Fasting Protocols Do Not Improve Intestinal Architecture and Immune Parameters in C57BL/6 Male Mice Fed a High Fat Diet
Abstract
1. Introduction
2. Materials and Methods
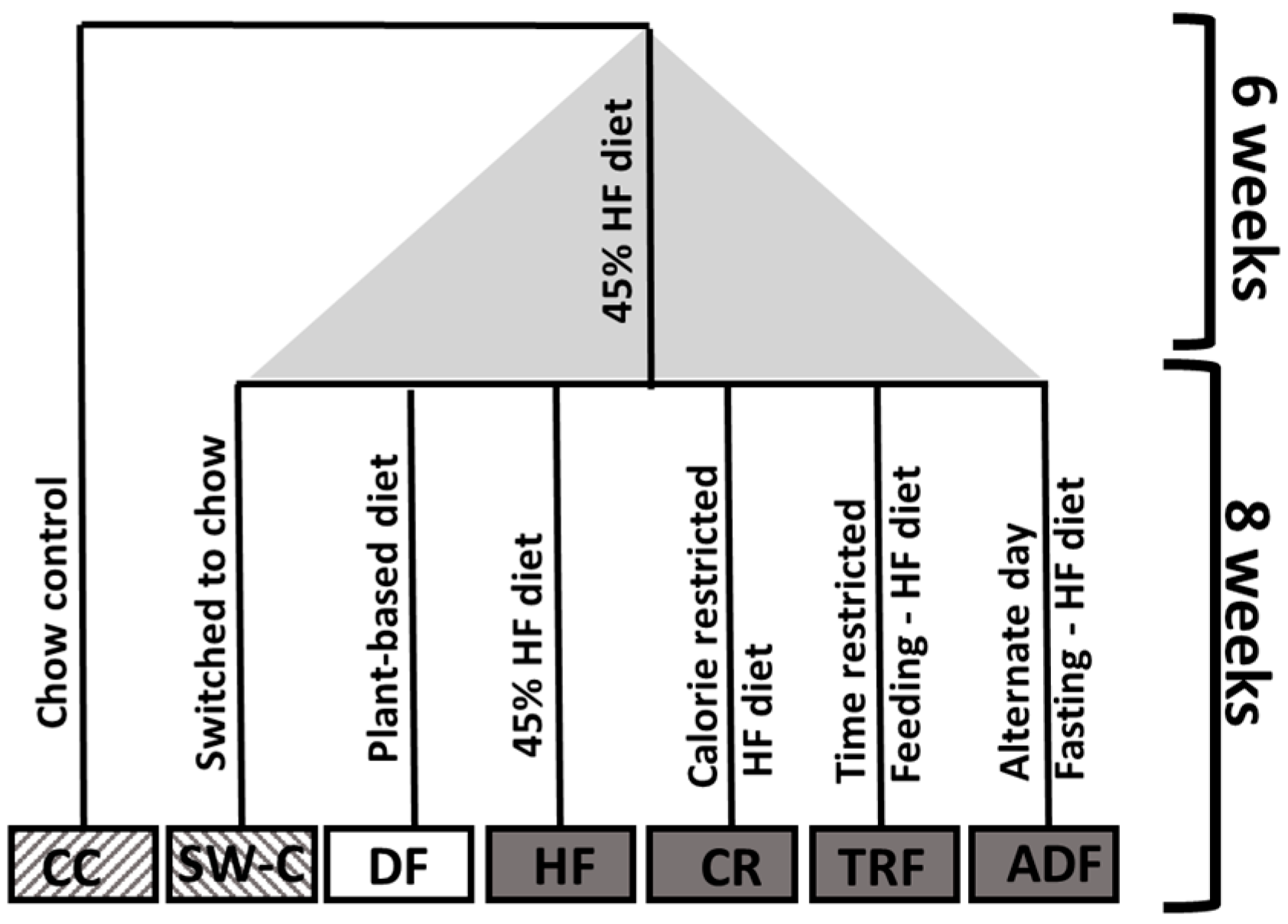
2.1. Experimental Animals and Diet
2.2. Tissue Sampling and Staining
2.3. Periodic Acid-Schiff (PAS)-Alcian Blue (AB)
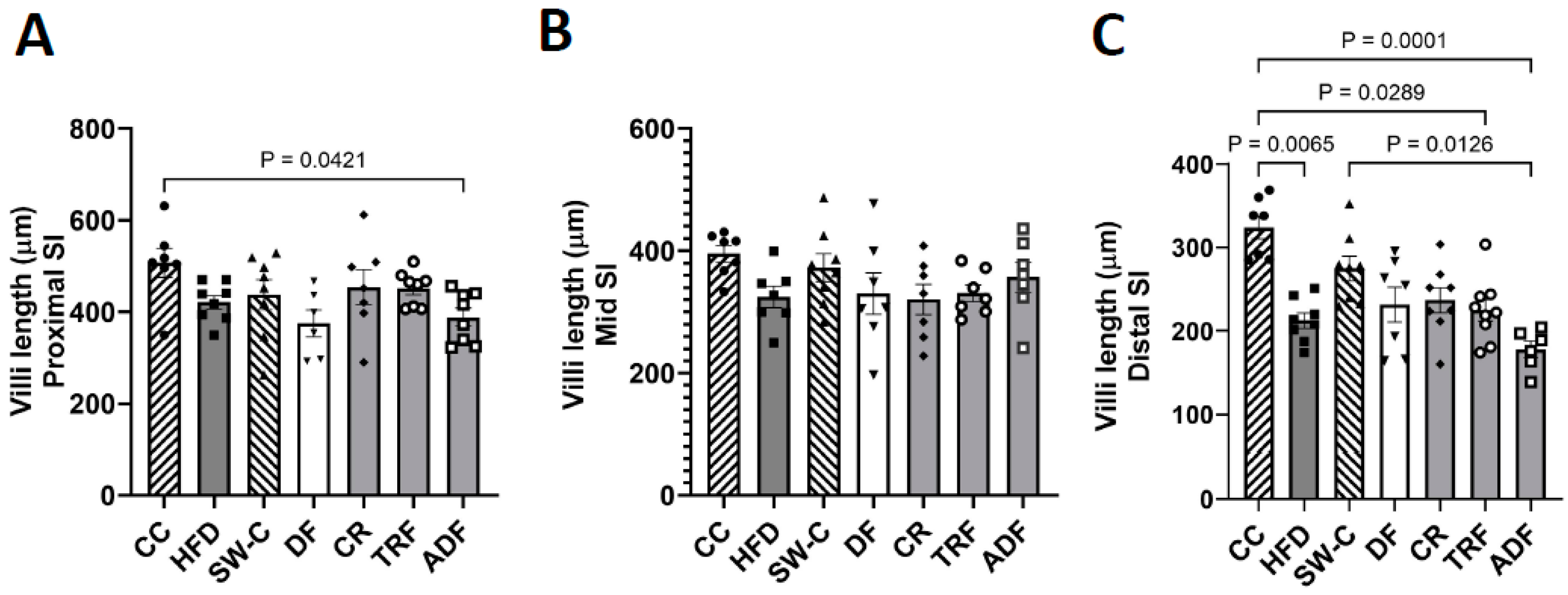
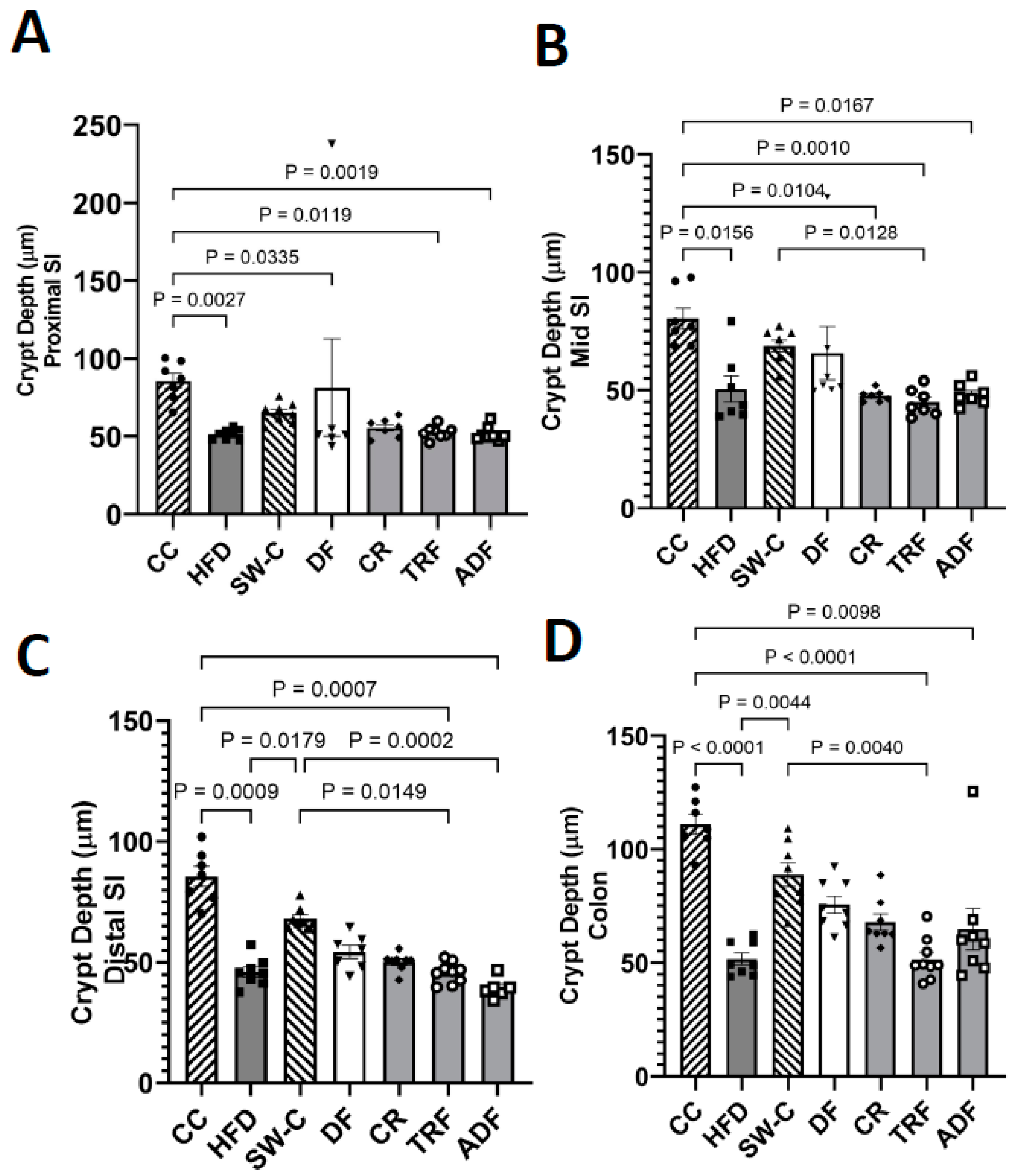
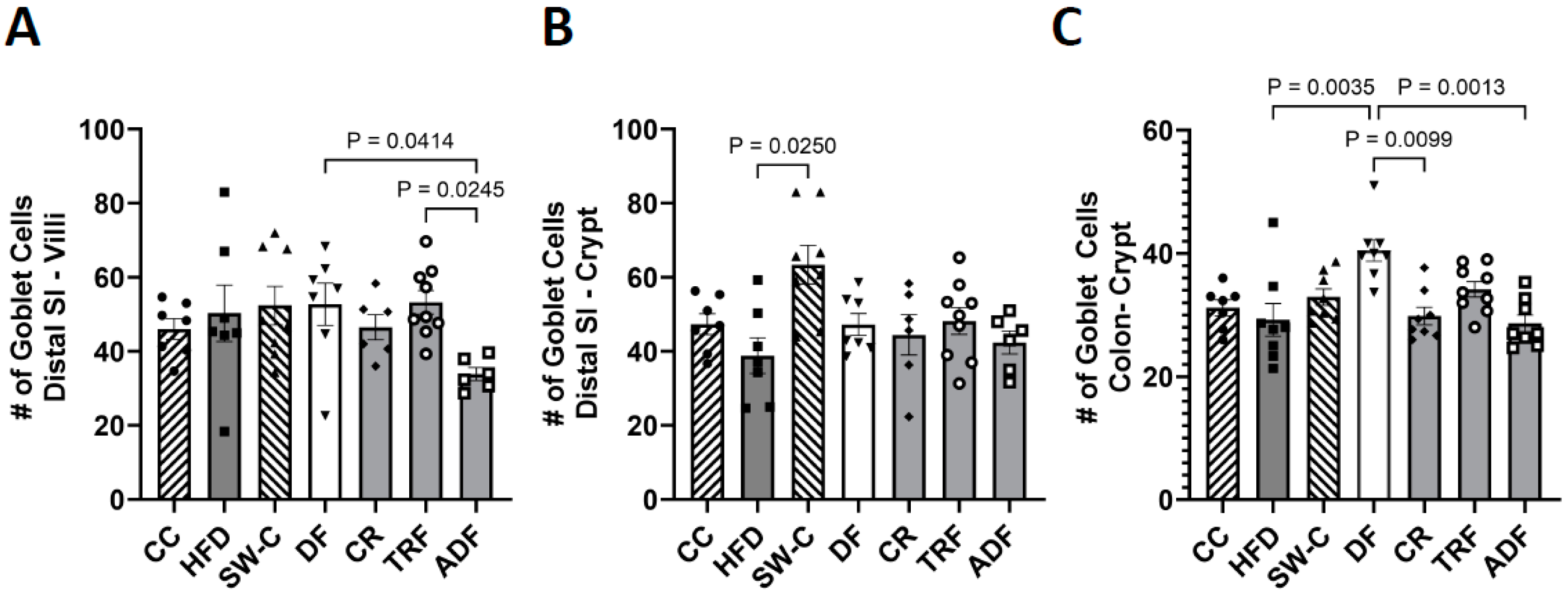
2.4. Measurement of Villus Height, Crypt Depth, and Goblet Cells Count
2.5. Real-Time qRT-PCR
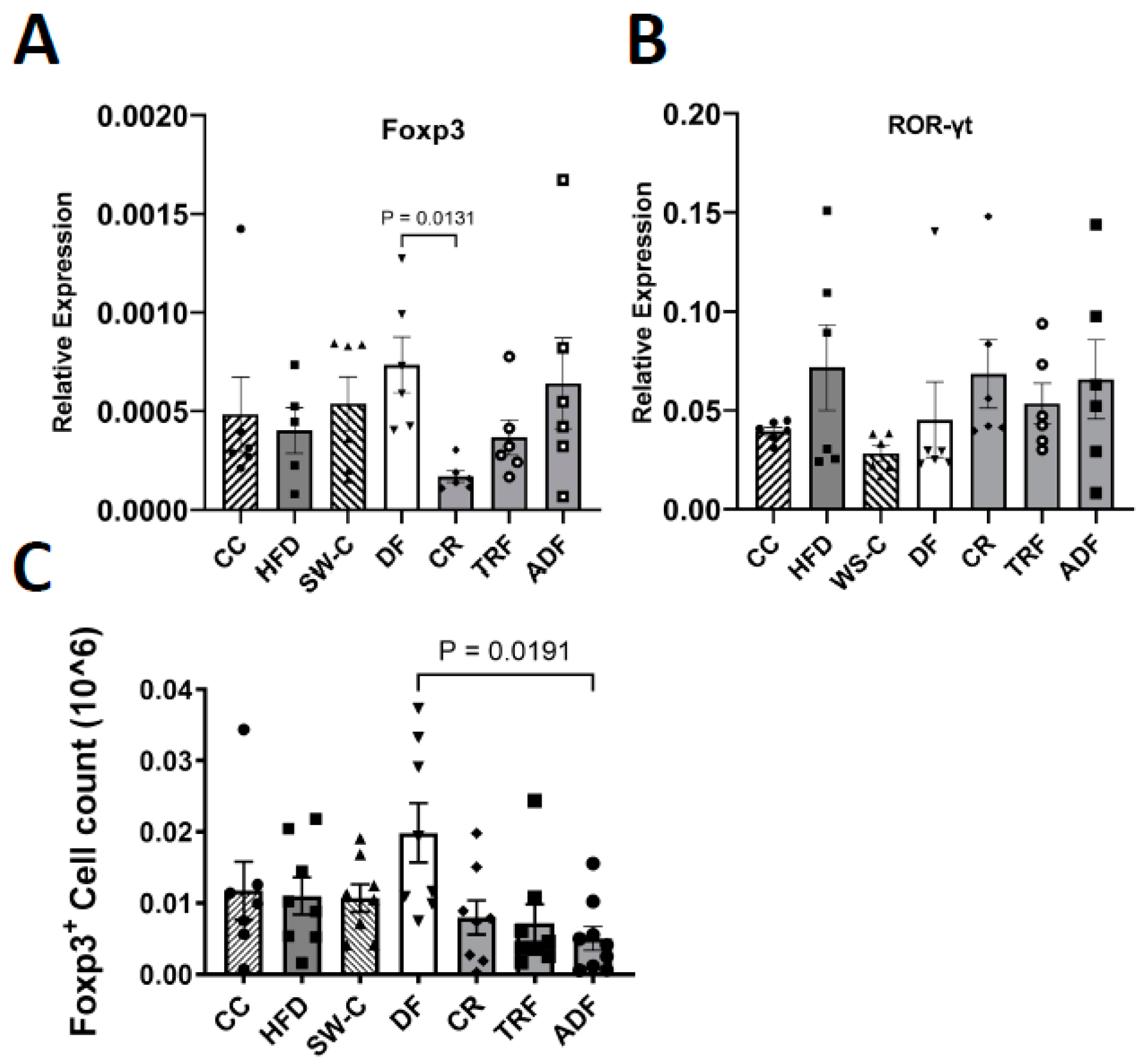
2.6. Cell Isolation and Flow Cytometry
2.7. Statistical Analysis
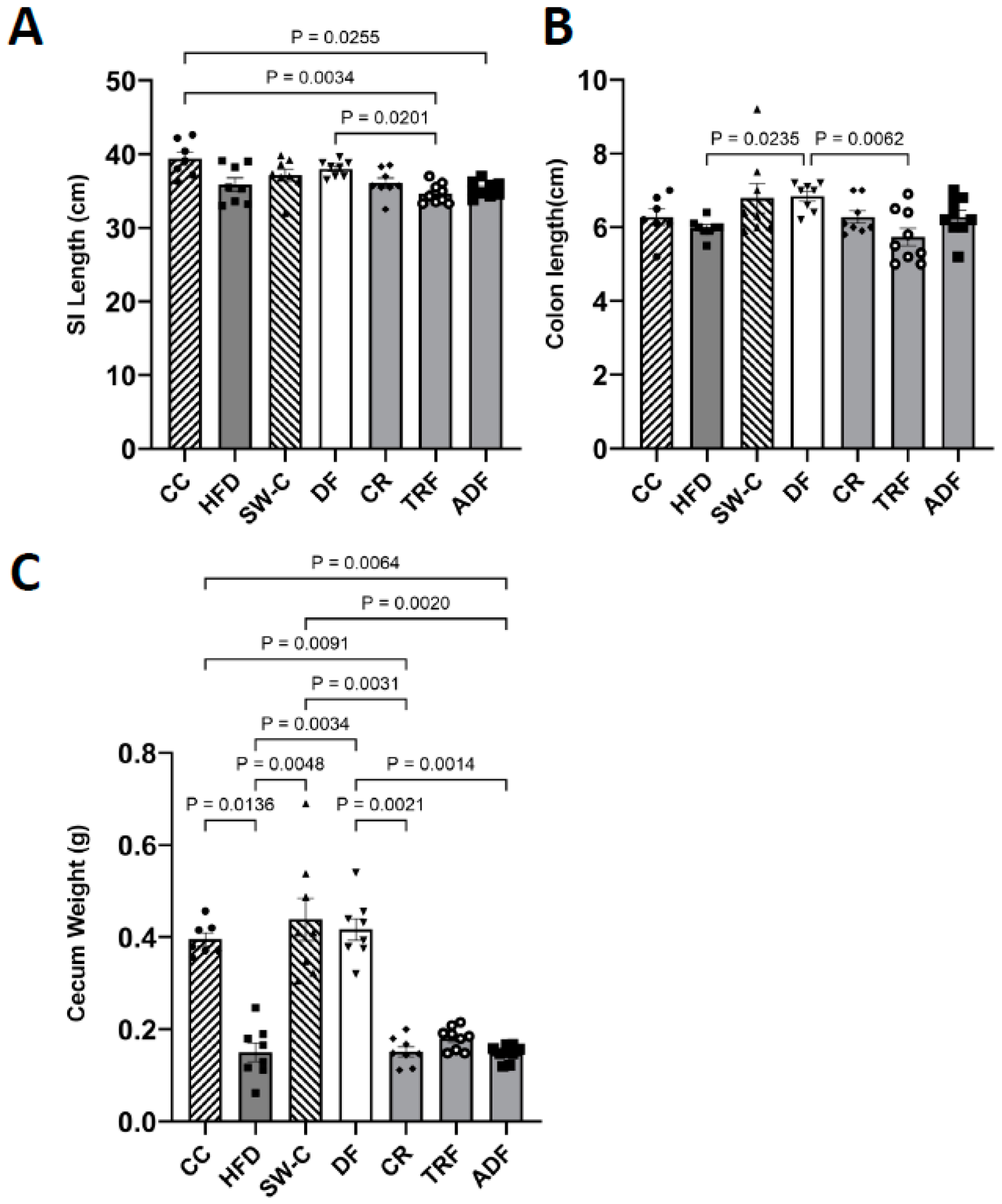
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abeyasekera, K.N. Benefits of Intermittent Fasting: A Systematic Review of Randomized Clinical Trials; Dominican University of California: San Rafael, CA, USA, 2020. [Google Scholar]
- Smith, N.J.; Caldwell, J.L.; van der Merwe, M.; Sharma, S.; Butawan, M.; Puppa, M.; Bloomer, R.J. A comparison of dietary and caloric restriction models on body composition, physical performance, and metabolic health in young mice. Nutrients 2019, 11, 350. [Google Scholar] [CrossRef]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4-and 6-h time-restricted feeding on weight and cardiometabolic health: A randomized controlled trial in adults with obesity. Cell Metab. 2020, 32, 366–378. [Google Scholar] [CrossRef]
- Tawfik, M.K.; Badran, D.I.; Keshawy, M.M.; Makary, S.; Abdo, M. Alternate-day fat diet and exenatide modulate the brain leptin JAK2/STAT3/SOCS3 pathway in a fat diet-induced obesity and insulin resistance mouse model. Arch. Med. Sci. 2022, 1–12. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: A randomized clinical trial. JAMA Intern. Med. 2017, 177, 930–938. [Google Scholar] [CrossRef]
- Flanagan, E.W.; Most, J.; Mey, J.T.; Redman, L.M. Calorie Restriction and Aging in Humans. Annu. Rev. Nutr. 2020, 40, 105–133. [Google Scholar] [CrossRef]
- Redman, L.M.; Smith, S.R.; Burton, J.H.; Martin, C.K.; Il’yasova, D.; Ravussin, E. Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metab. 2018, 27, 805–815. [Google Scholar] [CrossRef]
- Barnard, N.D.; Kahleova, H.; Levin, S.M. The use of plant-based diets for obesity treatment. Int. J. Dis. Reversal Prev. 2019, 1, 12-pp. [Google Scholar] [CrossRef]
- Saintila, J.; López, T.E.L.; Calizaya-Milla, Y.E.; Huancahuire-Vega, S.; White, M. Nutritional knowledge, anthropometric profile, total cholesterol and motivations in vegetarians and non-vegetarians. Nutr. Clínica Dietética Hosp. 2021, 41, 91–98. [Google Scholar]
