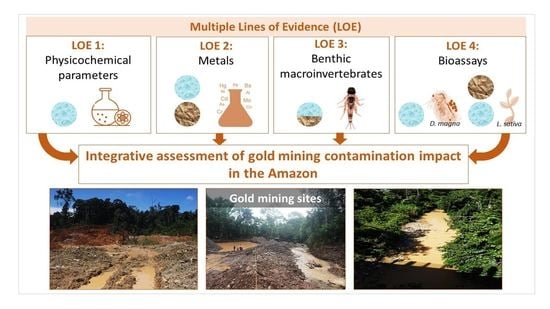
An Integrative Approach to Assess the Environmental Impacts of Gold Mining Contamination in the Amazon
Abstract
:1. Introduction
2. Materials and Methods
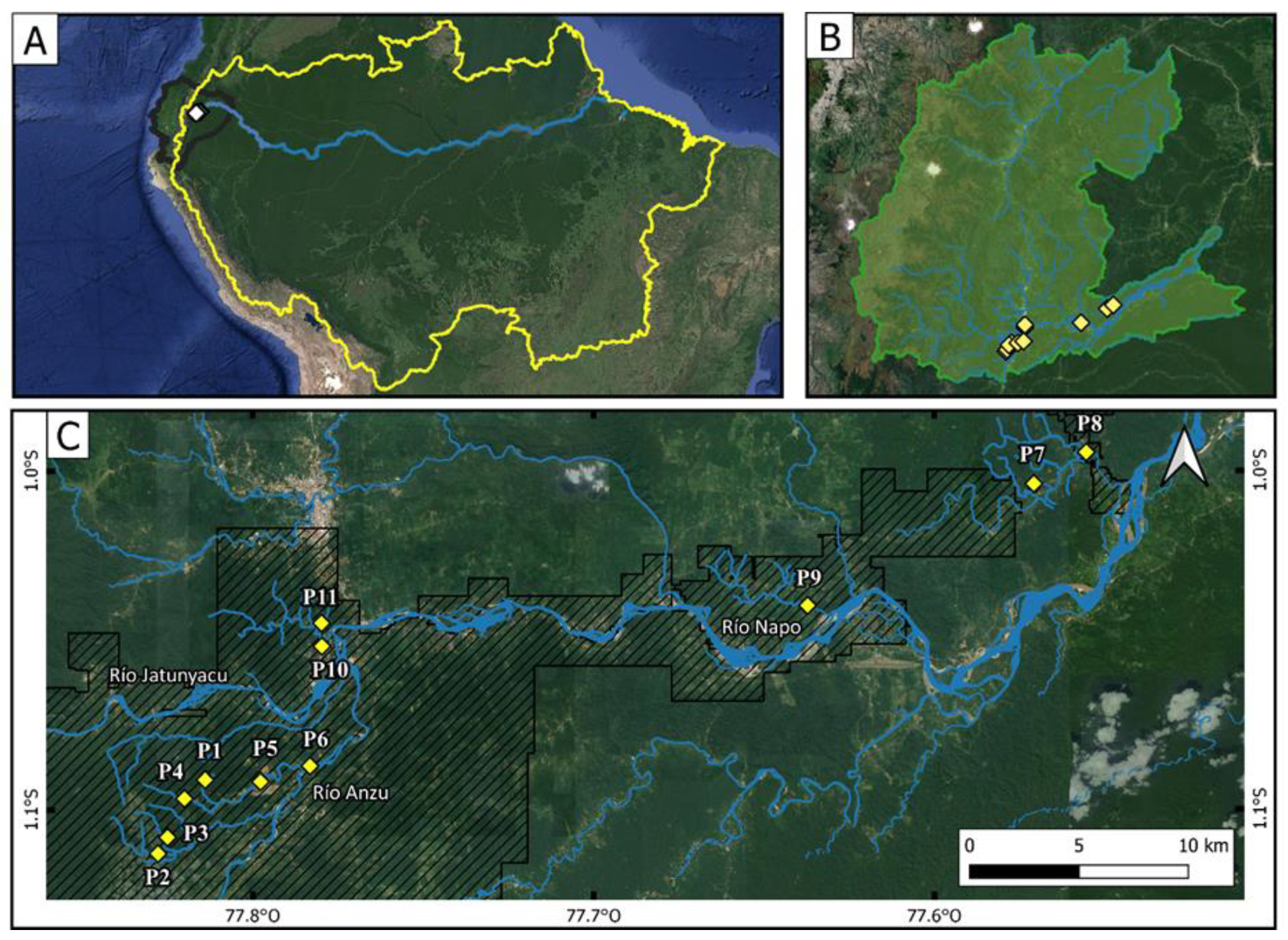
2.1. Study Area and Sampling
2.2. Measurements of Physicochemical Parameters
2.3. Metal Analyses
2.4. Calculation of the Andean–Amazon Biotic Index for Macroinvertebrates
2.5. Bioassays
2.6. Data Analyses
3. Results
3.1. Physicochemical Parameters
3.2. Metal Concentrations
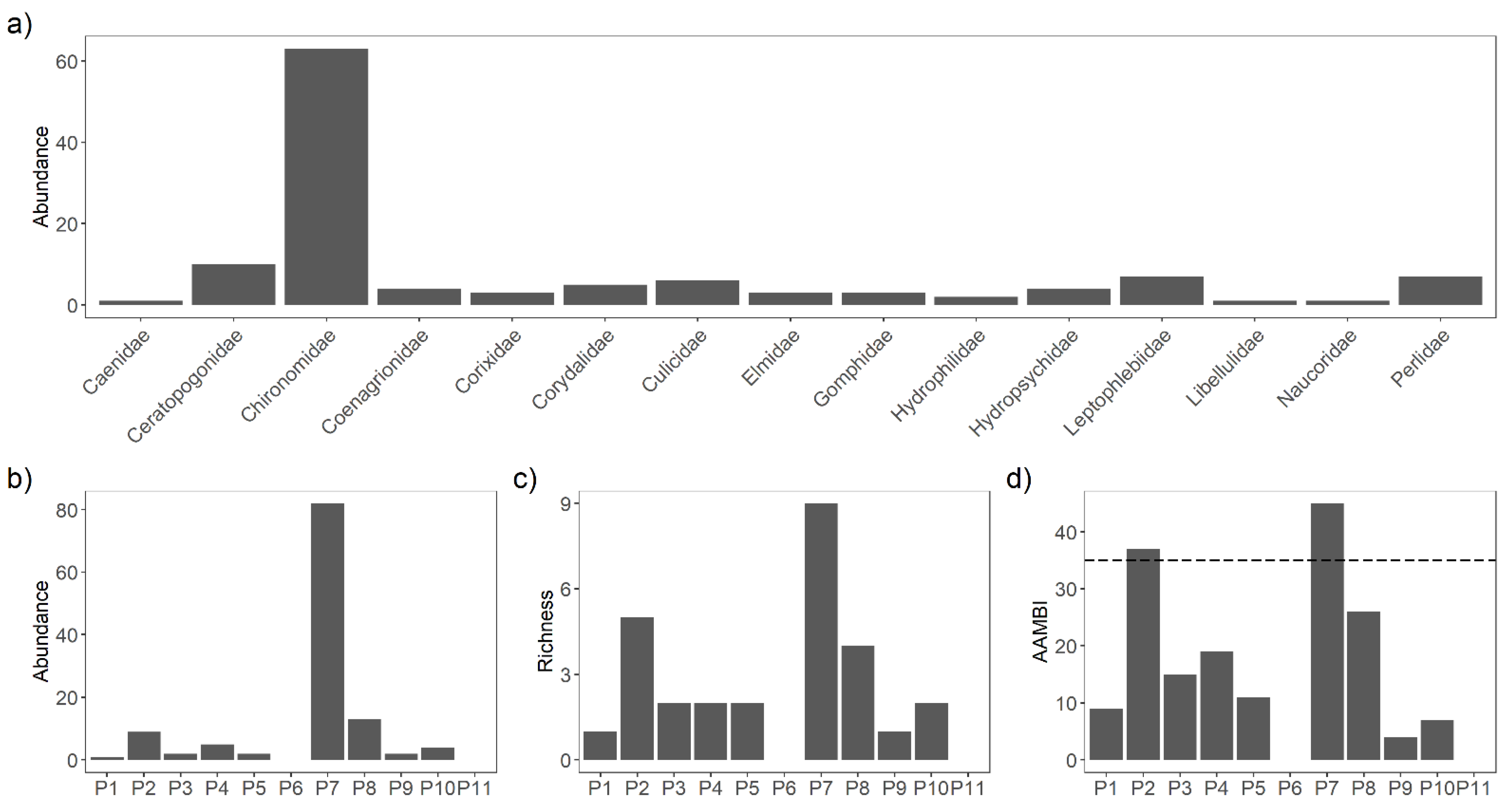
3.3. Macroinvertebrates
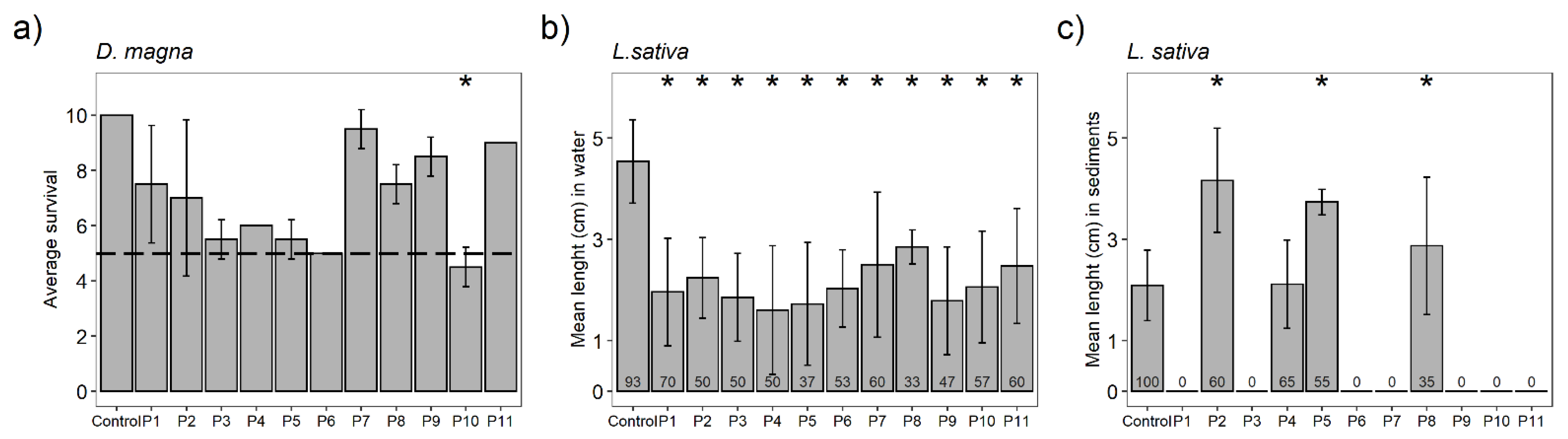
3.4. Toxicity Bioassays
3.5. Integrative Analysis of the Four LOEs
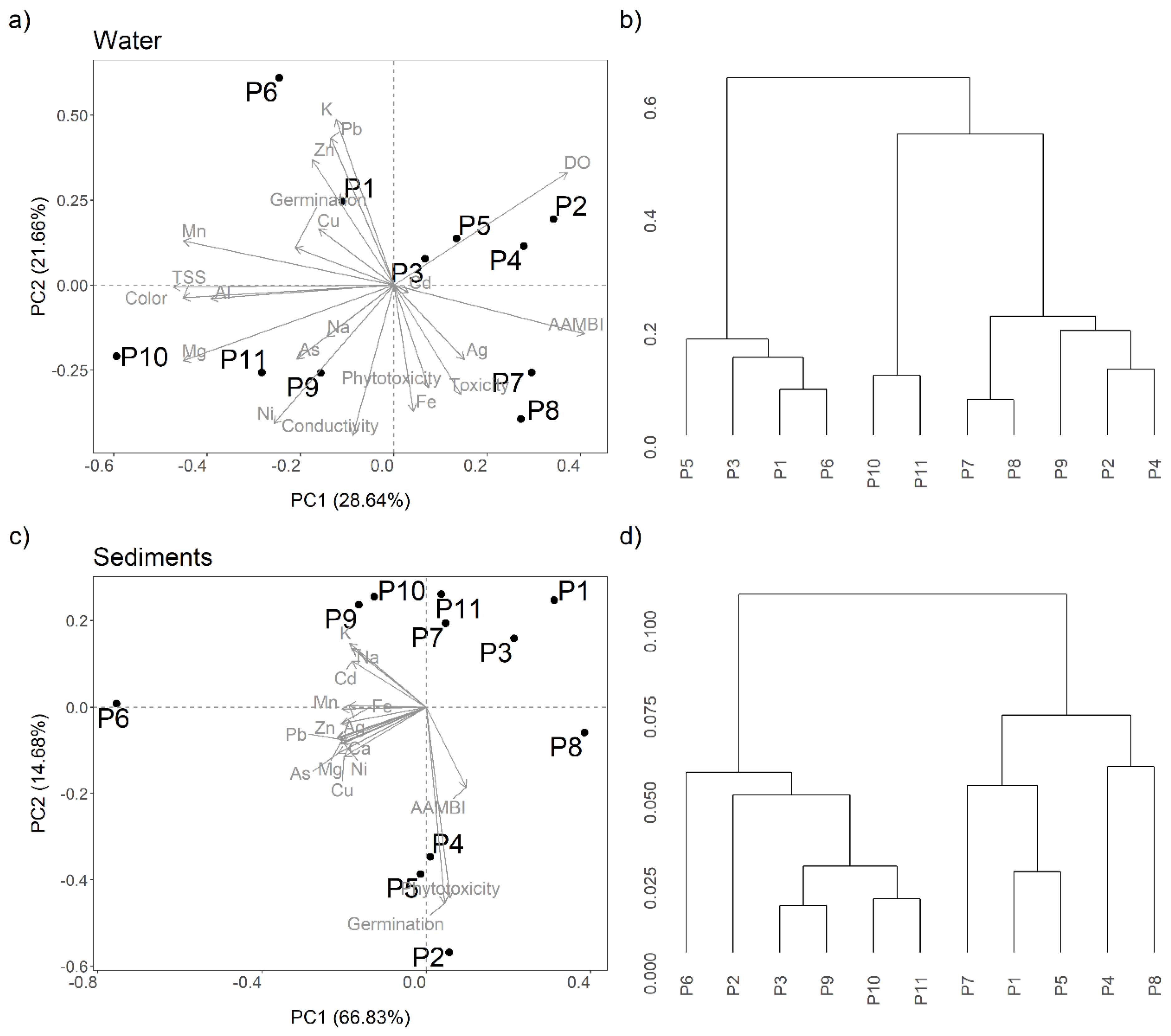
3.6. PCA and Hierarchical Cluster Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Couto, T.B.A.; Messager, M.L.; Olden, J.D. Safeguarding Migratory Fish via Strategic Planning of Future Small Hydropower in Brazil. Nat. Sustain. 2021, 1–8. [Google Scholar] [CrossRef]
