Paternal Fenitrothion Exposures in Rats Causes Sperm DNA Fragmentation in F0 and Histomorphometric Changes in Selected Organs of F1 Generation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Animals
2.3. Experimental Design
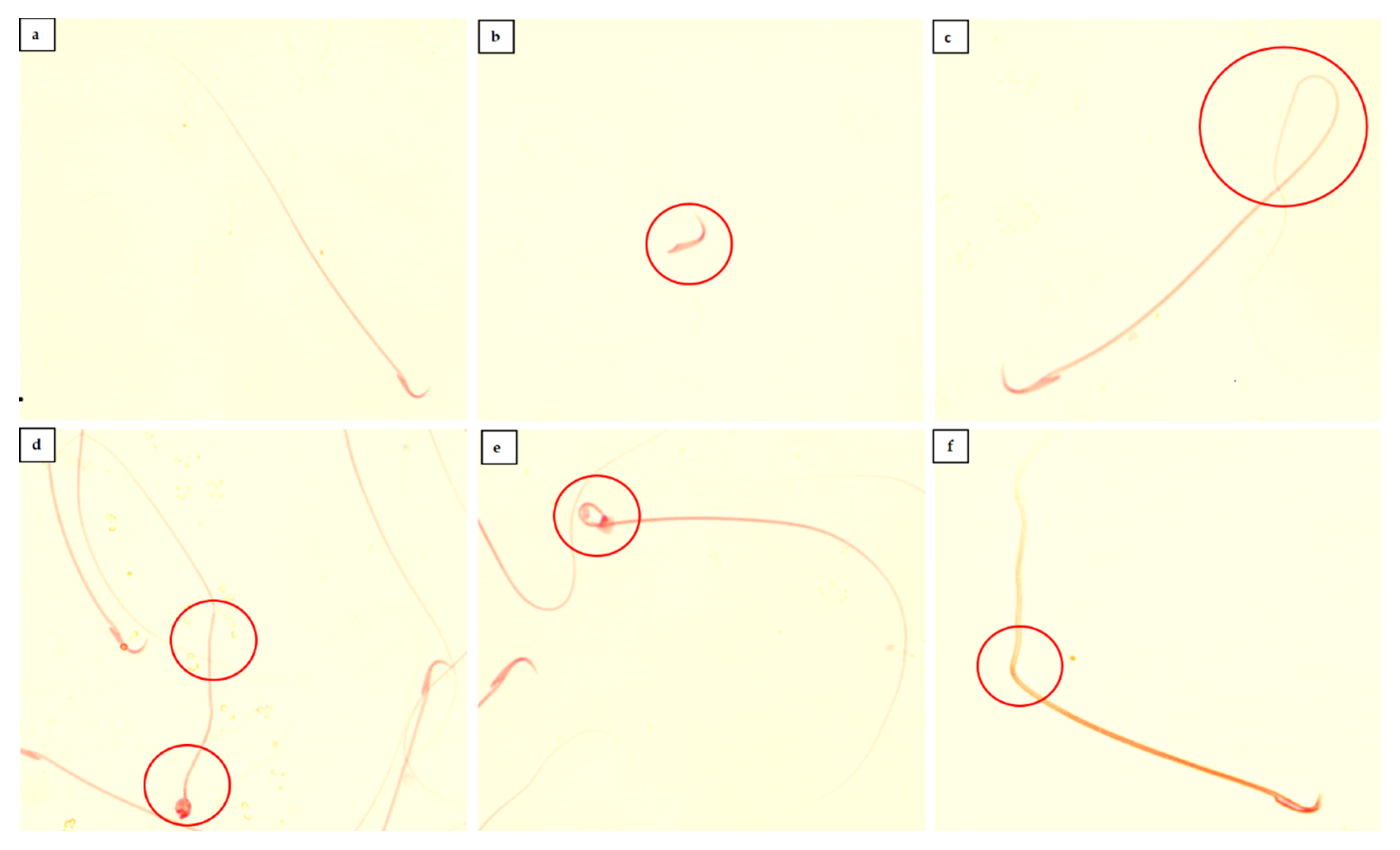
2.4. Sperm Characteristics Analysis
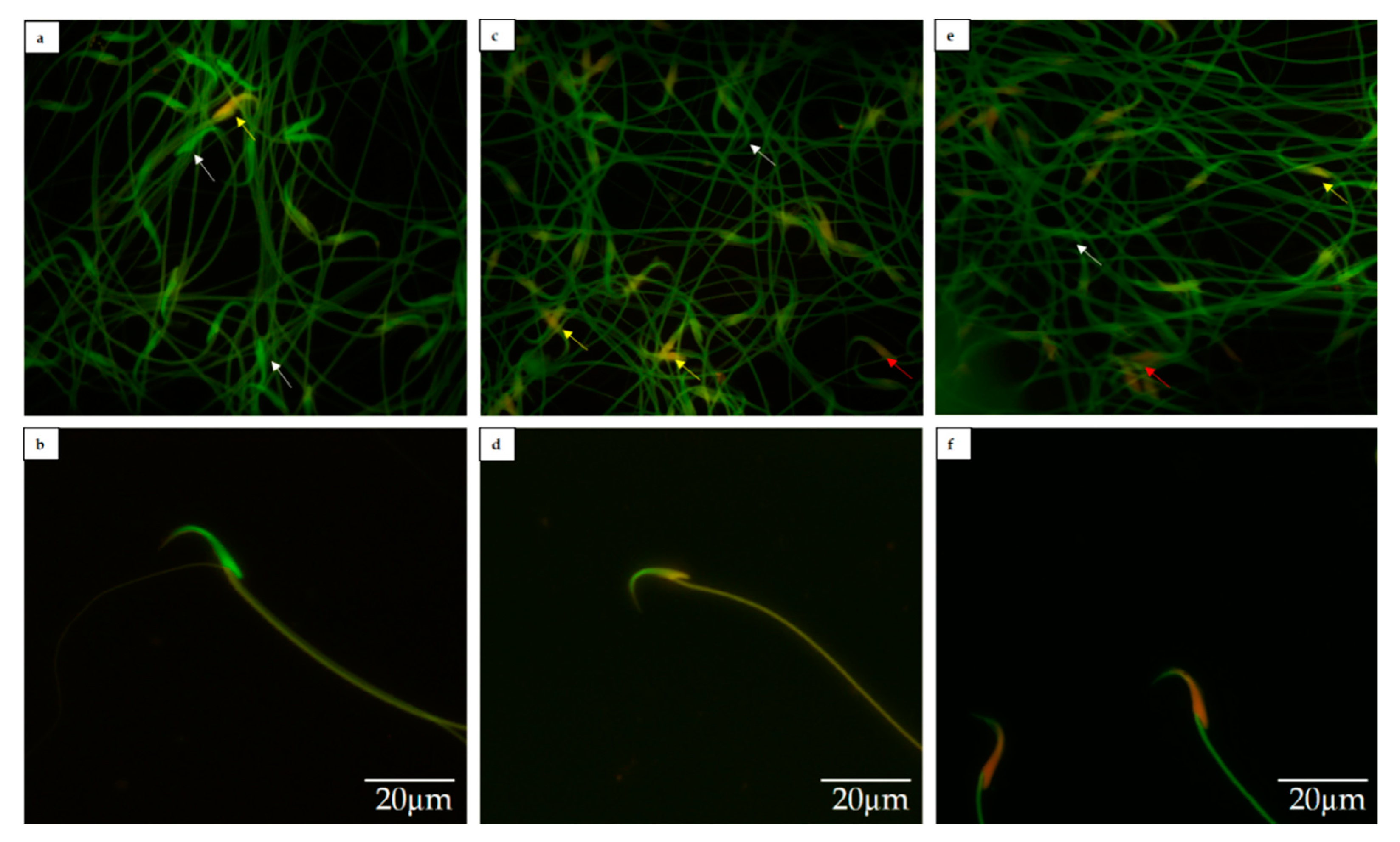
2.5. Sperm DNA Fragmentation Analysis
2.6. Developmental Landmarks Evaluation
2.7. Histomorphometry of Progeny’s Organ Analysis
2.8. Statistical Analysis
3. Results
3.1. Sperm Characteristics
3.2. Sperm DNA Fragmentation
3.3. Developmental Landmarks Evaluation
3.4. Absolute and Relative Weight of Organs
3.5. Histomorphometry Analysis
4. Discussion
4.1. Sperm Characteristics
4.2. DNA Fragmentation
4.3. Developmental Landmarks
4.4. Histomorphometry Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| F0 | Paternal |
| F1 | First-generation progeny |
| FNT | Fenitrothion |
| FNT-10 | Group receiving 10 mg/kg Fenitrothion |
| FNT-20 | Group receiving 20 mg/kg Fenitrothion |
| pControl | Progeny of control group |
| pFNT-10 | Progeny of paternal receiving 10 mg/kg Fenitrothion |
| pFNT-20 | Progeny of paternal receiving 20 mg/kg Fenitrothion |
| DNA | Deoxyribonucleic acid |
| OP | Organophosphate |
| AChE | Acetylcholinesterase |
| ACh | Acetylcholine |
| ROS | Reactive oxygen species |
| HPG | Hypothalamic-pituitary gonadal |
| KTX | Ketamine and xylazine cocktail |
| HBSS | Hank’s balanced salt solution |
| WHO | World Health Organization |
| IRDG | Industrial reproductive toxicology discussion group |
| AGD | Anogenital distance |
| PND | Postnatal day |
| H&E | Haematoxylin and eosin |
| MJTBS | Mean Johnsen testicular biopsy score |
| ANOVA | One-way analysis of variance |
| SEM | Mean ± standard error of the mean |
| PUFA | Polyunsaturated fatty acid |
| FAAH | Fatty acid amide hydrolase |
| DFI | DNA fragmentation index |
| BUP | Bupropion hydrochloride |
| AR | Androgen receptor |
| 3MNP | 3-methyl-4-nitrophenol |
| OC | Organochlorine |
| LH | Luteinizing hormone |
References
- Figueroa, Z.I.; Young, H.A.; Mumford, S.L.; Meeker, J.D.; Barr, D.B.; Gray, G.M.; Perry, M.J. Pesticide interactions and risks of sperm chromosomal abnormalities. Int. J. Hyg. Envir. Health 2019, 222, 1021–1029. [Google Scholar] [CrossRef]
- Mitra, A.; Maitra, S.K. Reproductive toxicity of organophosphate pesticides. Ann. Clin. Toxicol. 2018, 1, 1004. [Google Scholar]
- Tomer, V.; Sangha, J.K.; Ramya, H.G. Pesticide: An appraisal on human health implications. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. Vol. 2014, 85, 451–463. [Google Scholar] [CrossRef]
- Testai, E.; Buratti, F.M.; Di Consiglio, E. Chlorpyrifos. In Hayes’ Handb. Pestic. Toxicol., 3rd ed.; Krieger, R., Ed.; Academic Press: Cambridge, MA, USA, 2010; Volume 1, pp. 1505–1526. [Google Scholar]
- Malhat, F.M. Residues and dissipation of fenitrothion in green bean (Phaseolus vulgaris) and soil. Int. J. Sch. Res. Not. 2012, 1, 1–4. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D. Farmers’ exposure to pesticides: Toxicity types and ways of prevention. Toxics 2016, 4, 1. [Google Scholar] [CrossRef]