- Ostfeld, R.J. Definition of a plant-based diet and overview of this special issue. J. Geriatr. Cardiol. 2017, 14, 315. [Google Scholar]
- Ivanova, S.; Delattre, C.; Karcheva-Bahchevanska, D.; Benbasat, N.; Nalbantova, V.; Ivanov, K. Plant-based diet as a strategy for weight control. Foods 2021, 10, 3052. [Google Scholar] [CrossRef]
- Tran, E.; Dale, H.F.; Jensen, C.; Lied, G.A. Effects of plant-based diets on weight status: A systematic review. Diabetes Metab. Syndr. Obes. 2020, 13, 3433. [Google Scholar] [CrossRef] [PubMed]
- Djekic, D.; Shi, L.; Brolin, H.; Carlsson, F.; Särnqvist, C.; Savolainen, O.; Cao, Y.; Bäckhed, F.; Tremaroli, V.; Landberg, R. Effects of a vegetarian diet on cardiometabolic risk factors, gut microbiota, and plasma metabolome in subjects with ischemic heart disease: A randomized, crossover study. J. Am. Heart Assoc. 2020, 9, e016518. [Google Scholar] [CrossRef] [PubMed]
- McMacken, M.; Shah, S. A plant-based diet for the prevention and treatment of type 2 diabetes. J. Geriatr. Cardiol. 2017, 14, 342. [Google Scholar]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab. 2017, 26, 672–685. [Google Scholar] [CrossRef]
- Pinto, F.C.S.; Silva, A.A.M.; Souza, S.L. Repercussions of intermittent fasting on the intestinal microbiota community and body composition: A systematic review. Nutr. Rev. 2022, 80, 613–628. [Google Scholar] [CrossRef]
- Teng, K.; Huang, F.; Liu, Y.; Wang, Y.; Xia, T.; Yun, F.; Zhong, J. Food and gut originated bacteriocins involved in gut microbe-host interactions. Crit. Rev. Microbiol. 2022, 1–13. [Google Scholar] [CrossRef]
- Zhu, G.; Hu, J.; Xi, R. The cellular niche for intestinal stem cells: A team effort. Cell Regen. 2021, 10, 1–16. [Google Scholar] [CrossRef]
- Kong, S.; Zhang, Y.H.; Zhang, W. Regulation of intestinal epithelial cells properties and functions by amino acids. Biomed. Res. Int. 2018, 2018, 2819154. [Google Scholar] [CrossRef]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative effects of a high-fat diet on intestinal permeability: A review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef]
- Di, W.; Lv, Y.; Xia, F.; Sheng, Y.; Liu, J.; Ding, G. Improvement of intestinal stem cells and barrier function via energy restriction in middle-aged C57BL/6 mice. Nutr. Res. 2020, 81, 47–57. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Xu, Y.; Luo, T.; Ge, Y.; Jiang, Y.; Shi, Y.; Sun, J.; Le, G. Dietary methionine restriction improves the gut microbiota and reduces intestinal permeability and inflammation in high-fat-fed mice. Food Funct. 2019, 10, 5952–5968. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ding, F.; Di, W.; Lv, Y.; Xia, F.; Sheng, Y.; Yu, J.; Ding, G. Impact of a high-fat diet on intestinal stem cells and epithelial barrier function in middle-aged female mice. Mol. Med. Rep. 2020, 21, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Singh, J.; Boparai, R.K.; Zhu, J.; Mantri, S.; Khare, P.; Khardori, R.; Kondepudi, K.K.; Chopra, K.; Bishnoi, M. Isomalto-oligosaccharides, a prebiotic, functionally augment green tea effects against high fat diet-induced metabolic alterations via preventing gut dysbacteriosis in mice. Pharmacol. Res. 2017, 123, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Muthuramalingam, K.; Singh, V.; Choi, C.; Choi, S.I.; Kim, Y.M.; Unno, T.; Cho, M. Dietary intervention using (1, 3)/(1, 6)-β-glucan, a fungus-derived soluble prebiotic ameliorates high-fat diet-induced metabolic distress and alters beneficially the gut microbiota in mice model. Eur. J. Nutr. 2020, 59, 2617–2629. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Gushgari, L.R.; Vojdani, E. Interaction between food antigens and the immune system: Association with autoimmune disorders. Autoimmun. Rev. 2020, 19, 102459. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Zhu, Y.; Liu, X.; Gu, Y.; Dai, X.; Li, B. Transcriptional and posttranslational regulation of Th17/Treg balance in health and disease. Eur. J. Immunol. 2021, 51, 2137–2150. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, F.; Gao, Y.; Li, Z.; Zhang, J.; Xing, Y.; Deng, Z.; Yao, Z.; Tsun, A.; Li, B. FOXP3 and RORγt: Transcriptional regulation of Treg and Th17. Int. Immunopharmacol. 2011, 11, 536–542. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Tang, R.; Zhang, G.; Zeng, H.; Wood, R.J.; Liu, Z. High fat diet alters gut microbiota and the expression of paneth cell-antimicrobial peptides preceding changes of circulating inflammatory cytokines. Mediat. Inflamm. 2017, 2017, 9474896. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, S.-E.; Kim, A.; Kang, S.; Park, M.-Y.; Sung, M.-K. Dietary fat intake and age modulate the composition of the gut microbiota and colonic inflammation in C57BL/6J mice. BMC Microbiol. 2019, 19, 1–11. [Google Scholar] [CrossRef]
- Roller, M.; Rechkemmer, G.; Watzl, B. Prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis modulates intestinal immune functions in rats. J. Nutr. 2004, 134, 153–156. [Google Scholar] [CrossRef]
- Hoentjen, F.; Welling, G.W.; Harmsen, H.J.M.; Zhang, X.; Snart, J.; Tannock, G.W.; Lien, K.; Churchill, T.A.; Lupicki, M.; Dieleman, L.A. Reduction of colitis by prebiotics in HLA-B27 transgenic rats is associated with microflora changes and immunomodulation. Inflamm. Bowel Dis. 2005, 11, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Valcheva, R.; Hotte, N.; Gillevet, P.; Sikaroodi, M.; Thiessen, A.; Madsen, K.L. Soluble dextrin fibers alter the intestinal microbiota and reduce proinflammatory cytokine secretion in male IL-10--deficient mice. J. Nutr. 2015, 145, 2060–2066. [Google Scholar] [CrossRef] [PubMed]