- Paiva, P.F.P.R.; de Lourdes Pinheiro Ruivo, M.; da Silva Júnior, O.M.; de Nazare Martins Maciel, M.; Braga, T.G.M.; de Andrade, M.M.N.; dos Santos Junior, P.C.; da Rocha, E.S.; de Freitas, T.P.M.; da Silva Leite, T.V.; et al. Deforestation in Protect Areas in the Amazon: A Threat to Biodiversity. Biodivers. Conserv. 2020, 29, 19–38. [Google Scholar] [CrossRef]
- Sonter, L.J.; Herrera, D.; Barrett, D.J.; Galford, G.L.; Moran, C.J.; Soares-Filho, B.S. Mining Drives Extensive Deforestation in the Brazilian Amazon. Nat. Commun. 2017, 8, 1013. [Google Scholar] [CrossRef] [Green Version]
- Asner, G.P.; Tupayachi, R. Accelerated Losses of Protected Forests from Gold Mining in the Peruvian Amazon. Environ. Res. Lett. 2016, 12, 094004. [Google Scholar] [CrossRef]
- Webb, J.; Mainville, N.; Mergler, D.; Lucotte, M.; Betancourt, O.; Davidson, R.; Cueva, E.; Quizhpe, E. Mercury in Fish-Eating Communities of the Andean Amazon, Napo River Valley, Ecuador. EcoHealth 2004, 1, SU59–SU71. [Google Scholar] [CrossRef]
- Ferrante, L.; Fearnside, P.M. Brazil’s New President and ‘Ruralists’ Threaten Amazonia’s Environment, Traditional Peoples and the Global Climate. Environ. Conserv. 2019, 46, 261–263. [Google Scholar] [CrossRef]
