Effect of Low- and High-Frequency Auricular Stimulation with Electro-Acupuncture on Cutaneous Microcirculation: A Cross-Over Study in Healthy Subjects
Abstract
1. Introduction
Aim of the Study
2. Materials and Methods
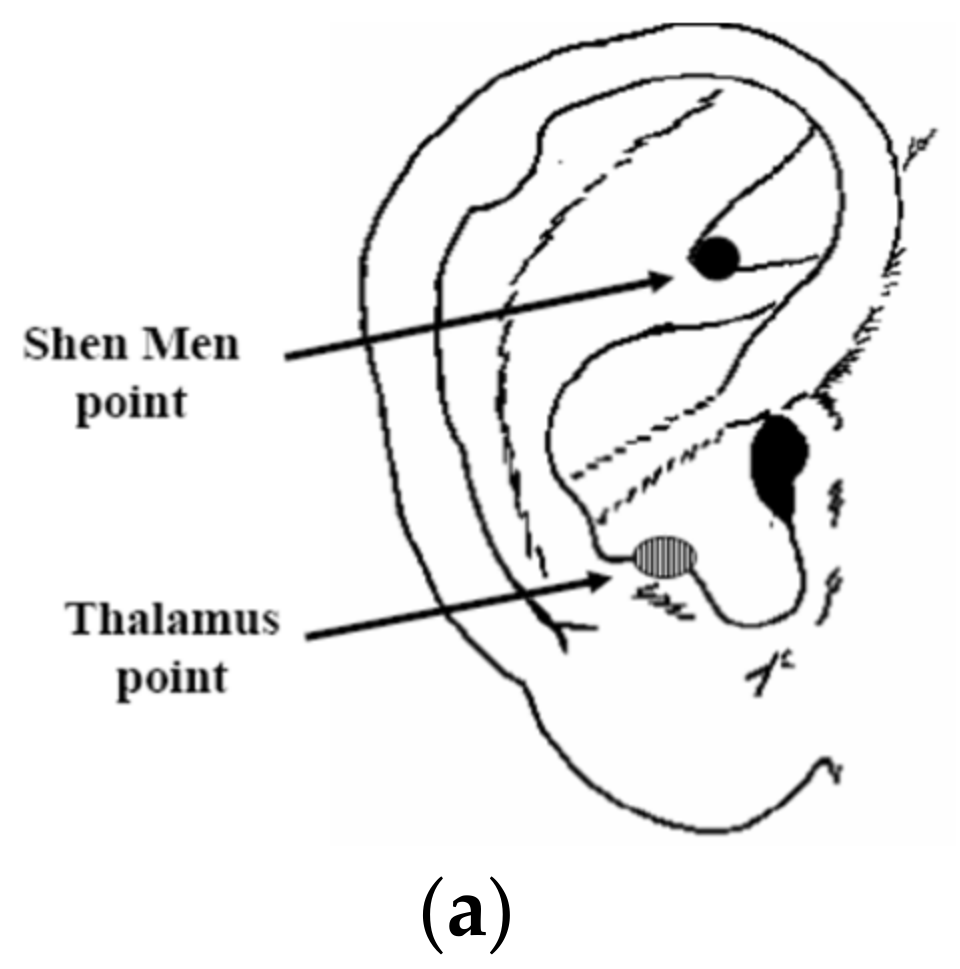
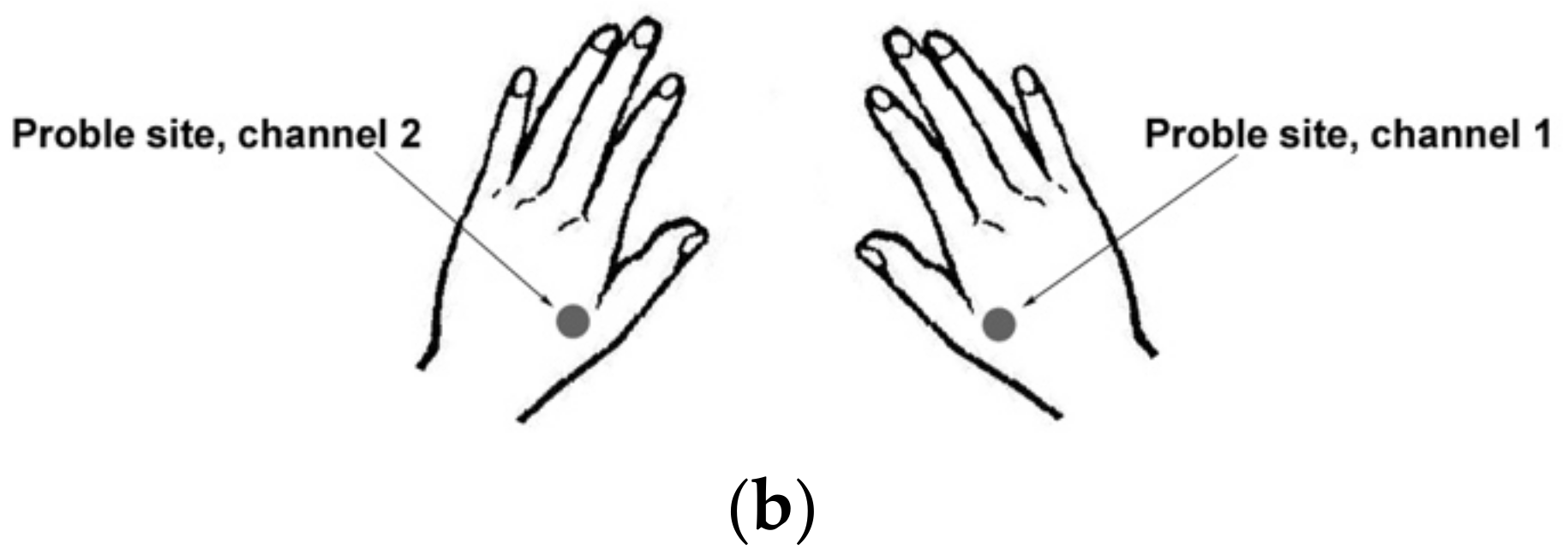
Experimental Protocol
3. Results
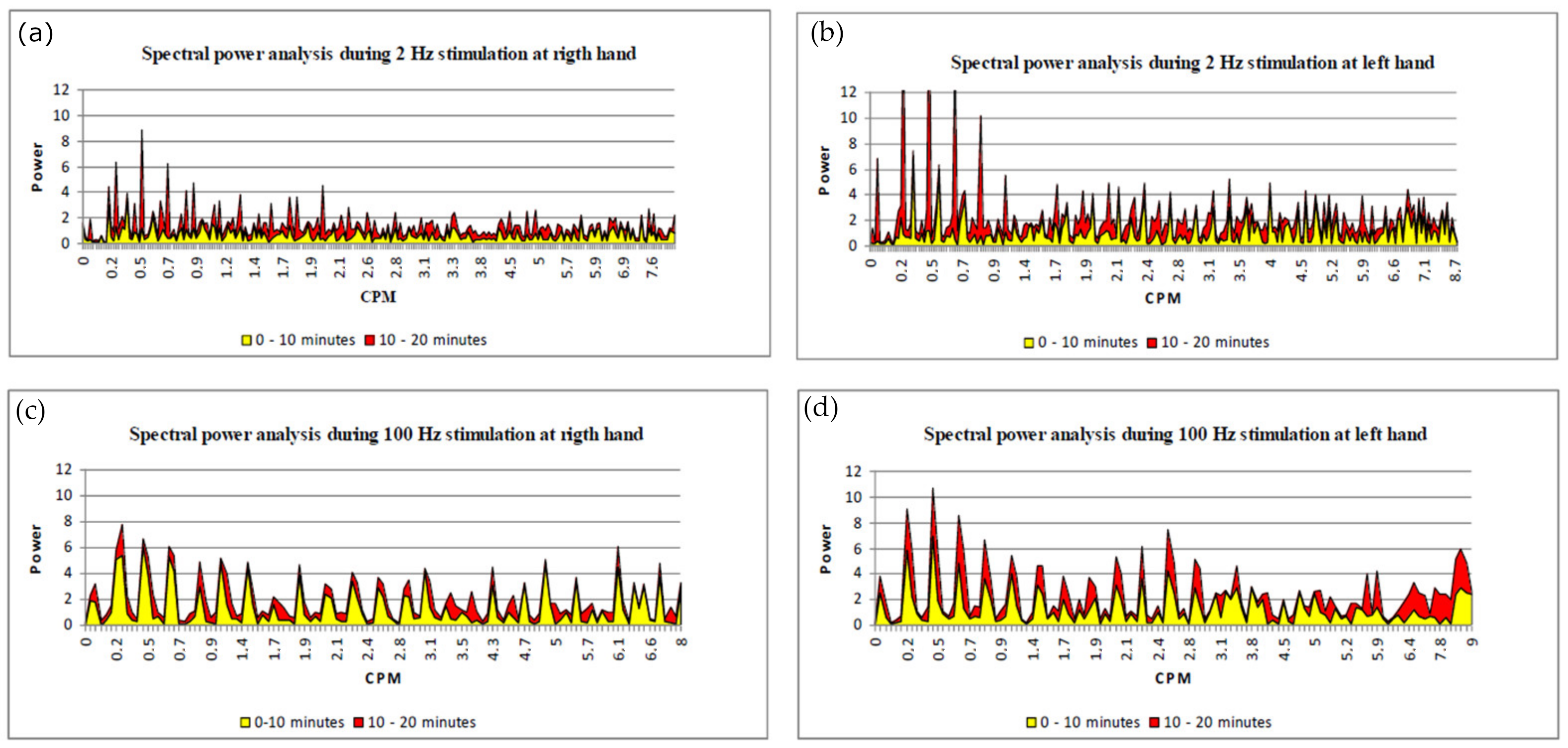
Vasomotion Analysis (Spectral Power Analysis)
4. Statistical Analysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Guven, G.; Hilty, M.P.; Ince, C. Microcirculation: Physiology, Pathophysiology, and Clinical Application. Blood Purif. 2020, 49, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Intaglietta, M. Vasomotion and flowmotion: Physiological mechanisms and clinical evidence. Vasc. Med. Rev. 2017, 1, 101–112. [Google Scholar] [CrossRef]
- Nilsson, H.; Aalkjaer, C. Vasomotion: Mechanisms and physiological importance. Mol. Interv. 2003, 3, 79–89. [Google Scholar] [CrossRef]
- Komori, M.; Takada, K.; Tomizawa, Y.; Nishiyama, K.; Kondo, I.; Kawamata, M.; Ozaki, M. Microcirculatory Responses to Acu-puncture Stimulation and Phototherapy. Anesth. Analg. 2009, 108, 635–640. [Google Scholar] [CrossRef]
- Lee, S.; Lee, M.S.; Choi, J.Y.; Lee, S.W.; Jeong, S.Y.; Ernst, E. Acupuncture and heart rate variability: A systematic review. Auton. Neurosci. 2010, 155, 5–13. [Google Scholar] [CrossRef]
- Li, P.; Pitsillides, K.F.; Rendig, S.V.; Pan, H.L.; Longhurst, J.C. Reversal of reflex-induced myocardial ischemia by median nerve stimulation: A feline model of electroacupuncture. Circulation 1998, 97, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Rowshan, K.; Crisostomo, M.; Tjen-A-Looi, S.C.; Longhurst, J.C. Effect of electroacupuncture on pressor reflex during gastric distension. Am. J. Physiol. Integr. Comp. Physiol. 2002, 283, R1335–R1345. [Google Scholar] [CrossRef]