- van der Merwe, M.; Sharma, S.; Caldwell, J.L.; Smith, N.J.; Gomes, C.K.; Bloomer, R.J.; Buddington, R.K.; Pierre, J.F. Time of feeding alters obesity-associated parameters and gut bacterial communities, but not fungal populations, in C57bl/6 male mice. Curr. Dev. Nutr. 2020, 4, nzz145. [Google Scholar] [CrossRef]
- Feldman, A.T.; Wolfe, D. Tissue processing and hematoxylin and eosin staining. In Histopathology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 31–43. [Google Scholar]
- Mayssara, A.; Abo Hassanin Supervised, A.; Munawarah, S.H.; Misnaniarti, M.; Isnurhadi, I.; Komunitas, J.K.; Rumbai, P.; City, P.; Komitmen, P.; Kbpkp, P.; et al. TRI Reagent® RT—RNA, DNA, protein isolation reagent-Manufacturer’s protocol. Pap. Knowl. Towar. Media Hist. Doc. 2011, 7, 1–33. [Google Scholar]
- Pillai, A.B.; George, T.I.; Dutt, S.; Strober, S. Host natural killer T cells induce an interleukin-4–dependent expansion of donor CD4+ CD25+ Foxp3+ T regulatory cells that protects against graft-versus-host disease. Blood J. Am. Soc. Hematol. 2009, 113, 4458–4467. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Qu, L.; Dou, T.; Ma, M.; Shen, M.; Guo, J.; Hu, Y.; Wang, K. Genome-wide association studies for small intestine length in an F2 population of chickens. Ital. J. Anim. Sci. 2018, 17, 294–300. [Google Scholar] [CrossRef]
- De Santis, S.; Cavalcanti, E.; Mastronardi, M.; Jirillo, E.; Chieppa, M. Nutritional keys for intestinal barrier modulation. Front. Immunol. 2015, 6, 612. [Google Scholar] [CrossRef]
- Chivers, D.J.; Hladik, C.M. Morphology of the gastrointestinal tract in primates: Comparisons with other mammals in relation to diet. J. Morphol. 1980, 166, 337–386. [Google Scholar] [CrossRef]
- Hunt, J.E.; Hartmann, B.; Schoonjans, K.; Holst, J.J.; Kissow, H. Dietary Fiber Is Essential to Maintain Intestinal Size, L-Cell Secretion, and Intestinal Integrity in Mice. Front. Endocrinol. 2021, 12, 640602. [Google Scholar] [CrossRef]
- Delmée, E.; Cani, P.D.; Gual, G.; Knauf, C.; Burcelin, R.; Maton, N.; Delzenne, N.M. Relation between colonic proglucagon expression and metabolic response to oligofructose in high fat diet-fed mice. Life Sci. 2006, 79, 1007–1013. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Hillemeier, C. An overview of the effects of dietary fiber on gastrointestinal transit. Pediatrics 1995, 96, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Farness, P.L.; Schneeman, B.O. Effects of dietary cellulose, pectin and oat bran on the small intestine in the rat. J. Nutr. 1982, 112, 1315–1319. [Google Scholar] [CrossRef]
