Abstract
Sleep disturbance is one of the neurobehavioral complications of lead neurotoxicity. The present study evaluated the impacts of chronic lead exposure on alteration of the sleep–wake cycle in association with changes of clock gene expression in the hypothalamus. Sprague–Dawley rats with chronic lead exposure consumed drinking water that contained 250 ppm of lead acetate for five weeks. Electroencephalography and electromyography were recorded for scoring the architecture of the sleep–wake cycle in animals. At six Zeitgeber time (ZT) points (ZT2, ZT6, ZT10, ZT14, ZT18, and ZT22), three clock genes, including rPer1, rPer2, and rBmal1b, were analyzed. The rats with chronic lead exposure showed decreased slow wave sleep and increased wakefulness in the whole light period (ZT1 to ZT12) and the early dark period (ZT13 to ZT15) that was followed with a rebound of rapid-eye-movement sleep at the end of the dark period (ZT22 to ZT24). The disturbance of the sleep–wake cycle was associated with changes in clock gene expression that was characterized by the upregulation of rPer1 and rPer2 and the feedback repression of rBmal1b. We concluded that chronic lead exposure has a negative impact on the sleep–wake cycle in rats that predominantly disrupts sleep homeostasis. The disruption of sleep homeostasis was associated with a toxic effect of lead on the clock gene expression in the hypothalamus.
1. Introduction
Lead, a toxic metal, is one of the most common cumulative and preventable toxic pollutants of our environment that can induce various adverse clinical consequences in children and adults [,,]. As lead can be stored in bones for decades, the toxic effects of lead may be retained throughout the life course [,]. Lead and other heavy metals in bones may serve as a source of exposure during different stages of one’s life and have a potential cumulative effect on adverse clinical outcomes [,]. In addition, lead may persist in the environment; populations can continue to be exposed in the region where it was previously used []. Age, country of birth, education level, gender, ethnicity, income, and occupation have shown significant differences depending on the degree of lead exposure, with higher levels of exposure resulting in worse outcomes []. Chronic lead exposure has been recognized to have substantial impacts on the renal, hematological, reproductive, cardiovascular, cerebrovascular, and neurological systems in humans [,,,,]. In the central nervous system (CNS), the detrimental neurotoxicity of lead is commonly associated with neuropsychological and neurobehavioral difficulties in intelligence, memory, attention, executive tasks, processing speed, language, visuospatial skills, motor abilities, and mood and affection [,,]. Early life exposure to low levels of lead may affect brain development, particularly in impairment of learning and memory function []. High-level lead exposure may result in an increase in the prevalence of brain cancer []. Several mechanisms for lead neurotoxicity have been proposed that included disruption of long-term potentiation in the hippocampus [], hyperphosphorylation of tau protein [], neuroinflammatory response [,], oxidative stress [,], mitochondrial dysfunctions [], abnormal zinc homeostasis [], deregulation of calcium signaling [], and abnormal neural transmission and gene expression [,]. Moreover, lead-induced damage in the prefrontal cerebral cortex, hippocampus, and cerebellum can lead to a variety of neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, and schizophrenia [,]. Clinically, chronic lead exposure can manifest with fatigue, headache, irritability, speech problems, cognitive and memory impairment, seizure disorders, and even sleep disturbances [,,,].
Sleep disturbance is one of the neurobehavioral complications of lead toxicity [,,]. The cumulative effect of chronic lead stored in bones throughout the life course may influence sleep architecture [,,,]. Combined lead and zinc exposure have caused severe sleep disturbance and daytime symptoms in copper smelt workers []. Elevated blood lead levels in early childhood are associated with an increased risk for sleep problems and excessive daytime sleepiness in later childhood [,]. Chronic lead exposure in early life may change the morphology, cellular density, and relative optical density in the cells of the suprachiasmatic nucleus (SCN) of the hypothalamus []. This issue of public and environmental health not only indicates that lead neurotoxicity should be concerned from the stage of infancy and even in the prenatal period [] but also emphasizes that monitoring the synergistic toxic effects of other heavy metals in the population with a high risk of chronic lead exposure is important [].
The circadian rhythm of stereotyped complex behaviors among lead-exposed rats showed a depressed response in light periods but an elevated response in dark periods []. These findings may indicate that behavioral pattern of the circadian rhythm might be disrupted by chronic lead exposure. Whereas sleep activity is the most important component of neurobehavioral functions, the effect of chronic lead intoxication on the sleep–wake cycle and clock gene expression in the hypothalamus is still unclear. In the present study, we aim to evaluate the impacts of chronic lead exposure on alteration of the sleep–wake cycle in association with changes of clock gene expression in the hypothalamus in a chronic lead-exposed animal model.
2. Materials and Methods
2.1. Study Design
This study is based on an experimental animal model of chronic lead toxication that aimed to explore the toxic effect of lead in the change of sleep–wake cycle and circadian genes. Experimental procedures in the present study were accomplished in accordance with the guidelines on animal experimentation, endorsed by the constituted research ethics committee of Kaohsiung Medical University, Kaohsiung, Taiwan (the project identification code 97033 was approved by the Institutional Animal Care and Use Committee of Kaohsiung Medical University; approval date on 1 August 2008).
2.2. Experimental Animals
Specific pathogen-free male Sprague–Dawley rats (postnatal week six; body weight: 250–280 g) were purchased from BioLASCO Taiwan Co. Ltd. (Taipei, Taiwan) and housed in an environmentally controlled room (22–24 °C; 12:12 h light/dark cycle; lights on at 07:00 am) at the Center for Laboratory Animals at Kaohsiung Medical University. Standard laboratory rat chow and tap water were available ad libitum. All efforts were made to reduce the number of animals used and to minimize animal suffering during the experiment.
2.3. Experimental Animal Model of Chronic Lead Toxication
The model of chronic lead poisoning in rats has been well established in our laboratory []. The animals with chronic lead exposure consumed drinking water that contained 250 ppm of lead acetate for five weeks. The water regimen of lead acetate solution was to dissolve 125 mg of lead acetate powder into 500 mL of reverse osmosis water. Animals that were fed with deionized water and surgical preparations without chronic lead exposure manipulation served as the control group [].