- Rorato, A.C.; Camara, G.; Escada, M.I.S.; Picoli, M.C.A.; Moreira, T.; Verstegen, J.A. Brazilian Amazon Indigenous Peoples Threatened by Mining Bill. Environ. Res. Lett. 2020, 15, 1040a3. [Google Scholar] [CrossRef]
- Roy, B.A.; Zorrilla, M.; Endara, L.; Thomas, D.C.; Vandegrift, R.; Rubenstein, J.M.; Policha, T.; Rios-Touma, B.; Read, M. New Mining Concessions Could Severely Decrease Biodiversity and Ecosystem Services in Ecuador. Trop. Conserv. Sci. 2018, 11, 1940082918780427. [Google Scholar] [CrossRef]
- Tarras-Wahlberg, N.H.; Flachier, A.; Fredriksson, G.; Lane, S.; Lundberg, B.; Sangfors, O. Environmental Impact of Small-Scale and Artisanal Gold Mining in Southern Ecuador. AMBIO J. Hum. Environ. 2000, 29, 484–491. [Google Scholar] [CrossRef]
- Adler Miserendino, R.; Bergquist, B.A.; Adler, S.E.; Guimarães, J.R.D.; Lees, P.S.J.; Niquen, W.; Velasquez-López, P.C.; Veiga, M.M. Challenges to Measuring, Monitoring, and Addressing the Cumulative Impacts of Artisanal and Small-Scale Gold Mining in Ecuador. Resour. Policy 2013, 38, 713–722. [Google Scholar] [CrossRef]
