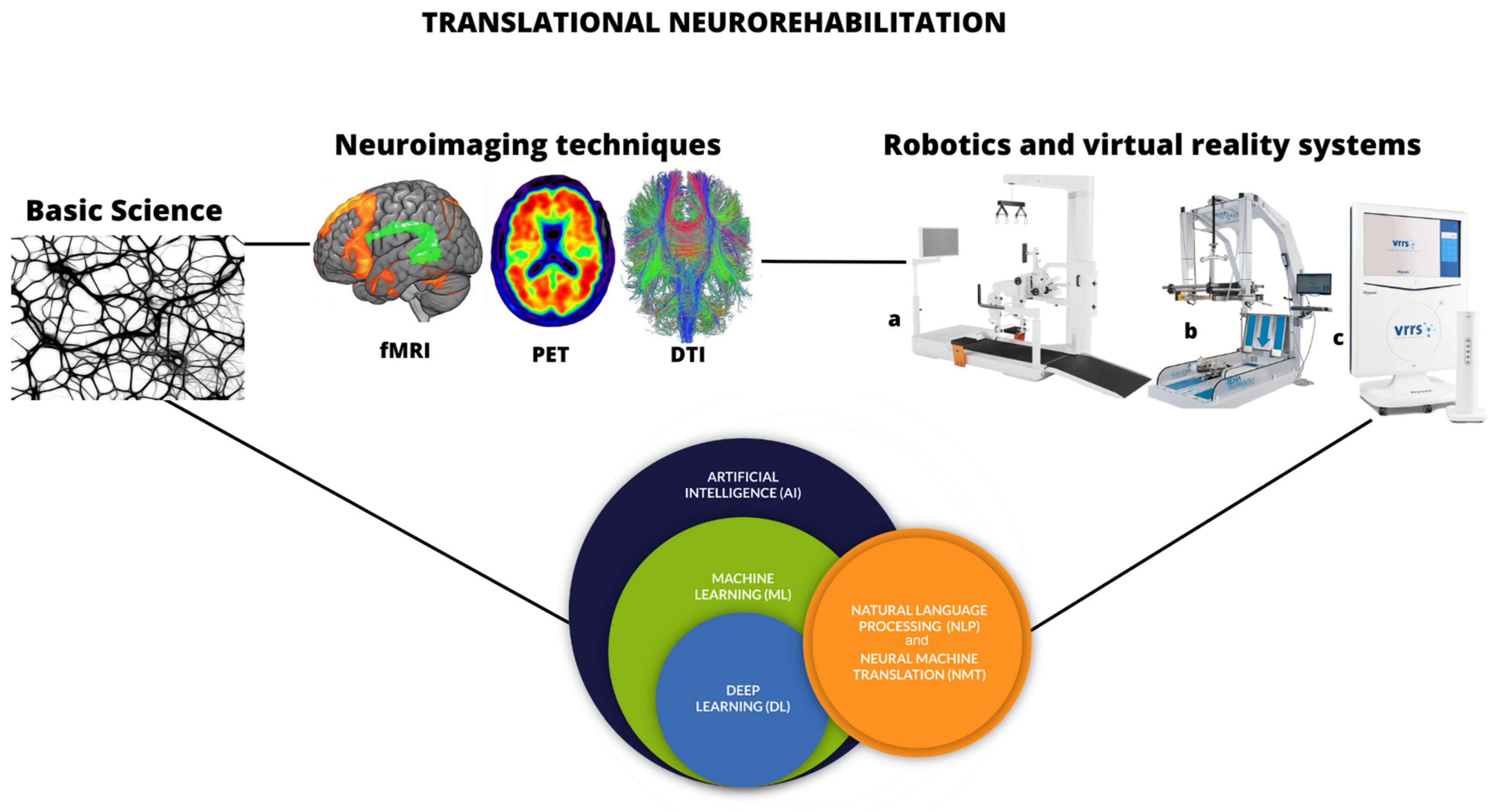
1. Introduction
2. Back to Basic Research: The Primary Stone of Translational Neurorehabilitation
3. The Role of Neuroimaging in the Neurorehabilitation Context
4. Innovative Technologies in Neurorehabilitation
5. Artificial Intelligence and Machine Learning in the Neurorehabilitation
6. Current Limitations in Translational Neurorehabilitation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davies, C.; Hamilton, O.K.L.; Hooley, M.; Ritakari, T.E.; Stevenson, A.J.; Wheater, E.N.W. Translational neuroscience: The state of the nation (A PhD student perspective). Brain Commun. 2020, 2, fcaa038. [Google Scholar] [CrossRef]
- Chiappalone, M.; Semprini, M. Using robots to advance clinical translation in neurorehabilitation. Sci. Robot. 2022, 7, eabo1966. [Google Scholar] [CrossRef]
- Bertani, R.; Melegari, C.; De Cola, M.C.; Bramanti, A.; Bramanti, P.; Calabrò, R.S. Effects of robot-assisted upper limb rehabilitation in stroke patients: A systematic review with meta-analysis. Neurol. Sci. 2017, 38, 1561–1569. [Google Scholar]
- Charette, C.; Déry, J.; Blanchette, A.K.; Faure, C.; Routhier, F.; Bouyer, L.J.; Lamontagne, M.E. A Systematic Review of the Determinants of Implementation of a Locomotor Training Program Using a Powered Exoskeleton for Individuals with a Spinal Cord Injury. Clin Rehabil. 2023, 37, 1119–1138. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.R.S.; Veeravagu, A.; Grant, G. Neuroplasticity after Traumatic Brain Injury. In Translational Research in Traumatic Brain Injury; Laskowitz, D., Grant, G., Eds.; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2016; Chapter 8. [Google Scholar]
- Nishiyama, J. Plasticity of dendritic spines: Molecular function and dysfunction in neurodevelopmental disorders. Psychiatry Clin. Neurosci. 2019, 73, 541–550. [Google Scholar] [CrossRef]
- Zanatta, F.; Farhane-Medina, N.Z.; Adorni, R.; Steca, P.; Giardini, A.; D’Addario, M.; Pierobon, A. Combining robot-assisted therapy with virtual reality or using it alone? A systematic review on health-related quality of life in neurological patients. Health Qual. Life Outcomes 2023, 21, 18. [Google Scholar] [CrossRef]
- Bonanno, M.; De Luca, R.; De Nunzio, A.M.; Quartarone, A.; Calabrò, R.S. Innovative Technologies in the Neurorehabilitation of Traumatic Brain Injury: A Systematic Review. Brain Sci. 2022, 12, 1678. [Google Scholar] [CrossRef]
- Prigatano, G.P.; Braga, L.W.; Johnson, S.F.; Souza, L.M.N. Neuropsychological rehabilitation, neuroimaging and neuroplasticity: A clinical commentary. NeuroRehabilitation 2021, 49, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cai, W.; Liu, S.; Zhang, F.; Fulham, M.; Feng, D.; Pujol, S.; Kikinis, R. Multimodal neuroimaging computing: A review of the applications in neuropsychiatric disorders. Brain Inform. 2015, 2, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Obermeyer, J.M.; Ho, E.; Gracias, A.; Shoichet, M.S. Influencing neuroplasticity in stroke treatment with advanced biomaterials-based approaches. Adv. Drug Deliv. Rev. 2019, 148, 204–218. [Google Scholar] [CrossRef]