2.4. Electroencephalography and Electromyography Recordings
Both animal groups received anesthesia via intraperitoneally injection of Zoletil® 50 (30 mg/kg, Virbac, Taipei, Taiwan) that contained tiletamine and zolazepam (1:1), then rats underwent surgical interventions for preparation of recordings of the sleep–wake cycle by electroencephalography (EEG) and electromyography (EMG) on the first day of week five. After anesthesia, the head of the rat was fixed to a stereotaxic headholder (Kopf, Tujunga, CA, USA), and the body of rats were kept at 37 °C by placing them on a heating pad. The procedure of EEG recording and scoring for sleep stages was performed according to previous reports [,,,]. To perform the EEG recording, three EEG screw electrodes were then implanted on the surface of the cortex with the following coordinates: (1) right frontal (2.0 mm anterior to bregma and 3.5 mm from the midline); (2) right hippocampus (3.5 mm posterior to bregma, 2.5 mm from the midline); and (3) left occipital (1.0 mm posterior to lambda and 3.5 mm from the midline) [,,]. The insulated wires from EEG electrodes were routed to a Teflon pedestal (Plastics One, Roanoke, VA, USA). The Teflon pedestal was then cemented to the skull using dental acrylic (Cranioplasty cement and cyanoacrylate gel, Plastics One, Roanoke, VA, USA). For evaluation of EMG activity during the sleep stage, two electrodes were simultaneously inserted into the posterior neck muscles [,]. The surgical wounds of animals were then treated topically with povidone-iodine ointment. Animals were returned to the 12 h light/dark cages individually and the rats were allowed a one-week interval for recovery and then prepared for the further EEG and EMG recordings. The drinking water containing lead acetate was continuously fed to the animals until EEG and EMG recording.
The EEG and EMG recordings for scoring the distribution of the sleep–wake cycle in animals under chronic lead exposure were carried out on the first day of week six [,]. In brief, the animals were connected to the EEG and EMG recording apparatus via a flexible tether for 24 h. The signal of body movement was determined by custom-made infrared-based motion detectors (Biobserve GmbH, Bonn, Germany), and the movement activity was converted to a voltage output. The signals from the EEG and EMG electrodes were transmitted to an amplifier (model V75-01; Colbourn Instruments, Lehigh Valley, PA, USA) [,,,]. These conditioned signals, including EEG, EMG, and body movements, were then converted with 16-bit precision at a sampling rate of 128 Hz (NI PCI-6033E; National Instruments, Austin, TX, USA). The digitalized EEG and EMG waveforms and integrated values for body movement were stored as binary computer files for further analyses as described in our previous reports [,,,].
Postacquisition judgment of the sleep–wake state was evaluated by visual scoring of 12-s epochs (ICELUS, Professor M. R. Opp, University of Michigan) authored in LabView for Windows (National Instruments, Austin, TX, USA) [,,]. The sleep architecture of animals was categorized to slow wave sleep (SWS), rapid-eye-movement (REM) sleep, and wakefulness according to our previous reports [,]. Based on these criteria, SWS is characterized by large-amplitude slow waves and high-power density values within the delta frequency band (0.5–4.0 Hz) during EEG recording [,,]. Moreover, EMG activities showed decreased muscle tone and lack of gross body movements. In the REM sleep, the amplitude of EEG is decreased, the density of EEG mainly occurs in the theta range (6.0–9.0 Hz), the activity of EMG often displays muscle atonia and low amplitudes, and phasic body twitches may appear [,,]. In the stage of wakefulness, animals are usually vigorous showing protracted body movement and robust activity and amplitudes in the EMG. The amplitude of the EEG is similar to that observed in REM sleep; however, values of power density in the delta frequency band are usually greater than those in the theta frequency band [,,]. For evaluation of architecture of the sleep–wake cycle, the percentage of SWS, REM sleep, and wakefulness were calculated at each Zeitgeber time (ZT) point.
2.5. Measurement of Blood Lead Level, Serum Creatinine Level and Daily Urine Amount
For confirmation of chronic lead intoxication in rats, blood lead levels were measured after the recording of the sleep–wake cycle. The rats were sacrificed at week six, and the blood sample was collected from the inferior vena cava to determine the concentration of blood lead and serum creatinine []. The 24 h urine sample was collected from metabolic cages for the measurement of urine amount []. Blood lead levels were measured by a Zeeman-effect graphite furnace atomic absorption spectrometry (Perkin–Elmer 5100 PC with AS 60 autosampler, EquipX, San Jose, CA, USA) in the same laboratory [,]. For intralaboratory quality controls, with the use of commercial standard materials (Betherning Institute, Bio-Rad, Hercules, CA, USA), all coefficients of variation were less than 3% for measurements at high (70.5 to 82.7 μg/dL) and medium levels (37.1 to 45.3 μg/dL), and were less than 5% for those at low levels (5.6 to 8.9 μg/dL). The detection limit of this instrument was 0.1 μg/dL. In addition, our laboratory has been participating in the interlaboratory blood-lead testing program in Taiwan [,]; the results of our measurements are all within the reference ranges, indicating that our blood lead measurements are accurate.
2.6. Analysis of Clock Gene Expression
The rats were anesthetized intraperitoneally with an injection of Zoletil® 50, the brain was then rapidly removed under visual inspection and placed on a piece of gauze moistened with ice-cold 0.9% saline. The hypothalamus was routinely dissected and stored at −80 °C for further investigating the clock gene expression.
Eight rats (four in the lead-exposure group and four in the control group) at each pre-determined ZT point (ZT2, ZT6, ZT10, ZT14, ZT18, and ZT22) were scarified for analyzing whether expressional changes of clock genes took place during chronic lead exposure. Three clock genes, including the rat period 1 gene (rPer1), rat period 1 gene (rPer2) and rat brain and muscle ARNT-like 1b gene (rBmal1b) were selected for analysis of mRNA expression. In brief, the tissue of hypothalamus was immediately transferred to RNA stabilization reagent kits, RNAlater® (Ambion, Valencia, CA, USA). Total RNA was extracted and purified by the spin column-based method (RNeasy Mini Kit®, 74104, QIAGEN, Hilden, Germany) following retrotranscription to cDNA with RevertAid First Strand cDNA Synthesis Kit® (K1622, Waltham, MA, USA). All procedures were according to the manufacturer’s protocol as per our previous reports [,]. Subsequently, the cDNA was employed for TaqMan real-time quantitative PCR analysis by StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The sequence of probe and primers used in this study were as follows: rPer1 (probe: 5′-CTTCAGCC-3′, forward: 5′-TGCTTTAGATCGGCAGTGGT-3′, reverse: 5′-GTGGGCTTGACACCTCTTCT-3′); rPer2 (probe: 5′-CTTCAGCC-3′, forward: 5′-GCTGGAGGACTACTTTGCATTT-3′, reverse: 5′-CCAGTGGCAAGAGTCAAAGC-3′) and rBmal1b (probe: 5′-TCCTCTCC-3′, forward: 5′-TGTCTGGAGTCCCTCCATTT-3′, reverse: 5′-AGTACGCCTCCCCCTGAT-3′).