- Banco Central de Ecuador (BCE). Reporte de Minería 2020. Available online: https://contenido.bce.fin.ec/documentos/Estadisticas/Hidrocarburos/ReporteMinero062020.pdf (accessed on 10 January 2021).
- Capparelli, M.V.; Moulatlet, G.M.; de Abessa, D.M.S.; Lucas-Solis, O.; Rosero, B.; Galarza, E.; Tuba, D.; Carpintero, N.; Ochoa-Herrera, V.; Cipriani-Avila, I. An Integrative Approach to Identify the Impacts of Multiple Metal Contamination Sources on the Eastern Andean Foothills of the Ecuadorian Amazonia. Sci. Total Environ. 2020, 709, 136088. [Google Scholar] [CrossRef]
- Perez-Escobar, O.A.; Camara-Leret, R.; Antonelli, A.; Bateman, R.; Bellot, S.; Chomicki, G.; Cleef, A.; Diazgranados, M.; Dodsworth, S.; Jaramillo, C.; et al. Mining Threatens Colombian Ecosystems. Science 2018, 359, 1475. [Google Scholar] [CrossRef] [Green Version]
- Agencia de regulación y Control Minero (ARCOM). Geoportal de Catastro Minero. Available online: https://gis-sigde.maps.arcgis.com/apps/webappviewer/index.html?id=8b53f9388c034b5e8e3147f03583d7ec (accessed on 8 May 2021).