- Stark, A.; Nyska, A.; Madar, Z. Metabolic and morphometric changes in small and large intestine in rats fed high-fiber diets. Toxicol. Pathol. 1996, 24, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Rémésy, C.; Behr, S.R.; Levrat, M.-A.; Demigne, C. Fiber fermentability in the rat cecum and its physiological consequences. Nutr. Res. 1992, 12, 1235–1244. [Google Scholar] [CrossRef]
- Kim, Y.; Hwang, S.W.; Kim, S.; Lee, Y.-S.; Kim, T.-Y.; Lee, S.-H.; Kim, S.J.; Yoo, H.J.; Kim, E.N.; Kweon, M.-N. Dietary cellulose prevents gut inflammation by modulating lipid metabolism and gut microbiota. Gut Microbes 2020, 11, 944–961. [Google Scholar] [CrossRef]
- Bird, A.R.; Vuaran, M.; Brown, I.; Topping, D.L. Two high-amylose maize starches with different amounts of resistant starch vary in their effects on fermentation, tissue and digesta mass accretion, and bacterial populations in the large bowel of pigs. Br. J. Nutr. 2007, 97, 134–144. [Google Scholar] [CrossRef]
- Chen, Y.C.; Nakthong, C.; Chen, T.C. Improvement of laying hen performance by dietary prebiotic chicory oligofructose and inulin. Int. J. Poult. Sci. 2005, 4, 103–108. [Google Scholar]
- Zhu, L.; Lu, X.; Liu, L.; Voglmeir, J.; Zhong, X.; Yu, Q. Akkermansia muciniphila protects intestinal mucosa from damage caused by S. pullorum by initiating proliferation of intestinal epithelium. Vet. Res. 2020, 51, 1–9. [Google Scholar] [CrossRef]
- Xi, M.; Li, J.; Hao, G.; An, X.; Song, Y.; Wei, H.; Ge, W. Stachyose increases intestinal barrier through Akkermansia muciniphila and reduces gut inflammation in germ-free mice after human fecal transplantation. Food Res. Int. 2020, 137, 109288. [Google Scholar] [CrossRef]
- Kabeerdoss, J.; Jayakanthan, P.; Pugazhendhi, S.; Ramakrishna, B.S. Alterations of mucosal microbiota in the colon of patients with inflammatory bowel disease revealed by real time polymerase chain reaction amplification of 16S ribosomal ribonucleic acid. Indian J. Med. Res. 2015, 142, 23. [Google Scholar] [PubMed]
- Jeffery, I.B.; O’toole, P.W.; Öhman, L.; Claesson, M.J.; Deane, J.; Quigley, E.M.M.; Simrén, M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 2012, 61, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Gulhane, M.; Murray, L.; Lourie, R.; Tong, H.; Sheng, Y.H.; Wang, R.; Kang, A.; Schreiber, V.; Wong, K.Y.; Magor, G.; et al. High fat diets induce colonic epithelial cell stress and inflammation