- Vermeire, T.; MacPhail, R.; Waters, M. Integrated human and ecological risk assessment: A case study of Organophosphorous pesticides in the environment. Hum. Ecol. Risk Asses. Int. J. 2003, 9, 343–357. [Google Scholar] [CrossRef]
- Elhalwagy, M.E.A.; Darwish, N.S.; Zaher, E.M. Prophylactic effect of green tea polyphenols against liver and kidney injury induced by fenitrothion insecticide. Pest. Biochem. Phy. 2008, 91, 81–89. [Google Scholar] [CrossRef]
- Jayusman, P.A.; Budin, S.B.; Taib, I.S.; Ghazali, A.R. The effects of tocotrienol-rich fraction on oxidative liver damage induced by fenitrothion. Sains Malaysiana. 2017, 46, 1603–1609. [Google Scholar] [CrossRef]
- Budin, S.B.; Saimin, H.; Taib, I.S.; Jayusman, P.A.; Mohamed, J. A histological studies of rats’ lung subacutely treated with fenitrothion. Int. J. Collab. Res. Int. Med. Public Health 2012, 4, 744–752. [Google Scholar]
- Yusoff, N.A.; Juremi, I.I.; Budin, S.B.; Taib, I.S. Repeated administration of fenitrothion alters renal functions via oxidative stress mechanism without inhibiting acetylcholinesterase activity in rats. Life Sci. Med. Biomed. 2020, 4, 1–6. [Google Scholar] [CrossRef]
- Taib, I.S.; Budin, S.B.; Ghazali, A.R.; Jayusman, P.A.; Louis, S.R.; Mohamed, J. Fenitrothion induced oxidative stress and morphological alterations of sperm and testes in male Sprague-dawley rats. Clinics 2013, 68, 93–100. [Google Scholar] [CrossRef]
- Blanco-Muñoz, J.; Morales, M.M.; Lacasaña, M.; Aguilar-Garduño, C.; Bassol, S.; Cebrián, M.E. Exposure to organophosphate pesticides and male hormone profile in floriculturist of the state of Morelos, Mexico. Human Reprod. 2010, 25, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, N.A.; Taib, I.S.; Budin, S.B.; Mohamed, M. Fenitrothion impaired sexual behaviour and reproductive performance in male Sprague-Dawley rats. Sains Malays. 2020, 49, 1333–1344. [Google Scholar] [CrossRef]
- Mascarenhas, M.N.; Flaxman, S.R.; Boerma, T.; Vanderpoel, S.; Stevens, G.A. National, regional, and global trends in infertility prevalence since 1990: A systematic analysis of 277 health surveys. PLoS Med. 2012, 9, e1001356. [Google Scholar] [CrossRef] [PubMed]
- Zegers-Hochschilda, F.; Adamson, D.; Dyer, D.G.; Racowsky, S.; Mouzone, C.J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.A.; et al. The international glossary on infertility and fertility care. Fertil. Steril. 2017, 108, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Kehinde, B.A.; Abolhassani, F.; Yazdekhasti, H.; Abbasi, N.; Heydari, L.; Daneshi, E.; Rajabi, Z.; Hamada, A.; Agarwal, A.; Abbasi, M. The effects of unilateral varicose ovarian vein on antioxidant capacity and oocyte quality in rat ovary. Iran J. Basic Med. Sci. 2016, 19, 863–869. [Google Scholar]
- D’Souza, U.J.A. Pesticide toxicity and oxidative stress: A review. Borneo J. Med. Sci. 2017, 11, 9–19. [Google Scholar]