- Cruz, A.C.F.; Gusso-Choueri, P.; de Araujo, G.S.; Campos, B.G.; de Abessa, D.M.S. Levels of Metals and Toxicity in Sediments from a Ramsar Site Influenced by Former Mining Activities. Ecotoxicol. Environ. Saf. 2019, 171, 162–172. [Google Scholar] [CrossRef]
- Adams, S.M.; Greeley, M.S. Ecotoxicological Indicators of Water Quality: Using Multi-Response Indicators to Assess the Health of Aquatic Ecosystems. Water Air Soil Pollut. 2000, 123, 103–115. [Google Scholar] [CrossRef]
- Chapman, P.M.; McDonald, B.G.; Lawrence, G.S. Weight-of-Evidence Issues and Frameworks for Sediment Quality (And Other) Assessments. Hum. Ecol. Risk Assess. Int. J. 2002, 8, 1489–1515. [Google Scholar] [CrossRef]
- Backhaus, T.; Brack, W.; Van den Brink, P.J.; Deutschmann, B.; Hollert, H.; Posthuma, L.; Segner, H.; Seiler, T.-B.; Teodorovic, I.; Focks, A. Assessing the Ecological Impact of Chemical Pollution on Aquatic Ecosystems Requires the Systematic Exploration and Evaluation of Four Lines of Evidence. Environ. Sci. Eur. 2019, 31, 98. [Google Scholar] [CrossRef] [Green Version]
- Galarza, E.; Cabrera, M.; Espinosa, R.; Espitia, E.; Moulatlet, G.M.; Capparelli, M.V. Assessing the Quality of Amazon Aquatic Ecosystems with Multiple Lines of Evidence: The Case of the Northeast Andean Foothills of Ecuador. Bull. Environ. Contam. Toxicol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Mora, A.; Jumbo-Flores, D.; González-Merizalde, M.; Bermeo-Flores, S.A.; Alvarez-Figueroa, P.; Mahlknecht, J.; Hernández-Antonio, A. Heavy Metal Enrichment Factors in Fluvial Sediments of an Amazonian Basin Impacted by Gold Mining. Bull. Environ. Contam. Toxicol. 2019, 102, 210–217. [Google Scholar] [CrossRef]
- Barraza, F.; Maurice, L.; Uzu, G.; Becerra, S.; López, F.; Ochoa-Herrera, V.; Ruales, J.; Schreck, E. Distribution, Contents and Health Risk Assessment of Metal(Loid)s in Small-Scale Farms in the Ecuadorian Amazon: An Insight into Impacts of Oil Activities. Sci. Total Environ. 2018, 622–623, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Maurice, L.; López, F.; Becerra, S.; Jamhoury, H.; Le Menach, K.; Dévier, M.-H.; Budzinski, H.; Prunier, J.; Juteau-Martineau, G.; Ochoa-Herrera, V.; et al. Drinking Water Quality in Areas Impacted by Oil Activities in Ecuador: Associated Health Risks and Social Perception of Human Exposure. Sci. Total Environ. 2019, 690, 1203–1217. [Google Scholar] [CrossRef]
- Dominguz, E.; Fernadéz, H.R. Macroinvertebrados Bentónicos Sudamericanos. In Sistemática y Biología; Miguel Lillo Foundation: Tucumán, Argentina, 2009; Volume 656. [Google Scholar]
- American Health Public Association (APHA). Standard Methods for the Examination of Water and Wastewater 2005. Available online: https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/scientific/technical-documents/white-papers/apha-water-testing-standard-methods-introduction-white-paper.pdf (accessed on 10 January 2021).
- American Public Health Association; American Water Works Association; Water Pollution Control Federation. Water Works Association; Water Pollution Control Federation. Water Environment Federation 2340 Hardness. In Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2018. [Google Scholar]
- Creed, J.T.; Brockhoff, C.A.; Martin, T.D. US EPA Method 200.8: Determination of Trace Elements in Waters and Wastes by Inductively Coupled Plasma-Mass Spectrometry; Revision 5.4.1994. Available online: https://www.epa.gov/sites/production/files/2015-06/documents/epa-200.8.pdf (accessed on 10 January 2021).
- Hamada, N.; Thorp, J.H.; Rogers, C.D. Keys to Neotropical Hexapoda. In Thorp and Covich’s Freshwater Invertebrates; Academic Press: Cambridge, MA, USA, 2018; Volume 3. [Google Scholar]
- Encalada, A.C.; Guayasamin, J.M.; Suárez, E.; Mena, C.F.; Lessmann, J.; Sampedro, C.; Martín, P.E.; Ochoa-Herrera, V.; Swing, K.; Celinéak, M.; et al. Los Ríos de Las Cuencas Andino-Amazónicas: Herramientas y Guía de Invertebrados Para El Diseño Efectivo de Programas de Monitoreo; Trama Diseño: Quito, Ecuador, 2019. [Google Scholar]
- United States Environmental Protection Services (US EPA). Ecological Effects Test Guidelines; US EPA: Washington, DC, USA, 1996. [Google Scholar]
- Ministerio del Ambiente (MAE). Reforma Texto Unificado de Legislación Secundaria, Medio Ambiente, Libro VI 2015. Available online: https://www.ambiente.gob.ec/wp-content/uploads/downloads/2018/05/Acuerdo-61.pdf (accessed on 10 January 2021).
- United States Environmental Protection Services (US EPA). National Recommended Water Quality Criteria—Aquatic Life Criteria Table. 2015. Available online: https://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-life-criteria-table (accessed on 10 January 2021).
- Canadian Council of Ministers of the Environment (CCME). Canadian Environmental Quality Guidelines; CCME: Winnipeg, MB, Canada, 2002; Volume 2. [Google Scholar]
- Long, E.R.; Macdonald, D.D.; Smith, S.L.; Calder, F.D. Incidence of Adverse Biological Effects within Ranges of Chemical Concentrations in Marine and Estuarine Sediments. Environ. Manag. 1995, 19, 81–97. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2020; Available online: http://www.r-project.org/ (accessed on 10 January 2021).