Real-time quantitative PCR was performed in the TaqMan® Universal PCR Master Mix (2X) (Applied Biosystems). All reactions were carried out in a 20 μL final volume containing 300 nM of each primer, 250 nM of the probe, 1 μL of TaqMan® 20× probe/primer assay ix, and 1 μL of cDNA for the optimal performance. The “comparative Ct method” was applied to relatively quantify target genes expressed. The relative changes were determined from the Ct values of samples in chronic lead-exposure and control groups, and normalized to an endogenous housekeeping gene, β-actin.
2.7. Statistical Analysis
The concentration of blood lead and serum creatinine, urine output and the architecture of the sleep–wake cycle were presented as mean ± standard deviation. We assumed the difference of blood lead level between exposure and control groups was 10 μg/dL, and difference of EEG between the sleep–wake cycle was 15%, then the power calculated would be more than 0.6 when n = 6 for each group of rats. The quantitative gene expressions of rPer1, rPer2, and rBmal1b between the lead-exposure and control groups were presented as the ratio of mRNA levels of clock genes to the housekeeping gene, β-actin. Three consecutive ZT points and twelve ZT points for light and dark periods were grouped for statistical analysis. Differences in the variables between lead-exposure and control groups were conducted using a Mann–Whitney U test. The statistical analysis was performed using the IBM SPSS statistics version 22.0 (IBM, Armonk, NY, USA). A p < 0.05 was considered statistically significant.
3. Results
3.1. The Blood Lead Level and Biochemical Assay
The body weight, blood level of lead, serum level of creatinine, and daily urine amount in lead-exposure and control animals are presented in Table 1. There was no difference in body weight between two groups. Compared to the normal control rats, increased urine output was noted in lead-treated animals. Additionally, significant increases in blood level of lead (15.30 ± 3.22 μg/dL; ranged from 12.5 to 21.3 μg/dL) and serum level of creatinine were found in the lead-exposure group.

Table 1.
Body weight, urine amount, and biochemical data in lead-exposure and control animals.
3.2. Alterations of Sleep–Wake Activities in Rats with Chronic Lead Poisoning
According to EEG and EMG recordings, the sleep–wake cycle in animals with lead exposure and controls is shown in Figure 1. Comparison between lead-exposure and control groups in a 12 h interval of light and dark periods revealed that rats with chronic lead exposure had a significant decrease in SWS (42.68 ± 2.29% versus 48.72 ± 1.87%, p < 0.001) and increase in wakefulness (43.17 ± 2.80% versus 35.82 ± 2.53%, p < 0.001) compared with controls in the light period. Otherwise, the REM sleep in the light period did not show statistically significant between the lead-exposure and control groups (14.14 ± 1.61% versus 15.46 ± 1.77%, p = 0.141). However, there was no significant difference between the lead-exposure and control groups in the SWS, REM sleep and wakefulness in the dark period.
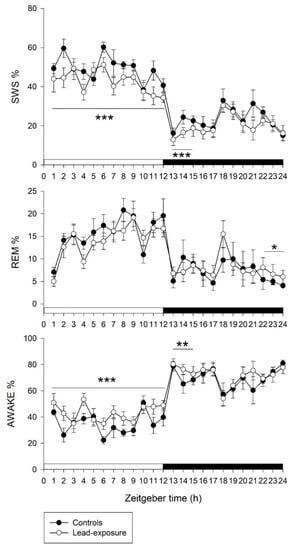
Figure 1.
A 24 h panel of sleep–wake cycle between lead-exposure and control rats. Values on y-axis represent percentage of each sleep stage. Values on x-axis represent circadian Zeitgeber time points. SWS, slow wave sleep; REM, REM sleep; awake, wakefulness period. Data are represented as mean ± standard deviation. The two groups within 12h-interval or 3h-interval were compared by using a Mann–Whitney U test. * p < 0.05, ** p < 0.01 and *** p < 0.001 versus the control group.
We further evaluated the sleep–wake architecture during the dark period in a 3 h interval. Based on statistical analysis, a significant decrease in SWS (16.10 ± 2.2 versus 21.10 ± 1.3, p < 0.001) and an increase in wakefulness (76.50 ± 2.6% versus 70.80 ± 2.0%, p = 0.005) were found at the ZT13-15 point in lead-exposure animals compared with controls. Moreover, a significant increase in REM sleep (6.80 ± 1.60% versus 4.79 ± 1.28%, p = 0.015) was noted at the ZT22-24 point in the lead-exposure group compared with the control group.
3.3. Alterations of the Clock Gene Expression in Rats with Chronic Lead Poisoning
The expressional changes of three clock genes (rPer1, rPer2, and rBmal1b) at each ZT points in the tissue from hypothalamus are shown in Figure 2. In the rats with chronic lead exposure, there was a significant upregulation of rPer1 expression compared with control animals at ZT6 (0.0533 ± 0.0041 versus 0.0366 ± 0.0035, p = 0.022), ZT10 (0.0830 ± 0.0041 versus 0.0652 ± 0.0042, p = 0.030) and ZT14 (0.2190 ± 0.0239 versus 0.0796 ± 0.0047, p = 0.008). The expression of rPer2 in lead-exposure animals had significant upregulation compared with controls (0.0282 ± 0.0025 versus 0.0123 ± 0.0009, p = 0.0046) at the ZT14 point. Otherwise, there was a significant downregulation of rBmal1b expression in lead-exposed rats at the ZT18 point (0.0019 ± 0.0000 versus 0.0025 ± 0.0001, p = 0.0195).
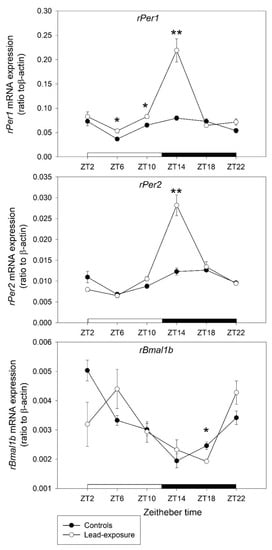
Figure 2.
Clock gene mRNA expression in the hypothalamus between lead-exposure rat and control rats. Values on y-axis represent normalized as ratio of mRNA levels of clock genes to the housekeeping gene, β-actin. Values on x-axis represent circadian Zeitgeber time (ZT) points. Data are represented as mean ± standard deviation. * p < 0.05 and ** p < 0.01 versus the control group in Mann–Whitney U test.