that is reversed by IL-22. Sci. Rep. 2016, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sumigray, K.D.; Terwilliger, M.; Lechler, T. Morphogenesis and compartmentalization of the intestinal crypt. Dev. Cell 2018, 45, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Bruens, L.; Ellenbroek, S.I.J.; van Rheenen, J.; Snippert, H.J. In vivo imaging reveals existence of crypt fission and fusion in adult mouse intestine. Gastroenterology 2017, 153, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Ijaz, M.U.; Ahmad, M.I.; Khan, I.A.; Brohi, S.A.; Shah, A.U.; Shinwari, K.I.; Zhao, D.; Xu, X.; Zhou, G. Meat proteins in a high-fat diet have a substantial impact on intestinal barriers through mucus layer and tight junction protein suppression in C57BL/6J mice. Food Funct. 2019, 10, 6903–6914. [Google Scholar] [CrossRef] [PubMed]
- Andoh, A.; Bamba, T.; Sasaki, M. Physiological and anti-inflammatory roles of dietary fiber and butyrate in intestinal functions. J. Parenter. Enter. Nutr. 1999, 23, S70–S73. [Google Scholar] [CrossRef]
- Howard, M.D.; Gordon, D.T.; Garleb, K.A.; Kerley, M.S. Dietary fructooligosaccharide, xylooligosaccharide and gum arabic have variable effects on cecal and colonic microbiota and epithelial cell proliferation in mice and rats. J. Nutr. 1995, 125, 2604–2609. [Google Scholar]
- Rehman, H.; Rosenkranz, C.; Böhm, J.; Zentek, J. Dietary inulin affects the morphology but not the sodium-dependent glucose and glutamine transport in the jejunum of broilers. Poult. Sci. 2007, 86, 118–122. [Google Scholar] [CrossRef]
- Enss, M.L.; Schmidt-Wittig, U.; Höner, K.; Kownatzki, R.; Gärtner, K. Mechanical challenge causes alterations of rat colonic mucosa and released mucins. Alterations of mucosa and mucins. J. Exp. Anim. Sci. 1994, 36, 128–140. [Google Scholar]
- Hino, S.; Takemura, N.; Sonoyama, K.; Morita, A.; Kawagishi, H.; Aoe, S.; Morita, T. Small intestinal goblet cell proliferation induced by ingestion of soluble and insoluble dietary fiber is characterized by an increase in sialylated mucins in rats. J. Nutr. 2012, 142, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Piel, C.; Montagne, L.; Sève, B.; Lallès, J.-P. Increasing digesta viscosity using carboxymethylcellulose in weaned piglets stimulates ileal goblet cell numbers and maturation. J. Nutr. 2005, 135, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Paturi, G.; Nyanhanda, T.; Butts, C.A.; Herath, T.D.; Monro, J.A.; Ansell, J. Effects of potato fiber and potato-resistant starch on biomarkers of colonic health in rats fed diets containing red meat. J. Food Sci. 2012, 77, H216–H223. [Google Scholar] [CrossRef] [PubMed]
