Temperature and Aging Affect Glyphosate Toxicity and Fatty Acid Composition in Allonychiurus kimi (Lee) (Collembola)
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Animals
2.2. Chemical and Toxicity Test
2.3. Fatty Acid Analysis
2.4. Glyphosate Degradation in Soil
2.5. Data Analysis
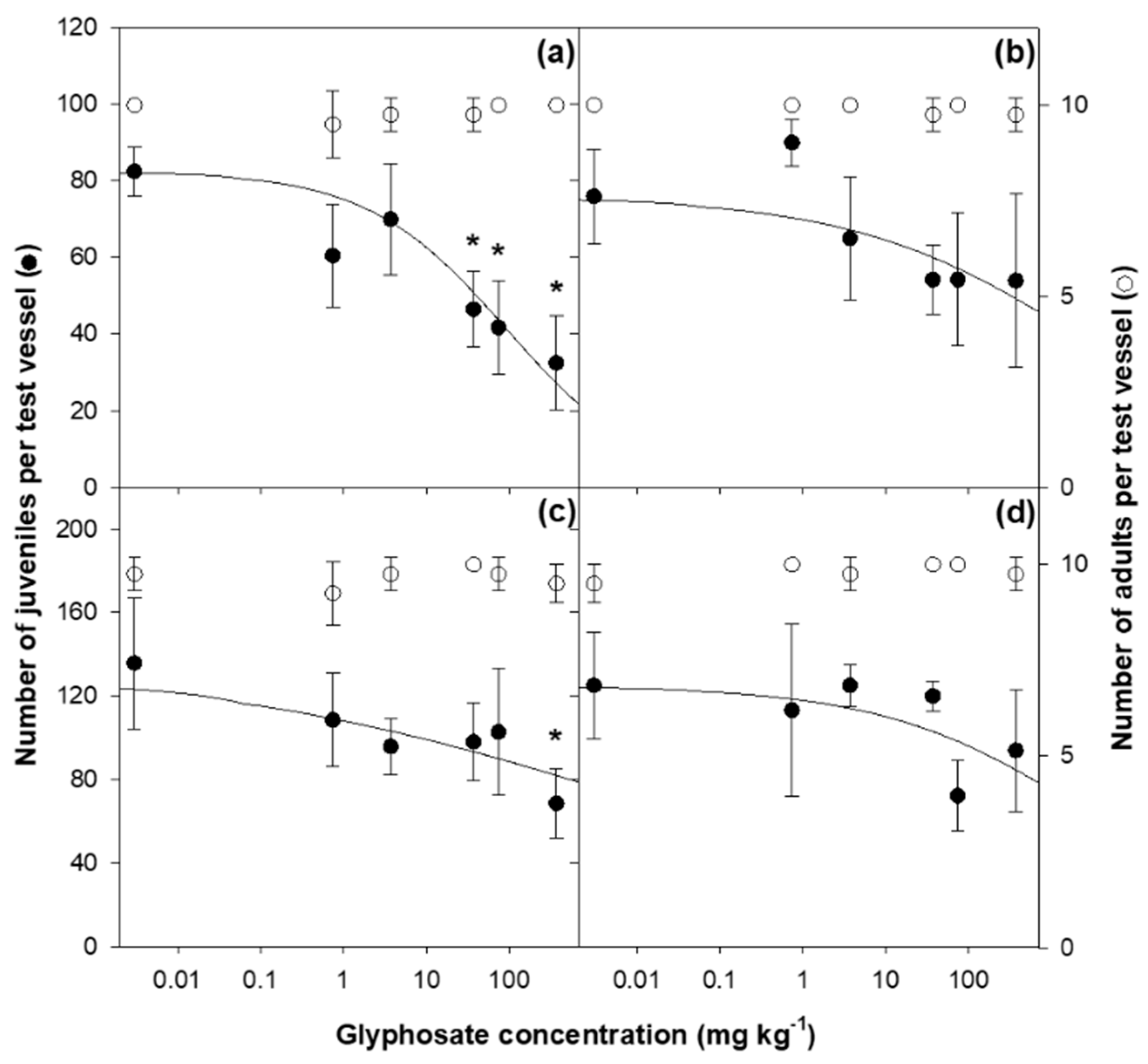
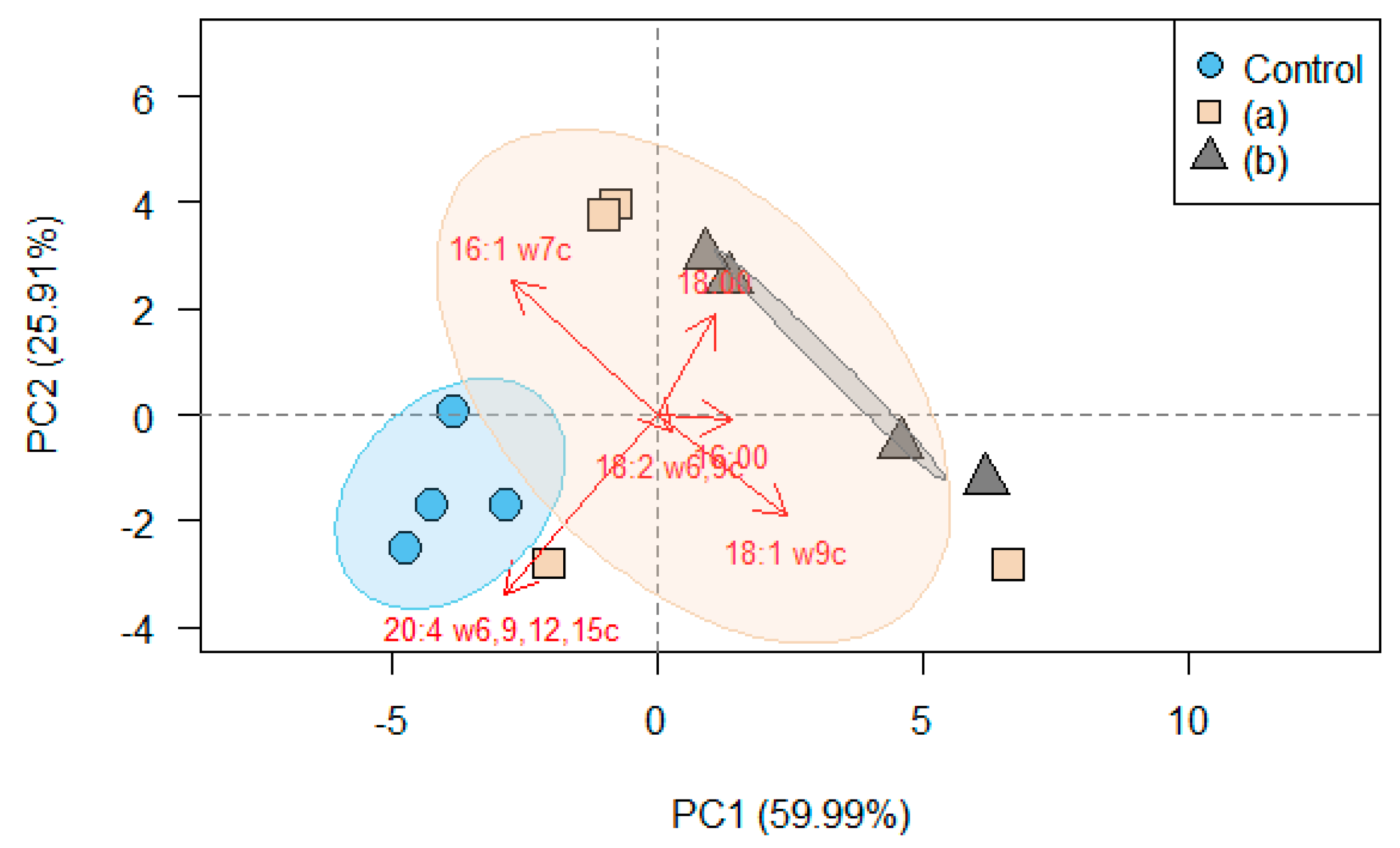
3. Results
3.1. Toxicity Test
3.2. Fatty Acid Composition
3.3. Glyphosate Degradation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duke, S.O. The history and current status of glyphosate. Pest Manag. Sci. 2018, 74, 1027–1034. [Google Scholar] [CrossRef]
- Giesy, J.P.; Dobson, S.; Solomon, K.R. Ecotoxicological risk assessment for Roundup® herbicide. Rev. Environ. Contam. Toxicol. 2000, 167, 35–120. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Gimsing, A.L. Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: A review. Pest Manag. Sci. 2008, 64, 441–456. [Google Scholar] [CrossRef]
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 1–15. [Google Scholar] [CrossRef]
- Lane, M.; Lorenz, N.; Saxena, J.; Ramsier, C.; Dick, R.P. The effect of glyphosate on soil microbial activity, microbial community structure, and soil potassium. Pedobiologia 2012, 55, 335–342. [Google Scholar] [CrossRef]
- Silva, V.; Montanarella, L.; Jones, A.; Fernández-Ugalde, O.; Mol, H.G.J.; Ritsema, C.J.; Geissen, V. Distribution of glyphosate and aminomethylphosphonic acid (AMPA) in agricultural topsoils of the European Union. Sci. Total Environ. 2018, 621, 1352–1359. [Google Scholar] [CrossRef]
- Primost, J.E.; Marino, D.J.G.; Aparicio, V.C.; Costa, J.L.; Carriquiriborde, P. Glyphosate and AMPA, “pseudo-persistent” pollutants under real-world agricultural management practices in the Mesopotamic Pampas agroecosystem, Argentina. Environ. Pollut. 2017, 229, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O.; Lydon, J.; Koskinen, W.C.; Moorman, T.B.; Chaney, R.L.; Hammerschmidt, R. Glyphosate effects on plant mineral nutrition, crop rhizosphere microbiota, and plant disease in glyphosate-resistant crops. J. Agric. Food Chem. 2012, 60, 10375–10397. [Google Scholar] [CrossRef]
- Grube, M.; Kalnenieks, U.; Muter, O. Metabolic response of bacteria to elevated concentrations of glyphosate-based herbicide. Ecotoxicol. Environ. Saf. 2019, 173, 373–380. [Google Scholar] [CrossRef]
- Nguyen, D.B.; Rose, M.T.; Rose, T.J.; Morris, S.G.; van Zwieten, L. Impact of glyphosate on soil microbial biomass and respiration: A meta-analysis. Soil Biol. Biochem. 2016, 92, 50–57. [Google Scholar] [CrossRef]
