The Blessed Union of Glycobiology and Immunology: A Marriage That Worked
Abstract
1. Introduction
2. Cell Surface Glycoconjugates: A Hallmark of All Living Cells
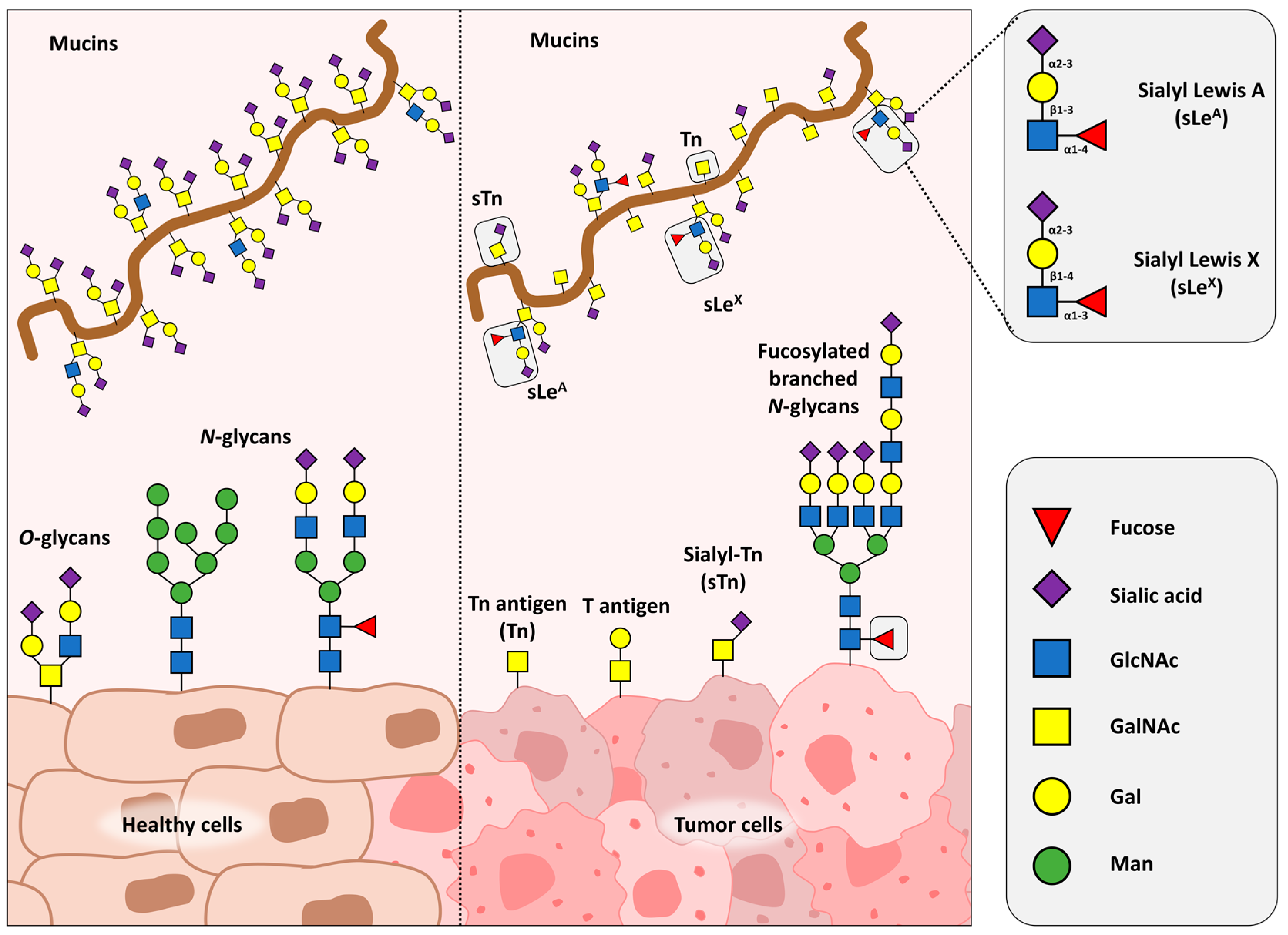
3. N-Glycans in Cancer Immunotherapy
4. Lectins as Decoders of Biological Information in Cellular Glycoconjugates
4.1. Lectins as Tools for N- and O-Glycan Detection and Purification
| Lectin | Main Specificity | Reference |
|---|---|---|
| Vicia villosa (VVL) |  | [74] |
| Helix pomatia agglutinin (HPA) |  | [75] |
| Peanut agglutinin (PNA) | 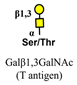 | [76] |
| Sambucus nigra (SNA) | 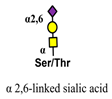 | [77] |
| Maackia amurensis (MAA) | 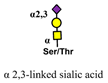 | [78] |
| Concanavalin A (ConA) |  | [79] |
| Phaseolus vulgaris-E (PHA-E) | 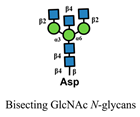 | [80] |
| Phaseolus vulgaris-L (PHA-L) |  | [81] |
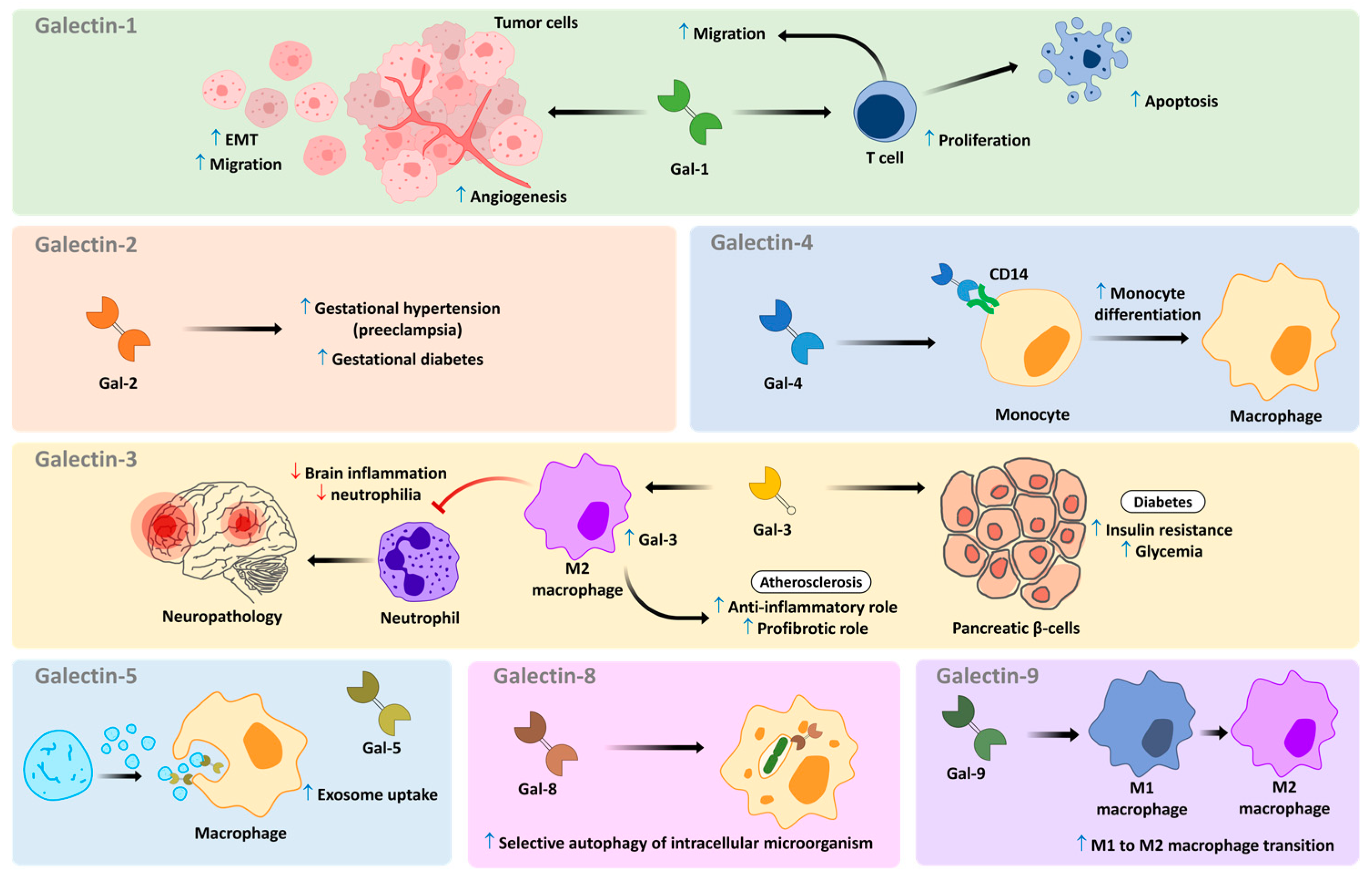
4.2. Galectins
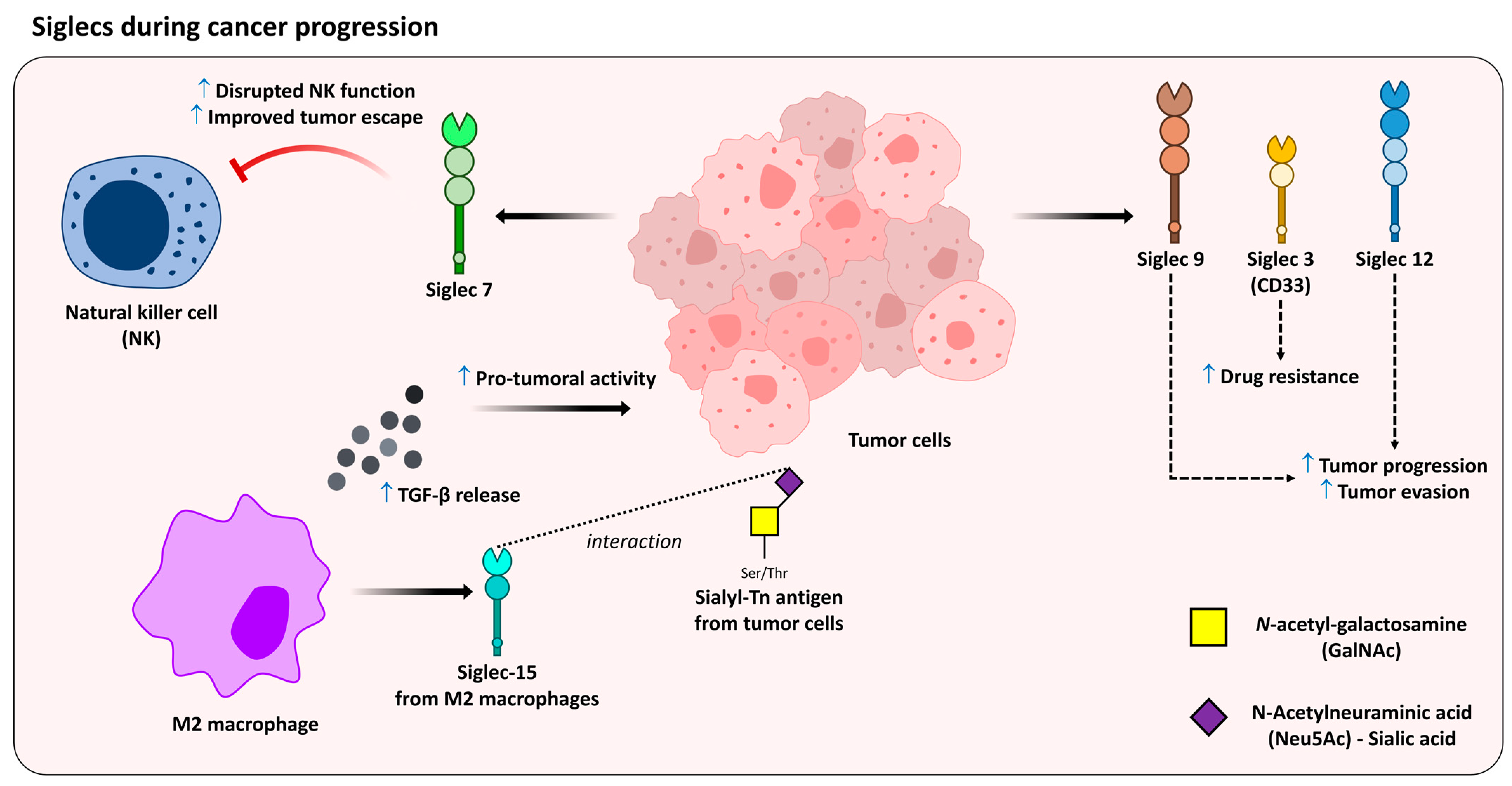
4.3. Siglecs
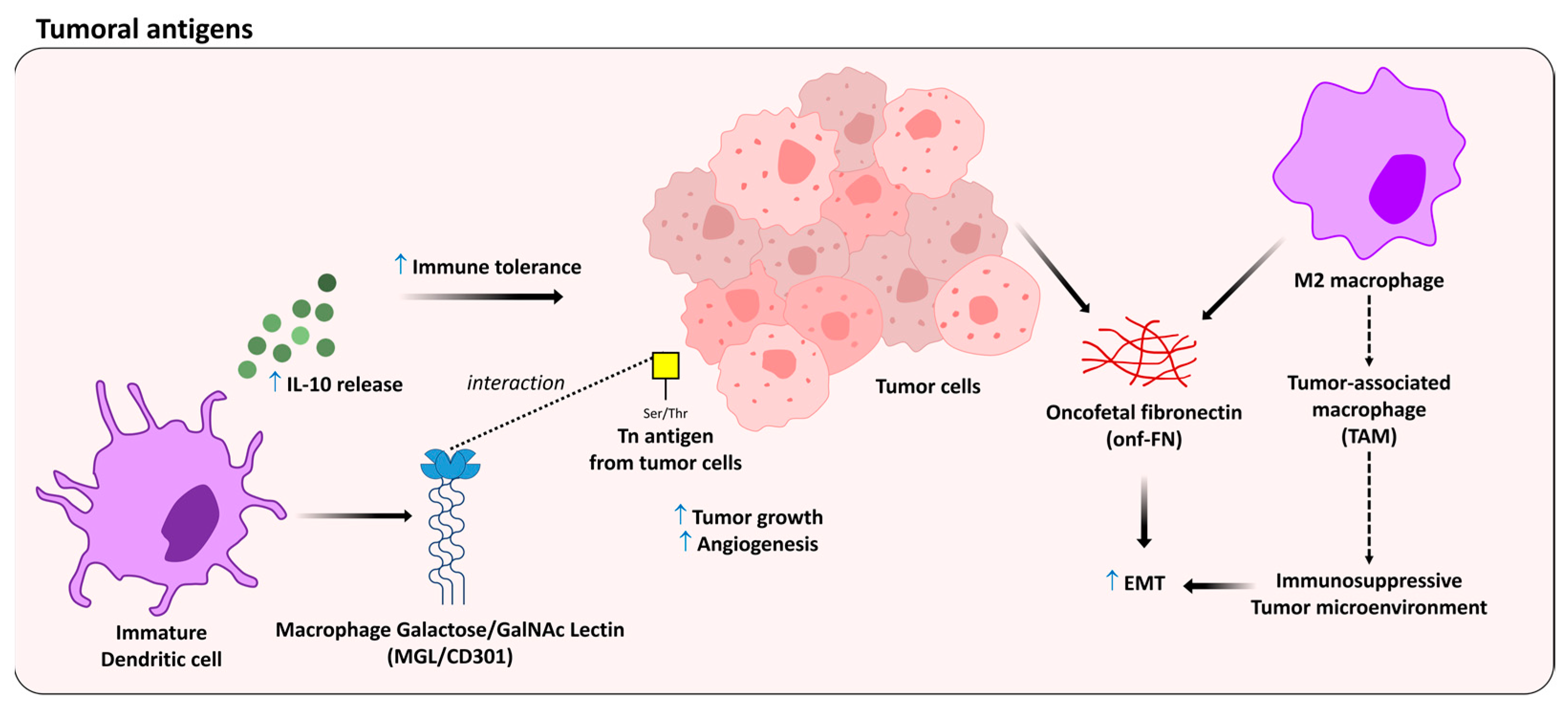
5. Oncofetal Antigens as Modulators of the Immune Response
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomas, D.; Rathinavel, A.K.; Radhakrishnan, P. Altered glycosylation in cancer: A promising target for biomarkers and therapeutics. Biochim. Biophys. Acta (BBA) Rev. Cancer 2021, 1875, 188464. [Google Scholar] [CrossRef]
- Go, S.; Yoshikawa, M.; Inokuchi, J.-I. Glycoconjugates in the mammalian auditory system. J. Neurochem. 2011, 116, 756–763. [Google Scholar] [CrossRef]
- Gabius, H.-J.; Manning, J.C.; Kopitz, J.; André, S.; Kaltner, H. Sweet complementarity: The functional pairing of glycans with lectins. Cell. Mol. Life Sci. 2016, 73, 1989–2016. [Google Scholar] [CrossRef]
- Chlubnová, I.; Sylla, B.; Nugier-Chauvin, C.; Daniellou, R.; Legentil, L.; Kralová, B.; Ferrières, V. Natural glycans and glycoconjugates as immunomodulating agents. Nat. Prod. Rep. 2011, 28, 937–952. [Google Scholar] [CrossRef]
- Esmail, S.; Manolson, M.F. Advances in understanding N-glycosylation structure, function, and regulation in health and disease. Eur. J. Cell Biol. 2021, 100, 151186. [Google Scholar] [CrossRef]
- Sun, X.; Zhan, M.; Sun, X.; Liu, W.; Meng, X. C1GALT1 in health and disease (Review). Oncol. Lett. 2021, 22, 589. [Google Scholar] [CrossRef]
- Neuberger, A. Carbohydrates in protein: The carbohydrate component of crystalline egg albumin. Biochem. J. 1938, 32, 1435–1451. [Google Scholar] [CrossRef]
- Mendonça-Previato, L.; Todeschini, A.R.; Heise, N.; Previato, J.O. Protozoan parasite-specific carbohydrate structures. Curr. Opin. Struct. Biol. 2005, 15, 499–505. [Google Scholar] [CrossRef]
- Villena, S.N.; Pinheiro, R.O.; Pinheiro, C.S.; Nunes, M.P.; Takiya, C.M.; DosReis, G.A.; Previato, J.O.; Mendonça-Previato, L.; Freire-De-Lima, C.G. Capsular polysaccharides galactoxylomannan and glucuronoxylomannan from Cryptococcus neoformans induce macrophage apoptosis mediated by Fas ligand. Cell. Microbiol. 2008, 10, 1274–1285. [Google Scholar] [CrossRef]
- Dobrica, M.-O.; Lazar, C.; Branza-Nichita, N. N-Glycosylation and N-Glycan Processing in HBV Biology and Pathogenesis. Cells 2020, 9, 1404. [Google Scholar] [CrossRef]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr. 2015, 3, 1. [Google Scholar] [CrossRef]
- Kumar, A.S.; Mody, K.; Jha, B. Bacterial exopolysaccharides—A perception. J. Basic Microbiol. 2007, 47, 103–117. [Google Scholar] [CrossRef]
- Spiro, R.G. Protein glycosylation: Nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology 2002, 12, 43R–56R. [Google Scholar] [CrossRef] [PubMed]
- Wandall, H.H.; Nielsen, M.A.I.; King-Smith, S.; de Haan, N.; Bagdonaite, I. Global functions of O-glycosylation: Promises and challenges in O-glycobiology. FEBS J. 2021, 288, 7183–7212. [Google Scholar] [CrossRef]
- Nio-Kobayashi, J. Tissue- and cell-specific localization of galectins, β-galactose-binding animal lectins, and their potential functions in health and disease. Anat. Sci. Int. 2016, 92, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Handa, K.; Hakomori, S.-I. Changes of glycoconjugate expression profiles during early development. Glycoconj. J. 2016, 34, 693–699. [Google Scholar] [CrossRef]
- Rudd, P.M.; Elliott, T.; Cresswell, P.; Wilson, I.A.; Dwek, R.A. Glycosylation and the Immune System. Science 2001, 291, 2370–2376. [Google Scholar] [CrossRef]
- Arnold, J.N.; Dwek, R.A.; Rudd, P.M.; Sim, R.B. Mannan binding lectin and its interaction with immunoglobulins in health and in disease. Immunol. Lett. 2006, 106, 103–110. [Google Scholar] [CrossRef]
- Dos Reis, J.S.; Santos, M.A.R.D.C.; Mendonça, D.P.; Nascimento, S.I.M.D.; Barcelos, P.M.; de Lima, R.G.C.; da Costa, K.M.; Freire-De-Lima, C.G.; Morrot, A.; Previato, J.O.; et al. Glycobiology of Cancer: Sugar Drives the Show. Medicines 2022, 9, 34. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Schjoldager, K.T.; Narimatsu, Y.; Joshi, H.J.; Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 729–749. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Toscano, M.A.; Jackson, S.S.; Vasta, G.R. Functions of cell surface galectin-glycoprotein lattices. Curr. Opin. Struct. Biol. 2007, 17, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Elola, M.T.; Blidner, A.G.; Ferragut, F.; Bracalente, C.; Rabinovich, G.A. Assembly, organization and regulation of cell-surface receptors by lectin–glycan complexes. Biochem. J. 2015, 469, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Baum, L.G.; Cobb, B.A. The direct and indirect effects of glycans on immune function. Glycobiology 2017, 27, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Conejo-García, J.R. Shaping the Immune Landscape in Cancer by Galectin-Driven Regulatory Pathways. J. Mol. Biol. 2016, 428, 3266–3281. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Froehlich, J.W.; Lebrilla, C.B. Determination of glycosylation sites and site-specific heterogeneity in glycoproteins. Curr. Opin. Chem. Biol. 2009, 13, 421–426. [Google Scholar] [CrossRef]