4. Discussion
Based on a clinically relevant experimental model, the present study demonstrated that rats with lead exposure for five weeks decreased SWS and increased wakefulness in the whole light period (ZT1 to ZT12) and the early dark period (ZT13 to ZT15) that was followed by a rebound of REM sleep at the end of the dark period (ZT22 to ZT24). The disturbance of sleep–wake cycle was associated with changes in clock gene expression that were characterized by upregulation of rPer1 and rPer2 and feedback repression of rBmal1b. The average blood level of lead-exposure animals was 15.30 ± 3.22 μg/dL, which ranged from 12.5 to 21.3 μg/dL. The exposure blood lead level in relation to human is considered as the low-to-medium level, which is thought to resemble the blood lead levels in chronic lead-exposed workers in Taiwan (the mean blood lead level was around 25 μg/dL) according to our previous human studies [,,]. Our major findings indicate that chronic lead exposure predominantly disrupts homeostasis of the sleep–wake cycle in rats with a subsequent compensation by circadian process. In addition, increases in serum creatinine level and daily urine output may suggest chronic lead exposure may damage the renal interstitium that cause interstitial nephritis in rats, leading to impaired ability to concentrate urine and nocturnal polyuria [,,].
The CNS is particularly susceptible to lead neurotoxicity that can result in a variety of neurological disorders [,,]. It has been reported that lead exposure disrupts the temporal organization of behavioral patterns, circadian rhythm of locomotor activity and the sleep–wake cycle [,]. Shorter sleep duration and excessive daytime sleepiness may represent an unrecognized adverse consequence of chronic lead exposure in youth [,]. In the present study, we found that animals with chronic lead poisoning decreased SWS and increased wakefulness in the light period and the early part of the dark period. The homeostatic pressure built up in response to decreased SWS at the same periods. Evidently, the duration of deep sleep in the whole light/dark period was reduced under chronic lead neurotoxicity. The SWS, which is the major component of non-rapid eye movement (NREM) sleep, has a strong link to homeostatic process []. Decreased SWS and hyperarousal may lead to irritability, fatigue, sleep deprivation, as well as increased daytime sleepiness []. We noted especially that rebound promotion of REM sleep occurred at the end of the dark period in the chronic lead-exposure rats. The phenomenon of daytime sleepiness with REM sleep rebound decreased after SWS and sleep deprivation, which may be related to the activated compensatory mechanism of circadian and homeostatic control process in the SCN of hypothalamus [,].
The dysregulation of sleep homeostasis can be compensated by the circadian process, which is a well-documented phenomenon in humans with chronic primary insomnia []. This circadian modulation is weaker and less clear in rodents than in humans []. However, under constant homeostatic sleep pressure, an endogenous circadian modulation of the duration of waking and NREM sleep has been observed in rats [,]. Whereas the active promotion of REM sleep was observed in response to a decrease in SWS and sleep deprivation at the end of the dark period, there was no compensatory increase in SWS in the subsequent dark period in the chronic lead-exposure rats. SWS deficits might mean that the integrity of homeostatic regulatory mechanism and its related restorative functions are impaired []. The abnormal molecular circuitry of circadian clocks through the expressional changes of clock genes might play an important role in the chronic lead-exposure rats [].
rPer1 and rPer2 are members of the period family of genes [,,]. The genes in this family encode components of the circadian rhythms of locomotor activity, metabolism, sleep–wake cycle, and behavior [,,]. The SCN, the primary circadian pacemaker in the mammalian brain, is composed of subgroups of cells that are different in circadian phase and oscillation magnitude of the rPer1 and rPer2 genes [,]. Quantitative in situ hybridization of rPer1 and rPer2 mRNAs showed a robust circadian rhythm in the SCN with a characteristic peak/trough profile in each gene [,]. The peak level of rPer1 mRNA was presented in the daytime and that of rPer2 mRNA was at the transition time of day to night in both light–dark and constant dark conditions [,]. Prolonged light exposure during daytime positively modulates daily levels of Per1 and Per2 mRNA in the SCN []. BMAL1 was suggested to be a partner of circadian locomotor output cycles kaput (CLOCK) and a component of a molecular feedback loop of circadian oscillation [,]. In the classic view, CLOCK and BMAL1 increase the transcription of Period (Per), Cryptochrome (Cry), and other clock-controlled genes during the daytime [,,]. The levels of Per and Cry proteins increase during the night, after which they dimerize and translocate to the nucleus to repress CLOCK–BMAL1-mediated transcription [,,].
In the present study, we found that a significant upregulation of rPer1 expression started at ZT6 to ZT14 with a heightened expressional level at Z14 point in the chronic lead-exposure rats. The expression of rPer2 also was highly upregulated at the ZT14 point. On the other hand, there was a significant downregulation of rBmal1b expression at the ZT18 point in chronic lead-exposure animals. The expression of rPer1 and rPer2 was heightened during the early dark period. This phenomenon was contrary to the normal circadian pattern that would be found in the daytime with the peak level of rPer1 and rPer2 mRNAs [,,]. Then, the abnormally high expression of rPer1 and rPer2 during the early dark period inhibited rBmal1b expression at the late stage of the dark period from the negative feedback loop. The abnormal expression of rPer1, rPer2, and rBmal1b may result in the disturbance of the circadian sleep–wake cycle and disruption of sleep homeostasis in chronic lead-exposure rats. Thus, we suggested that chronic lead exposure may exert toxic effects on the cells of SCN in the hypothalamus that result in abnormal changes of clock gene expression in rats. However, further studies are warranted to address these issues.
The molecular and cellular mechanism of lead neurotoxicity to the cells of SCN is still unclear. Several mechanisms have been proposed, such as ion mimicry, mitochondrial dysfunction, redox imbalance, and neuroinflammation []. The homeostatic dysregulation following lead neurotoxicity may be based on the similarity of ionized lead to calcium []. Lead exposure can disrupt calcium homeostasis caused by the abnormal calcium transportation []. Recent evidence has suggested that the toxic effects of lead might be brought on by occupying calcium-binding sites on calmodulin [,]. These effects might influence circadian regulation of voltage-dependent calcium channel activity that plays an important role in maintaining rhythmic clock gene expression associated with SCN oscillators []. Moreover, both adenosine and nitric oxide (NO) are known for their role in sleep homeostasis []. Adenosine causes a hyperpolarization of membrane potential and a reduction in postsynaptic potentials in the hypothalamus, which are calcium dependent through glutamate receptors []. The abnormal expression of glutamate receptor in the hippocampus and cerebral cortex might be involved in the sleep–wake homeostasis through wake-promoting glutaminergic activators [] and sleep-promoting glutaminergic inhibitors []. In addition, the homeostatic dysregulation following lead neurotoxicity may be involved in NO synthesis, which has a role in homeostasis in the basal forebrain of rats []. Lead exposure has been shown to inhibit NO synthase (NOS) activity in capillary and synaptosomal fractions of mouse brains []. This evidence showed that the adenosine, inducible NOS, and NO production may play a crucial role in the temporal and spatial sequence of sleep homeostatic cascade [].