- Jakob, V.; Küderle, A.; Kluge, F.; Klucken, J.; Eskofier, B.M.; Winkler, J.; Winterholler, M.; Gassner, H. Validation of a Sensor-Based Gait Analysis System with a Gold-Standard Motion Capture System in Patients with Parkinson’s Disease. Sensors 2021, 21, 7680. [Google Scholar] [CrossRef]
- Sridhar, S.; Mishra, S.; Gulyás, M.; Padmanabhan, P.; Gulyás, B. An Overview of Multimodal Neuroimaging Using Nanoprobes. Int. J. Mol. Sci. 2017, 18, 311. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Z.; Qiu, S.; Zhao, H.; Wang, C.; Shi, X.; Lin, F. A Wearable Gait Analysis and Recognition Method for Parkinson’s Disease Based on Error State Kalman Filter. IEEE J. Biomed. Health Inform. 2022, 26, 4165–4175. [Google Scholar] [CrossRef]
- Salchow-Hömmen, C.; Skrobot, M.; Jochner, M.C.E.; Schauer, T.; Kühn, A.A.; Wenger, N. Review-Emerging Portable Technologies for Gait Analysis in Neurological Disorders. Front. Hum. Neurosci. 2022, 16, 768575. [Google Scholar] [CrossRef]
- Altimus, C.M.; Marlin, B.J.; Charalambakis, N.E.; Colón-Rodriquez, A.; Glover, E.J.; Izbicki, P.; Johnson, A.; Lourenco, M.V.; Makinson, R.A.; McQuail, J.; et al. The Next 50 Years of Neuroscience. J. Neurosci. 2020, 40, 101–106, Erratum in J. Neurosci. 2020, 40, 4264. [Google Scholar] [CrossRef]
- Andreassen, O.A.; Hindley, G.F.L.; Frei, O.; Smeland, O.B. New insights from the last decade of research in psychiatric genetics: Discoveries, challenges and clinical implications. World Psychiatry 2023, 22, 4–24. [Google Scholar] [CrossRef] [PubMed]
- Toricelli, M.; Pereira, A.A.R.; Souza Abrao, G.; Malerba, H.N.; Maia, J.; Buck, H.S.; Viel, T.A. Mechanisms of neuroplasticity and brain degeneration: Strategies for protection during the aging process. Neural Regen. Res. 2021, 16, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Nie, Z.; Shu, H.; Kuang, Y.; Chen, X.; Cheng, J.; Yu, S.; Liu, H. The Role of BDNF on Neural Plasticity in Depression. Front Cell Neurosci. 2020, 14, 82. [Google Scholar] [CrossRef]
- Di Liegro, C.M.; Schiera, G.; Proia, P.; Di Liegro, I. Physical Activity and Brain Health. Genes 2019, 10, 720. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, L.M.; Meng, Y.; Xhima, K.; Lipsman, N.; Hamani, C.; Aubert, I. The Neuroprotective Effects of Exercise: Maintaining a Healthy Brain Throughout Aging. Brain Plast. 2018, 4, 17–52. [Google Scholar] [CrossRef]
- Giordano, A.; Clarelli, F.; Cannizzaro, M.; Mascia, E.; Santoro, S.; Sorosina, M.; Ferrè, L.; Leocani, L.; Esposito, F. BDNF Val66Met Polymorphism Is Associated with Motor Recovery After Rehabilitation in Progressive Multiple Sclerosis Patients. Front. Neurol. 2022, 13, 790360. [Google Scholar] [CrossRef]