- Hagner, M.; Mikola, J.; Saloniemi, I.; Saikkonen, K.; Helander, M. Effects of a glyphosate-based herbicide on soil animal trophic groups and associated ecosystem functioning in a northern agricultural field. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.J.G.; Soares, A.M.V.M.; Loureiro, S. Joint effects of three plant protection products to the terrestrial isopod Porcellionides pruinosus and the collembolan Folsomia candida. Chemosphere 2010, 80, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Simões, T.; Novais, S.C.; Natal-da-Luz, T.; Leston, S.; Rosa, J.; Ramos, F.; Pouca, A.S.V.; Freitas, A.; Barbosa, J.; Roelofs, D.; et al. Fate and effects of two pesticide formulations in the invertebrate Folsomia candida using a natural agricultural soil. Sci. Total Environ. 2019, 675, 90–97. [Google Scholar] [CrossRef]
- von Mérey, G.; Manson, P.S.; Mehrsheikh, A.; Sutton, P.; Levine, S.L. Glyphosate and aminomethylphosphonic acid chronic risk assessment for soil biota. Environ. Toxicol. Chem. 2016, 35, 2742–2752. [Google Scholar] [CrossRef] [PubMed]
- Gevao, B.; Semple, K.T.; Jones, K.C. Bound pesticide residues in soils: A review. Environ. Pollut. 2000, 108, 3–14. [Google Scholar] [CrossRef]
- Alexander, M. Critical Review Aging, Bioavailability, and Overestimation of Risk from Environmental Pollutants. Environ. Sci. Technol. 2000, 34, 4259–4265. [Google Scholar] [CrossRef]
- Reedich, L.M.; Millican, M.D.; Koch, P.L. Temperature Impacts on Soil Microbial Communities and Potential Implications for the Biodegradation of Turfgrass Pesticides. J. Environ. Qual. 2017, 46, 490–497. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Fang, W.; Yan, D.; Han, D.; Huang, B.; Zhang, Y.; Li, Y.; Ouyang, C.; Cao, A.; et al. Soil properties, presence of microorganisms, application dose, soil moisture and temperature influence the degradation rate of Allyl isothiocyanate in soil. Chemosphere 2020, 244, 125540. [Google Scholar] [CrossRef]
- Jegede, O.O.; Owojori, O.J.; Römbke, J. Temperature influences the toxicity of deltamethrin, chlorpyrifos and dimethoate to the predatory mite Hypoaspis aculeifer (Acari) and the springtail Folsomia candida (Collembola). Ecotoxicol. Environ. Saf. 2017, 140, 214–221. [Google Scholar] [CrossRef]
- Garba, J.; Samsuri, A.W.; Othman, R.; Ahmad Hamdani, M.S. Adsorption-desorption and leaching potential of glyphosate and aminomethylphosphonic acid in acidic Malaysian soil amended with cow dung and rice husk ash. Environ. Monit. Assess. 2018, 190, 1–15. [Google Scholar] [CrossRef]