The homeostatic dysregulation following lead neurotoxicity could be caused by an imbalance between acetylcholine and dopamine. It has been reported that acetylcholine in the preoptic area and anterior hypothalamus is important in regulation of homeostasis of core body temperature during sleep []. Lead exposure might induce the inhibition of acetylcholine receptor through enhancement of extracellular calcium concentrations []. Lead poisoning and some psychostimulants, such as amphetamine and modafinil, can act on dopamine-rich brain areas, including the striatum and the nucleus accumbens, that have a strong influence on homeostasis through a wake-promoting effect [,].
Furthermore, lead poisoning may cause a neuroinflammatory response that involves the production and release of inflammatory cytokines, augmented oxidative stress, and diminished antioxidant activity, resulting in neuronal injury or neuronal loss in the CNS []. The neural cell adhesion molecules (NCAM) and cytokines might also play a role in the pathogenesis of dysregulation of homeostatic process following lead neurotoxicity. The NCAM is a potential early target in the neurotoxicity of lead []. NCAM is involved in activity-dependent development and synaptic plasticity, which occurs during homeostatic processes [], not only in the regulation of wakefulness and NREM sleep but also in the homeostasis of REM sleep []. The T cells are critical functional targets of lead immunotoxicity. A single exposure to lead might activate CD4(+) T cells [] and lead to the production of cytokines through CD40 and disrupt the sleep–wake regulatory circuit [].
Disturbed sleep homeostasis is a common problem in occupational health. Most issues have focused on chronic sleep deprivation due to rotating shift work. In the present study, we demonstrated that chronic lead exposure predominantly disrupts homeostasis of the sleep–wake cycle, resulting in sleep deprivation. We suggest that sleep disturbance can be an unrecognized consequence in individuals with chronic lead exposure. Exposure to even low levels of lead can cause damage over time, especially in children [,]. The greatest risk is to brain development, where irreversible neuropsychological and functional decline can occur in later life [,,]. Moreover, chronic low levels of lead exposure can damage the renal, hematological, cardiovascular, and nervous systems in both children and adults [,,,,]. We thus emphasize that the focus of environmental and occupational medicine on lead neurotoxicity should be extended from acute lead poisoning to unrecognized chronic lead exposure.
There are some limitations to the present study. First, we selected the common circadian genes, including rPer1, rPer2, and rBmal1b, to reveal the role of altered expressions of clock genes under chronic lead exposure in rats. Nevertheless, the expressional changes of other circadian rhythm genes, such as Clock, REV-ERBα, and CRY, are advantageous to understand the crucial relationship between the chronic lead intoxication and circadian rhythm genes. Second, the mechanism of lead toxicity in changes of clock gene expression is unclear. Understanding the molecular and cellular mechanisms of lead neurotoxicity is required to advance studies. Third, the SCN cell is relatively small and the investigation of changes in clock genes in the SCN is not easy. Quantitative in situ hybridization of mRNAs of clock genes may be considered. Fourth, the exposure level of lead in the animal studies is a limitation because rats have a short lifespan compared to humans. In the present study, the chronic lead-exposure level of rats achieved to 15 μg/dL that needed to use high concentration of lead water, 250 ppm, which was much higher than human drinking water. Overall, further studies are warranted to address these issues.
5. Conclusions
The present study indicates that a chronic low-to-medium level of lead exposure has a potential and negative impact on the sleep–wake cycle in rats that predominantly disrupts sleep homeostasis. The disruption of sleep homeostasis was associated with a toxic effect of lead on the clock gene expression in the hypothalamus. Since environmental and occupational exposure is a commonly known cause of lead poisoning, careful monitoring of the toxicity and blood level of lead in the population with a high risk of chronic lead exposure is particularly important.
Author Contributions
Conceptualization, C.-Y.H. and C.-T.L.; methodology, C.-Y.H., F.-C.C. and H.-Y.C.; software, F.-C.C.; validation, C.-Y.H. and Y.-C.C. and C.-T.L.; formal analysis, C.-Y.H. and H.-Y.C.; investigation, C.-Y.H. and C.-T.L.; data curation, C.-Y.H., Y.-C.C. and T.T.-Y.C.; writing—original draft preparation, C.-Y.H.; writing—review and editing, Y.-C.C. and C.-T.L.; visualization, Y.-C.C. and C.-T.L.; supervision, C.-T.L.; funding acquisition, C.-Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by research grants (KMU-EM-97-1.3b.3) from the Research Center of Excellence for Environmental Medicine, Kaohsiung Medical University, Taiwan to Chung-Yao Hsu, and the funders had no role in the study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Institutional Review Board Statement
The present study was endorsed by the constituted research ethics committee of Kaohsiung Medical University, Kaohsiung, Taiwan. The project identification code: 97033 was approved on 1 August 2008 by Institutional Animal Care and Use Committee of Kaohsiung Medical University.
Data Availability Statement
The data used to support the findings of this study are included within the article.