- Munkley, J.; Elliott, D.J. Hallmarks of glycosylation in cancer. Oncotarget 2016, 7, 35478–35489. [Google Scholar] [CrossRef]
- Taniguchi, N.; Kizuka, Y. Glycans and Cancer: Role of N-glycans in cancer biomarker, progression and metastasis, and therapeutics. Adv. Cancer Res. 2015, 126, 11–51. [Google Scholar]
- Nagae, M.; Kizuka, Y.; Mihara, E.; Kitago, Y.; Hanashima, S.; Ito, Y.; Takagi, J.; Taniguchi, N.; Yamaguchi, Y. Structure and mechanism of cancer-associated N-acetylglucosaminyltransferase-V. Nat. Commun. 2018, 9, 3380. [Google Scholar] [CrossRef]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front. Immunol. 2021, 12, 636568. [Google Scholar] [CrossRef]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef]
- Nardy, A.F.F.R.; Freire-De-Lima, L.; Freire-De-Lima, C.G.; Morrot, A. The Sweet Side of Immune Evasion: Role of Glycans in the Mechanisms of Cancer Progression. Front. Oncol. 2016, 6, 54. [Google Scholar] [CrossRef]
- Silva, M.C.; Fernandes, A.; Oliveira, M.; Resende, C.; Correia, A.; De-Freitas-Junior, J.C.M.; Lavelle, A.; Andrade-Da-Costa, J.; Leander, M.; Xavier-Ferreira, H.; et al. Glycans as Immune Checkpoints: Removal of Branched N-glycans Enhances Immune Recognition Preventing Cancer Progression. Cancer Immunol. Res. 2020, 8, 1407–1425. [Google Scholar] [CrossRef] [PubMed]
- Grigorian, A.; Demetriou, M. Mgat5 Deficiency in T Cells and Experimental Autoimmune Encephalomyelitis. ISRN Neurol. 2011, 2011, 374314. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-U.; Grigorian, A.; Pawling, J.; Chen, I.-J.; Gao, G.; Mozaffar, T.; McKerlie, C.; Demetriou, M. N-Glycan Processing Deficiency Promotes Spontaneous Inflammatory Demyelination and Neurodegeneration. J. Biol. Chem. 2007, 282, 33725–33734. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.S.; Alves, I.; Vicente, M.; Campar, A.; Silva, M.C.; Padrão, N.; Pinto, V.; Fernandes, A.; Dias, A.; Pinho, S.S. Glycans as Key Checkpoints of T Cell Activity and Function. Front. Immunol. 2018, 9, 2754. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.M.; Correia, A.; Pereira, M.S.; Almeida, C.R.; Alves, I.; Pinto, V.; Catarino, T.A.; Mendes, N.; Leander, M.; Oliva-Teles, M.T.; et al. Metabolic control of T cell immune response through glycans in inflammatory bowel disease. Proc. Natl. Acad. Sci. USA 2018, 115, E4651–E4660. [Google Scholar] [CrossRef]
- Alves, I.; Fernandes, A.; Santos-Pereira, B.; Azevedo, C.M.; Pinho, S.S. Glycans as a key factor in self and nonself discrimination: Impact on the breach of immune tolerance. FEBS Lett. 2022, 596, 1485–1502. [Google Scholar] [CrossRef]
- De Bousser, E.; Meuris, L.; Callewaert, N.; Festjens, N. Human T cell glycosylation and implications on immune therapy for cancer. Hum. Vaccines Immunother. 2020, 16, 2374–2388. [Google Scholar] [CrossRef]
- Morgan, R.; Gao, G.; Pawling, J.; Dennis, J.W.; Demetriou, M.; Li, B. N-Acetylglucosaminyltransferase V (Mgat5)-Mediated N-Glycosylation Negatively Regulates Th1 Cytokine Production by T Cells. J. Immunol. 2004, 173, 7200–7208. [Google Scholar] [CrossRef]
- Ghosh, C.; Luong, G.; Sun, Y. A snapshot of the PD-1/PD-L1 pathway. J. Cancer 2021, 12, 2735–2746. [Google Scholar] [CrossRef]
- Su, C.; Wang, H.; Liu, Y.; Guo, Q.; Zhang, L.; Li, J.; Zhou, W.; Yan, Y.; Zhou, X.; Zhang, J. Adverse Effects of Anti-PD-1/PD-L1 Therapy in Non-small Cell Lung Cancer. Front. Oncol. 2020, 10, 554313. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Twomey, J.D.; Zhang, B. Cancer Immunotherapy Update: FDA-Approved Checkpoint Inhibitors and Companion Diagnostics. AAPS J. 2021, 23, 39. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Wang, D.; Sun, K.; Wang, L.; Zhang, Y. Resistance Mechanisms of Anti-PD1/PDL1 Therapy in Solid Tumors. Front. Cell Dev. Biol. 2020, 8, 672. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Yang, X.; Liu, D.; Ye, S.; Yang, W.; Xie, Z.; Lei, X. Research progress of PD-L1 non-glycosylation in cancer immunotherapy. Scand. J. Immunol. 2022, 94, e13205. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Lee, H.-H.; Hsu, J.L.; Yu, D.; Hung, M.-C. The impact of PD-L1 N-linked glycosylation on cancer therapy and clinical diagnosis. J. Biomed. Sci. 2020, 27, 77. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-W.; Lim, S.-O.; Xia, W.; Lee, H.-H.; Chan, L.-C.; Kuo, C.-W.; Khoo, K.-H.; Chang, S.-S.; Cha, J.-H.; Kim, T.; et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun. 2016, 7, 12632. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-W.; Lim, S.-O.; Chung, E.M.; Kim, Y.-S.; Park, A.H.; Yao, J.; Cha, J.-H.; Xia, W.; Chan, L.-C.; Kim, T.; et al. Eradication of Triple-Negative Breast Cancer Cells by Targeting Glycosylated PD-L1. Cancer Cell 2018, 33, 187–201.e10. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-H.; Wang, Y.-N.; Xia, W.; Chen, C.-H.; Rau, K.-M.; Ye, L.; Wei, Y.; Chou, C.-K.; Wang, S.-C.; Yan, M.; et al. Removal of N-Linked Glycosylation Enhances PD-L1 Detection and Predicts Anti-PD-1/PD-L1 Therapeutic Efficacy. Cancer Cell 2019, 36, 168–178.e4. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Tan, S.; Jin, W.; Guan, J.; Wang, Q.; Sun, H.; Qi, J.; Yan, J.; Chai, Y.; Wang, Z.; et al. N-glycosylation of PD-1 promotes binding of camrelizumab. EMBO Rep. 2020, 21, e51444. [Google Scholar] [CrossRef]
- Lu, D.; Xu, Z.; Zhang, D.; Jiang, M.; Liu, K.; He, J.; Ma, D.; Ma, X.; Tan, S.; Gao, G.F.; et al. PD-1 N58-Glycosylation-Dependent Binding of Monoclonal Antibody Cemiplimab for Immune Checkpoint Therapy. Front. Immunol. 2022, 13, 826045. [Google Scholar] [CrossRef] [PubMed]