- Kumari, K.G.I.D.; Moldrup, P.; Paradelo, M.; Elsgaard, L.; de Jonge, L.W. Soil Properties Control Glyphosate Sorption in Soils Amended with Birch Wood Biochar. Water. Air. Soil Pollut. 2016, 227, 1–12. [Google Scholar] [CrossRef]
- OECD. Test No. 232: Collembolan Reproduction Test in Soil. Organisation for Economic Co-operation and Development (OECD); OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2016; ISBN 9789264264601. [Google Scholar]
- Son, J.; Ryoo, M.I.; Jung, J.; Cho, K. Effects of cadmium, mercury and lead on the survival and instantaneous rate of increase of Paronychiurus kimi (Lee) (Collembola). Appl. Soil Ecol. 2007, 35, 404–411. [Google Scholar] [CrossRef]
- Van Gestel, C.A.M.; Borgman, E.; Verweij, R.A.; Diez Ortiz, M. The influence of soil properties on the toxicity of molybdenum to three species of soil invertebrates. Ecotoxicol. Environ. Saf. 2011, 74, 1–9. [Google Scholar] [CrossRef]
- Connon, R.E.; Geist, J.; Pfeiff, J.; Loguinov, A.V.; D’Abronzo, L.S.; Wintz, H.; Vulpe, C.D.; Werner, I. Linking mechanistic and behavioral responses to sublethal esfenvalerate exposure in the endangered delta smelt; Hypomesus transpacificus (Fam. Osmeridae). BMC Genom. 2009, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hook, S.E.; Gallagher, E.P.; Batley, G.E. The role of biomarkers in the assessment of aquatic ecosystem health. Integr. Environ. Assess. Manag. 2014, 10, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, B.; Maria, V.L.; Römbke, J.; Amorim, M.J.B. Multigenerational exposure of Folsomia candida to ivermectin—Using avoidance, survival, reproduction, size and cellular markers as endpoints. Geoderma 2019, 337, 273–279. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, S.E.; Son, J.; Kim, Y.; Wee, J.; Cho, K. Toxicity effects and biomarkers of tebufenozide exposure in Yuukianura szeptyckii (Collembola: Neanuridae). Environ. Geochem. Health 2018, 40, 2773–2784. [Google Scholar] [CrossRef]
- Nakamori, T.; Fujimori, A.; Kinoshita, K.; Ban-nai, T.; Kubota, Y.; Yoshida, S. mRNA expression of a cadmium-responsive gene is a sensitive biomarker of cadmium exposure in the soil collembolan Folsomia candida. Environ. Pollut. 2010, 158, 1689–1695. [Google Scholar] [CrossRef]
- Stanley-Samuelson, D.W.; Jurenka, R.A.; Cripps, C.; Blomquist, G.J.; de Renobales, M. Fatty acids in insects: Composition, metabolism, and biological significance. Arch. Insect Biochem. Physiol. 1988, 9, 1–33. [Google Scholar] [CrossRef]
- Wee, J.; Lee, Y.; Son, J.; Ko, E.; Cho, K. Effects of four substances requiring preparation for accidents on the survival and reproduction of Paronychiurus kimi (Collembola: Onychiuridae). Korean J. Environ. Biol. 2019, 37, 749–758. [Google Scholar] [CrossRef]