- De Souza Neto, H.F.; da Pereira, W.V.S.; Dias, Y.N.; de Souza, E.S.; Teixeira, R.A.; de Lima, M.W.; Ramos, S.J.; do Amarante, C.B.; Fernandes, A.R. Environmental and Human Health Risks of Arsenic in Gold Mining Areas in the Eastern Amazon. Environ. Pollut. 2020, 265, 114969. [Google Scholar] [CrossRef]
- Teixeira, S.S.; Dall’Agnol, R.; Sahoo, P.K.; Salomão, G.N.; Guimarães, J.T.F.; Costa, M. Water Chemistry and Estimation of Background Levels of Elements in Surface Water Bodies from a Protected Area in the Vicinity of Fe Deposits, Southeastern Amazon. Environ. Forensics 2020, 21, 176–194. [Google Scholar] [CrossRef]
- Viana, L.F.; Cardoso, C.A.L.; Lima-Junior, S.E.; Súarez, Y.R.; Florentino, A.C. Bioaccumulation of Metal in Liver Tissue of Fish in Response to Water Toxicity of the Araguari-Amazon River, Brazil. Environ. Monit. Assess. 2020, 192, 781. [Google Scholar] [CrossRef]
- Jimenez-Oyola, S.; Escobar Segovia, K.; Garcia-Martinez, M.-J.; Ortega, M.; Bolonio, D.; Garcia-Garizabal, I.; Salgado, B. Human Health Risk Assessment for Exposure to Potentially Toxic Elements in Polluted Rivers in the Ecuadorian Amazon. Water 2021, 13, 613. [Google Scholar] [CrossRef]
- Lessmann, J.; Troya, M.J.; Flecker, A.S.; Funk, W.C.; Guayasamin, J.M.; Ochoa-Herrera, V.; Poff, N.L.; Suárez, E.; Encalada, A.C. Validating Anthropogenic Threat Maps as a Tool for Assessing River Ecological Integrity in Andean–Amazon Basins. PeerJ 2019, 7, e8060. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Garcia, A.; Marquez-Rodriguez, J.; Ferreras-Romero, M. Implications of Anthropogenic Disturbance Factors on the Trichoptera Assemblage in a Mediterranean Fluvial System: Are Trichoptera Useful for Identifying Land-Use Alterations? Ecol. Indic. 2012, 14, 114–123. [Google Scholar] [CrossRef]
- Smolders, A.J.P.; Lock, R.A.C.; van der Velde, G.; Medina Hoyos, R.I.; Roelofs, J.G.M. Effects of Mining Activities on Heavy Metal Concentrations in Water, Sediment, and Macroinvertebrates in Different Reaches of the Pilcomayo River, South America. Arch. Environ. Contam. Toxicol. 2003, 44, 0314–0323. [Google Scholar] [CrossRef] [PubMed]
- Kjelland, M.E.; Woodley, C.M.; Swannack, T.M.; Smith, D.L. A Review of the Potential Effects of Suspended Sediment on Fishes: Potential Dredging-Related Physiological, Behavioral, and Transgenerational Implications. Environ. Syst. Decis. 2015, 35, 334–350. [Google Scholar] [CrossRef] [Green Version]
- Bojsen, B.; Jacobsen, D. Effects of Deforestation on Macroinvertebrate Diversity and Assemblage Structure in Ecuadorian Amazon Streams. Archiv Für Hydrobiolgie 2003, 158, 317–342. [Google Scholar] [CrossRef]
- Bagur-Gonzalez, M.G.; Estepa-Molina, C.; Martin-Peinado, F.; Morales-Ruano, S. Toxicity Assessment Using Lactuca Sativa L. Bioassay of the Metal(Loid)s As, Cu, Mn, Pb and Zn in Soluble-in-Water Saturated Soil Extracts from an Abandoned Mining Site. J. Soils Sediments 2011, 11, 281–289. [Google Scholar] [CrossRef]