- Al-Gubory, K.H. Environmental pollutants and lifestyle factors induce oxidative stress and poor prenatal development. Reproductive. Biomed. Online 2014, 29, 17–31. [Google Scholar] [CrossRef]
- Tamura, H.; Yoshikawa, H.; Gaido, K.W.; Ross, S.M.; DeLisle, R.K.; Welsh, W.J.; Richard, A.M. Interaction of organophosphate pesticides and related compounds with the androgen. Environ. Health Perspect. 2003, 111, 545–552. [Google Scholar] [CrossRef]
- Li, C.M.; Taneda, S.; Suzuki, A.K.; Furuta, C.; Watanabe, G.; Taya, K. Anti-androgenic activity of 3-methyl-4-nitrophenol in diesel exhaust particles. Eur. J. Pharm. 2006, 543, 194–199. [Google Scholar] [CrossRef]
- Taib, I.S.; Budin, S.B.; Ghazali, A.R.; Jayusman, P.A.; Mohamed, J. Fenitrothion alters sperm characteristics in rats: Ameliorating effects of palm oil tocotrienol-rich fraction. Exp. Anim. 2014, 63, 383–393. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumar, D.; Salian, S.R.; Kalthur, G.; Uppangala, S.; Kumari, S.; Challapalli, S.; Chandraguthi, S.G.; Krishnamurthy, H.; Jain, N.; Kumar, P.; et al. Semen abnormalities, sperm DNA damage and global hypermethylation in health workers occupationally exposed to ionizing radiation. PLoS ONE 2013, 8, e69927. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.D.; Hawkey, A.B.; Hall, B.J.; Cauley, M.; Slade, S.; Yazdani, E.; Kenou, B.; White, H.; Wells, C.; Rezvani, A.H. Paternal THC exposure in rats causes long-lasting neurobehavioral effects in the offspring. Neurotoxicol. Teratol. 2019, 74, 106806. [Google Scholar] [CrossRef] [PubMed]
- Cordier, S. Evidence for a role of paternal exposures in developmental toxicity. Basic Clin. Pharmacol. Toxicol. 2008, 102, 176–181. [Google Scholar] [CrossRef]
- Donkin, I.; Barrès, R. Sperm epigenetics and influence of environmental factors. Mol. Metab. 2018, 14, 1–11. [Google Scholar] [CrossRef]
- Mobasheri, M.B.; Babatunde, K.A. Testicular miRNAs in relation to spermatogenesis, spermatogonial stem cells and cancer/testis genes. Sci. Afr. 2019, 3, e00067. [Google Scholar] [CrossRef]
- Kishigami, S.; Van Thuan, N.; Hikichi, T.; Ohta, H.; Wakayama, S.; Mizutani, E.; Wakayama, T. Epigenetic abnormalities of the mouse paternal zygotic genome associated with micro insemination of round spermatids. Dev. Biol. 2006, 289, 195–205. [Google Scholar] [CrossRef]
- Fernández-González, R.; Moreira, P.N.; Pérez-Crespo, M.; Sánchez-Martín, M.; Ramirez, M.A.; Pericuesta, E.; Bilbao, A.; Bermejo-Alvarez, P.; de Dios Hourcade, J.; de Fonseca, F.R. Long-term effects of mouse intracytoplasmic sperm injection with DNA-fragmented sperm on health and behavior of adult offspring. Biol. Reprod. 2008, 78, 761–772. [Google Scholar] [CrossRef]
- Dobrzyńska, M.M.; Tyrkiel, E.J.; Pachocki, K.A. Developmental toxicity in mice following paternal exposure to Di-N-butyl-phthalate (DBP). Biomed. Environ. Sci. 2011, 24, 569–578. [Google Scholar] [PubMed]