- Li, P.; Tjen-A-Looi, S.C.; Guo, Z.-L.; Fu, L.-W.; Longhurst, J.C. Long-loop pathways in cardiovascular electroacupuncture responses. J. Appl. Physiol. 2009, 106, 620–630. [Google Scholar] [CrossRef]
- Nishijo, K.; Mori, H.; Yosikawa, K.; Yazawa, K. Decreased heart rate by acupuncture stimulation in humans via facilitation of cardiac vagal activity and suppression of cardiac sympathetic nerve. Neurosci. Lett. 1997, 227, 165–168. [Google Scholar] [CrossRef]
- Sugimachi, M.; Kawada, T.; Yamamoto, H.; Kamiya, A.; Miyamoto, T.; Sunagawa, K. Modification of autonomic balance by electrical acupuncture does not affect baroreflex dynamic characteristics. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 1981–1984. [Google Scholar]
- Haker, E.; Egekvist, H.; Bjerring, P. Effect of sensory stimulation (acupuncture) on sympathetic and parasympathetic activities in healthy subjects. J. Auton. Nerv. Syst. 2000, 79, 52–59. [Google Scholar] [CrossRef]
- Imai, K.; Ariga, H.; Takahashi, T. Electroacupuncture improves imbalance of autonomic function under restraint stress in con-scious rats. Am. J. Chin. Med. 2009, 37, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Fu, L.W.; Tjen-A-Looi, S.C.; Li, P.; Longhurst, J.C. Afferent mechanisms underlying stimulation modality-related modulation of acupuncture-related cardiovascular responses. J. Appl. Physiol. 