- Choi, W.; Ryoo, M.; Kim, J.G. Biology of Paronychiurus kimi (Collembola: Onychiuridae) under the influence of temperature, humidity and nutrition. Pedobiologia 2002, 46, 548–557. [Google Scholar] [CrossRef]
- Son, J.; Lee, S.E.; Park, B.S.; Jung, J.; Park, H.S.; Bang, J.Y.; Kang, G.Y.; Cho, K. Biomarker discovery and proteomic evaluation of cadmium toxicity on a collembolan species, Paronychiurus kimi (Lee). Proteomics 2011, 11, 2294–2307. [Google Scholar] [CrossRef]
- Chamberlain, P.M.; Bull, I.D.; Black, H.I.J.; Ineson, P.; Evershed, R.P. Fatty acid composition and change in Collembola fed differing diets: Identification of trophic biomarkers. Soil Biol. Biochem. 2005, 37, 1608–1624. [Google Scholar] [CrossRef]
- Haubert, D.; Häggblom, M.M.; Scheu, S.; Ruess, L. Effects of fungal food quality and starvation on the fatty acid composition of Protaphorura fimata (Collembola). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 138, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Çetin, E.; Şahan, S.; Ülgen, A.; Şahin, U. DLLME-spectrophotometric determination of glyphosate residue in legumes. Food Chem. 2017, 230, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Jan, M.R.; Shah, J.; Muhammad, M.; Ara, B. Glyphosate herbicide residue determination in samples of environmental importance using spectrophotometric method. J. Hazard. Mater. 2009, 169, 742–745. [Google Scholar] [CrossRef] [PubMed]
- FOCUS. Guidance Document on Estimating Persistence and Degradation Kinetics from Environmental Fate Studies on Pesticides in EU Registration. Report of the FOCUS Work Group on Degradation Kinetics, EC Document Reference Sanco/10058/2005 Version 2.0. 2006, p. 434. Available online: https://esdac.jrc.ec.europa.eu/public_path/projects_data/focus/dk/docs/finalreportFOCDegKinetics.pdf (accessed on 30 May 2021).
- Haanstra, L.; Doelman, P.; Voshaar, J.H.O. The use of sigmoidal dose response curves in soil ecotoxicological research. Plant Soil 1985, 84, 293–297. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.4-2. 2015. Available online: http://CRAN.R-project.org/package=vegan (accessed on 30 May 2021).
- R Studio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2016. Available online: http://www.rstudio.com (accessed on 30 May 2021).
- Daam, M.A.; Garcia, M.V.; Scheffczyk, A.; Römbke, J. Acute and chronic toxicity of the fungicide carbendazim to the earthworm Eisenia fetida under tropical versus temperate laboratory conditions. Chemosphere 2020, 255, 126871. [Google Scholar] [CrossRef]