- Paturi, G.; Butts, C.; Monro, J.; Nones, K.; Martell, S.; Butler, R.; Sutherland, J. Cecal and colonic responses in rats fed 5 or 30% corn oil diets containing either 7.5% broccoli dietary fiber or microcrystalline cellulose. J. Agric. Food Chem. 2010, 58, 6510–6515. [Google Scholar] [CrossRef] [PubMed]
- Morowitz, M.; Di Caro, V.; Pang, D.; Cummings, J.; Firek, B.; Rogers, M.B.; Ranganathan, S.; Clark, R.S.B.; Aneja, R.K. Dietary supplementation with non-fermentable fiber alters the gut microbiota and confers protection in a murine model of sepsis. Crit. Care Med. 2017, 45, e516. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, A.; Xie, K.; Yu, Y. Dietary supplementation with high fiber alleviates oxidative stress and inflammatory responses caused by severe sepsis in mice without altering microbiome diversity. Front. Physiol. 2019, 9, 1929. [Google Scholar] [CrossRef]
- Yue, C.; Chu, C.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Dietary strategies to promote the abundance of intestinal Akkermansia muciniphila, a focus on the effect of plant extracts. J. Funct. Foods 2022, 93, 105093. [Google Scholar] [CrossRef]
- Kim, S.; Shin, Y.-C.; Kim, T.-Y.; Kim, Y.; Lee, Y.-S.; Lee, S.-H.; Kim, M.-N.; O, E.; Kim, K.S.; Kweon, M.-N. Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development. Gut Microbes 2021, 13, 1892441. [Google Scholar] [CrossRef]
- Heyman, M. How dietary antigens access the mucosal immune system. Proc. Nutr. Soc. 2001, 60, 417–426. [Google Scholar] [CrossRef]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 1–7. [Google Scholar] [CrossRef]
- Kim, K.-A.; Gu, W.; Lee, I.-A.; Joh, E.-H.; Kim, D.-H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE 2012, 7, e47713. [Google Scholar] [CrossRef]
- Khalil, A.; Villard, P.-H.; Dao, M.A.; Burcelin, R.; Champion, S.; Fouchier, F.; Savouret, J.-F.; Barra, Y.; Seree, E. Polycyclic aromatic hydrocarbons potentiate high-fat diet effects on intestinal inflammation. Toxicol. Lett. 2010, 196, 161–167. [Google Scholar] [CrossRef]
- Hur, S.J.; Kang, S.H.; Jung, H.S.; Kim, S.C.; Jeon, H.S.; Kim, I.H.; Lee, J.D. Review of natural products actions on cytokines in inflammatory bowel disease. Nutr. Res. 2012, 32, 801–816. [Google Scholar] [CrossRef]
- Lin, S.-M.; Zhou, X.-M.; Zhou, Y.-L.; Kuang, W.-M.; Chen, Y.-J.; Luo, L.; Dai, F.-Y. Intestinal morphology, immunity and microbiota response to dietary fibers in largemouth bass, Micropterus salmoide. Fish Shellfish. Immunol. 2020, 103, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Cretney, E.; Kallies, A.; Nutt, S.L. Differentiation and function of Foxp3+ effector regulatory T cells. Trends Immunol. 2013, 34, 74–80. [Google Scholar] [CrossRef]