- Yu, Q.; Jian, Z.; Yang, D.; Zhu, T. Perspective insights into hydrogels and nanomaterials for ischemic stroke. Front. Cell Neurosci. 2023, 16, 1058753. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Shen, J. Local Delivery Strategies for Peptides and Proteins into the CNS: Status Quo, Challenges, and Future Perspectives. Pharmaceuticals 2023, 16, 810. [Google Scholar] [CrossRef]
- Dąbrowski, J.; Czajka, A.; Zielińska-Turek, J.; Jaroszyński, J.; Furtak-Niczyporuk, M.; Mela, A.; Poniatowski, Ł.A.; Drop, B.; Dorobek, M.; Barcikowska-Kotowicz, M.; et al. Brain Functional Reserve in the Context of Neuroplasticity after Stroke. Neural Plast. 2019, 2019, 9708905. [Google Scholar] [CrossRef]
- Sandroff, B.M.; Rafizadeh, C.M.; Motl, R.W. Neuroimaging Technology in Exercise Neurorehabilitation Research in Persons with MS: A Scoping Review. Sensors 2023, 23, 4530. [Google Scholar] [CrossRef]
- Buxton, R.B. The physics of functional magnetic resonance imaging (fMRI). Rep. Prog. Phys. 2013, 76, 096601. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.P.; Kassubek, J. Diffusion tensor magnetic resonance imaging in the analysis of neurodegenerative diseases. J. Vis. Exp. 2013, 77, 50427. [Google Scholar] [CrossRef]
- van Graan, L.A.; Lemieux, L.; Chaudhary, U.J. Methods and utility of EEG-fMRI in epilepsy. Quant. Imaging Med. Surg. 2015, 5, 300–312. [Google Scholar] [CrossRef]
- Light, G.A.; Williams, L.E.; Minow, F.; Sprock, J.; Rissling, A.; Sharp, R.; Swerdlow, N.R.; Braff, D.L. Electroencephalography (EEG) and event-related potentials (ERPs) with human participants. Curr. Protoc. Neurosci. 2010, 52, 6.25.1–6.25.24. [Google Scholar] [CrossRef]
- Maceira-Elvira, P.; Popa, T.; Schmid, A.C.; Hummel, F.C. Wearable technology in stroke rehabilitation: Towards improved diagnosis and treatment of upper-limb motor impairment. J. Neuroeng. Rehabil. 2019, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.; de Natale, E.R.; Politis, M. Recent Advances in Neuroimaging Techniques to Assist Clinical Trials on Cell-Based Therapies in Neurodegenerative Diseases. Stem Cells. 2022, 40, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Sorrentino, G.; Cassio, A.; Mazzoli, D.; Andrenelli, E.; Bizzarini, E.; Campanini, I.; Carmignano, S.M.; Cerulli, S.; Chisari, C.; et al. Robotic-assisted gait rehabilitation following stroke: A systematic review of current guidelines and practical clinical recommendations. Eur. J. Phys. Rehabil. Med. 2021, 57, 460–471. [Google Scholar] [CrossRef]
- Stampacchia, G.; Gazzotti, V.; Olivieri, M.; Andrenelli, E.; Bonaiuti, D.; Calabro, R.S.; Carmignano, S.M.; Cassio, A.; Fundaro, C.; Companini, I.; et al. Gait robot-assisted rehabilitation in persons with spinal cord injury: A scoping review. NeuroRehabilitation 2022, 51, 609–647. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Cacciola, A.; Bertè, F.; Manuli, A.; Leo, A.; Bramanti, A.; Naro, A.; Milardi, D.; Bramanti, P. Robotic gait rehabilitation and substitution devices in neurological disorders: Where are we now? Neurol. Sci. 2016, 37, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Bruni, M.F.; Melegari, C.; De Cola, M.C.; Bramanti, A.; Bramanti, P.; Calabrò, R.S. What does best evidence tell us about robotic gait rehabilitation in stroke patients: A systematic review and meta-analysis. J. Clin. Neurosci. 2018, 48, 11–17. [Google Scholar] [CrossRef]