- Navarro, P.; Martínez-Bosch, N.; Blidner, A.G.; Rabinovich, G.A. Impact of Galectins in Resistance to Anticancer Therapies. Clin. Cancer Res. 2020, 26, 6086–6101. [Google Scholar] [CrossRef] [PubMed]
- Cagnoni, A.J.; Sáez, J.M.P.; Rabinovich, G.A.; Mariño, K.V. Turning-Off Signaling by Siglecs, Selectins, and Galectins: Chemical Inhibition of Glycan-Dependent Interactions in Cancer. Front. Oncol. 2016, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Wang, F. The physiological and pathological roles and applications of sialyl Lewis x, a common carbohydrate ligand of the three selectins. Glycoconj. J. 2020, 37, 277–291. [Google Scholar] [CrossRef]
- Cerliani, J.P.; Blidner, A.G.; Toscano, M.A.; Croci, D.O.; Rabinovich, G.A. Translating the ‘Sugar Code’ into Immune and Vascular Signaling Programs. Trends Biochem. Sci. 2017, 42, 255–273. [Google Scholar] [CrossRef]
- Cummings, R.D.; Liu, F.T.; Rabinovich, G.A.; Stowell, S.R.; Vasta, G.R. Galectins. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2022; pp. 491–504. [Google Scholar]
- Compagno, D.; Jaworski, F.M.; Gentilini, L.; Contrufo, G.; Pérez, I.G.; Elola, M.T.; Pregi, N.; Rabinovich, G.A.; Laderach, D.J. Galectins: Major Signaling Modulators Inside and Outside the Cell. Curr. Mol. Med. 2014, 14, 630–651. [Google Scholar] [CrossRef]
- Stanczak, M.A.; Läubli, H. Siglec receptors as new immune checkpoints in cancer. Mol. Asp. Med. 2022, 7, 101112. [Google Scholar] [CrossRef]
- Läubli, H.; Nalle, S.C.; Maslyar, D. Targeting the Siglec–Sialic Acid Immune Axis in Cancer: Current and Future Approaches. Cancer Immunol. Res. 2022, 10, 1423–1432. [Google Scholar] [CrossRef]
- Akimoto, Y.; Kawakami, H. Histochemical Staining Using Lectin Probes. Methods Mol. Biol. 2014, 1200, 153–163. [Google Scholar] [CrossRef]
- Stoddart, R.W.; Jones, C.J.P. Lectin Histochemistry and Cytochemistry-Light Microscopy: Avidin-Biotin Amplification on Resin- Embedded Sectlons. Methods Mol. Biol. 1998, 9, 21–40. [Google Scholar] [CrossRef]
- Hashim, O.H.; Jayapalan, J.J.; Lee, C.-S. Lectins: An effective tool for screening of potential cancer biomarkers. Peerj 2017, 5, e3784. [Google Scholar] [CrossRef] [PubMed]
- Sato, T. Lectin-Probed Western Blot Analysis. Methods Mol. Biol. 2014, 1200, 93–100. [Google Scholar] [CrossRef]
- Roth, Z.; Yehezkel, G.; Khalaila, I. Identification and Quantification of Protein Glycosylation. Int. J. Carbohydr. Chem. 2012, 2012, 10. [Google Scholar] [CrossRef]
- Moriwaki, K.; Miyoshi, E. Basic Procedures for Lectin Flow Cytometry. Methods Mol. Biol. 2014, 1200, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Goumenou, A.; Delaunay, N.; Pichon, V. Recent Advances in Lectin-Based Affinity Sorbents for Protein Glycosylation Studies. Front. Mol. Biosci. 2021, 8, 746822. [Google Scholar] [CrossRef] [PubMed]
- Pilobello, K.T.; Krishnamoorthy, L.; Slawek, D.; Mahal, L.K. Development of a Lectin Microarray for the Rapid Analysis of Protein Glycopatterns. Chembiochem 2005, 6, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Dang, K.; Zhang, W.; Jiang, S.; Lin, X.; Qian, A. Application of Lectin Microarrays for Biomarker Discovery. Chemistryopen 2020, 9, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.L.S. Lectin biosensors in cancer glycan biomarker detection. Adv. Clin. Chem. 2019, 93, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Shu, J.; Li, Z. Lectin microarrays for glycoproteomics: An overview of their use and potential. Expert Rev. Proteom. 2020, 17, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Trbojević-Akmačić, I.; Lageveen-Kammeijer, G.S.M.; Heijs, B.; Petrović, T.; Deriš, H.; Wuhrer, M.; Lauc, G. High-Throughput Glycomic Methods. Chem. Rev. 2022, 122, 15865–15913. [Google Scholar] [CrossRef]
- Syed, P.; Gidwani, K.; Kekki, H.; Leivo, J.; Pettersson, K.; Lamminmäki, U. Role of lectin microarrays in cancer diagnosis. Proteomics 2016, 16, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Puri, K.D.; Gopalakrishnan, B.; Surolia, A. Carbohydrate binding specificity of the Tn-antigen binding lectin from Vicia villosa seeds (VVLB4). FEBS Lett. 1992, 312, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.A.; Leathem, A.J. Expression of alpha-GalNAc glycoproteins by breast cancers. Br. J. Cancer 1995, 71, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Farrag, F.; Gewaily, M.; AbdElmaksoud, A.; Kassab, M. Comparative glycoconjugates histochemistry of proventriculus of chicken, ducks and geese. Alex. J. Veter. Sci. 2017, 7, 53. [Google Scholar] [CrossRef]
- Shibuya, N.; Goldstein, I.J.; Broekaert, W.F.; Nsimba-Lubaki, M.; Peeters, B.; Peumans, W.J. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(alpha 2-6)Gal/GalNAc sequence. J. Biol. Chem. 1987, 262, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Miller, E.; Agbandje-McKenna, M.; Samulski, R.J. α2,3 and α2,6 N-Linked Sialic Acids Facilitate Efficient Binding and Transduction by Adeno-Associated Virus Types 1 and 6. J. Virol. 2006, 80, 9093–9103. [Google Scholar] [CrossRef] [PubMed]
- Dodla, M.C.; Young, A.; Venable, A.; Hasneen, K.; Rao, R.R.; Machacek, D.W.; Stice, S.L. Differing Lectin Binding Profiles among Human Embryonic Stem Cells and Derivatives Aid in the Isolation of Neural Progenitor Cells. PLoS ONE 2011, 6, e23266. [Google Scholar] [CrossRef]
- Lu, G.; Holland, L.A. Profiling the N-Glycan Composition of IgG with Lectins and Capillary Nanogel Electrophoresis. Anal. Chem. 2018, 91, 1375–1383. [Google Scholar] [CrossRef]
- Kaneda, Y.; Whittier, R.F.; Yamanaka, H.; Carredano, E.; Gotoh, M.; Sota, H.; Hasegawa, Y.; Shinohara, Y. The High Specificities of Phaseolus vulgaris Erythro- and Leukoagglutinating Lectins for Bisecting GlcNAc or β1–6-Linked Branch Structures, Respectively, Are Attributable to Loop B. J. Biol. Chem. 2002, 277, 16928–16935. [Google Scholar] [CrossRef]