- Okahashi, N.; Sano, M.; Miyata, K.; Tamano, S.; Higuchi, H.; Kamita, Y.; Seki, T. Lack of evidence for endocrine disrupting effects in rats exposed to fenitrothion in utero and from weaning to maturation. Toxicology 2005, 206, 17–31. [Google Scholar] [CrossRef]
- Enciso, M.; Alfarawati, S.; Wells, D. Increased numbers of DNA-damaged spermatozoa in samples presenting an elevated rate of numerical chromosome abnormalities. Hum. Reprod. 2013, 28, 1707–1715. [Google Scholar] [CrossRef]
- World Health Organization. International Code of Conduct on the Distribution and Use of Pesticides: Guidelines for the Registration of Pesticides; FAO: Rome, Italy, 2010. [Google Scholar]
- Turner, K.J.; Barlow, N.J.; Struve, M.F.; Wallace, D.G.; Gaido, K.W.; Dorman, D.C.; Foster, P.M. Effects of in utero exposure to the organophosphate insecticide fenitrothion on androgen-dependent reproductive development in the Crl: CD (SD) BR rat. Toxicol. Sci. 2002, 68, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Tomizawa, M.; Suzuki, H.; Okamura, A.; Ohtani, K.; Nunome, M.; Noro, Y.; Wang, D.; Nakajima, T.; Kamijima, M. Fenitrothion action at the endocannabinoid system leading to spermatotoxicity in Wistar rats. Toxicol. Appl. Pharm. 2014, 279, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Watchoa, P.; Zelefack, F.; Nguelefack, T.B.; Ngouela, S.; Telefo, P.B.; Kamtchouing, P.; Tsamo, E.; Kamanyi, A. Effects of the aqueous and hexane extracts of Mondia whitei on the sexual behaviour and some fertility parameters of sexually inexperienced male rats. Afr. J. Tradit. Complement. Alter Med. 2007, 4, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; Sulaiman, S.A.; Sirajudeen, K.N.S. Protective effect of honey against cigarette smoke induced-impaired sexual behaviour and fertility of male rats. Toxicol. Ind. Health 2013, 29, 264–271. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human SEMEN; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Industrial Reproductive Toxicology Discussion Group. Rat Sperm Morphological Assessment 2000; Available online: http://www.irdg.co.uk/Sperm_morphology.pdf (accessed on 18 March 2020).
- Pourentezari, M.; Talebi, A.R.; Mangoli, E.; Anvari, M.; Rahimipour, M. Additional deleterious effects of alcohol consumption on sperm parameters and DNA integrity in diabetic mice. Andrologia 2016, 48, 564–569. [Google Scholar] [CrossRef]
- Mylchreest, E.; Wallace, D.G.; Cattley, R.C.; Foster, P.M.D. Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to di(n-butyl) phthalate during late gestation. Toxicol. Sci. 2000, 55, 143–151. [Google Scholar] [CrossRef]
- Nna, V.U.; Bakar, A.B.A.; Ahmad, A.; Mohamed, M. Down-regulation of steroidogenesis-related genes and its accompanying fertility decline in streptozotocin-induced diabetic male rats: Ameliorative effect of metformin. Andrology 2019, 7, 110–123. [Google Scholar] [CrossRef]
- Vadakkumpadan, F.; Arevalo, H.; Prassl, A.; Chen, J.; Kickinger, F.; Kohl, P.; Plank, G.; Trayanova, N. Image-based models of cardiac structure in health and disease. Wiley interdisciplinary reviews. Syst. Biol. Med. 2010, 2, 489–506. [Google Scholar]