2005, 98, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Hodges, G.J.; Del Pozzi, A.T. Noninvasive examination of endothelial, sympathetic, and myogenic contributions to regional differences in the human cutaneous microcirculation. Microvasc. Res. 2014, 93, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-S. Acupuncture: Neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003, 26, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, K.; Gokal, R.; Todorsky, W. Neuromodulating Influence of Two Electroacupuncture Treatments on Heart Rate Variability, Stress, and Vagal Activity. J. Altern. Complement. Med. 2020, 26, 928–936. [Google Scholar] [CrossRef]
- Ping, Y.W.; Shu, W.; Lin, H.; Quan, M.J.; Yan, S. Effects of different frequencies of electro-acupuncture at Shuigou(GV 26) on recovery of motor function in rats with focal cerebra ischemic injury. J. Tradit. Chin. Med. 2012, 32, 99–104. [Google Scholar]
- Min, S.; Lee, H.; Kim, S.Y.; Park, J.Y.; Chae, Y.; Lee, H.; Park, H.J. Local Changes in Microcirculation and the Analgesic Effects of Acupuncture: A Laser Doppler Perfusion Imaging Study. J. Altern. Complement. Med. 2015, 21, 46–52. [Google Scholar] [CrossRef]
- Stener-Victorin, E.; Fujisawa, S.; Kurosawa, M. Ovarian blood flow responses to electroacupuncture stimulation depend on estrous cycle and on site and frequency of stimulation in anesthetized rats. J. Appl. Physiol. 2006, 101, 84–91. [Google Scholar] [CrossRef]
- Inoue, M.; Hojo, T.; Nakajima, M.; Kitakoji, H.; Itoi, M.; Katsumi, Y. The Effect of Electrical Stimulation of the Pudendal Nerve on Sciatic Nerve Blood Flow in Animals. Acupunct. Med. 2008, 26, 145–148. [Google Scholar] [CrossRef]
- Inoue, M.; Kitakoji, H.; Yano, T.; Ishizaki, N.; Itoi, M.; Katsumi, Y. Acupuncture Treatment for Low Back Pain and Lower Limb Symptoms-The Relation between Acupuncture or Electroacupuncture Stimulation and Sciatic Nerve Blood Flow. Evid.-Based Complement. Altern. Med. 2008, 5, 133–143. [Google Scholar] [CrossRef]