Acknowledgments
We thank the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital for their insightful suggestions on the statistical analysis of this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Senut, M.C.; Cingolani, P.; Sen, A.; Kruger, A.; Shaik, A.; Hirsch, H.; Suhr, S.T.; Ruden, D. Epigenetics of early-life lead exposure and effects on brain development. Epigenomics 2012, 4, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Patrick, L. Lead toxicity, a review of the literature. Part 1: Exposure, evaluation, and treatment. Altern. Med. Rev. 2006, 11, 2–22. [Google Scholar] [PubMed]
- Obeng-Gyasi, E. Lead Exposure and Oxidative Stress-A Life Course Approach in U.S. Adults. Toxics 2018, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Lanphear, B.P.; Rauch, S.; Auinger, P.; Allen, R.W.; Hornung, R.W. Low-level lead exposure and mortality in US adults: A population-based cohort study. Lancet Public Health 2018, 3, e177–e184. [Google Scholar] [CrossRef]
- Kuh, D.; Ben-Shlomo, Y.; Lynch, J.; Hallqvist, J.; Power, C. Life course epidemiology. J. Epidemiol. Community Health 2003, 57, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Todd, A.C. Lead poisoning. West. J. Med. 1994, 161, 153–159. [Google Scholar] [PubMed]
- Mason, L.H.; Harp, J.P.; Han, D.Y. Pb neurotoxicity: Neuropsychological effects of lead toxicity. Biomed. Res. Int. 2014, 2014, 840547. [Google Scholar] [CrossRef]
- Chen, C.C.; Yen, H.W.; Lo, Y.H.; Chu, Y.H.; Chiu, Y.W.; Chuang, H.Y. The association of prolonged QT interval on electrocardiography and chronic lead exposure. J. Occup. Environ. Med. 2013, 55, 614–619. [Google Scholar] [CrossRef]
- Liu, H.L.; Chuang, H.Y.; Hsu, C.N.; Lee, S.S.; Yang, C.C.; Liu, K.T. Effects of Vitamin D Receptor, Metallothionein 1A, and 2A Gene Polymorphisms on Toxicity of the Peripheral Nervous System in Chronically Lead-Exposed Workers. Int. J. Environ. Res. Public Health 2020, 17, 2909. [Google Scholar] [CrossRef]
- Altmann, L.; Weinsberg, F.; Sveinsson, K.; Lilienthal, H.; Wiegand, H.; Winneke, G. Impairment of long-term potentiation and learning following chronic lead exposure. Toxicol. Lett. 1993, 66, 105–112. [Google Scholar] [CrossRef]
- Wu, W.T.; Lin, Y.J.; Liou, S.H.; Yang, C.Y.; Cheng, K.F.; Tsai, P.J.; Wu, T.N. Brain cancer associated with environmental lead exposure: Evidence from implementation of a National Petrol-Lead Phase-Out Program (PLPOP) in Taiwan between 1979 and 2007. Environ. Int. 2012, 40, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Khan, K.M.; Al-Khaledi, G.; Khan, I.; Attur, S. Early postnatal lead exposure induces tau phosphorylation in the brain of young rats. Acta Biol. Hung. 2012, 63, 411–425. [Google Scholar] [CrossRef]
- Boskabady, M.; Marefati, N.; Farkhondeh, T.; Shakeri, F.; Farshbaf, A.; Boskabady, M.H. The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review. Environ. Int. 2018, 120, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Virgolini, M.B.; Aschner, M. Molecular Mechanisms of Lead Neurotoxicity. Adv. Neurotoxicol. 2021, 5, 159–213. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Choi, H.; Hwang, U.K.; Kang, J.C.; Kang, Y.J.; Kim, K.I.; Kim, J.H. Toxic effects of lead exposure on bioaccumulation, oxidative stress, neurotoxicity, and immune responses in fish: A review. Environ. Toxicol. Pharmacol. 2019, 68, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zeng, A.; Zheng, W.; Du, Y. Upregulation of zinc transporter 2 in the blood-CSF barrier following lead exposure. Exp. Biol. Med. 2013, 239, 202–212. [Google Scholar] [CrossRef]
- Simons, T.J. Lead-calcium interactions in cellular lead toxicity. Neurotoxicology 1993, 14, 77–85. [Google Scholar]
- Sanders, T.; Liu, Y.; Buchner, V.; Tchounwou, P.B. Neurotoxic effects and biomarkers of lead exposure: A review. Rev. Environ. Health 2009, 24, 15–45. [Google Scholar] [CrossRef]
- Liu, K.S.; Hao, J.H.; Zeng, Y.; Dai, F.C.; Gu, P.Q. Neurotoxicity and biomarkers of lead exposure: A review. Chin. Med. Sci. J. 2013, 28, 178–188. [Google Scholar] [CrossRef]
- White, L.D.; Cory-Slechta, D.A.; Gilbert, M.E.; Tiffany-Castiglioni, E.; Zawia, N.H.; Virgolini, M.; Rossi-George, A.; Lasley, S.M.; Qian, Y.C.; Basha, M.R. New and evolving concepts in the neurotoxicology of lead. Toxicol. Appl. Pharmacol. 2007, 225, 1–27. [Google Scholar] [CrossRef]
- Jansen, E.C.; Dunietz, G.L.; Dababneh, A.; Peterson, K.E.; Chervin, R.D.; Baek, J.; O’Brien, L.; Song, P.X.K.; Cantoral, A.; Hu, H.; et al. Cumulative Childhood Lead Levels in Relation to Sleep During Adolescence. J. Clin. Sleep Med. 2019, 15, 1443–1449. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Pak, V.; Wang, Y.; Yan, C.; Pinto-Martin, J.; Dinges, D. Early Blood Lead Levels and Sleep Disturbance in Preadolescence. Sleep 2015, 38, 1869–1874. [Google Scholar] [CrossRef]
- Shafiq-ur-Rehman. Circadian rhythm of stereotyped complex behaviours in rats in environmental lead exposure. Prog. Neuropsychopharmacol. Biol. Psychiatry 1999, 23, 149–159. [Google Scholar] [CrossRef]
- Lilis, R.; Valciukas, J.A.; Weber, J.P.; Malkin, J. Effects of low-level lead and arsenic exposure on copper smelter workers. Arch. Environ. Health 1985, 40, 38–47. [Google Scholar] [CrossRef]
- Rojas-Castaneda, J.C.; Vigueras-Villasenor, R.M.; Rojas, P.; Chavez-Saldana, M.; Gutierrez-Perez, O.; Montes, S.; Rios, C. Alterations induced by chronic lead exposure on the cells of circadian pacemaker of developing rats. Int. J. Exp. Pathol. 2011, 92, 243–250. [Google Scholar] [CrossRef]