- Wu, J.; Nofziger, D.L. Incorporating Temperature Effects on Pesticide Degradation into a Management Model. J. Environ. Qual. 1999, 28, 92–100. [Google Scholar] [CrossRef]
- León Paumen, M.; de Voogt, P.; van Gestel, C.A.M.; Kraak, M.H.S. Comparative chronic toxicity of homo- and heterocyclic aromatic compounds to benthic and terrestrial invertebrates: Generalizations and exceptions. Sci. Total Environ. 2009, 407, 4605–4609. [Google Scholar] [CrossRef]
- Domínguez, A.; Brown, G.G.; Sautter, K.D.; De Oliveira, C.M.R.; De Vasconcelos, E.C.; Niva, C.C.; Bartz, M.L.C.; Bedano, J.C. Toxicity of AMPA to the earthworm Eisenia andrei Bouché, 1972 in tropical artificial soil. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Sullivan, D.S. Vegetation management and ecosystem disturbance: Impact of glyphosate herbicide on plant and animal diversity in terrestrial systems. Environ. Rev. 2003, 11, 37–59. [Google Scholar] [CrossRef]
- Ahmad, R.; Kookana, R.S.; Megharaj, M.; Alston, A.M. Aging reduces the bioavailability of even a weakly sorbed pesticide (carbaryl) in soil. Environ. Toxicol. Chem. 2004, 23, 2084. [Google Scholar] [CrossRef] [PubMed]
- Robertson, B.K.; Alexander, M. Sequestration of DDT and dieldrin in soil: Disappearance of acute toxicity but not the compounds. Environ. Toxicol. Chem. 1998, 17, 1034–1038. [Google Scholar] [CrossRef]
- Li, H.; Sun, Z.; Qiu, Y.; Yu, X.; Han, X.; Ma, Y. Integrating bioavailability and soil aging in the derivation of DDT criteria for agricultural soils using crop species sensitivity distributions. Ecotoxicol. Environ. Saf. 2018, 165, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Zelles, L.; Adrian, P.; Bai, Q.Y.; Stepper, K.; Adrian, M.V.; Fischer, K.; Maier, A.; Ziegler, A. Microbial activity measured in soils stored under different temperature and humidity conditions. Soil Biol. Biochem. 1991, 23, 955–962. [Google Scholar] [CrossRef]
- Bento, C.P.M.; Yang, X.; Gort, G.; Xue, S.; van Dam, R.; Zomer, P.; Mol, H.G.J.; Ritsema, C.J.; Geissen, V. Persistence of glyphosate and aminomethylphosphonic acid in loess soil under different combinations of temperature, soil moisture and light/darkness. Sci. Total Environ. 2016, 572, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Hofman, J.; Hovorková, I.; Semple, K.T. The variability of standard artificial soils: Behaviour, extractability and bioavailability of organic pollutants. J. Hazard. Mater. 2014, 264, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Muskus, A.M.; Krauss, M.; Miltner, A.; Hamer, U.; Nowak, K.M. Degradation of glyphosate in a Colombian soil is influenced by temperature, total organic carbon content and pH. Environ. Pollut. 2020, 259, 113767. [Google Scholar] [CrossRef]