- Bhattacharya, A.; Dhar, P.; Mehra, R.D. Preliminary morphological and biochemical changes in rat liver following postnatal exposure to sodium arsenite. Anat. Cell Biol. 2012, 45, 229. [Google Scholar] [CrossRef]
- Kotyk, T.; Dey, N.; Ashour, A.S.; Balas-Timar, D.; Chakraborty, S. Measurement of glomerulus diameter and Bowman’s space width of renal albino rats. Compt. Methods Prog. Biomed. 2016, 126, 143–153. [Google Scholar] [CrossRef]
- Dunnill, M.S. Postnatal growth of the lung. Thorax 1962, 17, 329–333. [Google Scholar] [CrossRef]
- Budin, S.B.; Kho, J.H.; Lee, J.H.; Ramalingam, A.; Jubaidi, F.F.; Latif, E.S.; Zainalabidin, S.; Taib, I.S.; Mohamed, J. Low-dose Nicotine Exposure Induced the Oxidative Damage of Reproductive Organs and Altered the Sperm Characteristics of Adolescent Male Rats. Malays. J. Med. Sci. 2017, 24, 50–57. [Google Scholar] [CrossRef]
- Ben Abdallah, F.; Fetoui, H.; Zribi, N.; Fakfakh, F.; Ammar-Keskes, L. Antioxidant supplementations in vitro improve rat sperm parameters and enhance antioxidant enzyme activities against dimethoate-induced sperm damages. Andrologia 2012, 44, 272–279. [Google Scholar] [CrossRef]
- Martenies, S.E.; Perry, M.J. Environmental and occupational pesticide exposure and human sperm parameters: A systematic review. Toxicology 2012, 307, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.; El-Maddawy, Z. Protective effect of vitamin E and selenium combination on DLM-induced reproductive toxicity in male rats. Exp. Toxicol. Pathol. 2012, 64, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.E.; Paro, R.; Borriello, L.; Simon, L.; Robinson, L.; Dincer, Z.; Riedel, G.; Battista, N.; Maccarrone, M. Long-term use of HU210 adversely affects spermatogenesis in rats by modulating the endocannabinoid system. Int. J. Androl. 2012, 35, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Peña, L.; Reyes, B.; López-Carrillo, L.; Recio, R.; Morán-Martínez, J.; Cebrián, M.; Quintanilla-Vega, B. Organophosphorus pesticide exposure alters sperm chromatin structure in Mexican agricultural workers. Toxicol. Appl. Pharm. 2004, 196, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Giri, A.; Giri, S. The antimalarial agent artesunate causes sperm DNA damage and hepatic antioxidant defense in mice. Mutat. Res. Genet. Toxicol. Envirion. Mutagen. 2015, 777, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Łuczaj, W.; Skrzydlewska, E. DNA damage caused by lipid peroxidation products. Cell Mol. Biol. Lett. 2003, 8, 391–413. [Google Scholar] [PubMed]
- Leduc, F.; Nkoma, G.B.; Boissonneault, G. Spermiogenesis and DNA repair: A possible etiology of human infertility and genetic disorders. Syst. Biol. Reprod. Med. 2008, 54, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Marcon, L.; Boissonneault, G. Transient DNA strand breaks during mouse and human spermiogenesis: New insights in stage specificity and link to chromatin remodeling. Biol. Reprod. 2004, 70, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Piña-Guzmán, B.; Solís-Heredia, M.J.; Quintanilla-Vega, B. Diazinon alters sperm chromatin structure in mice by phosphorylating nuclear protamines. Toxicol. Appl. Pharm. 2005, 202, 189–198. [Google Scholar] [CrossRef]