- Sen, S.; Wang, F.; Zhang, J.; He, Z.; Ma, J.; Gwack, Y.; Xu, J.; Sun, Z. SRC1 promotes Th17 differentiation by overriding Foxp3 suppression to stimulate RORγt activity in a PKC-θ—Dependent manner. Proc. Natl. Acad. Sci. USA 2018, 115, E458–E467. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Meng, T.; He, W.; Huang, H.; Liu, C.; Fu, X.; He, J.; Yin, Y.; Xiao, D. Dietary Chito-oligosaccharides Improve Intestinal Immunity via Regulating Microbiota and Th17/Treg Balance-Related Immune Signaling in Piglets Challenged by Enterotoxigenic E. coli. J. Agric. Food Chem. 2021, 69, 15195–15207. [Google Scholar] [CrossRef]
- Chen, K.; Chen, H.; Faas, M.M.; de Haan, B.J.; Li, J.; Xiao, P.; Zhang, H.; Diana, J.; de Vos, P.; Sun, J. Specific inulin-type fructan fibers protect against autoimmune diabetes by modulating gut immunity, barrier function, and microbiota homeostasis. Mol. Nutr. Food Res. 2017, 61, 1601006. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Macia, L.; Mackay, C.R. Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Rep. 2016, 15, 2809–2824. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
| Primer Name | Primer Sequence (5′–3′) |
|---|---|
| β-actin | Fwd: ACCTTCTACAATGAGCTGCG Rev: CTGGATGGCTACGTACATGG |
| ROR-γt | Fwd: AAGTACCACAATATGCGACCC Rev: TCTGAAGTAGGCGAACATGC |
| Foxp3+ | Fwd: TTTCTGAGGATGAGATTGCCC Rev: TTGTCGATGAGTCTTGCAGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ageeli, R.Y.; Sharma, S.; Puppa, M.; Bloomer, R.J.; Buddington, R.K.; van der Merwe, M. Fasting Protocols Do Not Improve Intestinal Architecture and Immune Parameters in C57BL/6 Male Mice Fed a High Fat Diet. Medicines 2023, 10, 18. https://doi.org/10.3390/medicines10020018
Ageeli RY, Sharma S, Puppa M, Bloomer RJ, Buddington RK, van der Merwe M. Fasting Protocols Do Not Improve Intestinal Architecture and Immune Parameters in C57BL/6 Male Mice Fed a High Fat Diet. Medicines. 2023; 10(2):18. https://doi.org/10.3390/medicines10020018
Chicago/Turabian StyleAgeeli, Raed Y., Sunita Sharma, Melissa Puppa, Richard J. Bloomer, Randal K. Buddington, and Marie van der Merwe. 2023. "Fasting Protocols Do Not Improve Intestinal Architecture and Immune Parameters in C57BL/6 Male Mice Fed a High Fat Diet" Medicines 10, no. 2: 18. https://doi.org/10.3390/medicines10020018
APA StyleAgeeli, R. Y., Sharma, S., Puppa, M., Bloomer, R. J., Buddington, R. K., & van der Merwe, M. (2023). Fasting Protocols Do Not Improve Intestinal Architecture and Immune Parameters in C57BL/6 Male Mice Fed a High Fat Diet. Medicines, 10(2), 18. https://doi.org/10.3390/medicines10020018