- Nguyen, N.K.; Dörfler, U.; Welzl, G.; Munch, J.C.; Schroll, R.; Suhadolc, M. Large variation in glyphosate mineralization in 21 different agricultural soils explained by soil properties. Sci. Total Environ. 2018, 627, 544–552. [Google Scholar] [CrossRef]
- Chamberlain, P.M.; Black, H.I.J. Fatty acid compositions of Collembola: Unusually high proportions of C 20 polyunsaturated fatty acids in a terrestrial invertebrate. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005, 140, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef]
- Stanley, D.; Kim, Y. Prostaglandins and other eicosanoids in insects: Biosynthesis and biological actions. Front. Physiol. 2019, 10, 1927. [Google Scholar] [CrossRef] [PubMed]
- Pandit, P.R.; Fulekar, M.H.; Karuna, M.S.L. Effect of salinity stress on growth, lipid productivity, fatty acid composition, and biodiesel properties in Acutodesmus obliquus and Chlorella vulgaris. Environ. Sci. Pollut. Res. 2017, 24, 13437–13451. [Google Scholar] [CrossRef]
- Paraszkiewicz, K.; Bernat, P.; Długoński, J. Effect of nickel, copper, and zinc on emulsifier production and saturation of cellular fatty acids in the filamentous fungus Curvularia lunata. Int. Biodeterior. Biodegrad. 2009, 63, 100–105. [Google Scholar] [CrossRef]
- Visser, B.; Willett, D.S.; Harvey, J.A.; Alborn, H.T. Concurrence in the ability for lipid synthesis between life stages in insects. R. Soc. Open Sci. 2017, 4, 160815. [Google Scholar] [CrossRef]
- EFSA. Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA J. 2015, 13, 107. [Google Scholar] [CrossRef]
- Karasali, H.; Pavlidis, G.; Marousopoulou, A. Investigation of the presence of glyphosate and its major metabolite AMPA in Greek soils. Environ. Sci. Pollut. Res. 2019, 26, 36308–36321. [Google Scholar] [CrossRef] [PubMed]
| Temperature (°C) | Aging Time (d) | EC50 | NOEC b | LOEC b |
|---|---|---|---|---|
| 20 | 0 | 93.5 (25.9–161.2) | 3.7 | 37.1 |
| 7 | - a | - | - | |
| 25 | 0 | - | 74.1 | 370.5 |
| 7 | - | - | - |
| 20 °C | 25 °C | ||||||
|---|---|---|---|---|---|---|---|
| Glyphosate Concentration (mg kg−1) | Glyphosate Concentration (mg kg−1) | ||||||
| Fatty Acids | 0.0 | 37.1 | 370.5 | 0.0 | 37.1 | 370.5 | |
| Palmitoleic acid | 16:1ω7 | 5.77 ± 1.06 | 5.60 ± 3.68 | 5.48 ± 0.17 | 5.16 ± 0.18 | 6.72 ± 1.34 | 0.00 ± 0.00 |
| Palmitic acid | 16:0 | 21.37 ± 1.15 | 24.31 ± 0.43 | 23.79 ± 0.26 | 22.87 ± 0.71 | 22.84 ± 0.11 | 24.83 ± 1.30 |
| Linoleic acid | 18:2ω6,9 | 14.90 ± 0.95 | 13.41 ± 1.98 | 13.60 ± 0.93 | 12.50 ± 1.07 | 13.03 ± 0.81 | 13.70 ± 0.57 |