- González-Marín, C.; Gosálvez, J.; Roy, R. Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int. J. Mol. Sci. 2012, 13, 14026–14052. [Google Scholar] [CrossRef] [PubMed]
- Tavares, R.S.; Silva, A.F.; Lourenço, B.; Almeida-Santos, T.; Sousa, A.P.; Ramalho-Santos, J. Evaluation of human sperm chromatin status after selection using a modified Diff–Quik stain indicates embryo quality and pregnancy outcomes following in vitro fertilization. Andrology 2012, 1, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Federman, D.D. The biology of human sex differences. N. Engl. J. Med. 2006, 354, 1507–1514. [Google Scholar] [CrossRef]
- Schjenken, J.E.; Green, E.S.; Overduin, T.S.; Mah, C.Y.; Russell, D.L.; Robertson, S.A. Endocrine disruptor compounds-a cause of impaired immune tolerance driving inflammatory disorders of pregnancy. Front. Endoc. 2021, 12, 607539. [Google Scholar] [CrossRef]
- Sanches, E.S.A.M.; Tsuzuki, F.; Joinhas, F.; Figueiras, G.B.; Moreira, E.G.; Salles, M.J.S. Paternal exposure to bupropion affects postnatal development in the offspring. Reprod. Fertil. Dev. 2019, 31, 1539–1544. [Google Scholar] [CrossRef]
- Ellison, C.A.; Tian, Y.; Knaak, J.B.; Kostyniak, P.J.; Olson, J.R. Human hepatic cytochrome P450-specific metabolism of the organophosphorus pesticides methyl parathion and diazinon. Drug Metab. Dispos. 2011, 40, 1–5. [Google Scholar] [CrossRef]
- Mira-Escolano, M.; Mendiola, J.; Mínguez-Alarcón, L.; Melgarejo, M.; Cutillas-Tolín, A.; Roca, M.; López-Espín, J.J.; Noguera-Velasco, J.A.; Torres-Cantero, A.M. Longer anogenital distance is associated with higher testosterone levels in women: A cross-sectional study. Int. J. Obstet. Gynecol. 2014, 121, 1359–1364. [Google Scholar] [CrossRef]
- Brinkworth, M.H. Paternal transmission of genetic damage: Findings in animals and humans. Int. J. Androl. 2000, 23, 123–135. [Google Scholar] [CrossRef]
- Tamashiro, K.L.K.; Wakayama, T.; Akutsu, H.; Yamazaki, Y.; Lachey, J.L.; Wortmsan, M.D.; Seeley, R.J.; D′Alessio, D.A.; Woods, S.C.; Yanagimachi, R. Cloned mice have an obese phenotype that is not transmitted to their offspring. Nat. Med. 2002, 63, 328–334. [Google Scholar] [CrossRef]
- Curtis, L.R. Organophosphate antagonism of the androgen receptor. Toxicol. Sci. 2001, 60, 1–2. [Google Scholar] [CrossRef][Green Version]
- Tehrani, F.R.; Noroozzadeh, M.; Zahediasl, S.; Ghasemi, A.; Piryaei, A.; Azizi, F. Prenatal testosterone exposure worsen the reproductive performance of male rat at adulthood. PLoS ONE 2013, 8, e71705. [Google Scholar]