- Wang, G.; Litscher, D.; Tian, Y.; Gaischek, I.; Jia, S.; Wang, L.; Zhang, W.; Litscher, G. Acupoint Activation: Response in Microcirculation and the Role of Mast Cells. Medicines 2014, 1, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Kashiba, H.; Ueda, Y. Acupuncture to the skin induces release of substance P and calcitonin gene-related peptide from peripheral terminals of primary sensory neurons in the rat. Am. J. Chin. Med. 1991, 19, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Jou, N.-T.; Ma, S.-X. Responses of Nitric Oxide-cGMP Release in Acupuncture Point to Electroacupuncture in Human Skin In Vivo Using Dermal Microdialysis. Microcirculation 2009, 16, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Loaiza, L.A.; Yamaguchi, S.; Ito, M.; Ohshima, N. Electro-acupuncture stimulation to muscle afferents in anesthetized rats modulates the blood flow to the knee joint through autonomic reflexes and nitric oxide. Auton. Neurosci. 2002, 97, 103–109. [Google Scholar] [CrossRef] [PubMed]
- La Marca, R.; Nedeljkovic, M.; Yuan, L.; Maercker, A.; Ehlert, U. Effects of auricular electrical stimulation on vagal activity in healthy men: Evidence from a three-armed randomized trial. Clin. Sci. 2010, 118, 537–546. [Google Scholar] [CrossRef]
- Fukuta, H.; Koshita, M.; Nakamura, E.; Nakamura, H.; Yamada, A.; Kawase, Y.; Ishigami, T.; Kurono, Y.; Iino, S.; Suzuki, H. Acupuncture modulates mechanical responses of smooth muscle produced by transmural nerve stimulation in gastric antrum of genetically hyperglycemic rats. J. Smooth Muscle Res. 2009, 45, 167–185. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.-Y.; Wang, D.; Wu, M.-Z.; He, J.-K.; Zhang, J.-L.; Zhao, B.; Hou, L.-W.; Wang, J.-Y.; Wang, L.; et al. Transcutaneous Auricular Vagus Nerve Stimulation: From Concept to Application. Neurosci. Bull. 2020, 37, 853–862. [Google Scholar] [CrossRef]
- Kaniusas, E.; Kampusch, S.; Tittgemeyer, M.; Panetsos, F.; Gines, R.F.; Papa, M.; Kiss, A.; Podesser, B.; Cassara, A.M.; Tanghe, E.; et al. Current Directions in the Auricular Vagus Nerve Stimulation I—A Physiological Perspective. Front. Neurosci. 2019, 13, 854. [Google Scholar] [CrossRef]
| 2 Hz Ear-Electroacupuncture | T0 | T1 | T2 |
|---|---|---|---|
| Systolic blood pressure (mm Hg) | 112.38 ± 1.17 | 104.23 ± 1.28 | 108.28 ± 1.18 |
| Diastolic blood pressure (mm Hg) | 66.67 ± 0.98 | 65.28 ± 0.95 | 66.18 ± 0.90 |
| Heart rate (beats/min) | 66.56 ± 1.28 | 60.44 ± 0.64 | 59.44 ± 0.64 |
| 100 Hz Ear-Electroacupuncture | T0 | T1 | T2 |
|---|---|---|---|
| Systolic blood pressure (mm Hg) | 109.34 ± 1.14 | 111.33 ± 1.22 | 110.11 ± 1.20 |
| Diastolic blood pressure (mm Hg) | 62.64 ± 0.86 | 64.22 ± 0.89 | 63.12 ± 0.99 |
| Heart rate (beats/min) | 64.42 ± 1.17 | 65.38 ± 0.59 | 64.46 ± 0.61 |
| 100 Hz Stimulation | T0 | T1 | T2 |
|---|---|---|---|
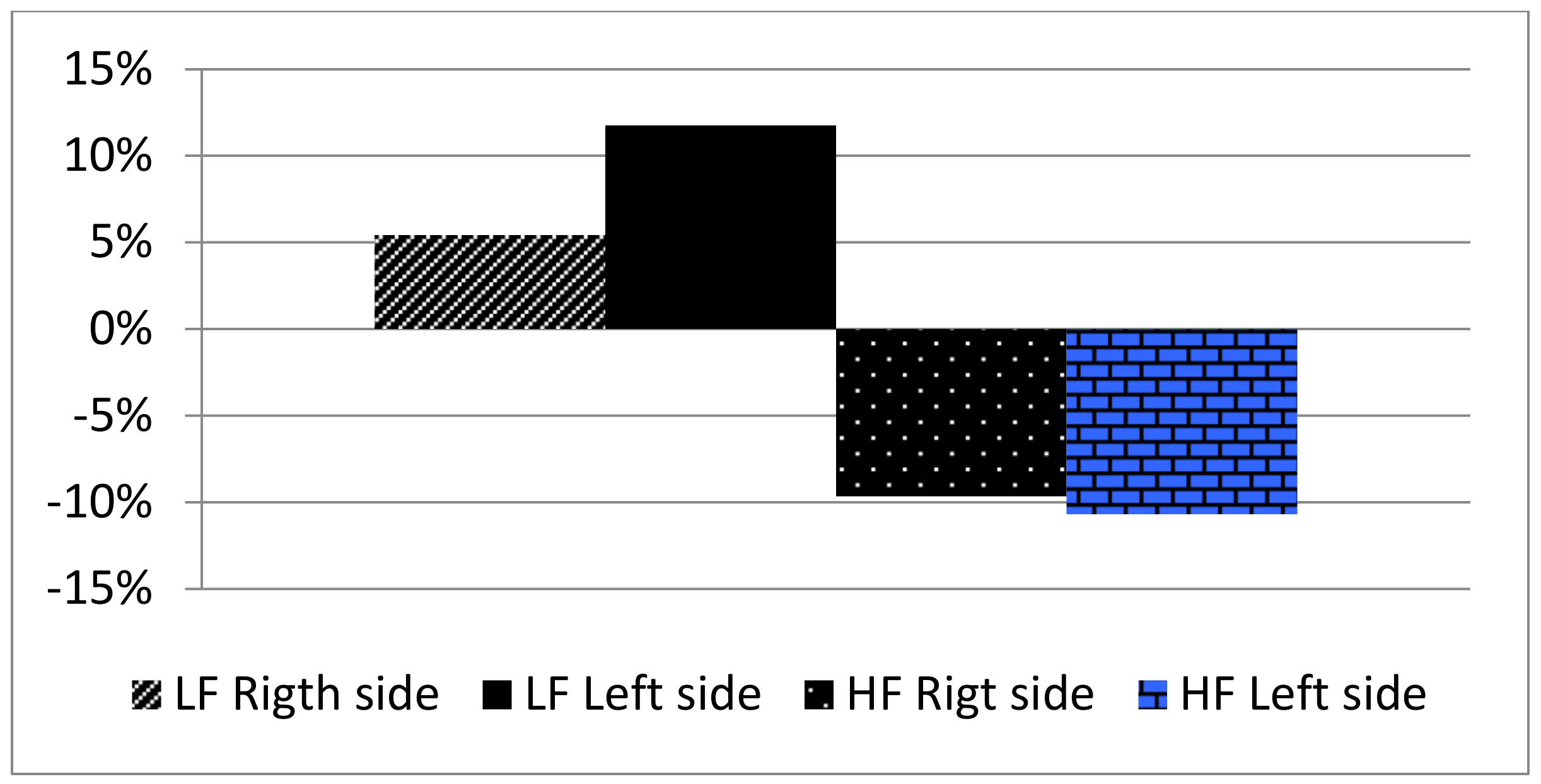
| PU right Mean | 10.25 | 9.64 | 9.26 |
| SD | 4.36 | 4.87 | 4.42 |
| % variation vs. T0 | −5.95 | −9.65 | |
| PU left Mean | 9.82 | 8.94 | 8.77 |
| SD | 3.15 | 3.00 | 4.07 |
| % variation vs. T0 | −8.96 | −10.69 |
| 2 Hz Stimulation | T0 | T1 | T2 |
|---|---|---|---|
| PU right Mean | 10.51 | 11.84 | 11.08 |
| SD | 3.91 | 5.49 | 5.79 |
| % variation vs. T0 | 12.65 | 5.42 | |
| PU left Mean | 10.73 | 11.38 | 11.99 |