- Nishi, N.; Shoji, H.; Seki, M.; Itoh, A.; Miyanaka, H.; Yuube, K.; Hirashima, M.; Nakamura, T. Galectin-8 modulates neutrophil function via interaction with integrin M. Glycobiology 2003, 13, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Heyl, K.A.; Karsten, C.M.; Slevogt, H. Galectin-3 binds highly galactosylated IgG1 and is crucial for the IgG1 complex mediated inhibition of C5aReceptor induced immune responses. Biochem. Biophys. Res. Commun. 2016, 479, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Giovannone, N.; Smith, L.K.; Treanor, B.; Dimitroff, C.J. Galectin-Glycan Interactions as Regulators of B Cell Immunity. Front. Immunol. 2018, 9, 2839. [Google Scholar] [CrossRef]
- Vasta, G.R.; Quesenberry, M.; Ahmed, H.; O’Leary, N. C-type lectins and galectins mediate innate and adaptive immune functions: Their roles in the complement activation pathway. Dev. Comp. Immunol. 1999, 23, 401–420. [Google Scholar] [CrossRef] [PubMed]
- Baum, L.G.; Pang, M.; Perillo, N.L.; Wu, T.; Delegeane, A.; Uittenbogaart, C.H.; Fukuda, M.; Seilhamer, J.J. Human thymic epithelial cells express an endogenous lectin, galectin-1, which binds to core 2 O-glycans on thymocytes and T lymphoblastoid cells. J. Exp. Med. 1995, 181, 877–887. [Google Scholar] [CrossRef]
- Manzi, M.; Bacigalupo, M.L.; Carabias, P.; Elola, M.T.; Wolfenstein-Todel, C.; Rabinovich, G.A.; Espelt, M.V.; Troncoso, M.F. Galectin-1 Controls the Proliferation and Migration of Liver Sinusoidal Endothelial Cells and Their Interaction with Hepatocarcinoma Cells. J. Cell. Physiol. 2015, 231, 1522–1533. [Google Scholar] [CrossRef]
- Perillo, N.L.; Marcus, M.E.; Baum, L.G. Galectins: Versatile modulators of cell adhesion, cell proliferation, and cell death. J. Mol. Med. 1998, 76, 402–412. [Google Scholar] [CrossRef]
- Earl, L.A.; Bi, S.; Baum, L.G. N- and O-Glycans Modulate Galectin-1 Binding, CD45 Signaling, and T Cell Death. J. Biol. Chem. 2010, 285, 2232–2244. [Google Scholar] [CrossRef]
- Zuñiga, E.; Rabinovich, G.A.; Iglesias, M.M.; Gruppi, A. Regulated expression of galectin-1 during B-cell activation and implications for T-cell apoptosis. J. Leukoc. Biol. 2001, 70, 73–79. [Google Scholar] [CrossRef]
- Blidner, A.G.; Méndez-Huergo, S.P.; Cagnoni, A.J.; Rabinovich, G.A. Re-wiring regulatory cell networks in immunity by galectin-glycan interactions. FEBS Lett. 2015, 589, 3407–3418. [Google Scholar] [CrossRef]
- Thiemann, S.; Baum, L.G. Galectins and Immune Responses—Just How Do They Do Those Things They Do? Annu. Rev. Immunol. 2016, 34, 243–264. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.A.; Bianco, G.A.; Ilarregui, J.M.; Croci, D.O.; Correale, J.; Hernandez, J.D.; Zwirner, N.; Poirier, F.; Riley, E.M.; Baum, L.G.; et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat. Immunol. 2007, 8, 825–834. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Ilarregui, J.M. Conveying glycan information into T-cell homeostatic programs: A challenging role for galectin-1 in inflammatory and tumor microenvironments. Immunol. Rev. 2009, 230, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Sotomayor, C.E.; Rabinovich, G.A. Galectin-1 Induces Central and Peripheral Cell Death: Implications in T-Cell Physiopathology. Dev. Immunol. 2000, 7, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.; Ilarregui, J.M.; Pesoa, S.A.; Croci, D.O.; Perretti, M.; Rabinovich, G.A. Multiple Functional Targets of the Immunoregulatory Activity of Galectin-1: Control of immune cell trafficking, dendritic cell physiology, and T-cell fate. Methods Enzym. 2010, 480, 199–244. [Google Scholar] [CrossRef]
- Croci, D.O.; Cerliani, J.P.; Dalotto-Moreno, T.; Méndez-Huergo, S.P.; Mascanfroni, I.D.; Dergan-Dylon, S.; Toscano, M.A.; Caramelo, J.J.; García-Vallejo, J.J.; Ouyang, J.; et al. Glycosylation-Dependent Lectin-Receptor Interactions Preserve Angiogenesis in Anti-VEGF Refractory Tumors. Cell 2014, 156, 744–758. [Google Scholar] [CrossRef]
- Croci, D.O.; Cerliani, J.P.; Pinto, N.A.; Morosi, L.G.; Rabinovich, G.A. Regulatory role of glycans in the control of hypoxia-driven angiogenesis and sensitivity to anti-angiogenic treatment. Glycobiology 2014, 24, 1283–1290. [Google Scholar] [CrossRef]
- Cagnoni, A.J.; Giribaldi, M.L.; Blidner, A.G.; Cutine, A.M.; Gatto, S.G.; Morales, R.M.; Salatino, M.; Abba, M.C.; Croci, D.O.; Mariño, K.V.; et al. Galectin-1 fosters an immunosuppressive microenvironment in colorectal cancer by reprogramming CD8 + regulatory T cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2102950118. [Google Scholar] [CrossRef]
- Bacigalupo, M.L.; Manzi, M.; Espelt, M.V.; Gentilini, L.D.; Compagno, D.; Laderach, D.J.; Wolfenstein-Todel, C.; Rabinovich, G.A.; Troncoso, M.F. Galectin-1 Triggers Epithelial-Mesenchymal Transition in Human Hepatocellular Carcinoma Cells. J. Cell. Physiol. 2015, 230, 1298–1309. [Google Scholar] [CrossRef]
- You, X.; Wu, J.; Zhao, X.; Jiang, X.; Tao, W.; Chen, Z.; Huang, C.; Zheng, T.; Shen, X. Fibroblastic galectin-1-fostered invasion and metastasis are mediated by TGF-β1-induced epithelial-mesenchymal transition in gastric cancer. Aging 2021, 13, 18464–18481. [Google Scholar] [CrossRef]
- Carabias, P.; Espelt, M.V.; Bacigalupo, M.L.; Rojas, P.; Sarrias, L.; Rubin, A.; Saffioti, N.A.; Elola, M.T.; Rossi, J.P.; Wolfenstein-Todel, C.; et al. Galectin-1 confers resistance to doxorubicin in hepatocellular carcinoma cells through modulation of P-glycoprotein expression. Cell Death Dis. 2022, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Strik, H.M.; Schmidt, K.; Lingor, P.; Tönges, L.; Kugler, W.; Nitsche, M.; Rabinovich, G.; Bähr, M. Galectin-1 expression in human glioma cells: Modulation by ionizing radiation and effects on tumor cell proliferation and migration. Oncol. Rep. 2007, 18, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, K.; Francuz, T.; Gallego-Colon, E.; Ruiz-Agamez, N.; Kubeczko, M.; Grochoła, I.; Wojnar, J. Galectin Targeted Therapy in Oncology: Current Knowledge and Perspectives. Int. J. Mol. Sci. 2018, 19, 210. [Google Scholar] [CrossRef] [PubMed]