- Wilson, V.S.; Lambright, C.R.; Furr, J.R.; Howdeshell, K.L.; Gray, L. The herbicide linuron reduces testosterone production from the fetal rat testis during both utero and in vitro exposures. Toxicol. Lett. 2009, 186, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Mclntyre, B.S.; Barlow, N.J.; Foster, P.M. Male rats exposed to linuron in utero exhibit permanent changes in anogenital distance, nipple retention and epididymal malformations that result in subsequent testicular atrophy. Toxicol. Sci. 2002, 65, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Zaya, R.; Hennick, C.; Pearl, C. In vitro expression of androgen and estrogen receptors in prepubertal and adult rat epididymis. Gen. Comp. Endoc. 2012, 178, 573–586. [Google Scholar] [CrossRef]
- Cooper, R.L.; Goldman, J.M.; Rehnberg, G.L. Neuroendocrine control of reproductive function in the aging female rodent. J. Am. Ger. Soc. 1986, 34, 735–751. [Google Scholar] [CrossRef]
- Gray, L.E.; Ostby, J.; Ferrell, J.; Rehnberg, G.; Linder, R.; Cooper, R.; Goldman, J.; Slott, V.; Laskey, J. A dose-response analysis of Methoxychlor-induced alterations of reproductive development and function in the rat. Fund. Appl. Toxicol. 1989, 12, 92–108. [Google Scholar] [CrossRef]
- Xia, D.; Parvizi, N.; Zhou, Y. Paternal fenvalerate exposure influences reproductive functions in the offspring. Reprod. Sci. 2013, 20, 1308–1315. [Google Scholar] [CrossRef]
- Aminsharifi, A.; Shakeri, S.; Ariafar, A.; Moeinjahromi, B.; Kumar, P.V.; Karbalaeedoost, S. Preventive role of exogenous testosterone on cisplatin-induced gonadal toxicity: An experimental placebo-controlled prospective trial. Fertil. Steril. 2010, 93, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, N.A.; Budin, S.B.; Taib, I.S. Pesticide exposures induce male-mediated reproductive toxicity: A review. J. Agric. Sci. 2017, 9, 122–135. [Google Scholar] [CrossRef][Green Version]
- Basal, W.T.; Ahmed, A.R.T.; Mahmoud, A.A.; Omar, A.R. Lufenuron induces reproductive toxicity and genotoxic effects in pregnant albino rats and their fetuses. Sci. Rep. 2020, 10, 19544. [Google Scholar] [CrossRef] [PubMed]

| Score | Stage of Spermatogenesis |
|---|---|
| 1 | Tubular sclerosis; absence of seminiferous epithelial cells. |
| 2 | Sertoli cells only; no germ cells. |
| 3 | Only spermatogonia. |
| 4 | Arrest of spermatogenesis at the primary spermatocyte stage; no spermatids. |
| 5 | Many spermatocytes; no spermatids. |
| 6 | No late spermatids; arrest of spermatogenesis at the spermatid stage. |
| 7 | Many early spermatids; no late spermatids. |
| 8 | Few late spermatids. |
| 9 | Disorganized tubular epithelium with many late spermatids. |
| 10 | Full spermatogenesis. |
| Parameter | Control | FNT-10 | FNT-20 |
|---|---|---|---|
| Sperm Count (×106) | 65.48 ± 1.89 | 53.00 ± 1.31 a | 46.52 ± 1.12 a,b |
| Sperm Motility (%) | 43.59 ± 1.34 | 20.74 ± 0.67 a | 14.10 ± 0.67 a,b |
| Sperm Viability (%) | 60.48 ± 1.20 | 43.19 ± 1.55 a | 35.62 ± 1.19 a,b |
| Abnormal Sperm Morphology (%) | 18.48 ± 1.30 | 26.10 ± 0.67 a | 33.83 ± 0.33 a,b |
| Sperm DNA Fragmentation (%) | 6.90 ± 0.61 | 12.00 ± 0.52 a | 20.91 ± 0.38 a,b |