| SD | 3.22 | 4.98 | 5.98 |
| % variation vs. T0 | 6.05 | 11.74 |
| T1 | T2 | ||||
|---|---|---|---|---|---|
| Expected Values | Observed Values | Expected Values | Observed Values | ||
| High-frequency stimulation (200 Hz) | 12.65 | −5.95 | High-frequency stimulation (200 Hz) | 5.42 | −9.65 |
| Low-frequency stimulation (2 Hz) | 12.65 | 12.65 | Low-frequency stimulation (2 Hz) | 5.42 | 5.42 |
| Chi-squared | 1.69886 × 10−7 | Chi-squared | 9.60001 × 10−11 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagliardi, V.; Gagliardi, G.; Ceccherelli, F.; Lovato, A. Effect of Low- and High-Frequency Auricular Stimulation with Electro-Acupuncture on Cutaneous Microcirculation: A Cross-Over Study in Healthy Subjects. Medicines 2023, 10, 17. https://doi.org/10.3390/medicines10020017
Gagliardi V, Gagliardi G, Ceccherelli F, Lovato A. Effect of Low- and High-Frequency Auricular Stimulation with Electro-Acupuncture on Cutaneous Microcirculation: A Cross-Over Study in Healthy Subjects. Medicines. 2023; 10(2):17. https://doi.org/10.3390/medicines10020017
Chicago/Turabian StyleGagliardi, Veronica, Giuseppe Gagliardi, Francesco Ceccherelli, and Antonello Lovato. 2023. "Effect of Low- and High-Frequency Auricular Stimulation with Electro-Acupuncture on Cutaneous Microcirculation: A Cross-Over Study in Healthy Subjects" Medicines 10, no. 2: 17. https://doi.org/10.3390/medicines10020017
APA StyleGagliardi, V., Gagliardi, G., Ceccherelli, F., & Lovato, A. (2023). Effect of Low- and High-Frequency Auricular Stimulation with Electro-Acupuncture on Cutaneous Microcirculation: A Cross-Over Study in Healthy Subjects. Medicines, 10(2), 17. https://doi.org/10.3390/medicines10020017