- Charkiewicz, K.; Goscik, J.; Raba, G.; Laudanski, P. Syndecan 4, galectin 2, and death receptor 3 (DR3) as novel proteins in pathophysiology of preeclampsia. J Matern. Neonatal Med. 2019, 34, 2965–2970. [Google Scholar] [CrossRef]
- Hepp, P.; Unverdorben, L.; Hutter, S.; Kuhn, C.; Ditsch, N.; Groß, E.; Mahner, S.; Jeschke, U.; Knabl, J.; Heidegger, H.H. Placental Galectin-2 Expression in Gestational Diabetes: A Systematic, Histological Analysis. Int. J. Mol. Sci. 2020, 21, 2404. [Google Scholar] [CrossRef]
- Nachtigal, M.; Al-Assaad, Z.; Mayer, E.P.; Kim, K.; Monsigny, M. Galectin-3 expression in human atherosclerotic lesions. Am. J. Pathol. 1998, 152, 1199–1208. [Google Scholar]
- Nachtigal, M.; Ghaffar, A.; Mayer, E.P. Galectin-3 Gene Inactivation Reduces Atherosclerotic Lesions and Adventitial Inflammation in ApoE-Deficient Mice. Am. J. Pathol. 2008, 172, 247–255. [Google Scholar] [CrossRef]
- MacKinnon, A.C.; Farnworth, S.L.; Hodkinson, P.S.; Henderson, N.C.; Atkinson, K.M.; Leffler, H.; Nilsson, U.J.; Haslett, C.; Forbes, S.J.; Sethi, T. Regulation of Alternative Macrophage Activation by Galectin-3. J. Immunol. 2008, 180, 2650–2658. [Google Scholar] [CrossRef]
- Li, Y.; Li, T.; Zhou, Z.; Xiao, Y. Emerging roles of Galectin-3 in diabetes and diabetes complications: A snapshot. Rev. Endocr. Metab. Disord. 2022, 23, 569–577. [Google Scholar] [CrossRef]
- Zangbede, F.O.Q.; Chauhan, A.; Sharma, J.; Mishra, B.B. Galectin-3 in M2 Macrophages Plays a Protective Role in Resolution of Neuropathology in Brain Parasitic Infection by Regulating Neutrophil Turnover. J. Neurosci. 2018, 38, 6737–6750. [Google Scholar] [CrossRef]
- Lv, R.; Bao, Q.; Li, Y. Regulation of M1-type and M2-type macrophage polarization in RAW264.7 cells by Galectin-9. Mol. Med. Rep. 2017, 16, 9111–9119. [Google Scholar] [CrossRef]
- Huflejt, M.; Leffler, H. Galectin-4 in normal tissues and cancer. Glycoconj. J. 2003, 20, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Hokama, A.; Mizoguchi, E.; Sugimoto, K.; Shimomura, Y.; Tanaka, Y.; Yoshida, M.; Rietdijk, S.T.; de Jong, Y.P.; Snapper, S.B.; Terhorst, C.; et al. Induced Reactivity of Intestinal CD4+ T Cells with an Epithelial Cell Lectin, Galectin-4, Contributes to Exacerbation of Intestinal Inflammation. Immunity 2004, 20, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Mudter, J.; Neurath, M.F. Il-6 signaling in inflammatory bowel disease: Pathophysiological role and clinical relevance. Inflamm. Bowel Dis. 2007, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Barrès, C.; Blanc, L.; Bette-Bobillo, P.; André, S.; Mamoun, R.; Gabius, H.-J.; Vidal, M. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood 2010, 115, 696–705. [Google Scholar] [CrossRef]
- Hong, S.-H.; Shin, J.-S.; Chung, H.; Park, C.-G. Galectin-4 Interaction with CD14 Triggers the Differentiation of Monocytes into Macrophage-like Cells via the MAPK Signaling Pathway. Immune Netw. 2019, 19, e17. [Google Scholar] [CrossRef]
- Bell, S.L.; Lopez, K.L.; Cox, J.S.; Patrick, K.L.; Watson, R.O. Galectin-8 Senses Phagosomal Damage and Recruits Selective Autophagy Adapter TAX1BP1 To Control Mycobacterium tuberculosis Infection in Macrophages. Mbio 2021, 12, e0187120. [Google Scholar] [CrossRef]
- Bochner, B.S.; Zimmermann, N. Role of siglecs and related glycan-binding proteins in immune responses and immunoregulation. J. Allergy Clin. Immunol. 2015, 135, 598–608. [Google Scholar] [CrossRef]
- Murugesan, G.; Weigle, B.; Crocker, P.R. Siglec and anti-Siglec therapies. Curr. Opin. Chem. Biol. 2021, 62, 34–42. [Google Scholar] [CrossRef]
- Gonzalez-Gil, A.; Schnaar, R.L. Siglec Ligands. Cells 2021, 10, 1260. [Google Scholar] [CrossRef]
- Van Houtum, E.J.H.; Büll, C.; Cornelissen, L.A.M.; Adema, G.J. Siglec Signaling in the Tumor Microenvironment. Front. Immunol. 2021, 12, 790317. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.A.H.; Bertozzi, C.R. The clinical impact of glycobiology: Targeting selectins, Siglecs and mammalian glycans. Nat. Rev. Drug Discov. 2021, 20, 217–243. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Paulson, J.C. Siglecs as Immune Cell Checkpoints in Disease. Annu. Rev. Immunol. 2020, 38, 365–395. [Google Scholar] [CrossRef]
- Crocker, P.R. Siglecs: Sialic-acid-binding immunoglobulin-like lectins in cell–cell interactions and signalling. Curr. Opin. Struct. Biol. 2002, 12, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Angata, T.; von Gunten, S.; Schnaar, R.L.; Varki, A. I-Type Lectins. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2022; pp. 475–490. [Google Scholar]
- Perdicchio, M.; Ilarregui, J.M.; Verstege, M.I.; Cornelissen, L.A.M.; Schetters, S.T.T.; Engels, S.; Ambrosini, M.; Kalay, H.; Veninga, H.; Haan, J.M.M.D.; et al. Sialic acid-modified antigens impose tolerance via inhibition of T-cell proliferation and de novo induction of regulatory T cells. Proc. Natl. Acad. Sci. USA 2016, 113, 3329–3334. [Google Scholar] [CrossRef]
- Merli, M.; Ferrario, A.; Maffioli, M.; Arcaini, L.; Passamonti, F. Investigational therapies targeting lymphocyte antigens for the treatment of non-Hodgkin’s lymphoma. Expert Opin. Investig. Drugs 2015, 24, 897–912. [Google Scholar] [CrossRef]