- Allen, K.A. Is Prenatal Lead Exposure a Concern in Infancy? What Is the Evidence? Adv. Neonatal Care 2015, 15, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Giusi, G.; Alo, R.; Crudo, M.; Di Vito, A.; Facciolo, R.M.; Canonaco, M. Environmental stressors and neurobiological features of marine teleosts: Histamine receptors as targets. Crit. Rev. Toxicol. 2010, 40, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.Y.; Tain, Y.L.; Lee, Y.T.; Hsu, C.Y.; Chiou, T.T.; Huang, P.C.; Lee, C.T. Renin angiotensin system blockade ameliorates lead nephropathy. Biochem. Biophys. Res. Commun. 2013, 438, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Yi, P.L.; Jou, S.B.; Wu, Y.J.; Chang, F.C. Manipulation of Epileptiform Electrocorticograms (ECoGs) and Sleep in Rats and Mice by Acupuncture. J. Vis. Exp. 2016, 118, e54896. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Chang, F.C.; Ng, H.Y.; Kuo, C.C.; Lee, Y.T.; Lu, C.Y.; Lee, C.T. Disrupted circadian rhythm in rats with nephrectomy-induced chronic kidney disease. Life Sci. 2012, 91, 127–131. [Google Scholar] [CrossRef]
- Le Bon, O. Relationships between REM and NREM in the NREM-REM sleep cycle: A review on competing concepts. Sleep Med. 2020, 70, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Jou, S.B.; Tsai, C.J.; Fang, C.Y.; Yi, P.L.; Chang, F.C. Effects of N6-(4-hydroxybenzyl) adenine riboside in stress-induced insomnia in rodents. J. Sleep Res. 2021, 30, e13156. [Google Scholar] [CrossRef] [PubMed]
- Yi, P.L.; Tsai, C.H.; Lu, M.K.; Liu, H.J.; Chen, Y.C.; Chang, F.C. Interleukin-1beta mediates sleep alteration in rats with rotenone-induced parkinsonism. Sleep 2007, 30, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Yi, P.L.; Chen, Y.J.; Lin, C.T.; Chang, F.C. Occurrence of epilepsy at different zeitgeber times alters sleep homeostasis differently in rats. Sleep 2012, 35, 1651–1665. [Google Scholar] [CrossRef][Green Version]
- Baracchi, F.; Opp, M.R. Sleep–wake behavior and responses to sleep deprivation of mice lacking both interleukin-1 beta receptor 1 and tumor necrosis factor-alpha receptor 1. Brain Behav. Immun. 2008, 22, 982–993. [Google Scholar] [CrossRef]
- Vanderheyden, W.M.; George, S.A.; Urpa, L.; Kehoe, M.; Liberzon, I.; Poe, G.R. Sleep alterations following exposure to stress predict fear-associated memory impairments in a rodent model of PTSD. Exp. Brain Res. 2015, 233, 2335–2346. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Lien, Y.H.; Lai, L.W.; Chen, J.B.; Lin, C.R.; Chen, H.C. Increased renal calcium and magnesium transporter abundance in streptozotocin-induced diabetes mellitus. Kidney Int. 2006, 69, 1786–1791. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.K. Renal effects of environmental and occupational lead exposure. Indian J. Occup. Environ. Med. 2008, 12, 103–106. [Google Scholar] [CrossRef]
- Wedeen, R.P. The role of lead in renal failure. Clin. Exp. Dial. Apheresis 1982, 6, 113–146. [Google Scholar] [CrossRef]
- Dantzler, W.H.; Layton, A.T.; Layton, H.E.; Pannabecker, T.L. Urine-concentrating mechanism in the inner medulla: Function of the thin limbs of the loops of Henle. Clin. J. Am. Soc. Nephrol. 2014, 9, 1781–1789. [Google Scholar] [CrossRef]
- Campbell, I.G.; Darchia, N.; Higgins, L.M.; Dykan, I.V.; Davis, N.M.; de Bie, E.; Feinberg, I. Adolescent changes in homeostatic regulation of EEG activity in the delta and theta frequency bands during NREM sleep. Sleep 2011, 34, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.K.; Snyder, E.; Hall, J.; Randazzo, A.C.; Griffin, K.; Groeger, J.; Eisenstein, R.; Feren, S.D.; Dickey, P.; Schweitzer, P.K. Slow wave sleep enhancement with gaboxadol reduces daytime sleepiness during sleep restriction. Sleep 2008, 31, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Wurts, S.W.; Edgar, D.M. Circadian and homeostatic control of rapid eye movement (REM) sleep: Promotion of REM tendency by the suprachiasmatic nucleus. J. Neurosci. 2000, 20, 4300–4310. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Fukuda, K.; Takeuchi, T.; Inugami, M.; Miyasita, A. Sleep onset REM period appearance rate is affected by REM propensity in circadian rhythm in normal nocturnal sleep. Clin. Neurophysiol. 2000, 111, 428–433. [Google Scholar] [CrossRef]
- Pigeon, W.R.; Perlis, M.L. Sleep homeostasis in primary insomnia. Sleep Med. Rev. 2006, 10, 247–254. [Google Scholar] [CrossRef]
- Yasenkov, R.; Deboer, T. Circadian modulation of sleep in rodents. Prog. Brain Res. 2012, 199, 203–218. [Google Scholar] [CrossRef]
- Yasenkov, R.; Deboer, T. Circadian regulation of sleep and the sleep EEG under constant sleep pressure in the rat. Sleep 2010, 33, 631–641. [Google Scholar] [CrossRef]
- Kaskie, R.E.; Gill, K.M.; Ferrarelli, F. Reduced frontal slow wave density during sleep in first-episode psychosis. Schizophr. Res. 2019, 206, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Allada, R.; Bass, J. Circadian Mechanisms in Medicine. N. Engl. J. Med. 2021, 384, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Okamura, H. Gradients in the circadian expression of Per1 and Per2 genes in the rat suprachiasmatic nucleus. Eur. J. Neurosci. 2002, 15, 1153–1162. [Google Scholar] [CrossRef]
- Riddle, M.; Mezias, E.; Foley, D.; LeSauter, J.; Silver, R. Differential localization of PER1 and PER2 in the brain master circadian clock. Eur. J. Neurosci. 2017, 45, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Rijo-Ferreira, F.; Takahashi, J.S. Genomics of circadian rhythms in health and disease. Genome Med. 2019, 11, 82. [Google Scholar] [CrossRef]
- Sahar, S.; Sassone-Corsi, P. Metabolism and cancer: The circadian clock connection. Nat. Rev. Cancer 2009, 9, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Takekida, S.; Shigeyoshi, Y.; Okamura, H. Per1 and Per2 gene expression in the rat suprachiasmatic nucleus: Circadian profile and the compartment-specific response to light. Neuroscience 1999, 94, 141–150. [Google Scholar] [CrossRef]