| Parameter | pControl | pFNT-10 | pFNT-20 |
|---|---|---|---|
| Anogenital Distance, AGD (mm) | |||
| PND0 | |||
| Male | 4.13 ± 0.30 | 3.87 ± 0.30 | 3.75 ± 0.16 |
| Female | 1.88 ± 0.23 | 1.88 ± 0.23 | 1.63 ± 0.18 |
| PND12 | |||
| Male | 14.88 ± 0.30 | 14.75 ± 0.31 | 14.63 ± 0.18 |
| Female | 8.75 ± 0.37 | 8.50 ± 0.42 | 8.38 ± 0.46 |
| PND35 | |||
| Male | 29.38 ± 0.38 | 29.25 ± 0.31 | 28.50 ± 0.63 |
| Female | 18.75 ± 0.41 | 18.88 ± 0.55 | 18.63 ± 0.42 |
| Number of Nipple and Areola | |||
| PND12 | |||
| Male | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Female | 12.13 ± 0.22 | 12.00 ± 0.19 | 11.88 ± 0.13 |
| Parameter | pControl | pFNT-10 | pFNT-20 |
|---|---|---|---|
| Leydig Cell Count | 208.44 ± 1.46 | 207.13 ± 1.50 | 201.94 ± 1.59 a |
| Seminiferous Tubule Diameter (µm) | 250.86 ± 1.27 | 248.24 ± 1.56 | 243.59 ± 2.33 a |
| Seminiferous Tubule Height (µm) | 78.67 ± 2.46 | 75.85 ± 2.28 | 71.33 ± 1.02 |
| Seminiferous Tubule with Germ Cell Loss (%) | 2.72 ± 0.29 | 2.81 ± 0.23 | 2.97 ± 0.26 |
| Johnsen Testicular Biopsy Score | 9.25 ± 0.25 | 9.13 ± 0.23 | 9.00 ± 0.27 |
| Parameter | Male | Female | ||||
|---|---|---|---|---|---|---|
| pControl | pFNT-10 | pFNT-20 | pControl | pFNT-10 | pFNT-20 | |
| Cardiomyocyte Size (×103 µm2) | 9.93 ± 0.36 | 10.03 ± 0.17 | 9.97 ± 0.30 | 9.54 ± 0.18 | 9.59 ± 0.11 | 9.60 ± 0.18 |
| Hepatocyte Size (%) | 98.13 ± 0.13 | 97.50 ± 0.09 | 95.63 ± 0.11 | 96.25 ± 0.16 | 95.63 ± 0.15 | 95.63 ± 0.11 |
| Glomerulus Diameter (µm) | 38.70 ± 1.08 | 31.49 ± 0.68 a | 29.78 ± 0.36 a | 34.69 ± 0.24 | 34.10 ± 0.36 | 34.03 ± 0.25 |
| Bowman’s Space (µm) | 535.41 ± 4.03 | 551.20 ± 0.85 a | 569.98 ± 2.40 a,b | 508.41 ± 2.47 | 502.21 ± 2.11 | 502.08 ± 1.58 |
| Number of Alveoli (×10−3) | 14.53 ± 0.31 | 14.38 ± 0.36 | 14.31 ± 0.24 | 13.68 ± 0.12 | 13.64 ± 0.14 | 13.65 ± 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusoff, N.A.; Taib, I.S.; Budin, S.B.; Mohamed, M. Paternal Fenitrothion Exposures in Rats Causes Sperm DNA Fragmentation in F0 and Histomorphometric Changes in Selected Organs of F1 Generation. Toxics 2021, 9, 159. https://doi.org/10.3390/toxics9070159
Yusoff NA, Taib IS, Budin SB, Mohamed M. Paternal Fenitrothion Exposures in Rats Causes Sperm DNA Fragmentation in F0 and Histomorphometric Changes in Selected Organs of F1 Generation. Toxics. 2021; 9(7):159. https://doi.org/10.3390/toxics9070159
Chicago/Turabian StyleYusoff, Nur Afizah, Izatus Shima Taib, Siti Balkis Budin, and Mahaneem Mohamed. 2021. "Paternal Fenitrothion Exposures in Rats Causes Sperm DNA Fragmentation in F0 and Histomorphometric Changes in Selected Organs of F1 Generation" Toxics 9, no. 7: 159. https://doi.org/10.3390/toxics9070159
APA StyleYusoff, N. A., Taib, I. S., Budin, S. B., & Mohamed, M. (2021). Paternal Fenitrothion Exposures in Rats Causes Sperm DNA Fragmentation in F0 and Histomorphometric Changes in Selected Organs of F1 Generation. Toxics, 9(7), 159. https://doi.org/10.3390/toxics9070159