- Jandus, C.; Boligan, K.F.; Chijioke, O.; Liu, H.; Dahlhaus, M.; Démoulins, T.; Schneider, C.; Wehrli, M.; Hunger, R.E.; Baerlocher, G.M.; et al. Interactions between Siglec-7/9 receptors and ligands influence NK cell–dependent tumor immunosurveillance. J. Clin. Investig. 2014, 124, 1810–1820. [Google Scholar] [CrossRef]
- Walter, R.B.; Gooley, T.A.; Van Der Velden, V.H.J.; Loken, M.R.; Van Dongen, J.J.M.; Flowers, D.A.; Bernstein, I.D.; Appelbaum, F.R. CD33 expression and P-glycoprotein–mediated drug efflux inversely correlate and predict clinical outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin monotherapy. Blood 2007, 109, 4168–4170. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, R.; Ohtsubo, K.; Takamatsu, S.; Taniguchi, N.; Angata, T. The interaction between Siglec-15 and tumor-associated sialyl-Tn antigen enhances TGF- secretion from monocytes/macrophages through the DAP12-Syk pathway. Glycobiology 2012, 23, 178–187. [Google Scholar] [CrossRef]
- Mitra, N.; Banda, K.; Altheide, T.K.; Schaffer, L.; Johnson-Pais, T.L.; Beuten, J.; Leach, R.J.; Angata, T.; Varki, N.; Varki, A. SIGLEC12, a Human-specific Segregating (Pseudo)gene, Encodes a Signaling Molecule Expressed in Prostate Carcinomas. J. Biol. Chem. 2011, 286, 23003–23011. [Google Scholar] [CrossRef] [PubMed]
- Ibarlucea-Benitez, I.; Weitzenfeld, P.; Smith, P.; Ravetch, J.V. Siglecs-7/9 function as inhibitory immune checkpoints in vivo and can be targeted to enhance therapeutic antitumor immunity. Proc. Natl. Acad. Sci. USA 2021, 10, 1178. [Google Scholar] [CrossRef]
- Nunes, M.P.; Fortes, B.; Silva-Filho, J.L.; Terra-Granado, E.; Santos, L.; Conde, L.; Oliveira, I.D.A.; Freire-De-Lima, L.; Martins, M.V.; Pinheiro, A.A.S.; et al. Inhibitory Effects of Trypanosoma cruzi Sialoglycoproteins on CD4+ T Cells Are Associated with Increased Susceptibility to Infection. PLoS ONE 2013, 8, e77568. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Nizet, V. The interplay between Siglecs and sialylated pathogens. Glycobiology 2014, 24, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Herzog, S.; Fragkou, P.C.; Arneth, B.M.; Mkhlof, S.; Skevaki, C. Myeloid CD169/Siglec1: An immunoregulatory biomarker in viral disease. Front. Med. 2022, 9, 979373. [Google Scholar] [CrossRef]
- Mikulak, J.; Di Vito, C.; Zaghi, E.; Mavilio, D. Host Immune Responses in HIV-1 Infection: The Emerging Pathogenic Role of Siglecs and Their Clinical Correlates. Front. Immunol. 2017, 8, 314. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Nizet, V. Siglecs at the Host–Pathogen Interface. Adv. Exp. Med. Biol. 2020, 1204, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, T.; Medeiros, M.M.; Mule, S.N.; Palmisano, G.; Stolf, B.S. The Role of Sialic Acids in the Establishment of Infections by Pathogens, With Special Focus on Leishmania. Front. Cell. Infect. Microbiol. 2021, 11, 671913. [Google Scholar] [CrossRef]
- Stowell, S.R.; Ju, T.; Cummings, R.D. Protein Glycosylation in Cancer. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 473–510. [Google Scholar] [CrossRef]
- Mereiter, S.; Balmaña, M.; Campos, D.; Gomes, J.; Reis, C.A. Glycosylation in the Era of Cancer-Targeted Therapy: Where Are We Heading? Cancer Cell 2019, 36, 6–16. [Google Scholar] [CrossRef]
- Bartish, M.; Del Rincón, S.V.; Rudd, C.E.; Saragovi, H.U. Aiming for the Sweet Spot: Glyco-Immune Checkpoints and γδ T Cells in Targeted Immunotherapy. Front. Immunol. 2020, 11, 564499. [Google Scholar] [CrossRef]
- Videla-Richardson, G.A.; Morris-Hanon, O.; Torres, N.I.; Esquivel, M.I.; Vera, M.B.; Ripari, L.B.; Croci, D.O.; Sevlever, G.E.; Rabinovich, G.A. Galectins as Emerging Glyco-Checkpoints and Therapeutic Targets in Glioblastoma. Int. J. Mol. Sci. 2021, 23, 316. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, V.L.; Rabinovich, G.A.; Griffioen, A.W. Vascular galectins: Regulators of tumor progression and targets for cancer therapy. Cytokine Growth Factor Rev. 2013, 24, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Manni, M.; Läubli, H. Targeting glyco-immune checkpoints for cancer therapy. Expert Opin. Biol. Ther. 2021, 21, 1063–1071. [Google Scholar] [CrossRef]
- Bärenwaldt, A.; Läubli, H. The sialoglycan-Siglec glyco-immune checkpoint—A target for improving innate and adaptive anti-cancer immunity. Expert Opin. Ther. Targets 2019, 23, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Compagno, D.; Tiraboschi, C.; Garcia, J.D.; Rondón, Y.; Corapi, E.; Velazquez, C.; Laderach, D.J. Galectins as Checkpoints of the Immune System in Cancers, Their Clinical Relevance, and Implication in Clinical Trials. Biomolecules 2020, 10, 750. [Google Scholar] [CrossRef]
- Sharma, A.; Seow, J.J.W.; Dutertre, C.-A.; Pai, R.; Blériot, C.; Mishra, A.; Wong, R.M.M.; Singh, G.S.N.; Sudhagar, S.; Khalilnezhad, S.; et al. Onco-fetal Reprogramming of Endothelial Cells Drives Immunosuppressive Macrophages in Hepatocellular Carcinoma. Cell 2020, 183, 377–394.e21. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, N.; Zhang, Y.-F.; Fu, H.; Feng, M.; Schneider, D.; Su, L.; Wu, X.; Zhou, J.; Mackay, S.; et al. Persistent Polyfunctional Chimeric Antigen Receptor T Cells That Target Glypican 3 Eliminate Orthotopic Hepatocellular Carcinomas in Mice. Gastroenterology 2020, 158, 2250–2265.e20. [Google Scholar] [CrossRef]
- Sun, C.; Lan, P.; Han, Q.; Huang, M.; Zhang, Z.; Xu, G.; Song, J.; Wang, J.; Wei, H.; Zhang, J.; et al. Oncofetal gene SALL4 reactivation by hepatitis B virus counteracts miR-200c in PD-L1-induced T cell exhaustion. Nat. Commun. 2018, 9, 1241. [Google Scholar] [CrossRef]
- Elcheva, I.A.; Wood, T.; Chiarolanzio, K.; Chim, B.; Wong, M.; Singh, V.; Gowda, C.P.; Lu, Q.; Hafner, M.; Dovat, S.; et al. RNA-binding protein IGF2BP1 maintains leukemia stem cell properties by regulating HOXB4, MYB, and ALDH1A1. Leukemia 2020, 34, 1354–1363. [Google Scholar] [CrossRef]