- Liu, J.; Li, P.; Zuo, S. Actuation and design innovations in earthworm-inspired soft robots: A review. Front. Bioeng. Biotechnol. 2023, 11, 1088105. [Google Scholar] [CrossRef]
- Bonanno, M.; De Nunzio, A.M.; Quartarone, A.; Militi, A.; Petralito, F.; Calabrò, R.S. Gait Analysis in Neurorehabilitation: From Research to Clinical Practice. Bioengineering 2023, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, W.; Yang, W.; Jiao, Z.; Yu, Y. Review of the Research Progress in Soft Robots. Appl. Sci. 2023, 13, 120. [Google Scholar] [CrossRef]
- Perpetuini, D.; Russo, E.F.; Cardone, D.; Palmieri, R.; De Giacomo, A.; Pellegrino, R.; Merla, A.; Calabrò, R.S.; Filoni, S. Use and Effectiveness of Electrosuit in Neurological Disorders: A Systematic Review with Clinical Implications. Bioengineering 2023, 10, 680. [Google Scholar] [CrossRef]
- Pennati, G.V.; Bergling, H.; Carment, L.; Borg, J.; Lindberg, P.G.; Palmcrantz, S. Effects of 60 Min Electrostimulation with the EXOPULSE Mollii Suit on Objective Signs of Spasticity. Front. Neurol. 2021, 12, 706610. [Google Scholar] [CrossRef]
- Lorenz, M.; Brade, J.; Diamond, L.; Sjölie, D.; Busch, M.; Tscheligi, M.; Klimant, P.; Heyde, C.E.; Hammer, N. Presence and User Experience in a Virtual Environment under the Influence of Ethanol: An Explorative Study. Sci. Rep. 2018, 8, 6407. [Google Scholar] [CrossRef]
- Maggio, M.G.; Maresca, G.; De Luca, R.; Stagnitti, M.C.; Porcari, B.; Ferrera, M.C.; Galletti, F.; Casella, C.; Manuli, A.; Calabrò, R.S. The Growing Use of Virtual Reality in Cognitive Rehabilitation: Fact, Fake or Vision? A Scoping Review. J. Natl. Med. Assoc. 2019, 111, 457–463. [Google Scholar] [CrossRef] [PubMed]
- De Luca, R.; Bonanno, M.; Rifici, C.; Pollicino, P.; Caminiti, A.; Morone, G.; Calabrò, R.S. Does Non-Immersive Virtual Reality Improve Attention Processes in Severe Traumatic Brain Injury? Encouraging Data from a Pilot Study. Brain Sci. 2022, 12, 1211. [Google Scholar] [CrossRef] [PubMed]
- De Luca, R.; Bonanno, M.; Marra, A.; Rifici, C.; Pollicino, P.; Caminiti, A.; Castorina, M.V.; Santamato, A.; Quartarone, A.; Calabrò, R.S. Can Virtual Reality Cognitive Rehabilitation Improve Executive Functioning and Coping Strategies in Traumatic Brain Injury? A Pilot Study. Brain Sci. 2023, 13, 578. [Google Scholar] [CrossRef] [PubMed]
- Varela-Aldás, J.; Buele, J.; Ramos Lorente, P.; García-Magariño, I.; Palacios-Navarro, G. A Virtual Reality-Based Cognitive Telerehabilitation System for Use in the COVID-19 Pandemic. Sustainability 2021, 13, 2183. [Google Scholar] [CrossRef]
- Goffredo, M.; Pagliari, C.; Turolla, A.; Tassorelli, C.; Di Tella, S.; Federico, S.; Pournajaf, S.; Jonsdottir, J.; De Icco, R.; Pellicciari, L.; et al. Non-Immersive Virtual Reality Telerehabilitation System Improves Postural Balance in People with Chronic Neurological Diseases. J. Clin. Med. 2023, 12, 3178. [Google Scholar] [CrossRef]