- Azzi, V.; Kanso, A.; Kazpard, V.; Kobeissi, A.; Lartiges, B.; El Samrani, A. Lactuca Sativa Growth in Compacted and Non-Compacted Semi-Arid Alkaline Soil under Phosphate Fertilizer Treatment and Cadmium Contamination. Soil Tillage Res. 2017, 165, 1–10. [Google Scholar] [CrossRef]
- Chan-Keb, C.A.; Agraz-Hernández, C.M.; Perez-Balan, R.A.; Gómez-Solano, M.I.; Maldonado-Montiel, T.D.N.J.; Ake-Canche, B.; Gutiérrez-Alcántara, E.J. Acute Toxicity of Water and Aqueous Extract of Soils from Champotón River in Lactuca Sativa L. Toxicol. Rep. 2018, 5, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Belz, R.G.; Patama, M.; Sinkkonen, A. Low Doses of Six Toxicants Change Plant Size Distribution in Dense Populations of Lactuca Sativa. Sci. Total Environ. 2018, 631–632, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Di Salvatore, M.; Carafa, A.M.; Carratù, G. Assessment of Heavy Metals Phytotoxicity Using Seed Germination and Root Elongation Tests: A Comparison of Two Growth Substrates. Chemosphere 2008, 73, 1461–1464. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Preconditioning Is Hormesis Part I: Documentation, Dose-Response Features and Mechanistic Foundations. Pharmacol. Res. 2016, 110, 242–264. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Bachmann, K.A.; Bailer, A.J.; Bolger, P.M.; Borak, J.; Cai, L.; Cedergreen, N.; Cherian, M.G.; Chiueh, C.C.; Clarkson, T.W.; et al. Biological Stress Response Terminology: Integrating the Concepts of Adaptive Response and Preconditioning Stress within a Hormetic Dose–Response Framework. Toxicol. Appl. Pharmacol. 2007, 222, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Pandey, P.; Hussain, J. Effect of Physicochemical and Biological Parameters on the Quality of River Water of Narmada, Madhya Pradesh, India. Water Sci. 2017, 31, 11–23. [Google Scholar] [CrossRef]
- Reeder, R.J.; Schoonen, M.A.A.; Lanzirotti, A. Metal Speciation and Its Role in Bioaccessibility and Bioavailability. Rev. Mineral. Geochem. 2006, 64, 59–113. [Google Scholar] [CrossRef]
- Gambrell, R.P.; Wiesepape, J.B.; Patrick, W.H.; Duff, M.C. The Effects of PH, Redox, and Salinity on Metal Release from a Contaminated Sediment. Water. Air. Soil Pollut. 1991, 57, 359–367. [Google Scholar] [CrossRef]
- Lucas-Solis, O.; Moulatlet, G.M.; Guamangallo, J.; Yacelga, N.; Villegas, L.; Galarza, E.; Rosero, B.; Zurita, B.; Sabando, L.; Cabrera, M.; et al. Preliminary Assessment of Plastic Litter and Microplastic Contamination in Freshwater Depositional Areas: The Case Study of Puerto Misahualli, Ecuadorian Amazonia. Bull. Environ. Contam. Toxicol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.R. The Role of Fluvial Geomorphic Processes in the Dispersal of Heavy Metals from Mine Sites. J. Geochem. Explor. 1997, 58, 101–118. [Google Scholar] [CrossRef]
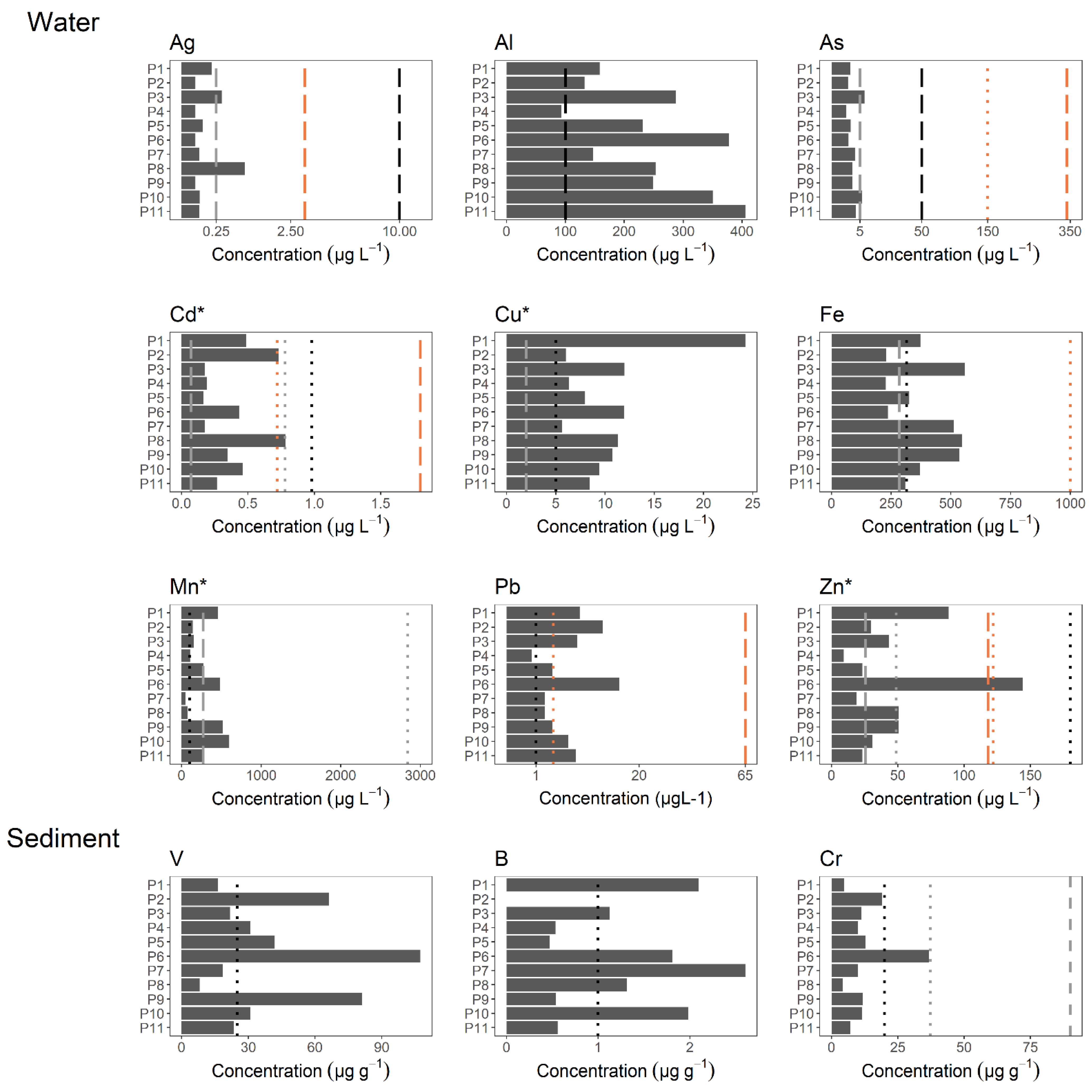