- Stern, P.L. Oncofetal Antigen. In Encyclopedia of Cancer; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 2610–2613. [Google Scholar]
- Buonaguro, F.M.; Pauza, D.; Tornesello, M.L.; Hainaut, P.; Franco, R.; Marincola, F.M. Cancer Diagnostic and Predictive Biomarkers. BioMed Res. Int. 2014, 2014, 980163. [Google Scholar] [CrossRef]
- Drake, P.M.; Cho, W.; Li, B.; Prakobphol, A.; Johansen, E.; Anderson, N.L.; Regnier, F.E.; Gibson, B.W.; Fisher, S.J. Sweetening the Pot: Adding Glycosylation to the Biomarker Discovery Equation. Clin. Chem. 2010, 56, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Peracaula, R.; Tabarés, G.; Royle, L.; Harvey, D.J.; Dwek, R.A.; Rudd, P.M.; de Llorens, R.R. Altered glycosylation pattern allows the distinction between prostate-specific antigen (PSA) from normal and tumor origins. Glycobiology 2003, 13, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Saldova, R.; Struwe, W.B.; Wynne, K.; Elia, G.; Duffy, M.J.; Rudd, P.M. Exploring the Glycosylation of Serum CA125. Int. J. Mol. Sci. 2013, 14, 15636–15654. [Google Scholar] [CrossRef] [PubMed]
- Freire-De-Lima, L. Sweet and sour: The impact of differential glycosylation in cancer cells undergoing epithelial-mesenchymal transition. Front. Oncol. 2014, 4, 59. [Google Scholar] [CrossRef]
- Backes, C.; Ludwig, N.; Leidinger, P.; Harz, C.; Hoffmann, J.; Keller, A.; Meese, E.; Lenhof, H.-P. Immunogenicity of autoantigens. BMC Genom. 2011, 12, 340. [Google Scholar] [CrossRef]
- McClintock, S.D.; Warner, R.L.; Ali, S.; Chekuri, A.; Dame, M.K.; Attili, D.; Knibbs, R.K.; Aslam, M.N.; Sinkule, J.; Morgan, A.C.; et al. Monoclonal antibodies specific for oncofetal antigen—Immature laminin receptor protein: Effects on tumor growth and spread in two murine models. Cancer Biol. Ther. 2015, 16, 724–732. [Google Scholar] [CrossRef][Green Version]
- Fu, C.; Zhao, H.; Wang, Y.; Cai, H.; Xiao, Y.; Zeng, Y.; Chen, H. Tumor-associated antigens: Tn antigen, sTn antigen, and T antigen. Hla 2016, 88, 275–286. [Google Scholar] [CrossRef]
- Bulteau, F.; Thépaut, M.; Henry, M.; Hurbin, A.; Vanwonterghem, L.; Vivès, C.; Le Roy, A.; Ebel, C.; Renaudet, O.; Fieschi, F.; et al. Targeting Tn-Antigen-Positive Human Tumors with a Recombinant Human Macrophage Galactose C-Type Lectin. Mol. Pharm. 2021, 19, 235–245. [Google Scholar] [CrossRef]
- Loureiro, L.R.; Carrascal, M.A.; Barbas, A.; Ramalho, J.S.; Novo, C.; Delannoy, P.; Videira, P.A. Challenges in Antibody Development against Tn and Sialyl-Tn Antigens. Biomolecules 2015, 5, 1783–1809. [Google Scholar] [CrossRef]
- Hakomori, S.-I. Tumor-Associated Carbohydrate Antigens Defining Tumor Malignancy: Basis for Development of Anti-Cancer Vaccines. Adv. Exp. Med. Biol. 2001, 491, 369–402. [Google Scholar] [CrossRef]
- Ibrahim, N.K.; Murray, J.L. Clinical Development of the STn-KLH Vaccine (Theratope®). Clin. Breast Cancer 2003, 3, S139–S143. [Google Scholar] [CrossRef] [PubMed]
- Julien, S.; Videira, P.A.; Delannoy, P. Sialyl-Tn in Cancer: (How) Did We Miss the Target? Biomolecules 2012, 2, 435–466. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.; Otto, V.I.; Cummings, R.D. The Tn Antigen-Structural Simplicity and Biological Complexity. Angew. Chem. Int. Ed. 2011, 50, 1770–1791. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.; Dausset, J.; Bernard, J.; Moullec, J. Acquired hemolytic anemia with polyagglutinability of erythrocytes by a new factor present in normal blood. Bull. Mem. La Soc. Med. Des Hop. Paris 1957, 73, 569–587. [Google Scholar]
- Dahr, W.; Uhlenbruck, G.; Gunson, H.H.; Hart, M. Molecular Basis of Tn-Polyagglutinability. Vox Sang. 1975, 29, 36–50. [Google Scholar] [CrossRef]
- Ju, T.; Wang, Y.; Aryal, R.P.; Lehoux, S.D.; Ding, X.; Kudelka, M.R.; Cutler, C.; Zeng, J.; Wang, J.; Sun, X.; et al. Tn and sialyl-Tn antigens, aberrant O-glycomics as human disease markers. Proteom. Clin. Appl. 2013, 7, 618–631. [Google Scholar] [CrossRef]
- Cornelissen, L.A.M.; Blanas, A.; Zaal, A.; Van Der Horst, J.C.; Kruijssen, L.J.W.; O’Toole, T.; Van Kooyk, Y.; Van Vliet, S.J. Tn Antigen Expression Contributes to an Immune Suppressive Microenvironment and Drives Tumor Growth in Colorectal Cancer. Front. Oncol. 2020, 10, 1622. [Google Scholar] [CrossRef]
- Springer, G.F. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J. Mol. Med. 1997, 75, 594–602. [Google Scholar] [CrossRef]
- Desai, P.R. Immunoreactive T and Tn antigens in malignancy: Role in carcinoma diagnosis, prognosis, and immunotherapy. Transfus. Med. Rev. 2000, 14, 312–325. [Google Scholar] [CrossRef]
- Da Costa, V.; Mariño, K.V.; Rodríguez-Zraquia, S.A.; Festari, M.F.; Lores, P.; Costa, M.; Landeira, M.; Rabinovich, G.A.; van Vliet, S.J.; Freire, T. Lung Tumor Cells with Different Tn Antigen Expression Present Distinctive Immunomodulatory Properties. Int. J. Mol. Sci. 2022, 23, 12047. [Google Scholar] [CrossRef]
- Da Costa, V.; van Vliet, S.J.; Carasi, P.; Frigerio, S.; García, P.A.; Croci, D.O.; Festari, M.F.; Costa, M.; Landeira, M.; Rodríguez-Zraquia, S.A.; et al. The Tn antigen promotes lung tumor growth by fostering immunosuppression and angiogenesis via interaction with Macrophage Galactose-type lectin 2 (MGL2). Cancer Lett. 2021, 518, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, T.; Hakomori, S.-I.; Singhal, A.K. Synthetic carbohydrate vaccines: Synthesis and immunogenicity of Tn antigen conjugates. Bioorganic Med. Chem. 1994, 2, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Amedei, A.; Asadzadeh, F.; Papi, F.; Vannucchi, M.G.; Ferrucci, V.; Bermejo, I.A.; Fragai, M.; De Almeida, C.V.; Cerofolini, L.; Giuntini, S.; et al. A Structurally Simple Vaccine Candidate Reduces Progression and Dissemination of Triple-Negative Breast Cancer. Iscience 2020, 23, 101250. [Google Scholar] [CrossRef] [PubMed]