| Oleic acid | 18:1ω9 | 39.65 ± 0.07 | 44.63 ± 2.96 | 41.40 ± 1.11 | 40.18 ± 0.86 | 39.54 ± 1.68 | 43.46 ± 1.40 |
| Stearic acid | 18:0 | 12.84 ± 0.39 | 12.54 ± 1.87 | 15.75 ± 0.11 | 13.38 ± 0.17 | 14.99 ± 0.37 | 15.49 ± 0.30 |
| Arachidonic acid | 20:4ω6,9,12,15 | 5.49 ± 1.58 | 2.51 ± 3.55 | 0.00 ± 0.00 | 5.93 ± 0.47 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Total cellular lipids (nmol·ind−1) | 6.90 ± 1.10 | 3.80 ± 1.90 | 3.70 ± 1.20 | 8.20 ± 4.40 | 3.70 ± 0.20 | 3.10 ± 0.60 | |
| C16:C18 a | 0.40 ± 0.04 | 0.39 ± 0.08 | 0.41 ± 0.00 | 0.42 ± 0.01 | 0.44 ± 0.00 | 0.34 ± 0.01 | |
| Unsaturation index b | 0.97 ± 0.07 | 0.84 ± 0.11 | 0.74 ± 0.01 | 0.94 ± 0.03 | 0.72 ± 0.05 | 0.71 ± 0.03 | |
| Fatty Acids | Glyphosate (G) | Temperature (T) | G × T | ||||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| Palmitoleic acid | 16:1ω7 | 3.02 | 0.12 | 0.76 | 0.42 | 8.00 | 0.02 |
| Palmitic acid | 16:0 | 7.91 | 0.02 | 0.64 | 0.45 | 4.05 | 0.08 |
| Linoleic acid | 18:2ω6,9 | 0.21 | 0.82 | 1.78 | 0.23 | 1.35 | 0.33 |
| Oleic acid | 18:1ω9c | 2.90 | 0.13 | 0.81 | 0.40 | 5.53 | 0.04 |
| Stearic acid | 18:0 | 9.41 | 0.01 | 3.72 | 0.10 | 2.82 | 0.14 |
| Arachidonic acid | 20:4ω6,9,12,15 | 14.14 | 0.01 | 0.78 | 0.41 | 1.05 | 0.41 |
| C16:C18 a | 1.15 | 0.38 | 0.00 | 0.98 | 3.03 | 0.12 | |
| Unsaturation index | 11.74 | 0.01 | 2.28 | 0.18 | 0.29 | 0.76 | |
| Temperature (°C) | k | DT50 (d) | DT90 (d) | χ2 Error (%) |
|---|---|---|---|---|
| 20 | 0.291 | 2.38 (1.74–3.02) | 7.91 (5.79–10.05) | 9.55 |
| 25 | 0.409 | 1.69 (1.27–2.11) | 5.63 (4.23–7.01) | 14.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wee, J.; Lee, Y.-S.; Kim, Y.; Son, J.; Cho, K. Temperature and Aging Affect Glyphosate Toxicity and Fatty Acid Composition in Allonychiurus kimi (Lee) (Collembola). Toxics 2021, 9, 126. https://doi.org/10.3390/toxics9060126
Wee J, Lee Y-S, Kim Y, Son J, Cho K. Temperature and Aging Affect Glyphosate Toxicity and Fatty Acid Composition in Allonychiurus kimi (Lee) (Collembola). Toxics. 2021; 9(6):126. https://doi.org/10.3390/toxics9060126
Chicago/Turabian StyleWee, June, Yun-Sik Lee, Yongeun Kim, Jino Son, and Kijong Cho. 2021. "Temperature and Aging Affect Glyphosate Toxicity and Fatty Acid Composition in Allonychiurus kimi (Lee) (Collembola)" Toxics 9, no. 6: 126. https://doi.org/10.3390/toxics9060126
APA StyleWee, J., Lee, Y.-S., Kim, Y., Son, J., & Cho, K. (2021). Temperature and Aging Affect Glyphosate Toxicity and Fatty Acid Composition in Allonychiurus kimi (Lee) (Collembola). Toxics, 9(6), 126. https://doi.org/10.3390/toxics9060126