| Sites | Physicochemical Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH | T (°C) | Turbidity (NTU) | TDS (mg L−1) | TSS (mg L−1) | Conductivity (μs/cm) | DO (% sat) | Color (Pt-Co) | DOC (mg L−1) | Hardness (mg CaCO3 L−1) | |
| P1 | 6.91 | 23.3 | 765 | 45.5 | 698 | 67.6 | 80.6 | 2800 | 4.31 | 11.8 |
| P2 | 6.7 | 25.6 | 10.2 | 16.25 | 3 | 25.3 | 81.5 | 82 | 1.85 | 3.9 |
| P3 | 6.55 | 30 | 277 | 14.95 | 523 | 25 | 75 | 375 | 2.4 | 5.3 |
| P4 | 6.67 | 28.3 | 24 | 33.2 | 19 | 53.8 | 76.2 | 63 | 3.86 | 7.6 |
| P5 | 7.17 | 31 | 246 | 31.2 | 171 | 52.1 | 78.4 | 1200 | 2.98 | 9.0 |
| P6 | 6.8 | 29.2 | 1457 | 27.3 | 953 | 45.8 | 76.6 | 3950 | 3.52 | 12.3 |
| P7 | 6.61 | 30 | 37.3 | 42.2 | 19 | 70.9 | 74.7 | 245 | 4.07 | 37.9 |
| P8 | 8.06 | 28.1 | 28.2 | 115 | 6 | 187.2 | 76.9 | 78 | 1.89 | 34.5 |
| P9 | 7.18 | 26.5 | 339 | 96.2 | 201 | 152.3 | 56.5 | 650 | 1.74 | 23.4 |
| P10 | 7.37 | 25.1 | 5026 | 57.85 | 3200 | 88.6 | 54.5 | 8500 | 2.27 | 37.4 |
| P11 | 7.79 | 26.7 | 1690 | 79.95 | 1024 | 127.3 | 50.8 | 5250 | 2.39 | 30.6 |
| CCME | 6.5–8.5 | 22.5–27.5 | - | 500 | - | 500 | >80 | - | - | - |
| TULSMA | 6.5–9.0 | 22.0–28.0 | 10 | 1000 | 130 | 1000 | >80 | - | - | - |
| US EPA | 6.5–9.0 | 22.0–28.0 | - | 500 | - | 500 | >80 | - | - | - |
| Sites | Physicochemical Parameters | Metal Concentrations | Toxicity (Bioassays) | Macroinvertebrates (AAMBI) | Total |
|---|---|---|---|---|---|
| P7 | 4.01 | 4.12 | 3.84 | 1.8 | 13.77 |
| P2 | 4.25 | 4.08 | 3.09 | 1.48 | 12.90 |
| P8 | 4.03 | 4.06 | 3.55 | 1.04 | 12.68 |
| P4 | 4.38 | 4.56 | 2.5 | 0.76 | 12.20 |
| P9 | 3.84 | 4.09 | 3.79 | 0.16 | 11.88 |
| P1 | 3.84 | 4.17 | 3.06 | 0.36 | 11.43 |
| P3 | 3.71 | 4.18 | 2.49 | 0.6 | 10.98 |
| P5 | 3.87 | 4.15 | 2.43 | 0.44 | 10.89 |
| P11 | 3.82 | 4.29 | 2.57 | 0 | 10.68 |
| P6 | 3.45 | 3.74 | 3.15 | 0 | 10.34 |
| P10 | 3.67 | 3.96 | 2.35 | 0.28 | 10.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
V. Capparelli, M.; Cabrera, M.; Rico, A.; Lucas-Solis, O.; Alvear-S, D.; Vasco, S.; Galarza, E.; Shiguango, L.; Pinos-Velez, V.; Pérez-González, A.; et al. An Integrative Approach to Assess the Environmental Impacts of Gold Mining Contamination in the Amazon. Toxics 2021, 9, 149. https://doi.org/10.3390/toxics9070149
V. Capparelli M, Cabrera M, Rico A, Lucas-Solis O, Alvear-S D, Vasco S, Galarza E, Shiguango L, Pinos-Velez V, Pérez-González A, et al. An Integrative Approach to Assess the Environmental Impacts of Gold Mining Contamination in the Amazon. Toxics. 2021; 9(7):149. https://doi.org/10.3390/toxics9070149
Chicago/Turabian StyleV. Capparelli, Mariana, Marcela Cabrera, Andreu Rico, Oscar Lucas-Solis, Daniela Alvear-S, Samantha Vasco, Emily Galarza, Lady Shiguango, Veronica Pinos-Velez, Andrés Pérez-González, and et al. 2021. "An Integrative Approach to Assess the Environmental Impacts of Gold Mining Contamination in the Amazon" Toxics 9, no. 7: 149. https://doi.org/10.3390/toxics9070149