- Matsui, D.; Takekida, S.; Okamura, H. Molecular oscillation of Per1 and Per2 genes in the rodent brain: An in situ hybridization and molecular biological study. Kobe J. Med. Sci. 2005, 51, 85–93. [Google Scholar] [PubMed]
- Challet, E.; Poirel, V.J.; Malan, A.; Pevet, P. Light exposure during daytime modulates expression of Per1 and Per2 clock genes in the suprachiasmatic nuclei of mice. J. Neurosci. Res. 2003, 72, 629–637. [Google Scholar] [CrossRef]
- Marchetti, C. Molecular targets of lead in brain neurotoxicity. Neurotox. Res. 2003, 5, 221–236. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, H.; Li, Q.; Wang, Q.; Zhang, W.; Chen, Y.; Wang, D.; Cai, Y. Effect of lead sulfide nanoparticles exposure on calcium homeostasis in rat hippocampus neurons. J. Inorg. Biochem. 2013, 126, 70–75. [Google Scholar] [CrossRef]
- Nahm, S.S.; Farnell, Y.Z.; Griffith, W.; Earnest, D.J. Circadian regulation and function of voltage-dependent calcium channels in the suprachiasmatic nucleus. J. Neurosci. 2005, 25, 9304–9308. [Google Scholar] [CrossRef]
- Kalinchuk, A.V.; McCarley, R.W.; Porkka-Heiskanen, T.; Basheer, R. The time course of adenosine, nitric oxide (NO) and inducible NO synthase changes in the brain with sleep loss and their role in the non-rapid eye movement sleep homeostatic cascade. J. Neurochem. 2011, 116, 260–272. [Google Scholar] [CrossRef]
- Obrietan, K.; Belousov, A.B.; Heller, H.C.; van den Pol, A.N. Adenosine pre- and postsynaptic modulation of glutamate-dependent calcium activity in hypothalamic neurons. J. Neurophysiol. 1995, 74, 2150–2162. [Google Scholar] [CrossRef]
- Ahnaou, A.; Ver Donck, L.; Drinkenburg, W.H. Blockade of the metabotropic glutamate (mGluR2) modulates arousal through vigilance states transitions: Evidence from sleep–wake EEG in rodents. Behav. Brain Res. 2014, 270, 56–67. [Google Scholar] [CrossRef]
- Cavas, M.; Scesa, G.; Navarro, J.F. Positive allosteric modulation of mGlu7 receptors by AMN082 affects sleep and wakefulness in the rat. Pharmacol. Biochem. Behav. 2013, 103, 756–763. [Google Scholar] [CrossRef]
- Greene, R.W. Role for neuronal nitric oxide synthase in sleep homeostasis and arousal. Proc. Natl. Acad. Sci. USA 2013, 110, 19982–19983. [Google Scholar] [CrossRef]
- Garcia-Arenas, G.; Claudio, L.; Perez-Severiano, F.; Rios, C. Lead acetate exposure inhibits nitric oxide synthase activity in capillary and synaptosomal fractions of mouse brain. Toxicol. Sci. 1999, 50, 244–248. [Google Scholar] [CrossRef]
- Crouzier, D.; Baubichon, D.; Bourbon, F.; Testylier, G. Acetylcholine release, EEG spectral analysis, sleep staging and body temperature studies: A multiparametric approach on freely moving rats. J. Neurosci. Methods 2006, 151, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Mike, A.; Pereira, E.F.; Albuquerque, E.X. Ca2+-sensitive inhibition by Pb2+ of alpha7-containing nicotinic acetylcholine receptors in hippocampal neurons. Brain Res. 2000, 873, 112–123. [Google Scholar] [CrossRef]
- Qiu, M.H.; Liu, W.; Qu, W.M.; Urade, Y.; Lu, J.; Huang, Z.L. The role of nucleus accumbens core/shell in sleep–wake regulation and their involvement in modafinil-induced arousal. PLoS ONE 2012, 7, e45471. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.; Szczerbak, G.; Nitka, D.; Kostrzewa, R.M.; Josko, J.; Brus, R. Cortical dopaminergic neurotransmission in rats intoxicated with lead during pregnancy. Nitric oxide and hydroxyl radicals formation involvement. Neurotoxicol. Teratol. 2008, 30, 428–432. [Google Scholar] [CrossRef]
- Hu, Q.; Fu, H.; Song, H.; Ren, T.; Li, L.; Ye, L.; Liu, T.; Dong, S. Low-level lead exposure attenuates the expression of three major isoforms of neural cell adhesion molecule. Neurotoxicology 2011, 32, 255–260. [Google Scholar] [CrossRef]
- Citri, A.; Malenka, R.C. Synaptic plasticity: Multiple forms, functions, and mechanisms. Neuropsychopharmacology 2008, 33, 18–41. [Google Scholar] [CrossRef] [PubMed]
- Black, M.A.; Deurveilher, S.; Seki, T.; Marsh, D.R.; Rutishauser, U.; Rafuse, V.F.; Semba, K. Role of polysialylated neural cell adhesion molecule in rapid eye movement sleep regulation in rats. Eur. J. Neurosci. 2009, 30, 2190–2204. [Google Scholar] [CrossRef]
- McCabe, M.J., Jr.; Singh, K.P.; Reiners, J.J., Jr. Low level lead exposure in vitro stimulates the proliferation and expansion of alloantigen-reactive CD4(high) T cells. Toxicol. Appl. Pharmacol. 2001, 177, 219–231. [Google Scholar] [CrossRef]
- Gast, H.; Muller, A.; Lopez, M.; Meier, D.; Huber, R.; Dechent, F.; Prinz, M.; Emmenegger, Y.; Franken, P.; Birchler, T.; et al. CD40 activation induces NREM sleep and modulates genes associated with sleep homeostasis. Brain Behav. Immun. 2013, 27, 133–144. [Google Scholar] [CrossRef]
- Chen, J.; Li, M.; Lv, Q.; Chen, G.; Li, Y.; Li, S.; Mo, Y.; Ou, S.; Yuan, Z.; Huang, M.; et al. Blood lead level and its relationship to essential elements in preschool children from Nanning, China. J. Trace Elem. Med. Biol. 2015, 30, 137–141. [Google Scholar] [CrossRef]
- Mendelsohn, A.L.; Dreyer, B.P.; Fierman, A.H.; Rosen, C.M.; Legano, L.A.; Kruger, H.A.; Lim, S.W.; Courtlandt, C.D. Low-level lead exposure and behavior in early childhood. Pediatrics 1998, 101, E10. [Google Scholar] [CrossRef]
- Chambial, S.; Shukla, K.K.; Dwivedi, S.; Bhardwaj, P.; Sharma, P. Blood Lead Level (BLL) in the Adult Population of Jodhpur: A Pilot Study. Indian J. Clin. Biochem. 2015, 30, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.C.; Oh, S.Y.; Kwon, S.O.; Park, M.S.; Kim, H.; Leem, J.H.; Ha, E.H. Blood lead level modifies the association between dietary antioxidants and oxidative stress in an urban adult population. Br. J. Nutr. 2013, 109, 148–154. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).