- Liew, S.L.; Santarnecchi, E.; Buch, E.R.; Cohen, L.G. Non-invasive brain stimulation in neurorehabilitation: Local and distant effects for motor recovery. Front. Hum. Neurosci. 2014, 8, 378. [Google Scholar] [CrossRef]
- Li, K.P.; Wu, J.J.; Zhou, Z.L.; Xu, D.S.; Zheng, M.X.; Hua, X.Y.; Xu, J.G. Noninvasive Brain Stimulation for Neurorehabilitation in Post-Stroke Patients. Brain Sci. 2023, 13, 451. [Google Scholar] [CrossRef]
- Banduni, O.; Saini, M.; Singh, N.; Nath, D.; Kumaran, S.S.; Kumar, N.; Srivastava, M.V.P.; Mehndiratta, A. Post-Stroke Rehabilitation of Distal Upper Limb with New Perspective Technologies: Virtual Reality and Repetitive Transcranial Magnetic Stimulation—A Mini Review. J. Clin. Med. 2023, 12, 2944. [Google Scholar] [CrossRef]
- Groiss, S.J.; Wojtecki, L.; Südmeyer, M.; Schnitzler, A. Deep brain stimulation in Parkinson’s disease. Ther. Adv. Neurol. Disord. 2009, 2, 20–28. [Google Scholar] [CrossRef]
- Zanos, S. Closed-Loop Neuromodulation in Physiological and Translational Research. Cold Spring Harb. Perspect. Med. 2019, 9, a034314. [Google Scholar] [CrossRef]
- Shah, R.S.; Chang, S.Y.; Min, H.K.; Cho, Z.H.; Blaha, C.D.; Lee, K.H. Deep brain stimulation: Technology at the cutting edge. J. Clin. Neurol. 2010, 6, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Haug, C.J.; Drazen, J.M. Artificial Intelligence and Machine Learning in Clinical Medicine. N. Engl. J. Med. 2023, 388, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Erickson, B.J. Basic Artificial Intelligence Techniques: Machine Learning and Deep Learning. Radiol. Clin. N. Am. 2021, 59, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Alsobhi, M.; Sachdev, H.S.; Chevidikunnan, M.F.; Basuodan, R.; K U, D.K.; Khan, F. Facilitators and Barriers of Artificial Intelligence Applications in Rehabilitation: A Mixed-Method Approach. Int. J. Environ. Res. Public Health 2022, 19, 15919. [Google Scholar] [CrossRef]
- Kristoffersson, A.; Lindén, M. A Systematic Review of Wearable Sensors for Monitoring Physical Activity. Sensors 2022, 22, 573. [Google Scholar] [CrossRef]
- Cecil, C.A.M.; Nigg, J.T. Epigenetics and ADHD: Reflections on Current Knowledge, Research Priorities and Translational Potential. Mol. Diagn. Ther. 2022, 26, 581–606. [Google Scholar] [CrossRef]
- Segato, A.; Marzullo, A.; Calimeri, F.; De Momi, E. Artificial intelligence for brain diseases: A systematic review. APL Bioeng. 2020, 4, 041503. [Google Scholar] [CrossRef]
- Chen, Z.S.; Kulkarni, P.P.; Galatzer-Levy, I.R.; Bigio, B.; Nasca, C.; Zhang, Y. Modern views of machine learning for precision psychiatry. Patterns 2022, 3, 100602. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, L.; Wang, X.; Monaghan, J.; McAlpine, D.; Zhang, Y. A survey on deep learning-based non-invasive brain signals: Recent advances and new frontiers. J. Neural. Eng. 2021, 18, 031002. [Google Scholar] [CrossRef]