- Richichi, B.; Thomas, B.; Fiore, M.; Bosco, R.; Qureshi, H.; Nativi, C.; Renaudet, O.; BenMohamed, L. A Cancer Therapeutic Vaccine based on Clustered Tn-Antigen Mimetics Induces Strong Antibody-Mediated Protective Immunity. Angew. Chem. Int. Ed. 2014, 53, 11917–11920. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, H.; Hakomori, S. The oncofetal domain of fibronectin defined by monoclonal antibody FDC-6: Its presence in fibronectins from fetal and tumor tissues and its absence in those from normal adult tissues and plasma. Proc. Natl. Acad. Sci. USA 1985, 82, 6517–6521. [Google Scholar] [CrossRef]
- Loridon-Rosa, B.; Vielh, P.; Matsuura, H.; Clausen, H.; Cuadrado, C.; Burtin, P. Distribution of oncofetal fibronectin in human mammary tumors: Immunofluorescence study on histological sections. Cancer Res 1990, 50, 1608–1612. [Google Scholar]
- Kaczmarek, J.; Castellani, P.; Nicolo, G.; Spina, B.; Allemanni, G.; Zardi, L. Distribution of oncofetal fibronectin isoforms in normal, hyperplastic and neoplastic human breast tissues. Int. J. Cancer 1994, 59, 11–16. [Google Scholar] [CrossRef]
- Mandel, U.; Therkildsen, M.H.; Reibel, J.; Sweeney, B.; Matsuura, H.; Hakomori, S.; Dabelsteen, E.; Clausen, H. Cancer-associated changes in glycosylation of fibronectin. Immunohistological localization of oncofetal fibronectin defined by monoclonal antibodies. Apmis 1992, 100, 817–826. [Google Scholar] [CrossRef]
- Matsuura, H.; Takio, K.; Titani, K.; Greene, T.; Levery, S.B.; Salyan, M.E.; Hakomori, S. The oncofetal structure of human fibronectin defined by monoclonal antibody FDC-6. Unique structural requirement for the antigenic specificity provided by a glycosylhexapeptide. J. Biol. Chem. 1988, 263, 3314–3322. [Google Scholar] [CrossRef]
- Freire-De-Lima, L.; Gelfenbeyn, K.; Ding, Y.; Mandel, U.; Clausen, H.; Handa, K.; Hakomori, S.-I. Involvement of O-glycosylation defining oncofetal fibronectin in epithelial-mesenchymal transition process. Proc. Natl. Acad. Sci. USA 2011, 108, 17690–17695. [Google Scholar] [CrossRef]
- Ding, Y.; Gelfenbeyn, K.; Freire-De-Lima, L.; Handa, K.; Hakomori, S.-I. Induction of epithelial-mesenchymal transition with O-glycosylated oncofetal fibronectin. FEBS Lett. 2012, 586, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Alisson-Silva, F.; Freire-De-Lima, L.; Donadio, J.L.; Lucena, M.C.; Penha, L.; Sá-Diniz, J.N.; Dias, W.B.; Todeschini, A.R. Increase of O-Glycosylated Oncofetal Fibronectin in High Glucose-Induced Epithelial-Mesenchymal Transition of Cultured Human Epithelial Cells. PLoS ONE 2013, 8, e60471. [Google Scholar] [CrossRef] [PubMed]
- Da Fonseca, L.M.; da Silva, V.A.; da Costa, K.M.; dos Reis, J.S.; Previato, J.O.; Previato, L.M.; Freire-De-Lima, L. Resistance to cisplatin in human lung adenocarcinoma cells: Effects on the glycophenotype and epithelial to mesenchymal transition markers. Glycoconj. J. 2022, 39, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.R.d.C.; dos Reis, J.S.; Santos, C.A.D.N.; da Costa, K.M.; Barcelos, P.M.; Francisco, K.Q.d.O.; Barbosa, P.A.G.N.; da Silva, E.D.S.; Freire-De-Lima, C.G.; Morrot, A.; et al. Expression of O-glycosylated oncofetal fibronectin in alternatively activated human macrophages. Immunol. Res. 2023, 71, 92–104. [Google Scholar] [CrossRef]
- Wang, H.-W.; Joyce, J.A. Alternative activation of tumor-associated macrophages by IL-4: Priming for protumoral functions. Cell Cycle 2010, 9, 4824–4835. [Google Scholar] [CrossRef]
- Aras, S.; Zaidi, M.R. TAMeless traitors: Macrophages in cancer progression and metastasis. Br. J. Cancer 2017, 117, 1583–1591. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Dong, X.; Hu, X.; Jiang, Y.; Li, L.; Du, T.; Yang, L.; Wen, T.; An, G.; et al. Tn antigen promotes human colorectal cancer metastasis via H-Ras mediated epithelial-mesenchymal transition activation. J. Cell. Mol. Med. 2019, 23, 2083–2092. [Google Scholar] [CrossRef]
- Dong, X.; Jiang, Y.; Liu, J.; Liu, Z.; Gao, T.; An, G.; Wen, T. T-Synthase Deficiency Enhances Oncogenic Features in Human Colorectal Cancer Cells via Activation of Epithelial-Mesenchymal Transition. BioMed Res. Int. 2018, 2018, 9532389. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Reis, J.S.; Diniz-Lima, I.; Santos, M.A.R.d.C.; Barcelos, P.M.; da Costa, K.M.; Valente, R.d.C.; Chaves, L.d.S.; de Campos, L.P.; dos Santos, A.C.; Correia de Lima, R.G.; et al. The Blessed Union of Glycobiology and Immunology: A Marriage That Worked. Medicines 2023, 10, 15. https://doi.org/10.3390/medicines10020015
dos Reis JS, Diniz-Lima I, Santos MARdC, Barcelos PM, da Costa KM, Valente RdC, Chaves LdS, de Campos LP, dos Santos AC, Correia de Lima RG, et al. The Blessed Union of Glycobiology and Immunology: A Marriage That Worked. Medicines. 2023; 10(2):15. https://doi.org/10.3390/medicines10020015
Chicago/Turabian Styledos Reis, Jhenifer Santos, Israel Diniz-Lima, Marcos André Rodrigues da Costa Santos, Pedro Marçal Barcelos, Kelli Monteiro da Costa, Raphael do Carmo Valente, Lorrane de Souza Chaves, Luma Petel de Campos, Ariely Costa dos Santos, Rafaela Gomes Correia de Lima, and et al. 2023. "The Blessed Union of Glycobiology and Immunology: A Marriage That Worked" Medicines 10, no. 2: 15. https://doi.org/10.3390/medicines10020015
APA Styledos Reis, J. S., Diniz-Lima, I., Santos, M. A. R. d. C., Barcelos, P. M., da Costa, K. M., Valente, R. d. C., Chaves, L. d. S., de Campos, L. P., dos Santos, A. C., Correia de Lima, R. G., Decote-Ricardo, D., Morrot, A., Previato, J. O., Mendonça-Previato, L., Freire-de-Lima, C. G., Fonseca, L. M. d., & Freire-de-Lima, L. (2023). The Blessed Union of Glycobiology and Immunology: A Marriage That Worked. Medicines, 10(2), 15. https://doi.org/10.3390/medicines10020015