- Zhang, Y.; Naparstek, S.; Gordon, J.; Watts, M.; Shpigel, E.; El-Said, D.; Badami, F.S.; Eisenberg, M.L.; Toll, R.T.; Gage, A.; et al. Machine learning-based identification of a psychotherapy-predictive electroencephalographic signature in PTSD. Nat. Ment. Health 2023, 1, 284–294. [Google Scholar] [CrossRef]
- Preatoni, E.; Nodari, S.; Lopomo, N.F. Supervised Machine Learning Applied to Wearable Sensor Data Can Accurately Classify Functional Fitness Exercises within a Continuous Workout. Front. Bioeng. Biotechnol. 2020, 8, 664. [Google Scholar] [CrossRef] [PubMed]
- Slemenšek, J.; Fister, I.; Geršak, J.; Bratina, B.; van Midden, V.M.; Pirtošek, Z.; Šafarič, R. Human Gait Activity Recognition Machine Learning Methods. Sensors 2023, 23, 745. [Google Scholar] [CrossRef]
- Siegelmann, H.T. Complex systems science and brain dynamics. Front. Comput. Neurosci. 2010, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Kenzie, E.S.; Parks, E.L.; Carney, N.; Wakeland, W. System dynamics modeling for traumatic brain injury: Mini-review of applications. Front. Bioeng. Biotechnol. 2022, 10, 854358. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Assessing Interactions among Social, Behavioral, and Genetic Factors in Health. Genes, Behavior, and the Social Environment: Moving Beyond the Nature/Nurture Debate; Hernandez, L.M., Blazer, D.G., Eds.; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Garro, F.; Chiappalone, M.; Buccelli, S.; De Michieli, L.; Semprini, M. Neuromechanical Biomarkers for Robotic Neurorehabilitation. Front. Neurorobot. 2021, 15, 742163. [Google Scholar] [CrossRef]
- Lo, K.; Stephenson, M.; Lockwood, C. The economic cost of robotic rehabilitation for adult stroke patients: A systematic review. JBI Database Syst. Rev. Implement Rep. 2019, 17, 520–547. [Google Scholar] [CrossRef]
- Ciesielski, T.H.; Aldrich, M.C.; Marsit, C.J.; Hiatt, R.A.; Williams, S.M. Transdisciplinary approaches enhance the production of translational knowledge. Transl Res. 2017, 182, 123–134. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonanno, M.; Calabrò, R.S. Bridging the Gap between Basic Research and Clinical Practice: The Growing Role of Translational Neurorehabilitation. Medicines 2023, 10, 45. https://doi.org/10.3390/medicines10080045
Bonanno M, Calabrò RS. Bridging the Gap between Basic Research and Clinical Practice: The Growing Role of Translational Neurorehabilitation. Medicines. 2023; 10(8):45. https://doi.org/10.3390/medicines10080045
Chicago/Turabian StyleBonanno, Mirjam, and Rocco Salvatore Calabrò. 2023. "Bridging the Gap between Basic Research and Clinical Practice: The Growing Role of Translational Neurorehabilitation" Medicines 10, no. 8: 45. https://doi.org/10.3390/medicines10080045
APA StyleBonanno, M., & Calabrò, R. S. (2023). Bridging the Gap between Basic Research and Clinical Practice: The Growing Role of Translational Neurorehabilitation. Medicines, 10(8), 45. https://doi.org/10.3390/medicines10080045






